Abstract
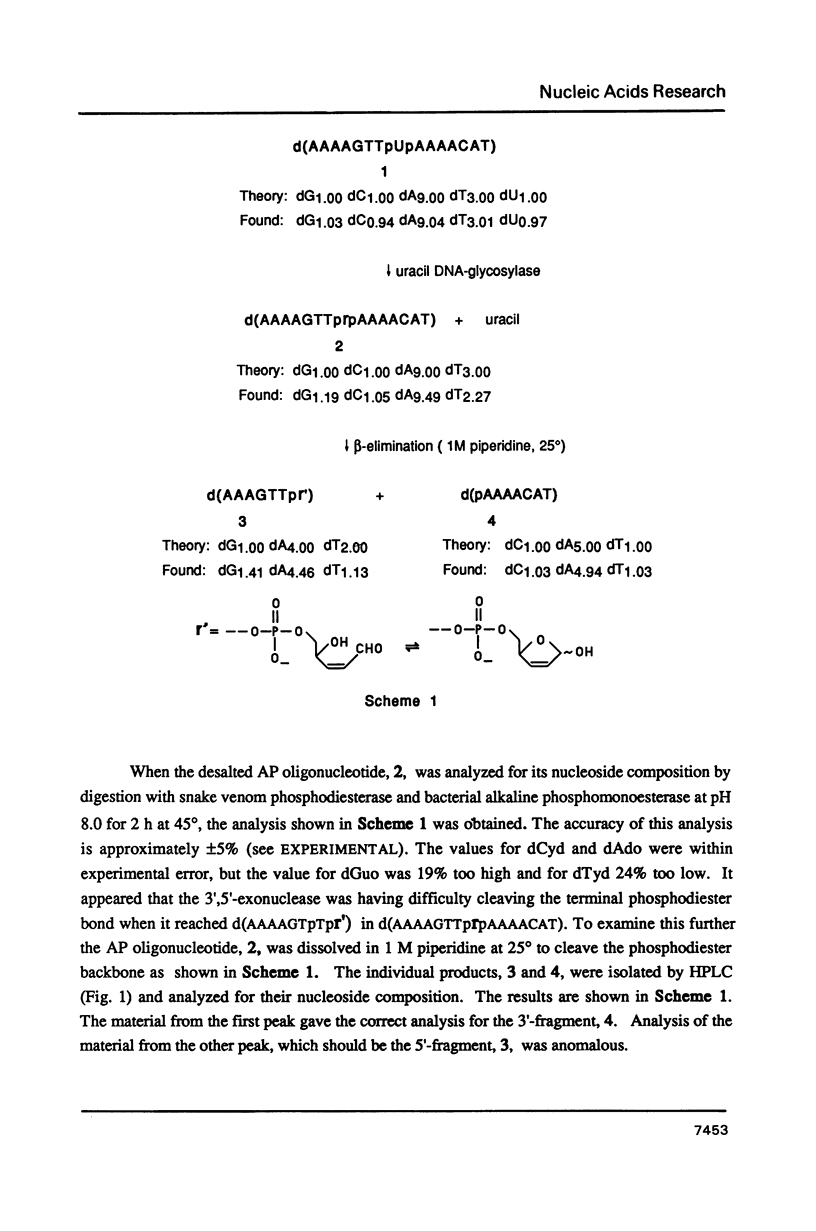
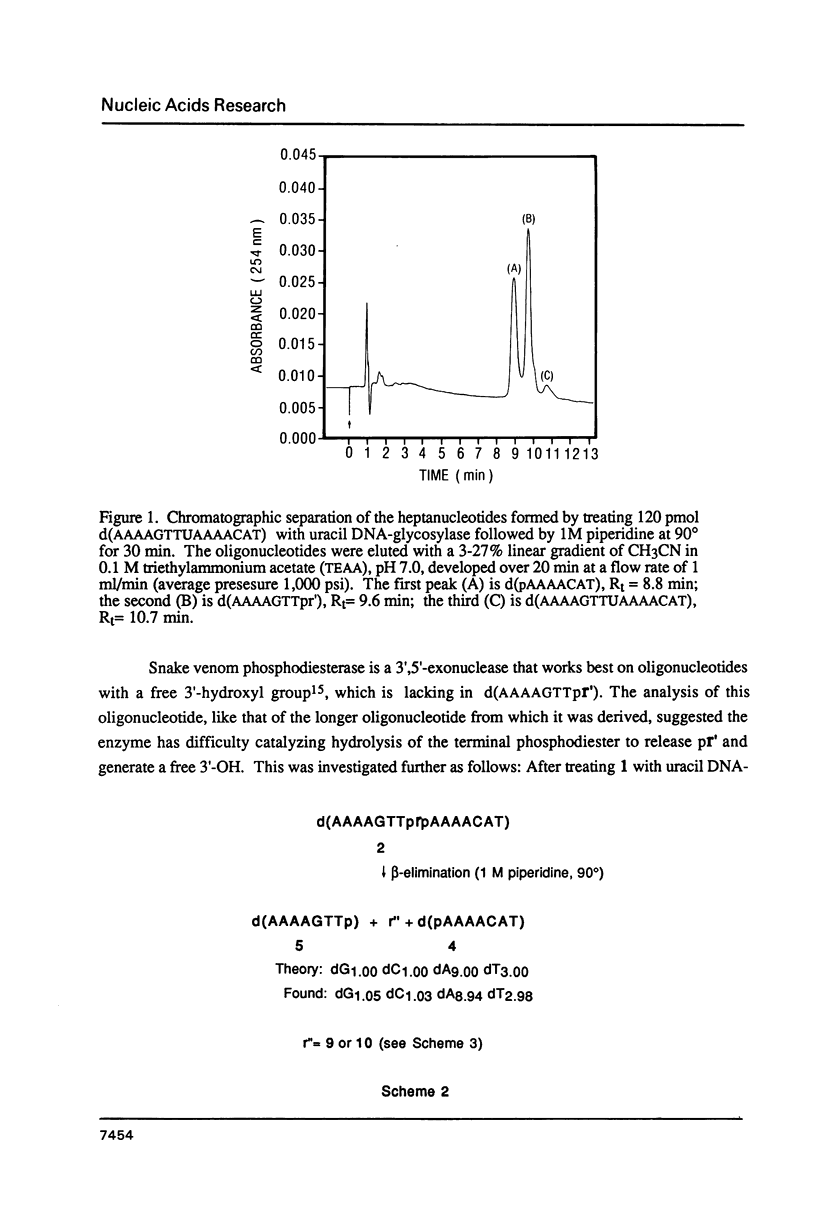
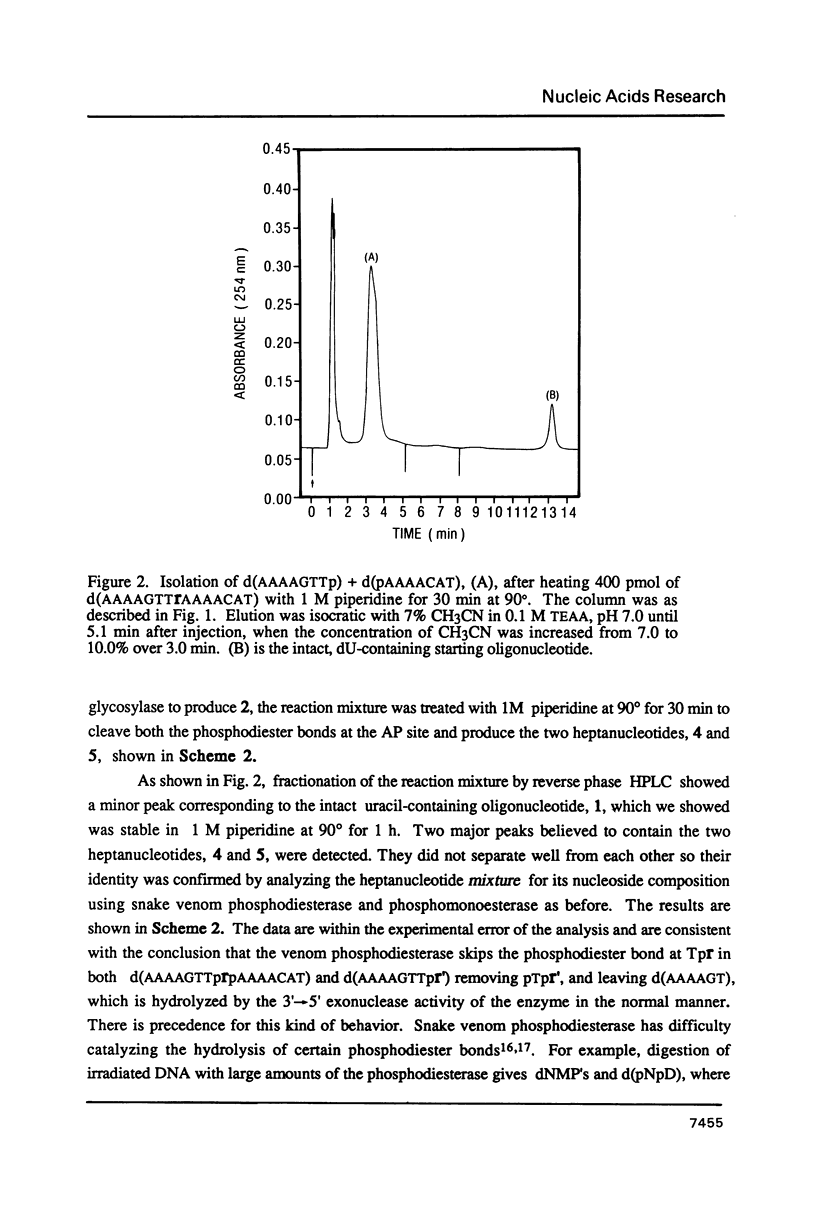
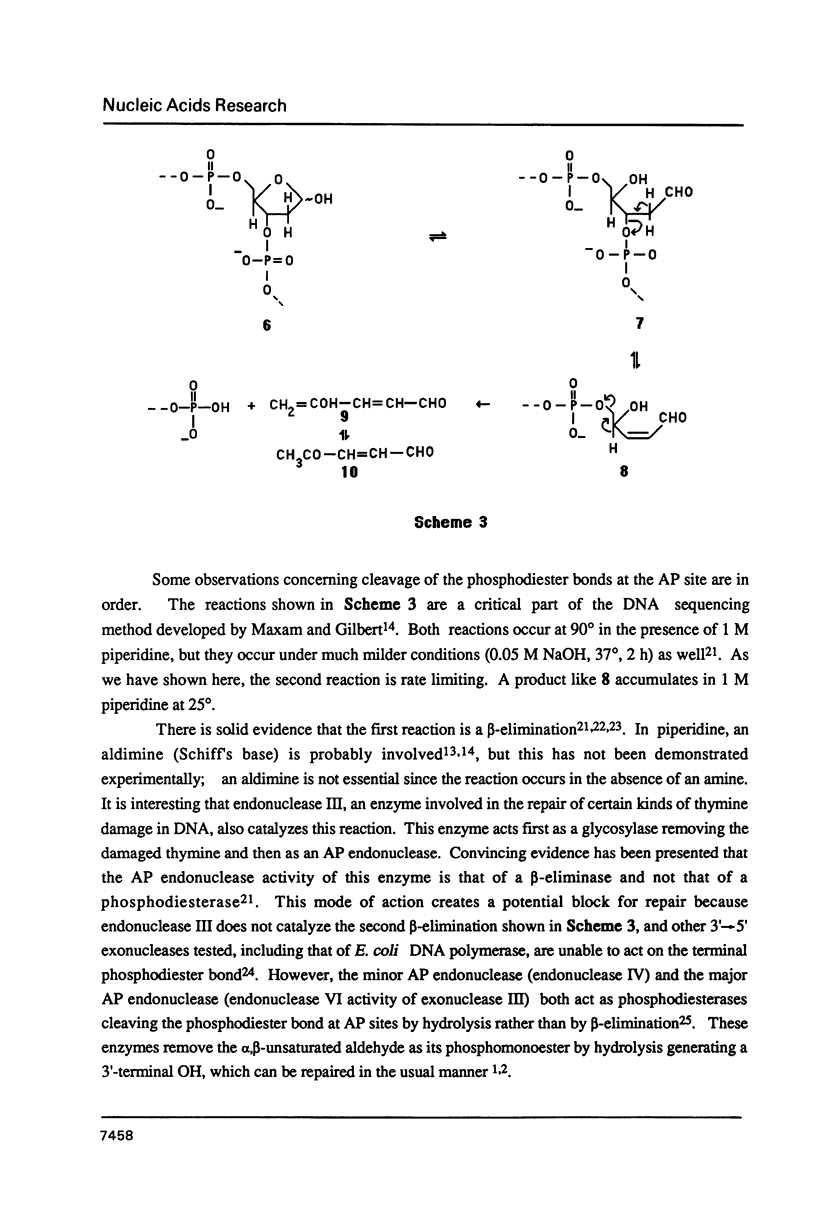
A general synthesis of a deoxyoligonucleotide with an AP site at a preselected sequence is described. Deoxyuridine is introduced during routine oligonucleotide syntheses of d(TTTUTTTT) and d(AAAAGTTUAAAACAT). Treatment with uracil DNA-glycosylase produces d(TTTrTTTT), where r = deoxyribose, and d(AAAAGTTprpAAAACAT). KM and Vmax are: d(TTTUTTTT), 7.3 X 10(-9)M and 2.0 X 10(-9) mumol/min; d(AAAAGTTUAAAACAT), 1.5 X 10(-8) M and 6.4 X 10(-9) mumol/min. Both d(AAAAGTTprpAAAACAT) and d(TTTprpTTTT) undergo rapid beta-elimination in 1 M piperidine at 25 degrees giving two oligonucleotide fragments, d(AAAAGTTpr') and d(pAAAACAT), where r' = -O-CH2-CHOH-CH=CH-CHO (or its hemiacetal form). The fragment, d(AAAAGTTpr'), which can be isolated by reverse phase chromatography, is resistant to the 3'----5' exonuclease activity of snake venom phosphodiesterase. Endonucleolytic hydrolysis of the penultimate phosphodiester occurs removing pTpr' and generating a normal 3'-OH end. In 1 M piperidine at 90 degrees two beta-eliminations occur producing the oligonucleotides d(AAAAGTTp) and d(pAAAACAT) from d(AAAAGTTprpAAAACAT); d(TTTp) and d(pTTTT) from d(TTTprpTTTT).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly V., Verly W. G. Escherichia coli endonuclease III is not an endonuclease but a beta-elimination catalyst. Biochem J. 1987 Mar 1;242(2):565–572. doi: 10.1042/bj2420565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot O. S., Khan S. A., Chambers R. W. A new system for studying molecular mechanisms of mutation by carcinogens. J Biol Chem. 1979 Dec 25;254(24):12684–12693. [PubMed] [Google Scholar]
- Borowy-Borowski H., Chambers R. W. A study of side reactions occurring during synthesis of oligodeoxynucleotides containing O6-alkyldeoxyguanosine residues at preselected sites. Biochemistry. 1987 May 5;26(9):2465–2471. doi: 10.1021/bi00383a010. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Sledziewska-Gojska E., Hirani-Hojatti S., Borowy-Borowski H. uvrA and recA mutations inhibit a site-specific transition produced by a single O6-methylguanine in gene G of bacteriophage phi X174. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7173–7177. doi: 10.1073/pnas.82.21.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delort A. M., Duplaa A. M., Molko D., Teoule R., Leblanc J. P., Laval J. Excision of uracil residues in DNA: mechanism of action of Escherichia coli and Micrococcus luteus uracil-DNA glycosylases. Nucleic Acids Res. 1985 Jan 25;13(2):319–335. doi: 10.1093/nar/13.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard F., Verly W. G. Properties of the main endonuclease specific for apurinic sites of Escherichia coli (endonuclease VI). Mechanism of apurinic site excision from DNA. Eur J Biochem. 1978 Jan 16;82(2):321–332. doi: 10.1111/j.1432-1033.1978.tb12026.x. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Phillips S., Edgell M. H., Gillam S., Jahnke P., Smith M. Mutagenesis at a specific position in a DNA sequence. J Biol Chem. 1978 Sep 25;253(18):6551–6560. [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Loeb L. A. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983 May 10;22(10):2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Loeb L. A. Apurinic sites as mutagenic intermediates. Cell. 1985 Mar;40(3):483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Hasegawa S., Fujimura S., Shima T., Sugimura T. Studies on poly (adenosine diphosphate ribose). V. Mechanism of hydrolysis of poly (adenosine diphosphate ribose) by snake venom phosphodiesterase. J Biol Chem. 1970 Jul 25;245(14):3606–3611. [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. Mechanism of DNA strand breakage by piperidine at sites of N7-alkylguanines. Biochim Biophys Acta. 1986 Oct 16;868(1):71–76. doi: 10.1016/0167-4781(86)90088-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Studies on polynucleotides. IV. Enzymic degradation; the stepwise action of venom phosphodiesterase on deoxyribo-oligonucleotides. J Biol Chem. 1959 Aug;234(8):2114–2117. [PubMed] [Google Scholar]
- Razin A., Hirose T., Itakura K., Riggs A. D. Efficient correction of a mutation by use of chemically synthesized DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4268–4270. doi: 10.1073/pnas.75.9.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L., BOLLUM F. J. NUCLEASE-RESISTANT SEQUENCES IN ULTRAVIOLET-IRRADIATED DEOXYRIBONUCLEIC ACID. Biochim Biophys Acta. 1964 Nov 15;91:446–461. doi: 10.1016/0926-6550(64)90075-1. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Letsinger R. L. Syringe method for stepwise chemical synthesis of oligonucleotides. Nucleic Acids Res. 1982 May 25;10(10):3249–3260. doi: 10.1093/nar/10.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur J. J., Rayner B., Imbach J. L. Preparation of a short synthetic apurinic oligonucleotide. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1204–1208. doi: 10.1016/0006-291x(86)90378-5. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Bos J. L., Marshall C. J., Phillips D. H. Mutations activating human c-Ha-ras1 protooncogene (HRAS1) induced by chemical carcinogens and depurination. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1222–1226. doi: 10.1073/pnas.83.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]