Abstract
Plasmids were assembled in which the coding region of the pyruvate kinase (PYK) gene of Saccharomyces cerevisiae was replaced by that of the B-galactosidase (LacZ) gene from Escherichia coli. Analysis of the resultant, chimaeric transcripts from low copy number, centromeric plasmids indicated that this substitution caused a dramatic reduction in the steady-state level of the messenger RNA (mRNA). This fluctuation cannot be wholly accounted for by the 2-fold decrease in mRNA stability observed. This is consistent with the existence of a transcriptional Downstream Activation Site (DAS) within the PYK coding region, analogous to the DAS reported within the yeast phosphoglycerate kinase gene (PGK; Kingsman, S M et al. (1985) Biotech. Gen. Eng. Rev. 3, 377). At these low levels of heterologous gene expression, comparison of the distribution of PYK and PYK/LacZ transcripts across polysome gradients revealed no significant effect mediated by their striking disparity in codon usage. Nevertheless, upon increasing B-galactosidase mRNA levels, via manipulation of plasmid copy number, a distinct decline in ribosome loading was observed for the heterologous PYK/LacZ transcript which was not mirrored by either endogenous PYK transcripts or other yeast mRNAs of high (Ribosomal protein 1) or moderate (Actin) codon bias. However, high levels of the PYK/LacZ mRNA did affect the translation of an endogenous mRNA with poor codon bias (TRP2). The possible basis for this phenomenon is discussed.
Full text
PDF



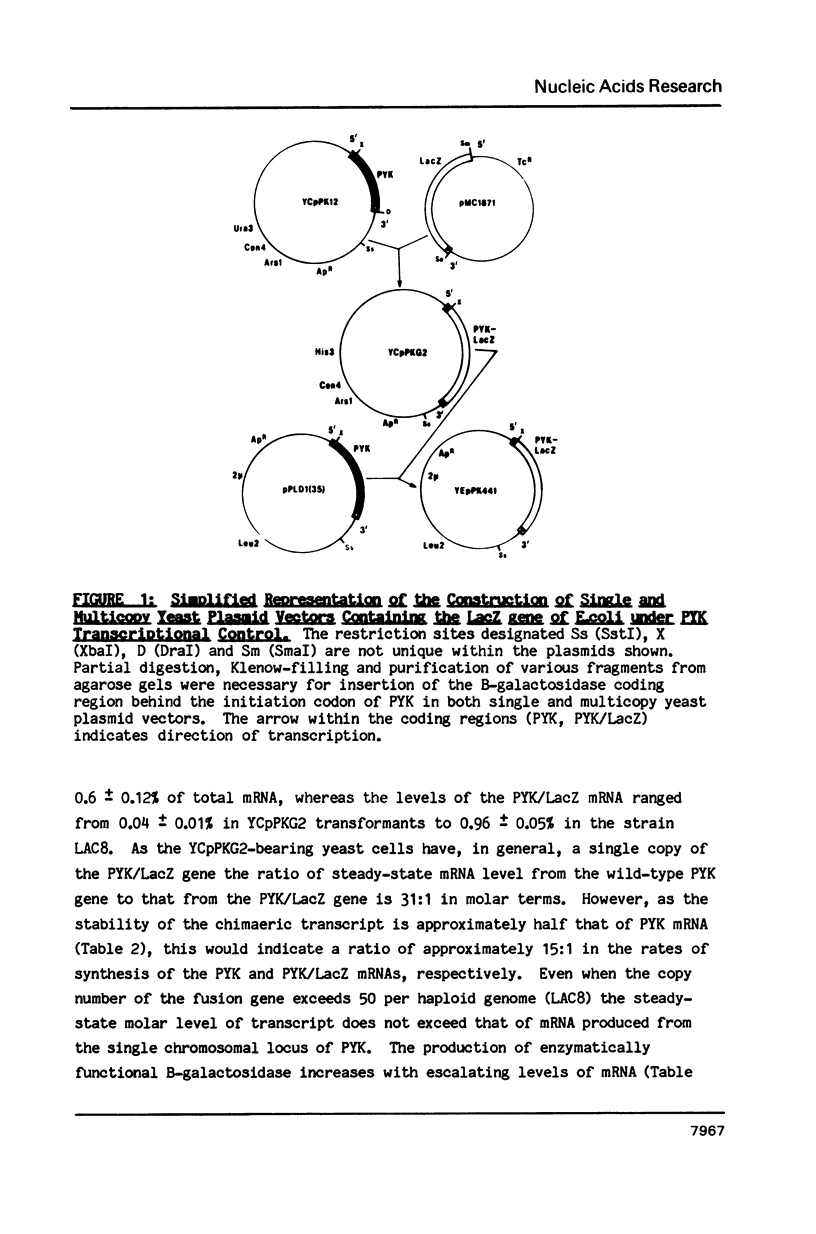
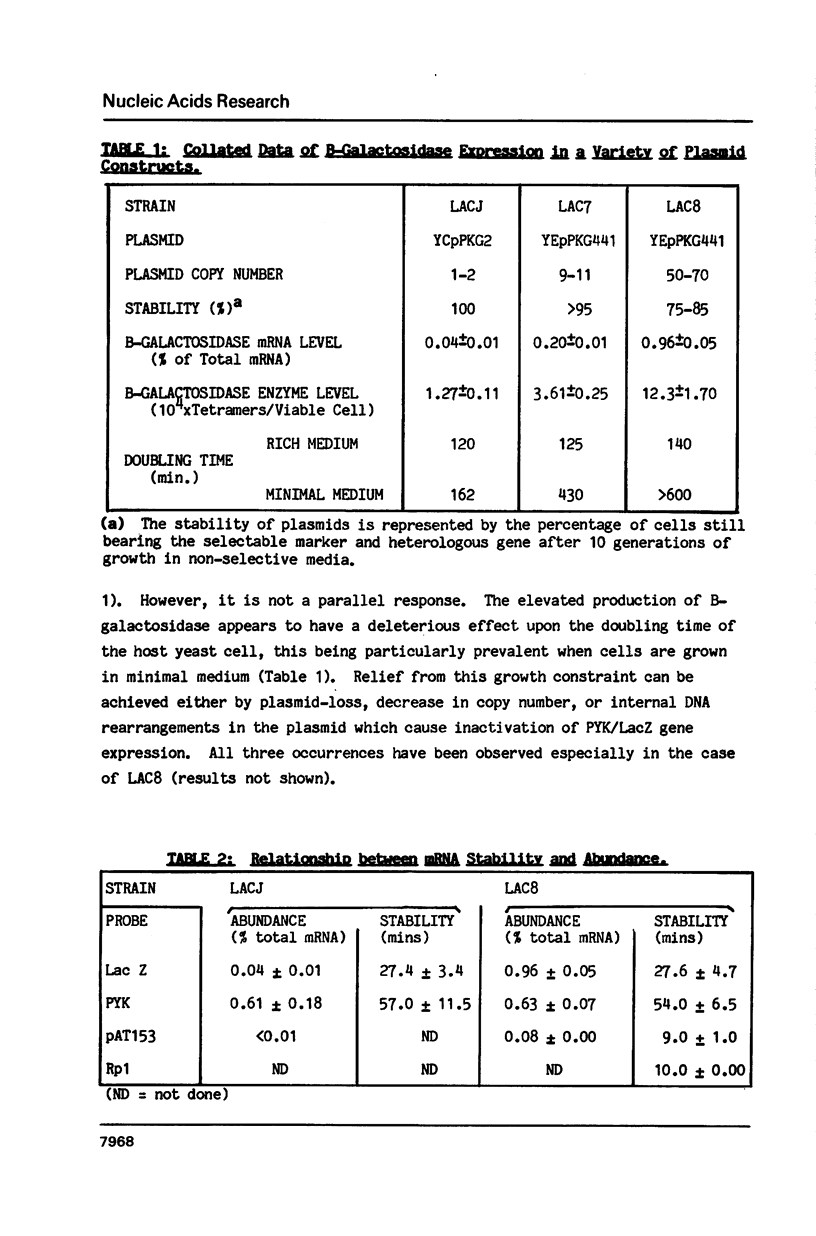
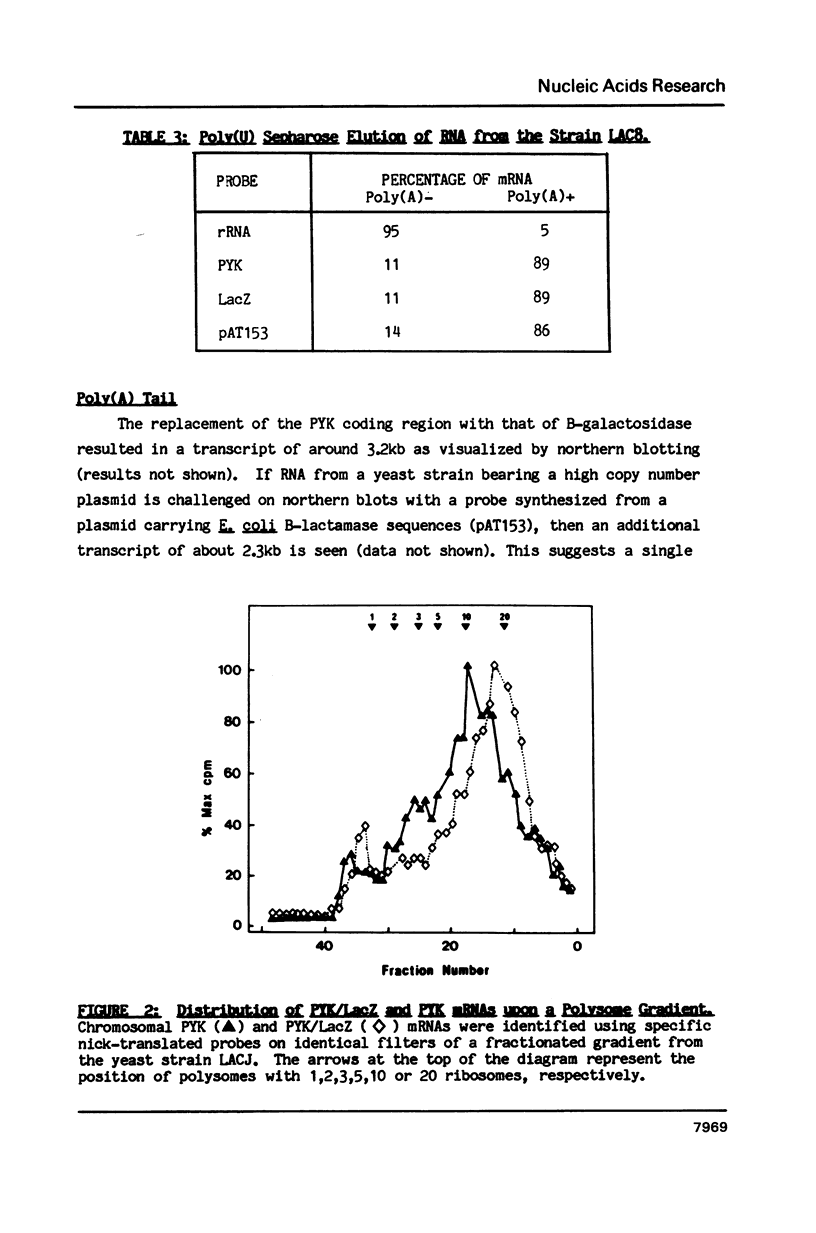
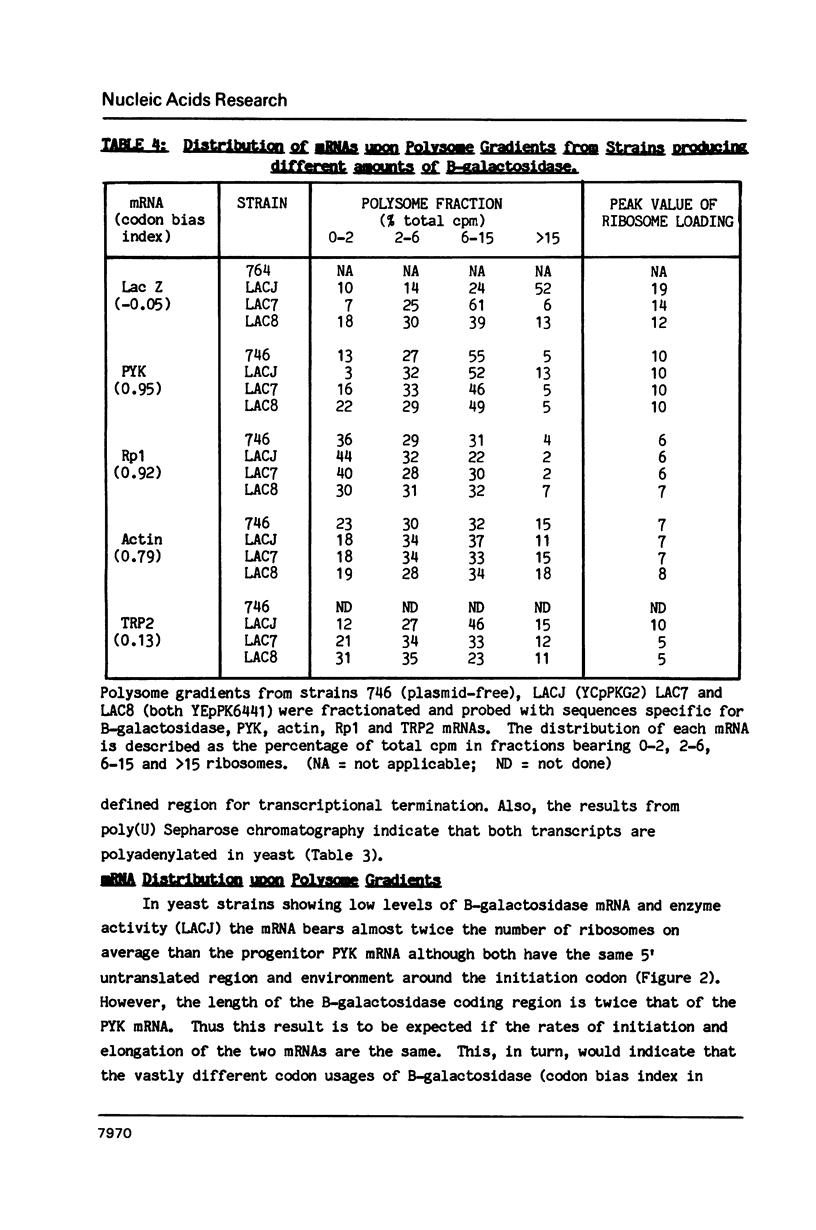




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Brown A. J., Hardman N. Utilization of polyadenylate mRNA during growth and starvation in Physarum polycephalum. Eur J Biochem. 1980 Sep;110(2):413–420. doi: 10.1111/j.1432-1033.1980.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Hitzeman R. A. Human, yeast and hybrid 3-phosphoglycerate kinase gene expression in yeast. Nucleic Acids Res. 1987 Jan 26;15(2):643–660. doi: 10.1093/nar/15.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Oppermann H., Hitzeman R. A. Homologous versus heterologous gene expression in the yeast, Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Dec 11;12(23):8951–8970. doi: 10.1093/nar/12.23.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. M., Schild D., Lovett S. T., Mortimer R. K. Regulation of RAD54- and RAD52-lacZ gene fusions in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1987 Mar;7(3):1078–1084. doi: 10.1128/mcb.7.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. A hierarchy of trans-acting factors modulates translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Sep;5(9):2349–2360. doi: 10.1128/mcb.5.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L. Codon usage and gene expression. Nucleic Acids Res. 1986 Apr 11;14(7):3075–3087. doi: 10.1093/nar/14.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Kingsman S. M., Kingsman A. J., Dobson M. J., Mellor J., Roberts N. A. Heterologous gene expression in Saccharomyces cerevisiae. Biotechnol Genet Eng Rev. 1985;3:377–416. doi: 10.1080/02648725.1985.10647819. [DOI] [PubMed] [Google Scholar]
- Larson G. P., Itakura K., Ito H., Rossi J. J. Saccharomyces cerevisiae actin--Escherichia coli lacZ gene fusions: synthetic-oligonucleotide-mediated deletion of the 309 base pair intervening sequence in the actin gene. Gene. 1983 Apr;22(1):31–39. doi: 10.1016/0378-1119(83)90061-6. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lycan D. E., Osley M. A., Hereford L. M. Role of transcriptional and posttranscriptional regulation in expression of histone genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Feb;7(2):614–621. doi: 10.1128/mcb.7.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arias A. E., Casadaban M. J. Fusion of the Saccharomyces cerevisiae leu2 gene to an Escherichia coli beta-galactosidase gene. Mol Cell Biol. 1983 Apr;3(4):580–586. doi: 10.1128/mcb.3.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas M. T., Chen C. Y., Hitzeman R. A., Riggs A. D. Active human-yeast chimeric phosphoglycerate kinases engineered by domain interchange. Science. 1986 Aug 15;233(4765):788–790. doi: 10.1126/science.3526552. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Kingsman A. J., Kingsman S. M. Factors affecting heterologous gene expression in Saccharomyces cerevisiae. Gene. 1985;33(2):215–226. doi: 10.1016/0378-1119(85)90096-4. [DOI] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. C., Bettany A. J., Purvis I. J., Brown A. J. Messenger RNA stability in Saccharomyces cerevisiae: the influence of translation and poly(A) tail length. Nucleic Acids Res. 1987 Mar 25;15(6):2417–2429. doi: 10.1093/nar/15.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. C., Purvis I. J., Bettany A. J., Brown A. J. The relationship between mRNA stability and length in Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Nov 11;14(21):8347–8360. doi: 10.1093/nar/14.21.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Paluh J. L., van Cleemput M., Moye W. S., Yanofsky C. Nucleotide sequence of Saccharomyces cerevisiae genes TRP2 and TRP3 encoding bifunctional anthranilate synthase: indole-3-glycerol phosphate synthase. J Biol Chem. 1984 Mar 25;259(6):3985–3992. [PubMed] [Google Scholar]


