Abstract
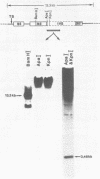
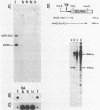
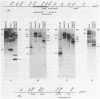
The intervening sequences in the large ribosomal RNA gene of Ascaris lumbricoides var. suum show many similarities to the type I insertions, previously found only in some insect species. They include structural features, but also a presumed transcriptional inactivity in vivo: No transcript of the rDNA intervening sequence in A. lumbricoides could be detected in Northern and dot blot hybridizations. However, the primary structure of the Pol I promoter region is well conserved in interrupted and uninterrupted genes. Moreover, genes with an intervening sequence are correctly initiated in a whole-cell in vitro extract from Ascaris oogonia. Hence, the presence of the intervening sequence alone does not seem to account for a transcriptional inhibition in rRNA genes. As with the type I insertions of insect rDNA, some copies of the A. lumbricoides intervening sequence are also present in locations outside the rDNA cluster. About 50% of the extraribosomal copies are found in a repetitive sequence of the genome, and additional copies are inserted in unique sequences. These striking analogies to type I insertions are discussed, and lead to the conclusion that the two phenomena are undoubtedly related. This is the first report proving the presence of a type I-like insertion element outside of the class Insecta.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back E., Felder H., Müller F., Tobler H. Chromosomal arrangement of the two main rDNA size classes of Ascaris lumbricoides. Nucleic Acids Res. 1984 Feb 10;12(3):1333–1347. doi: 10.1093/nar/12.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back E., Müller F., Tobler H. Structural organization of the two main rDNA size classes of Ascaris lumbricoides. Nucleic Acids Res. 1984 Feb 10;12(3):1313–1332. doi: 10.1093/nar/12.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
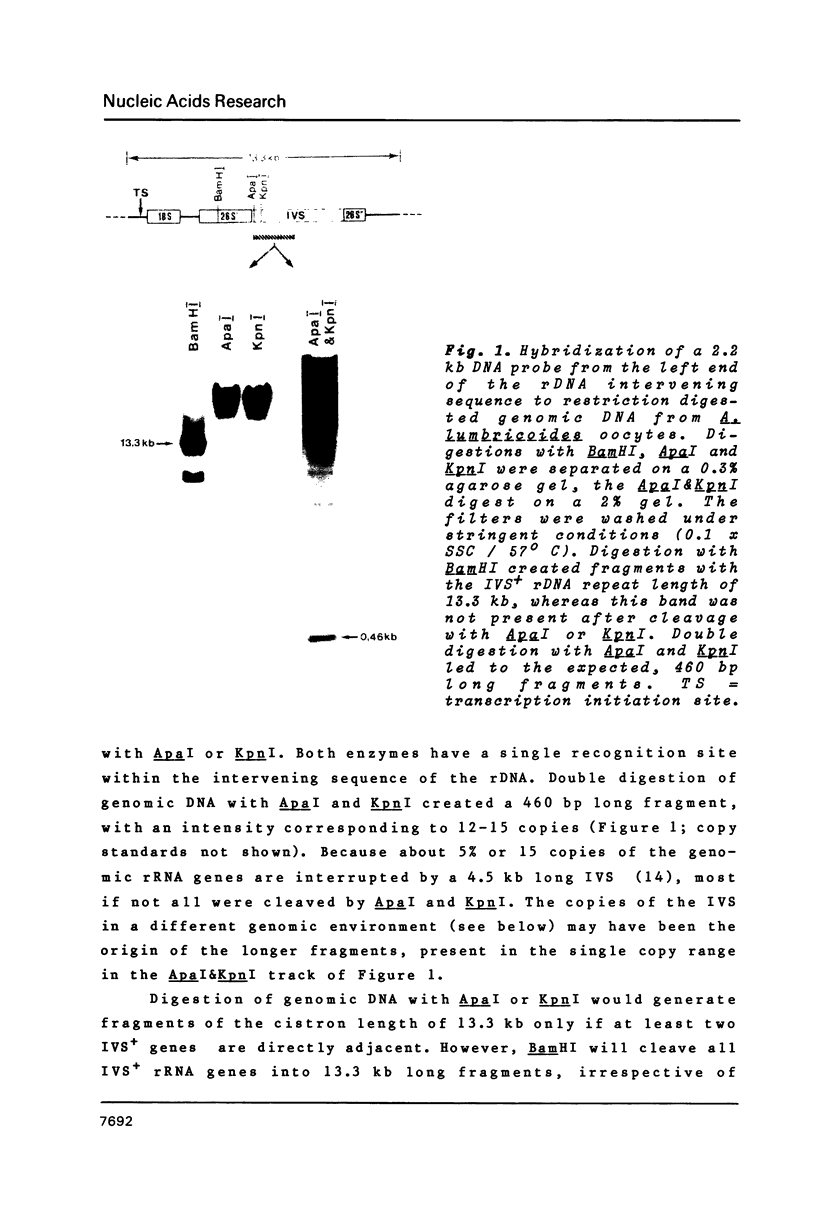
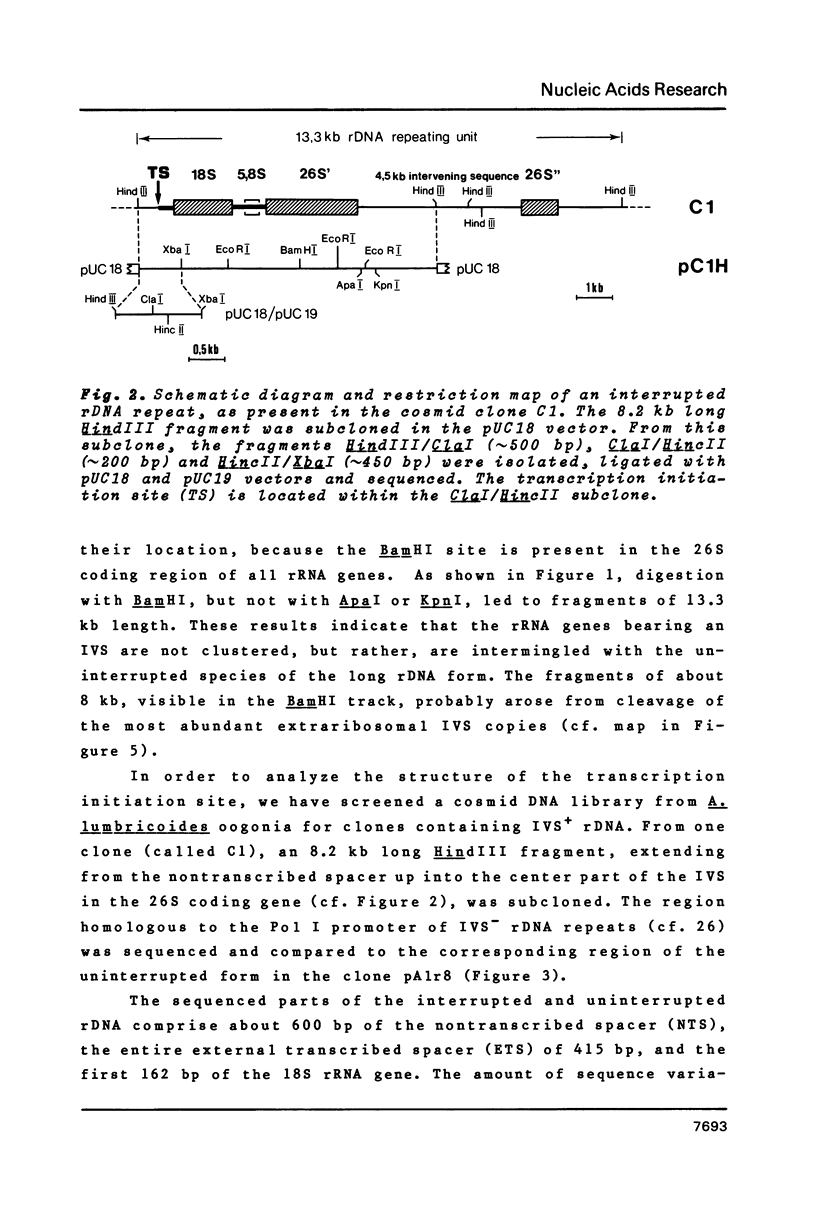
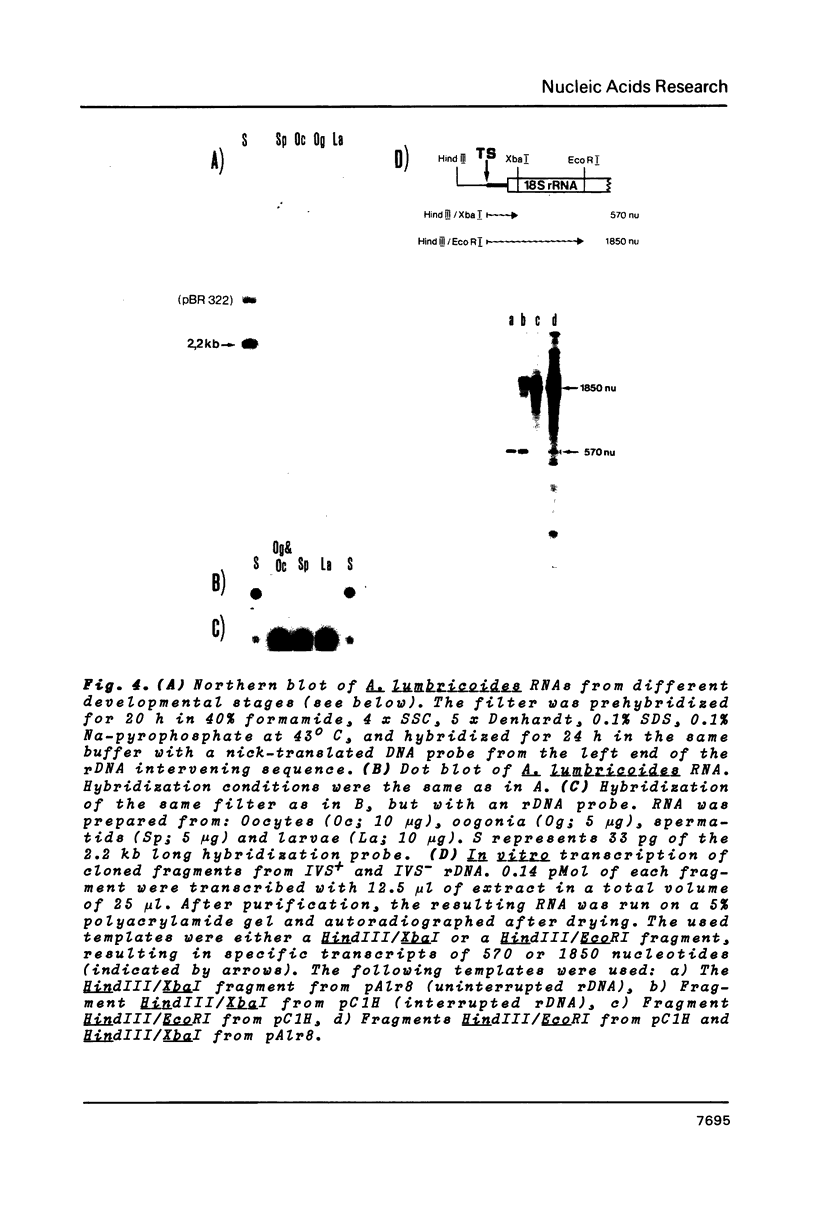
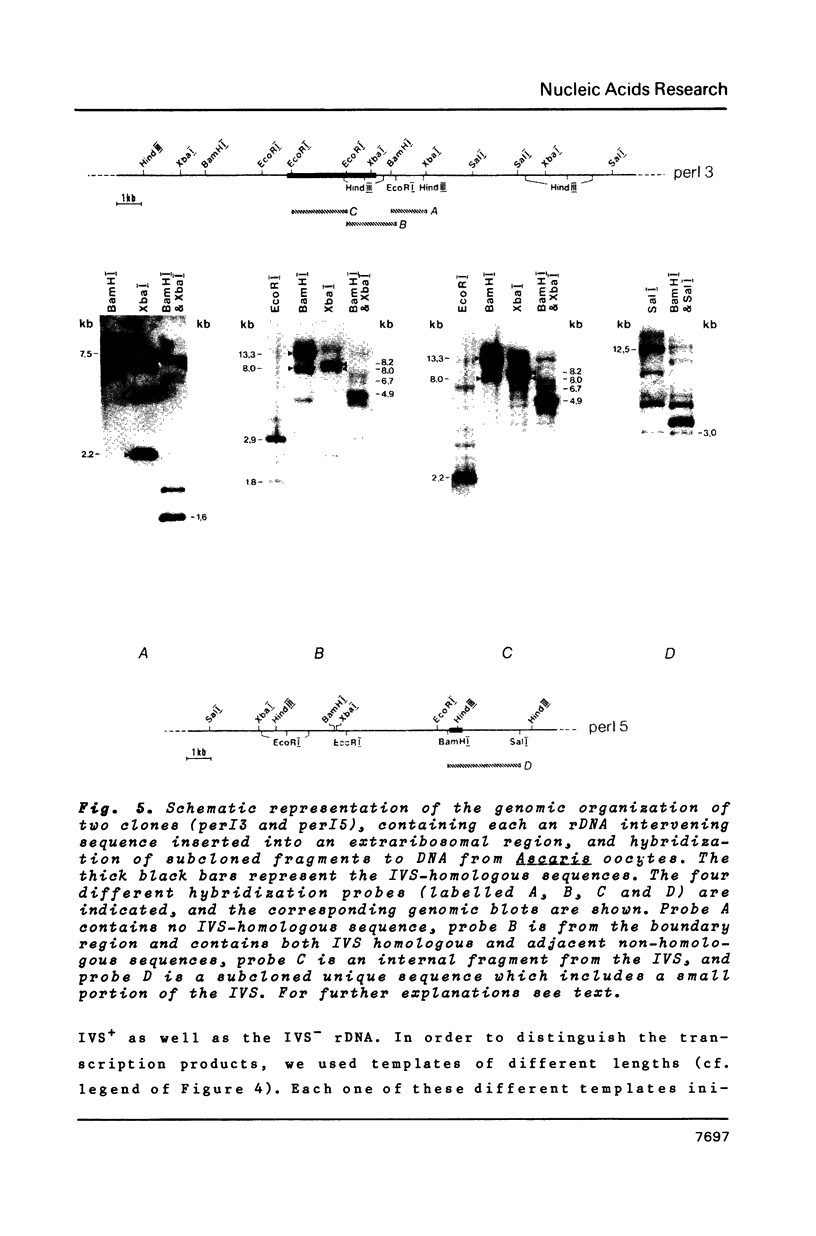
- Back E., Van Meir E., Müller F., Schaller D., Neuhaus H., Aeby P., Tobler H. Intervening sequences in the ribosomal RNA genes of Ascaris lumbricoides: DNA sequences at junctions and genomic organization. EMBO J. 1984 Nov;3(11):2523–2529. doi: 10.1002/j.1460-2075.1984.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckingham K. The ribosomal DNA of Calliphora erythrocephala. The cistron classes of total genomic DNA. J Mol Biol. 1981 Jun 25;149(2):141–169. doi: 10.1016/0022-2836(81)90296-5. [DOI] [PubMed] [Google Scholar]
- Browne M. J., Read C. A., Roiha H., Glover D. M. Site specific insertion of a type I rDNA element into a unique sequence in the Drosophila melanogaster genome. Nucleic Acids Res. 1984 Dec 11;12(23):9111–9122. doi: 10.1093/nar/12.23.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. G., Tague B. W., Ware V. C., Gerbi S. A. Xenopus laevis 28S ribosomal RNA: a secondary structure model and its evolutionary and functional implications. Nucleic Acids Res. 1984 Aug 10;12(15):6197–6220. doi: 10.1093/nar/12.15.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Long E. O., DiNocera P. P., Pardue M. L. Ribosomal insertion-like elements in Drosophila melanogaster are interspersed with mobile sequences. Cell. 1981 Aug;25(2):399–408. doi: 10.1016/0092-8674(81)90058-1. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Rebbert M. L. Nucleotide sequences at the boundaries between gene and insertion regions in the rDNA of Drosophilia melanogaster. Nucleic Acids Res. 1981 Oct 10;9(19):5011–5020. doi: 10.1093/nar/9.19.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Robins B. Bombyx mori 28S ribosomal genes contain insertion elements similar to the Type I and II elements of Drosophila melanogaster. EMBO J. 1985 Sep;4(9):2281–2285. doi: 10.1002/j.1460-2075.1985.tb03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Ogura T., Takada N., Miyajima N., Ishikawa H., Maekawa H. Introns and their flanking sequences of Bombyx mori rDNA. Nucleic Acids Res. 1984 Sep 11;12(17):6861–6869. doi: 10.1093/nar/12.17.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., Hogness D. S. A novel arrangement of the 18S and 28S sequences in a repeating unit of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):167–176. doi: 10.1016/0092-8674(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Miller O. L., Jr The rare transcripts of interrupted rRNA genes in Drosophila melanogaster are processed or degraded during synthesis. EMBO J. 1984 Jul;3(7):1541–1545. doi: 10.1002/j.1460-2075.1984.tb02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Thomas C. A., Jr Nuclear RNA transcripts from Drosophila melanogaster ribosomal RNA genes containing introns. Nucleic Acids Res. 1980 Jan 11;8(1):67–84. doi: 10.1093/nar/8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S. J., Glover D. M. A DNA segment from D. melanogaster which contains five tandemly repeating units homologous to the major rDNA insertion. Cell. 1980 Jan;19(1):103–119. doi: 10.1016/0092-8674(80)90392-x. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Rae P. M. Accurate transcription of truncated ribosomal DNA templates in a Drosophila cell-free system. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1501–1505. doi: 10.1073/pnas.79.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanidou R., Eickbush T. H., Kafatos F. C. Ribosomal DNA genes of Bombyx mori: a minor fraction of the repeating units contain insertions. Nucleic Acids Res. 1984 Jun 11;12(11):4703–4713. doi: 10.1093/nar/12.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell. 1979 Dec;18(4):1185–1196. doi: 10.1016/0092-8674(79)90231-9. [DOI] [PubMed] [Google Scholar]
- Long E. O., Rebbert M. L., Dawid I. B. Nucleotide sequence of the initiation site for ribosomal RNA transcription in Drosophila melanogaster: comparison of genes with and without insertions. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1513–1517. doi: 10.1073/pnas.78.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Walker P., Aeby P., Neuhaus H., Felder H., Back E., Tobler H. Nucleotide sequence of satellite DNA contained in the eliminated genome of Ascaris lumbricoides. Nucleic Acids Res. 1982 Dec 11;10(23):7493–7510. doi: 10.1093/nar/10.23.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Rae P. M. Coding region deletions associated with the major form of rDNA interruption in Drosophila. Nucleic Acids Res. 1981 Oct 10;9(19):4997–5010. doi: 10.1093/nar/9.19.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz-Pohl R., Glätzer K. H., Kunz W. Ribosomal RNA genes with an intervening sequence are clustered within the X chromosomal ribosomal DNA of Drosophila hydei. J Mol Biol. 1981 May 5;148(1):95–101. doi: 10.1016/0022-2836(81)90237-0. [DOI] [PubMed] [Google Scholar]
- Roiha H., Glover D. M. Duplicated rDNA sequences of variable lengths flanking the short type I insertions in the rDNA of Drosophila melanogaster. Nucleic Acids Res. 1981 Nov 11;9(21):5521–5532. doi: 10.1093/nar/9.21.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. L., Beckingham K. The intron boundaries and flanking rRNA coding sequences of Calliphora erythrocephala rDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1707–1724. doi: 10.1093/nar/12.3.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahli W., Germond J. E., ten Heggeler B., May F. E. Vitellogenin genes A1 and B1 are linked in the Xenopus laevis genome. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6832–6836. doi: 10.1073/pnas.79.22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Tartof K. D. X and Y chromosomal ribosomal DNA of Drosophila: comparison of spacers and insertions. Cell. 1978 Jun;14(2):269–278. doi: 10.1016/0092-8674(78)90113-7. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]