Abstract
Background
To avoid unnecessary blood transfusions, physiologic transfusion triggers, rather than exclusively hemoglobin-based transfusion triggers have been suggested. The objective of this study was to determine systemic and microvascular effects of using a perfluorocarbon-based oxygen carrier (PFCOC) to maintaining perfusion and oxygenation during extreme anemia.
Methods
The hamster (weight 55-65 g) window chamber model was used. Two isovolemic hemodilution steps were performed using 10% hydroxyethyl starch at normoxic conditions to hematocrit of 19% (5.5 gHb/dl), point where the transfusion trigger was reached. Two additional hemodilution exchanges using the PFCOC (Oxycyte™, Synthetic Blood International, Inc. Costa Mesa, CA) and increasing fraction of inspired oxygen to 1.0 were performed to reduce hematocrit to 11% (3.8 gHb/dl) and 6% (2.0 gHb/dl), respectively. No control group was used in the study, as this level of hemodilution is lethal with conventional plasma expanders. Systemic parameters, microvascular perfusion, functional capillary density and oxygen tensions across the microvascular network were measured.
Results
At 6% hematocrit, the PFCOC maintained mean arterial pressure, cardiac output, systemic oxygen delivery and consumption. As hematocrit was lowered from 11% to 6%, functional capillary density, calculated microvascular oxygen delivery and consumption decreased, and oxygen extraction ratio was close to 100%. Peripheral tissue oxygenation was not predicted by systemic oxygenation.
Conclusions
PFCOC in conjunction with hyperoxia was able to sustain organ function, and partially provide systemic oxygenation during extreme anemia over the observation period. The PFCOC can work as a bridge until red blood cells are available for transfusion, or where additional oxygen is required, notwithstanding possible limitations in peripheral tissue oxygenation.
1. Introduction
Allogenic blood transfusions treat insufficient oxygen carrying capacity, so called anemia. However, while oxygen transport capacity is invariably restored by blood transfusion, it has also been associated with increased morbidity and mortality 1-3. As a result of an increasing awareness about the risks associated with allogenic blood transfusion, elevated costs associated with this procedure 4, and controversies around the real implication of anemia in critically ill patients, several strategies aiming to reduce blood utilization have been proposed 1-3. Lately the blood transfusion controversy has increased, as fresh and stored erythrocytes do not similarly restore oxygenation 5. Moreover, transfusion-related adverse events, both short and long-term, are among the costliest contributors to healthcare expenditures, including illness, future outcomes, lost wages and impact on quality of life 4. To avoid unnecessary blood transfusions, the use of physiologic transfusion triggers and goal directed, rather than exclusively hemoglobin based transfusion triggers, has been suggested 6.
An oxygen carrying fluid that sustains life in the absence of blood may have many benefits. Although, an oxygen carrier is not available yet, the development of these solutions will have unprecedented medical applications. Perfluorocarbons are derived from hydrocarbons by replacing all the hydrogen atoms by fluorine atoms, available in very large quantities and at relatively low cost. Perfluorocarbons have a high solubility for gases, and are chemically and biologically inert. In principle, lead to a convenient, largely available, cost effective, pathogen free and storable oxygen carrier plasma expander. However, perfluorocarbons are not soluble in water, and must be emulsified using a surfactant. Perfluorocarbon based oxygen carriers (PFCOCs) carry oxygen as function of their oxygen solubility and the fraction of inspired oxygen (FiO2). Preclinical, and Phase I, II and III clinical trials with PFCOCs have been reported with a Perflubron emulsion (Oxygent™, Alliance Pharmaceutical Corp. San Diego, CA) 7,8, however this initiative was subsequently abandoned 9,10. Currently, Oxycyte™ (Synthetic Blood International, Inc. Costa Mesa, CA) remains in clinical trials, has completed Phase II clinical safety trial in Traumatic Brain Injury, and Phase II, dose escalation in Switzerland and Israel 11-13.
In the present study, we addressed the question of whether adjunctive therapy with PFCOC and increased FiO2 can compensate for severe decreases in native oxygen carrying capacity. We also addressed the physiological changes in perfusion, PO2s gradients and oxygen delivery and extraction generated by this compensation. The objective of the study was to determine systemic and microvascular changes induced by co-administration of PFCOC and increased FiO2 during lethal extreme anemia (6% hematocrit). We developed an experimental model in which systemic and microvascular hemodynamics, and tissue oxygenation can be concurrently studied. In order to drastically reduce native oxygen carrying capacity, our experimental hamster window chamber model was first subjected to moderate hemodilution at normoxic conditions, via two isovolemic exchanges to 18% hematocrit using a plasma expander [10% hydroxyethyl starch]. After moderate hemodilution, hematocrit was further decreased to 11% and 6% hematocrit using PFCOC (Oxycyte™, Synthetic Blood International, Inc.). Based on previous results with our experimental model, 11% is the transfusion trigger value for awake hamsters, as it is the minimal hematocrit supplying the tissue with sufficient oxygen necessary for organ function 14-16, and a further hematocrit reduction does not adequately maintain vital functions.
2. Materials and Methods
Animal Preparation
Investigations were performed in 55 - 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal window chamber. Animal handling and care followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee (Institutional Animal Care and Use Committee, University of California, San Diego, La Jolla, California). The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere 17,18. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion Criteria
Animals were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate > 340 beat/min, mean arterial blood pressure > 80 mmHg, systemic hematocrit > 45%, and arterial oxygen partial pressure (PO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under a x650 magnification did not reveal signs of edema or bleeding.
Plasma expander and PFCOC
The non oxygen carrier plasma expander used in the study was Pentaspan™ (B. Braun Medical, Irvine, CA). The PFCOC used in the study was Oxycyte™ (Synthetic Blood International). Oxycyte™ (Synthetic Blood International, Inc.) is a third-generation PFCOC, the PFC is 60% vol/vol F-tert-butylcyclohexane (molecular formula C10F20). Oxycyte™ (Synthetic Blood International, Inc.) does not have colloidal osmotic pressure, and was mixed with 20% hydroxyethyl starch solution (mean molecular mass 200 kDa, Leopold Pharma, Graz, Austria) in a proportion of 80% Oxycyte™ (Synthetic Blood International, Inc.) and 20% colloidal hydroxyethyl starch solution according to Nolte et al. 19.
Systemic Parameters
Mean arterial pressure and heart rate were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hematocrit was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hemoglobin content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden). The proportion of blood volume occupied by PFCOC, fluorocrit, was measured using standard hematocrit procedures.
Cardiac Output
Cardiac output (CO) was measured by a modified thermodilution technique 20. Animals instrumented for CO measurements were surgically prepared, and recovered identically to animals studied for microvascular measurements. However, the complexity of the setup for the thermodilution prohibited positioning them on the microscope. CO was measured 15-20 min after each exchange.
Blood Chemistry and Biophysical Properties
Arterial and venous blood were collected in heparinized glass capillaries and analyzed for PO2, PCO2, base-excess and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA). The comparatively low arterial PO2 and high PCO2 of hamsters is a consequence of their adaptation to a fossorial environment 21. Venous blood samples were only obtained after decreasing the hematocrit to 11%, which decreased blood viscosity and, therefore the resistance of the small catheters implanted in the animal. Blood samples for viscosity and colloidal osmotic pressure measurements were quickly withdrawn into heparinized 5 ml syringes at the end of the experiment. Viscosity was measured in a DV-II plus (Brookfield, Middleboro, MA). Colloidal osmotic pressure was measured using a 4420 Colloid Osmometer (Wescor, Logan, UT) 22.
Hemoglobin Oxygen Saturation
Oxygen equilibrium curves for red blood cells (RBCs) were measured using a Hemox Analyzer (TCS Scientific Corporation, New Hope, PA) 23.
Functional Capillary Density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least a single RBC in a 45 s period in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. FCD (cm−1), i.e., total length of RBC perfused capillaries divided by the area of the microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion 14,15.
Microhemodynamics
A video image-shearing method was used to measure vessel diameter (D) 24. Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Arteriolar and venular centerline velocities were measured on-line by using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B, Vista Electronics, San Diego, CA) 25. The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity 26. Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. This calculation assumes a parabolic velocity profile and has been found to be applicable to tubes of 15 - 80 μm internal diameters and for hematocrits in the range of 6 - 60% 26.
Microvascular PO2 distribution
High resolution non-invasive microvascular PO2 measurements were made using phosphorescence quenching microscopy 27,28. Phosphorescence quenching microscopy is based on the oxygen-dependent quenching of phosphorescence emitted by albumin-bound metalloporphyrin complex after pulsed light excitation. Phosphorescence quenching microscopy is independent of the dye concentration within the tissue and is well suited for detecting hypoxia because its decay time is inversely proportional to the PO2 level, causing the method to be more precise at low PO2’s. This technique is used to measure both intravascular and extravascular PO2 since the albumin-dye complex continuously extravasates the circulation into the interstitial fluid 27,28. Extravascular fluid PO2 (interstitial fluid) was measured in tissue regions in between functional capillaries. Phosphorescence quenching microscopy allows for precise localization of the PO2 measurements without subjecting the tissue to injury. These measurements provide a detailed understanding of microvascular oxygen distribution and indicate whether oxygen is delivered to the interstitial areas.
Microvascular Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage for transillumination with the intravital microscope (BX51WI, Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the tube environment before any measurement. Tissue image was projected onto a charge-coupled device camera (COHU 4815, Cohu Electronics, San Diego, CA) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. The same sites of study were followed throughout the experiment so that comparisons could be made directly to baseline levels.
Experimental group
Animals included in the study were divided in two groups. One group of animals was used to measure cardiac output, and another group was used to characterize microvascular hemodynamics and oxygenation.
Moderate Isovolemic Hemodilution
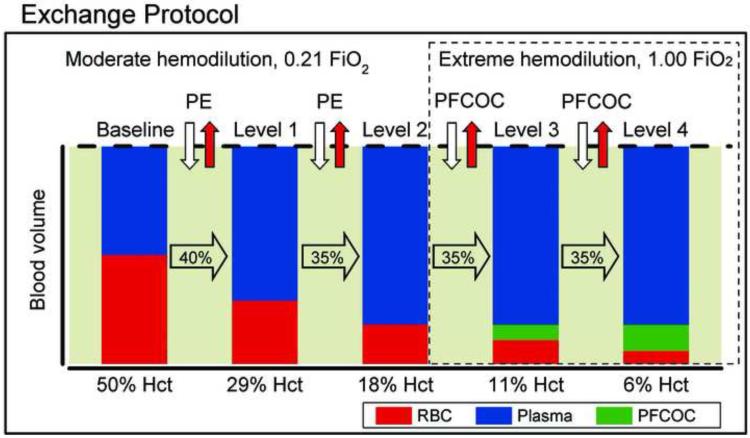
Both groups of animals were prepared identically and underwent an identical progressive hemodilution scheme. Progressive hemodilution was accomplished by two isovolemic exchange steps using hydroxyethyl starch at normoxic condition (FiO2 of 0.21). This protocol has been described in detail in previous reports 14,15. Blood volume was estimated as 7% of body weight. First exchange was 40% of blood volume (29% hematocrit, Level 1). Second exchange was 35% of blood volume, and decreased the hematocrit to 18% (5.5 gHb/dl, Level 2).
Extreme Isovolemic Hemodilution
After the moderate hemodilution, the protocol was continued by exchanging 35% of blood volume to 11% hematocrit (3.8 gHb/dl, Level 3) and lastly, a final exchange of 35% of blood volume to 6% hematocrit (2.0 gHb/dl, Level 4) using the PFCOC mixture described above. Animals were changed to hyperoxia (FiO2 of 1.0) as the third exchange was started. Each exchange and the respective observation time point post-exchanges were fully completed in 1 h, after last exchange animals were followed for 90 mins, thus total duration of the experiments was 5 hours. Systemic and microcirculation data were taken 10 min after a stabilization period post hemodilution step. Figure 1 shows the time line of the protocol.
Figure 1.
Hemodilution was attained by means of a progressive, stepwise, isovolemic, blood exchange-transfusion protocol in which the red blood cell (RBC) volume (dark bar) is continuously decreased and the plasma volume (clear bar) is increased while maintaining the total blood volume constant (represented by the dotted line). Volume of each exchange-transfusion step was calculated as a percentage of the blood volume, estimated as 7% of the body weight. Third and fourth hemodilution steps were achieved (35% of blood volume) using a mixture of perfluorocarbon oxygen carrier (PFCOC) and hydroxyethyl starch (HES) and the fraction of inspired oxygen (FiO2) was increased. PE, plasma expander (HES).
Tissue and Systemic Oxygen Delivery and Consumption
The methodology used in our studies allows a detailed analysis of tissue oxygen supply. Calculations were made using equation 1 for microvascular oxygen delivery (DmicO2) and equation 2 for consumption (VmicO2)14:
| (1) |
| (2) |
where RBCHb is the hemoglobin in RBCs [gHb/dl], γ is the oxygen carrying capacity of saturated hemoglobin [1.34 mlO2/gHb], SA is the arteriolar oxygen saturation, Hct, hematocrit, Fct, fluorocrit, (1 - Hct - Fct) is the fractional plasma volume [dlplasma/dl], αplasma is the solubility of oxygen in plasma (3.14 × 10−3 mlO2/dlplasma mmHg), αPFC is the solubility of oxygen in Oxycyte™ (2.4 × 10−2 mlO2/dlOxycyte mmHg), PAO2 is the arteriolar partial pressure of oxygen, A-V indicates arteriolar-venular difference, and Q is the microvascular flow.
Detailed analysis of systemic oxygen delivery (DO2) and consumption (VO2) was established using equations 3 and 4:
| (3) |
| (4) |
where SA is the arterial oxygen saturation, PAO2 is the arterial partial pressure of O2, A-V indicates arterio-venous difference and all other parameters were described above. In addition, fractional contribution to oxygen delivery of each phase, and the fractional contribution of each phase to oxygen consumption were calculated.
Statistical Analysis
Results are presented as mean ± standard deviation. In the box-whisker plot, the top of the box, the line within the box and the bottom of the box indicate the 75th percentile, the median and the 25th percentile, respectively. The upper and lower whiskers define the 95th and 5th percentile, respectively. Because animals instrumented for CO measurements were an independent group, from the animals used for tissue PO2, as the complexity of the setup for the thermodilution prohibited positioning them on the microscope, two sample sizes (CO and tissue PO2) were calculated independently. A priori calculation of the sample size for testing the changes in CO and tissue PO2 after hemodilution with PFCOC in hamsters was based on our previous results 15 where during hemodilution CO changed by 25% (SD 12%) after hemodilution with PFCOC. Therefore the number of animals used in the present study was based on a power analysis of CO using an alpha of 0.05 and a 1-beta of 0.9 estimated 9 animals to required to identify differences in CO. Additionally, tissue PO2 decreased by 17% (SD 8%) after hemodilution with PFCOC, therefore, the number of animals was based on a power analysis of PO2 using an alpha of 0.05 and a 1-beta of 0.9 estimated 6 animals. As the data was collected, interim analysis were implemented, and following animal care regulation at our institution, no more animals were included as statistical significance was reached. No attempts were made to adjust the significance level for the interim analyses. Data within the group was analyzed using an analysis of variance for non-parametric repeated measurements, ANOVA, Friedman test. When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Changes in PO2s microvascular oxygen delivery, systemic oxygen consumption and microvascular oxygen consumption, were evaluated using a two-tailed paired t test to compare 11% hematocrit (Level 3) against 6% hematocrit (Level 4). Closeness within a Gaussian population for all measured parameters values at baseline for each animal was quantified with Grubbs test. Oxygen distributions were analyzed using Kurtosis test and Skewness test 29. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. The same vessels and functional capillary fields were followed so that direct comparisons to their baseline levels could be performed. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P < 0.05.
3. Results
Eleven animals were entered into this study, and all animals tolerated and survived the entire protocol without visible signs of discomfort. Blood gases, mean arterial pressure and heart rate were measured in all animals. Six animals were used to study microvascular changes, and five animals were used to study CO changes. Blood gasses, mean arterial pressure and heart rate were no different between the animals used for microhemodynamics and CO (P>0.30). All animals included in the study passed the Grubbs’ test ensuring that all the measured parameter values at baseline were within a similar population (P<0.05).
Systemic and blood laboratory parameters are shown in Table 1. The first and second hemodilution exchanges with non-oxygen carrying plasma expander statistically reduced hematocrit and hemoglobin. Third and fourth hemodilution exchanges steps with PFCOC further decreased hematocrit and hemoglobin. Arterial blood gas analysis showed an increase in arterial PO2 at hemodilution exchanges with non-oxygen carrying plasma expander at 0.21 FiO2, however it was not statistically significant. Only after hemodilution exchange to 11% hematocrit with PFCOC and increased of FiO2 to 1.00, arterial PO2 statistically increased compared to baseline and to 18% hematocrit. Further decrease in hematocrit to 6% with PFCOC, increased arterial PO2 compared to 11% hematocrit. During the entire hemodilution protocol arterial PCO2 decreased, however no statistical difference was measured. At 11% and 6% hematocrit, PFCOC and increased of FiO2 to 1.00, prevented hyperventilation and maintained venular PCO2 within the specie physiological range. Additionally, the benefits of PFCOC and increased FiO2 to 1.00, were evident on the central venous PO2, which decreased after hemodilution from 11% to 6% hematocrit, however central venous oxygen saturation remained above 50%. Moreover, arterial pH did not present a significant change through the extreme hemodilution protocol, indicating the absence of acidosis, and as the arterial PCO2 did not show major deviations, suggest metabolic maintenance. The benefits of PFCOC and increased of FiO2 to 1.00 at 11% and 6% hematocrit are confirmed by the maintenance of acid-base balance, as judged by arterial PCO2 and pH, were not significantly altered at this degree of hemodilution.
Table 1.
Laboratory parameters before and after blood exchange
| Baseline |
Moderate Hemodilution |
Extreme Hemodilution |
||||
|---|---|---|---|---|---|---|
| 29% Hct | 18% Hct | 11% Hct | 6% Hct | |||
| n | 11 | 11 | 11 | 11 | 11 | |
| Hct, % | 49.3 ± 1.0 | 29.3 ± 1.4 | 18.2 ± 1.0 | 11.1 ± 0.8†‡ |
|
5.9 ± 0.5†‡ |
| Hb, g/dl | 14.8 ± 0.3 | 9.0 ± 0.6 | 5.5 ± 0.3 | 3.8 ± 0.5†‡ |
|
2.0 ± 0.4†‡ |
| Fct, % | 9.5 ± 0.7 |
|
19.0 ± 0.8 | |||
|
|
|
|||||
| PaO2, mmHg | 62.4 ± 7.3 | 66.0 ± 5.6 | 76.2 ± 4.2 | 575.5 ± 19.3†‡ |
|
676.6 ± 13.6†‡ |
| PvO2, mmHg | 48.3 ± 3.9 |
|
39.4 ± 4.6 | |||
| SvO2, % | 76 ± 5 |
|
64 ± 7 | |||
| PaCO2, mmHg | 51.1 ± 7.3 | 47.9 ± 4.3 | 46.6 ± 4.0 | 46.0 ± 4.6 | 43.7 ± 6.6 | |
| Arterial pH | 7.358 ± 0.017 | 7.362 ± 0.018 | 7.389 ± 0.028 | 7.356 ± 0.021 | 7.330 ± 0.015 | |
| BE, mmol/l | 2.9 ± 1.7 | 1.3 ± 1.7 | 2.1 ± 1.6 | 0.7 ± 1.3 | −0.8 ± 1.5 | |
|
|
|
|||||
Values are means ± SD. Baseline included all the animals in the study. BE, base excess; Fct, fluorocrit; Hb, hemoglobin; Hct, hematocrit; PaCO2, arterial partial carbon dioxide pressure; PaO2, arterial partial oxygen pressure; PvO2, mixed venous partial oxygen pressure.
P<0.05 compared to baseline.
P<0.05 compared to 18% Hct.
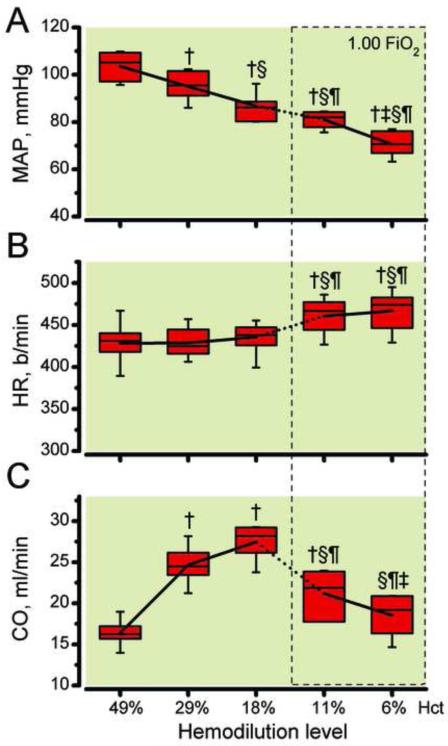
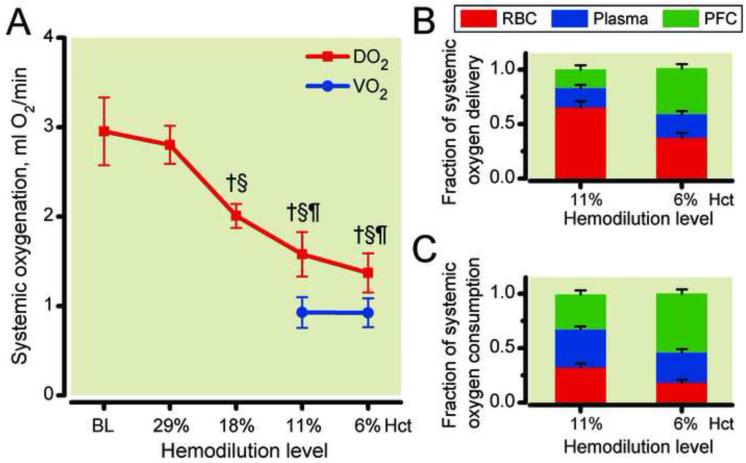
Systemic hemodynamic changes are presented in Figure 2. mean arterial pressure decreased progressively from 103.5 ± 9.8 mmHg at baseline to 95.0 ± 6.9 mmHg at 29% hematocrit, to 87.5 ± 5.8 mmHg at 19% hematocrit, to 81.0 ± 4.5 mmHg at 11% hematocrit and to 70.5 ± 5.6 mmHg at 6% hematocrit (Figure 2A). Heart rate remained unchanged during hemodilution with the plasma expander until 18% hematocrit, continuing the hemodilution with PFCOC statistically increased CO at 11% and 6% hematocrit compared to baseline and previous hemodilution levels (Figure 2B). CO statistically increased after initial reduction of hematocrit to 29% and 18%, respectably. Continuing the hemodilution to 11% hematocrit with PFCOC maintained CO statistically increased compared to baseline. The final hemodilution to reach 6% hematocrit using PFCOC statistically reduced CO compared to previous hemodilution levels. However, CO thought the entire progressive hemodilution remained above baseline levels (Figure 2C).
Figure 2.
A. Mean arterial pressure (MAP), B. heart rate (HR) and C. cardiac output (CO) as hemodilution progresses to 6% hematocrit. FiO2, fraction of inspired oxygen. †, P < 0.05 compared to baseline; §, P < 0.05 compared to 29% hematocrit; ¶, P < 0.05 compared to 18% hematocrit; ‡, P < 0.05 compared to 11% hematocrit.
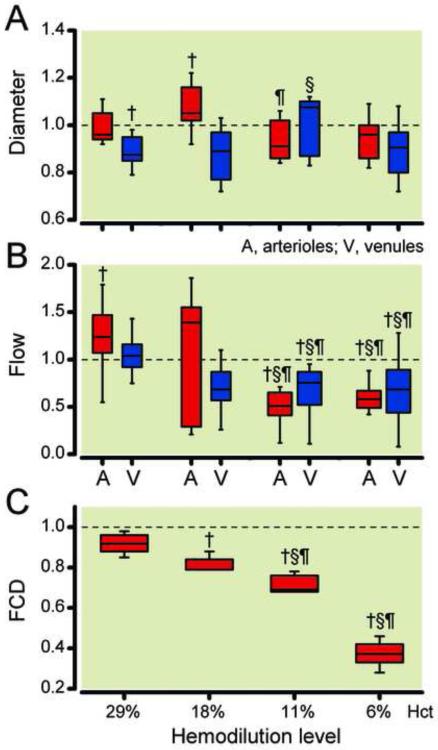
Changes in microcirculation diameters and flows and statistical significance are presented in Figure 3. Arteriolar diameter statistically increased from baseline after hemodilution to 29% hematocrit, and was not different from baseline after continuing the hemodilution to 11% and 6% hematocrit with PFCOC. Venular diameter statistically decreased from baseline after hemodilution to 29% hematocrit. This venular constriction was statistically reverted after hemodilution to 11% hematocrit with PCFOC (Figure 3A). Arteriolar microvascular flows were statistically increased after hemodilution to 29% hematocrit. Continuing the hemodilution to 11% hematocrit with PCFOC statistically decreased microvascular flows (arterioles and venules) at 11% and 6% hematocrit, respectively (Figure 3B). Microvascular flows at 11% and 6% hematocrit were statistically lower than previous hemodilution levels. FCD decreased to 0.92 ± 0.06 of baseline after hemodilution to 29% hematocrit, continuing the hemodilution to 18% hematocrit statistically decreased FCD to 0.79 ± 0.12 of baseline (Figure 3C). Hemodilution to 11% hematocrit with PFCOC statistically decreased FCD to 0.69 ± 0.10 of baseline, and continuing the hemodilution to 11% hematocrit further decreased FCD to 0.37 ± 0.08 of baseline.
Figure 3.
Relative changes from baseline in arteriolar (A) and venular (V) diameter (Panel A), blood flow (Panel B) and functional capillary density (FCD, Panel C) at different degrees of hemodilution. Broken line represents baseline level. Diameters (μm, mean ± SD) were for baseline (arterioles: 65.1 ± 6.1, n = 104; venules: 67.9 ± 8.9, n = 112). n = number of vessels studied. Red blood cell velocities (mm/s, mean ± SD) were for baseline (arterioles: 4.3 ± 0.7, venules: 2.3 ± 0.5). Calculated flows (nl/s, mean ± SD) were for baseline (arterioles: 14.9 ± 3.1; venules: 7.7 ± 2.2). Functional capillary density (1/cm, mean ± SD) was for Baseline (114 ± 12). FiO2, fraction of inspired oxygen. †, P < 0.05 compared to baseline; §, P < 0.05 compared to 29% hematocrit; ¶, P < 0.05 compared to 18% hematocrit; ‡, P < 0.05 compared to 11% hematocrit.
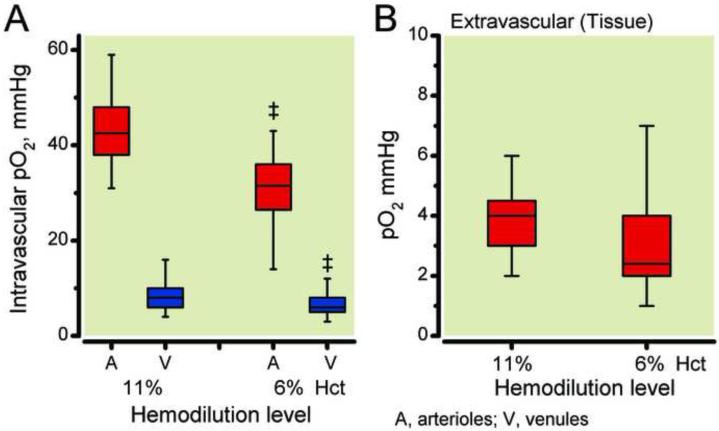
Intravascular oxygen tension values and distribution curves are presented in Figure 4A. Arteriolar pO2 was 42.0 ± 6.8 mmHg at 11% hematocrit and statistically decreased to 29.0 ± 6.6 mmHg at 6% hematocrit. Venular oxygen tension was 7.6 ± 2.5 mmHg at 11% hematocrit and statistically decreased to 6.2 ± 2.0 mmHg at 6% hematocrit. Tissue PO2 and distribution curves are illustrated in Figure 4B. Tissue PO2 was 3.8 ± 1.1 mmHg at 11% hematocrit and statistically decreased to 2.8 ± 1.4 mmHg at 6% hematocrit. Arteriolar oxygen distribution at 11% hematocrit and 6% hematocrit were not symmetrical (Skewness: 11% hematocrit: 0.94, and 6% hematocrit: 1.01) and skew to higher values, both distributions did not closely match a Gaussian distribution (Kurtosis: 11% hematocrit: −1.79, and 6% hematocrit: −0.92). Venular oxygen distribution at 11% hematocrit and 6% hematocrit were highly symmetrical (Skewness: 11% hematocrit: 0.35, and 6% hematocrit: 0.20) and closely match a Gaussian distribution (Kurtosis: 11% hematocrit: −0.36, and 6% hematocrit: −0.04). Tissue oxygen distribution at 11% hematocrit and 6% hematocrit were both highly symmetrical (Skewness: 11% hematocrit: 0.71, and 6% hematocrit: 0.78), however only 6% hematocrit closely match a Gaussian distribution (Kurtosis: 11% hematocrit: 1.52, and 6% hematocrit: −0.20).
Figure 4.
Intravascular partial oxygen pressure after hemodilution to 11% hematocrit (3.8 gHb/dl, Level 3) and 6% hematocrit (2.0 gHb/dl, Level 4). Panel A presents whisker boxes plot of arteriolar (A, n=104) and venular (V, n=112) oxygen tensions. n = number of vessels studied. Panel B presents whisker boxes plot of interstitial tissue oxygen pressure after hemodilution to 11% hematocrit (3.8 gHb/dl) and 6% hematocrit (2.0 gHb/dl), 98 spots studied. No significant difference in interstitial tissue pO2 between 11% hematocrit and 6% hematocrit. ‡, P < 0.05 compared to 11% hematocrit.
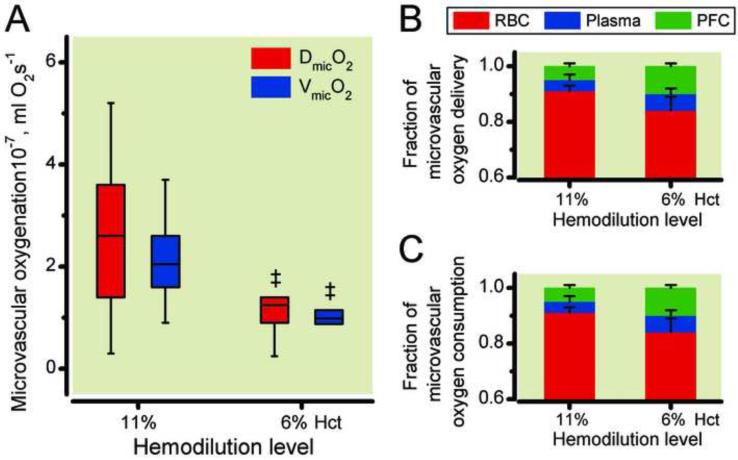
Systemic oxygen delivery and consumption and the fractional contribution of each phase are illustrated in Figure 5. At 11% hematocrit, fluorocrit 9.5%, therefore plasma volume represented the remaining 79.4%, while at 6% hematocrit, fluorocrit 19.0% and plasma volume 75.2%. DO2 was 1.67 ± 0.20 mlO2/min at 11% hematocrit and statistically decreased to 1.37 ± 0.14 mlO2/min at 6% hematocrit. As shown in Figure 5B, the fractional contribution of each phase to delivery was 65% (RBC), 17% (PFCOC) and 18% (Plasma) at 11% hematocrit and changed to 37% (RBC), 42% (PFCOC) and 22% (Plasma) at 6% hematocrit. In contrast with DO2, VO2 statistically increased from 0.80 ± 0.08 mlO2/min at 11% hematocrit to 1.00 ± 0.10 mlO2/min at 6% hematocrit (unpaired t-test). The fractional contribution to consumption was 32% (RBC), 32% (PFCOC) and 35% (Plasma) at 11% hematocrit and 18% (RBC), 54% (PFCOC) and 28% (Plasma) at 6% hematocrit. Figure 6 presents the changes in DmicO2 and VmicO2. Between 11% hematocrit and 6% hematocrit there was a significant decrease in DmicO2 and VmicO2. Reduction in hematocrit from 11% to 6% using the PFCOC did not maintain delivery or consumption of oxygen in the microcirculation.
Figure 5.
Systemic oxygen delivery (DO2) and consumption (VO2) after hemodilution (Panel A) and fractional contribution of each phase to oxygen delivery (Panel B) and consumption (Panel C). †, P < 0.05 compared to baseline (BL). PFC, perfluorocarbon phase; RBC, red blood cells phase. §, P < 0.05 compared to 29% hematocrit; ¶, P < 0.05 compared to 18% hematocrit; ‡, P < 0.05 compared to 11% hematocrit. Calculations of oxygen transport were obtained using measured blood gas parameters and hemodynamics. The difference in DO2 between 11% hematocrit and 6% hematocrit is not statistically significant.
Figure 6.
Microvascular oxygen delivery (DmicO2) and consumption (VmicO2) after hemodilution (Panel A) and fractional contribution of each phase to oxygen delivery (Panel B, error bars not shown) and consumption (Panel C, error bars not shown). PFC, perfluorocarbon phase; RBC, red blood cells phase. ‡, P < 0.05 compared to 11% hematocrit. Microvascular consumption was calculated as the difference of averaged arterioles and venules for each animal. The differences in DmicO2 and VmicO2 between 11% hematocrit and 6% hematocrit are statistically significant.
4. Discussion
The principal finding of this study is that by using a PFCOC emulsion combined with hyperoxia, hemodilution can be continued to 1/3 of the transfusion trigger hematocrit (6% hematocrit, 2.0 gHb/dl) partially sustaining systemic oxygen delivery and consumption. However, local microvascular tissue PO2s were physiologically low, and oxygen delivery and extraction remained partially dependent on the oxygen transported by the remaining erythrocytes. PFCOC combined with hyperoxia, increases arterial PO2, directly affecting the amount of oxygen dissolved in the blood, therefore not only affecting blood oxygen content, but also directly changing the longitudinal and circumferential arterial oxygen gradients. Since oxygen diffusivity cannot be affected, the changes in blood oxygen solubility produced by PFCOC affected the local oxygen gradients, which is the only mechanism that drives oxygen to leave the intravascular compartment. After moderate hemodilution, the increase of CO and microvascular blood flow, due to reduced blood viscosity, maintained oxygen supply to the tissues. Sufficient oxygenation of the tissues is limited by the functional reserve of the heart, as at a given hematocrit an increase in cardiac output is needed to deliver oxygen needs 30,31. However, continuing the progressive hemodilution regulatory mechanisms tend to fail, even with PFCOC emulsion combined with hyperoxia. Important physiologic parameters, such as tachycardia, hypotension, and decrease in CO, that indicate the need for a blood transfusion were observed as hemodilution progressed beyond the transfusion trigger. During the initial experimental design, we planned to include a control group hemodiluted with a colloid plasma expander in combination with hyperoxia. In the pilot study, the awake hamsters that tolerated hemodilution to 8% hematocrit, showed signs of stress and hypotension (mean arterial pressure < 40 mmHg) and no animal survived hemodilution to 6% hematocrit without PFCOC.
Avoiding allogeneic blood transfusions has become an important issue, as patients demand treatment without allogeneic blood transfusions. Thus, in recent years, a variety of methods and procedures have been developed to minimize allogeneic blood transfusions. Therefore, the current study defines a potential application for PFCOC emulsions and hyperoxia for clinical scenarios where bleeding is not completely controlled and blood for transfusion is not available. It is worth noticing that this possible application would be limited to a few patients in such conditions, and that it is far from the universal blood substitute once erroneously envisioned. The metabolic acidosis developed during progressive hemodilution may represent lactic acidosis, due to tissue hypoxia or a loss of buffering capacity of the blood, although lactate levels were not measured. The progressive hemodilution with PFCOC with increased FiO2 of 1.0 prevented metabolic acidosis and maintained arterial PCO2 unaltered (Table 1). This indicates improved perfusion and oxygen delivery to tissue during extreme hemodilution, which was partially verified by the microvascular measurements. Thereby, as found in this study, changes in pH, based excess, and HCO3− do not correlate with the decrease in hemoglobin concentration, as PFCOC was capable of sustaining oxygen delivery above oxygen demand. Similar clinical observation has been reported with perflubron emulsion, delaying blood transfusions in orthopedic surgery 7.
Current results indicate that CO and microvascular blood flow are the major determinants of systemic and microvascular oxygen delivery respectively. CO increased after the initial hemodilution and started to decrease near the transfusion trigger, and the use of the PFCOC and hyperoxia maintained CO above baseline even when blood’s oxygen carrying capacity was only 12% of baseline. Changes in CO were produced by changes in heart rate and stroke volume, specifically during the initial hemodilution phase, decreasing hematocrit to 29% and 18% increased stroke volume by 50% and 61% from baseline, respectively. Fluid shifts from the interstitial space as result of hydroxyethyl starch hyperoncotic properties could be responsible for the initial elevation in stroke volume, as capillaries hydrostatic pressure decreases, and corresponds to the observed reduction in FCD, mostly dependent in capillary hydrostatic pressure and plasma viscosity 14. Maintaining normovolemia is of primordial importance during extreme hemodilution, to support the compensatory mechanisms, such as the increase in CO, and the decrease of mean arterial pressure. It also prevents relevant tachycardia with potential consecutive myocardial ischemia. As hematocrit decreased from 18% to 11%, maintained CO above baseline CO however decreased in similar proportion than oxygen delivery, and stroke volume was not different from baseline. The cardiac response depends on the maintenance of cardiac filling and adequate myocardial oxygenation, which itself is almost exclusively dependent on coronary blood flow 30. The tachycardia measured at hematocrit 11 and 6% corresponds to a 25% increase on heart rate, compared to baseline, shortening of diastolic phase and is prone to induce myocardial ischemia by decreasing coronary filling and perfusion. Adequate oxygenation of the myocardium is beyond the scope of this study, but further research to particularly identify the sensitivity of the myocardium to blood oxygen content at reduced hematocrit is needed, because no extra myocardial work is needed to maintain higher CO and oxygen delivery if blood is hemodiluted due to reduced viscosity. In addition, PFCOC combined with hyperoxia at extreme anemic states may require supplementary support to maintain perfusion pressure with catecholamines, to allow the effects of the increased physically dissolved oxygen to arrive to peripheral tissues.
Microvascular DmicO2 was critical at 11% and 6% hematocrit, when the microvascular oxygen extraction ratio (VmicO2/DmicO2) increased from 38% at baseline 32, to 92% at 11% hematocrit and to 95% at 6% hematocrit, respectively. It has been documented that microvascular blood flow decreases after extreme hemodilution (11% hematocrit) as a response to the drastic decrease in oxygen carrying capacity and blood viscosity 14,16. This is characterized by a selective vasoconstriction and a decrease in FCD, as observed in this study. In previous studies when extremely low hematocrit levels are achieved by hemodilution with low viscosity plasma expanders, vascular hindrance increased, relative to baseline, indicating probable vasoconstriction accompanied by a redistribution of blood flow to vital organs 16. In light of the microvascular vasoconstriction and reduced blood flow, hydroxyethyl starch in conjunction with hyperoxia did not maintain CO or vascular perfusion above baseline at 11% hematocrit 15.
Previous studies have shown that VO2 remained relatively constant and independent of DO2, as DO2 was sustained above VO2 33. The increase on FiO2 with the PFCOC emulsion increases significantly oxygen delivery to the tissue, but interestingly, most of the oxygen is due to the hemoglobin in the RBCs. The explanation maybe related to the increase in oxygen solubility in blood that allows hemoglobin to remain partially saturated until it arrives to the hypoxic tissues. The mechanism by which the PFCOC was able to sustain life and even increase oxygen extraction ratio after the last hemodilution step was by increasing oxygen extraction from 48% at 11% hematocrit to 73% at 6% hematocrit. From 11% to 6% hematocrit, there was a reduction in systemic oxygen delivery of 0.30 mlO2/min. Despite this reduction, oxygen consumption increased by 0.20 mlO2/min due to a reduction in oxygen content in venous blood of 0.50 mlO2/min.
It is interesting to note the finding that despite apparently sufficient systemic oxygen delivery, local signs of hypoxia persist. The localized measurements of tissue PO2 highlight the importance of microvascular information when compared with systemic measurements, which showed that oxygen extraction was satisfied by oxygen delivery. Microvascular results show that the tissue was extracting most of the oxygen delivered, indicating that during extreme anemia the tissues consumed as much oxygen as is available. Under conditions in which oxygen supply becomes limited but microvascular regulation is intact, such as may occur during dysoxia (local hypoxia), corrections of global hemodynamic and oxygen-derived variables may be erroneously expected to maintain tissue oxygenation. As reported by Ott and Cooley 34, there are clinically relevant situations when major surgeries need to be performed safely in individuals who refuse blood transfusion and preoperative and intraoperative techniques that decrease surgical blood loss, decrease oxygen consumption, and where increased oxygen delivery are required. Therefore, in order to increase oxygen delivery after severe normovolemic hemodilution, techniques such as FiO2 to 1.0 to increase oxygen content, sedation and paralysis to decrease oxygen consumption, and finally, drugs to increase CO and cause peripheral vasodilation to increase oxygen delivery are clinically implemented. Additionally, the clinical relevance of applying PFCOC in situations of extreme anemia, in conjunction with the currently used techniques, is supported by the metabolic benefit observed with PFCOC and FiO2 of 1.0, even though oxygen carrying capacity was decreased from 11% to 6% hematocrit.
Conventional procedures to restore oxygen carrying capacity at extreme anemic conditions are based on the correction of systemic oxygen delivery. The current work shows that such correction can be inadequate, as regional hypoxia was observed in the peripheral tissues. Lack of knowledge about the basic mechanisms controlling oxygen transport in the microcirculation and the insufficiency of clinical techniques for assessment of the adequacy of tissue oxygenation were the principal causes of failure during the early stages of the development of blood substitutes. Regional assessment of vital organ oxygen delivery at 11% hematocrit has been previously studied, concluding that all vital organs are under-oxygenated, receiving 50% of the basal oxygen supply 16. Assessment of adequate tissue oxygenation lacks universal consensus, as all approaches measuring blood flow and arterial oxygen content are to determine oxygen supply and extraction. Assessment of regional dysoxia has been carried out mainly by lactate, gastric tonometry and oxygen electrodes. Interstitial tissue pH measurements are considered indicators of the activity of anaerobic metabolism associated with tissue dysoxia, although to what extent measured levels are influenced by the balance between lactate production and clearance and by lactate produced from sources other than anaerobic metabolism, is difficult to determine 23.
Limitations of the study. Despite the use of microvascular and global oxygen transport variables to address endpoints achieved with PFCOC-hyperoxia, the current study lacks the demonstration of improvement in organ function. Understanding the function of the brain in relation to PFCOC combined with hyperoxia will be an important step toward physiologic transfusion triggers. Anemia sensitive neurologic monitoring in animals requires further development. Analyses of evoked potentials have shown to be sensitive to cerebral ischemia and critical oxygenation levels. Further studies have to show their usefulness in anemia treated with perfluorocarbon emulsions. In addition to reduced oxygen transport, other factors may play a major role in producing cell and organ dysfunction; the fact that global oxygen transport variables are increased does not necessarily correlate with the outcome, and future studies addressing these points are needed. For assessing the progression of tissue dysfunction, oxygen delivery and extraction at the local level (microcirculation) rather than at the systemic level could be more important. Due to the resistance of the small catheters required in this experimental model, central venous blood gases were only collected after at 11% hematocrit. Therefore, in conscious hamsters, a total oxygen consumption was stable at 11% and 6% hematocrit, and hemodilution with PFCOC with FiO2 of 1.0 did not improve or further compromise oxygen transport capacity. Paradoxically, oxygen delivery decreased progressively during the hemodilution with PFCOC with FiO2 of 1.0, a consequence of the reduction of CO. In addition, local microvascular tissues blood flows and oxygen delivery were progressively reduced. These results suggest that the effects of hemodilution with PFCOC with FiO2 of 1.0 are counteracted and/or masked by local and remote control mechanisms, owing to the simultaneously reduced arterial oxygen content. The effects of reduced arterial oxygen content without alterations in viscosity could shed some light on the problem of the influence of viscosity during hemodilution. This is indicated by experimental results where reductions in arterial oxygen content are tolerated if produced by hemodilution, rather than if it is produced by carboxyhemoglobinemia 35. Although this study has restricted direct clinical significance, it presents a comprehensive experimental study, with the objective of defining the mechanistic principles for translational developments. Translational research of PFCOC sets the cornerstone for transfusion medicine. Designing appropriate clinical studies depends on good, robust, and reproducible preclinical data, to support the investment in prospective studies large enough to be adequately powered to reveal an effect that can change clinical practice. The results attained in the current in conscious animals will be use as the basis to design future studies in anesthetized condition to specifically address the role of perfluorocarbon emulsion in conjunction to increase inhaled oxygen at clinically relevant anemic condition. There are several elements that can also be learned in future studies include longer survival time, neurological markers and colloid controls. Likely applying all the currently experimental technology to the analysis of hemodynamic, oxygenation and metabolism will provide information to define suitable clinical indications and precise potential analytical challenges associated with PFCOC use.
The objective of this study was to observe systemic and microcirculatory effects of PFCOC-hyperoxia used to sustain oxygen delivery during extreme anemic conditions. This study demonstrates that infusion of an 8.0 g/kg dose of PFCOC combined with increased FiO2 was well-tolerated and resulted in hemodynamic changes. Our investigations were carried out using an extreme hemodilution protocol aimed to determine the efficacy of oxygen carrying plasma expanders, at the critical hematocrit (18%) progressive hemodilution limits metabolic oxygen supply-dependent. A critical parameter that determines microvascular function is FCD, which remained above 40%; a value that is low, but not pathological, according to findings of Kerger et al 36. It also was able to provide sufficient oxygen delivery to maintain systemic metabolic needs, although localized analysis of oxygenation showed that the oxygen extraction rate of peripheral tissues was close to 100% of the oxygen supplied. The degree of hemodilution achieved in the current study cannot be attained with a plasma expander, as previously published 15. Preservation or even temporary upholding of systemic oxygen delivery and CO after drastic hemodilution with PFCOC-hyperoxia defines a potential application for PFCOC emulsions as a bridge until RBCs are available for transfusion, although clear signs of microvascular dysoxia were observed. Additionally, in conditions where metabolic and immunologic complications increased oxygen extraction 37, as sepsis, PFCOC emulsion plus hyperoxia may provide the additional oxygen required until the problem is controlled. Even during prehospital asanguineous fluid resuscitation, PFCOC-hyperoxia could aim to restoration of macro-circulation oxygenation. Once the trauma victim is hospitalized, the necessary increase in oxygen carrying capacity can be achieved by blood transfusion; however, this is often not yet available in the prehospital setting. In the absence of RBC, hyperoxic ventilation in conjunction with PFCOC can help to augment arterial oxygen content. These results suggest the possibility of maintaining oxygenation below the transfusion trigger until allogeneic blood transfusions can be accomplished.
Acknowledgments
The authors thank for the surgical preparation of the animals used in the study: Froilan P. Barra, BSc (Technician, Microhemodynamics, Bioengineering University of California, San Diego, School of Engineering, La Jolla, California), and Cynthia Walser, BSc (Technician, Microhemodynamics, Bioengineering University of California, San Diego, School of Engineering, La Jolla, California).
Funding:
Supported by funds provided by the University of California, San Diego, School of Engineering, La Jolla, California, United States and grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, Maryland (Bioengineering Research Partnership grant R24-HL64395, and research project grant program R01-HL62354 and R01-HL62318).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 2.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E, Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 4.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 6.Spahn DR, Madjdpour C. Physiologic transfusion triggers: Do we have to use (our) brain? Anesthesiology. 2006;104:905–6. doi: 10.1097/00000542-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Spahn DR, van Brempt R, Theilmeier G, Reibold JP, Welte M, Heinzerling H, Birck KM, Keipert PE, Messmer K, European Perflubron Emulsion Study Group Perflubron emulsion delays blood transfusions in orthopedic surgery. Anesthesiology. 1999;91:1195–208. doi: 10.1097/00000542-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Spahn DR, Waschke KF, Standl T, Motsch J, Van Huynegem L, Welte M, Gombotz H, Coriat P, Verkh L, Faithfull S, Keipert P. Use of perflubron emulsion to decrease allogeneic blood transfusion in high-blood-loss non-cardiac surgery: Results of a European phase 3 study. Anesthesiology. 2002;97:1338–49. doi: 10.1097/00000542-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hill SE, Leone BJ, Faithfull NS, Flaim KE, Keipert PE, Newman MF. Perflubron emulsion (AF0144) augments harvesting of autologous blood: A phase II study in cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:555–60. doi: 10.1053/jcan.2002.126947. [DOI] [PubMed] [Google Scholar]
- 10.Olofsson C, Ahl T, Johansson T, Larsson S, Nellgard P, Ponzer S, Fagrell B, Przybelski R, Keipert P, Winslow N, Winslow RM. A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol-modified Hemoglobin (Hemospan) in patients undergoing major orthopedic surgery. Anesthesiology. 2006;105:1153–63. doi: 10.1097/00000542-200612000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Sun D, Levasseur JE, Merenda A, Hamm RJ, Zhu J, Spiess BD, Bullock MR. Perfluorocarbon emulsions improve cognitive recovery after lateral fluid percussion brain injury in rats. Neurosurgery. 2008;63:799–806. doi: 10.1227/01.NEU.0000325493.51900.53. discussion 806-7. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZJ, Price CD, Bosco G, Tucci M, El-Badri NS, Mangar D, Camporesi EM. The effect of isovolemic hemodilution with oxycyte, a perfluorocarbon emulsion, on cerebral blood flow in rats. PLoS One. 2008;3:e2010. doi: 10.1371/journal.pone.0002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woitzik J, Weinzierl N, Schilling L. Early administration of a second-generation perfluorochemical decreases ischemic brain damage in a model of permanent middle cerebral artery occlusion in the rat. Neurol Res. 2005;27:509–15. doi: 10.1179/016164105X15677. [DOI] [PubMed] [Google Scholar]
- 14.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol. 2004;287:H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol. 2004;287:H320–30. doi: 10.1152/ajpheart.01166.2003. [DOI] [PubMed] [Google Scholar]
- 16.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol. 2006;291:H2445–52. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 17.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–17. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 18.Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–34. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 19.Nolte D, Pickelmann S, Lang M, Keipert P, Messmer K. Compatibility of different colloid plasma expanders with perflubron emulsion: An intravital microscopic study in the hamster. Anesthesiology. 2000;93:1261–70. doi: 10.1097/00000542-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Cabrales P, Acero C, Intaglietta M, Tsai AG. Measurement of the cardiac output in small animals by thermodilution. Microvasc Res. 2003;66:77–82. doi: 10.1016/s0026-2862(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 21.Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med. 2005;39:1229–37. doi: 10.1016/j.freeradbiomed.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Webb AR, Barclay SA, Bennett ED. In vitro colloid osmotic pressure of commonly used plasma expanders and substitutes: A study of the diffusibility of colloid molecules. Int Care Med. 1989;15:116–20. doi: 10.1007/BF00295988. [DOI] [PubMed] [Google Scholar]
- 23.Cabrales P, Nacharaju P, Manjula BN, Tsai AG, Acharya SA, Intaglietta M. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 24.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5:309–12. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 25.Intaglietta M, Tompkins WR. On-line microvascular blood cell flow velocity measurement by simplified correlation technique. Microvas Res. 1972;4:217–20. [Google Scholar]
- 26.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 27.Torres Filho IP, Intaglietta M. Microvessel pO2 measurements by phosphorescence decay method. Am J Physiol. 1993;265:H1434–8. doi: 10.1152/ajpheart.1993.265.4.H1434. [DOI] [PubMed] [Google Scholar]
- 28.Kerger H, Groth G, Kalenka A, Vajkoczy P, Tsai AG, Intaglietta M. PO2 measurements by phosphorescence quenching: Characteristics and applications of an automated system. Microvasc Res. 2003;65:32–8. doi: 10.1016/s0026-2862(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 29.Royston P. Which measures of skewness and kurtosis are best? Stat Med. 1992;11:333–43. doi: 10.1002/sim.4780110306. [DOI] [PubMed] [Google Scholar]
- 30.Crystal GJ, Kim SJ, Salem MR. Right and left ventricular oxygen uptake during hemodilution and beta-adrenergic stimulation. Am J Physiol. 1993;265:H1769–77. doi: 10.1152/ajpheart.1993.265.5.H1769. [DOI] [PubMed] [Google Scholar]
- 31.Meisner FG, Kemming GI, Habler OP, Kleen MS, Tillmanns JH, Hutter JW, Bottino DA, Thein E, Meier JM, Wojtczyk CJ, Pape A, Messmer K. Diaspirin crosslinked hemoglobin enables extreme hemodilution beyond the critical hematocrit. Crit Care Med. 2001;29:829–38. doi: 10.1097/00003246-200104000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Cabrales P, Tsai AG, Intaglietta M. Nitric oxide regulation of microvascular oxygen exchange during hypoxia and hyperoxia. J Appl Physiol. 2006;100:1181–7. doi: 10.1152/japplphysiol.01105.2005. [DOI] [PubMed] [Google Scholar]
- 33.Schlichtig R, Kramer DJ, Pinsky MR. Flow redistribution during progressive hemorrhage is a determinant of critical oxygen delivery. J Appl Physiol. 1991;70:169–78. doi: 10.1152/jappl.1991.70.1.169. [DOI] [PubMed] [Google Scholar]
- 34.Ott DA, Cooley DA. Cardiovascular surgery in Jehovah’s Witnesses. Report of 542 operations without blood transfusion. JAMA. 1977;238:1256–8. [PubMed] [Google Scholar]
- 35.Biro GP, Beresford-Kroeger D. Myocardial blood flow and oxygen-supply following dextran-haemodilution and methaemoglobinaemia in the dog. Cardiovasc Res. 1979;13:459–68. doi: 10.1093/cvr/13.8.459. [DOI] [PubMed] [Google Scholar]
- 36.Kerger H, Tsai AG, Saltzman DJ, Winslow RM, Intaglietta M. Fluid resuscitation with oxygen vs. non-O2 carriers after 2 h of hemorrhagic shock in conscious hamsters. Am J Physiol. 1997;272:H525–37. doi: 10.1152/ajpheart.1997.272.1.H525. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DP, Beyer C, Samsel RW, Wood LD, Schumacker PT. Pathological supply dependence of O2 uptake during bacteremia in dogs. J Appl Physiol. 1987;63:1487–92. doi: 10.1152/jappl.1987.63.4.1487. [DOI] [PubMed] [Google Scholar]