Abstract
Emerging evidence indicates that once established, synapses and dendrites can be maintained for long periods, if not for the organism’s entire lifetime. In contrast to the wealth of knowledge regarding axon, dendrite, and synapse development, we understand comparatively little about the cellular and molecular mechanisms that enable long-term synapse and dendrite maintenance. Here, we review how the actin cytoskeleton and its regulators, adhesion receptors, and scaffolding proteins mediate synapse and dendrite maintenance. We examine how these mechanisms are reinforced by trophic signals passed between the pre-and postsynaptic compartments. We also discuss how synapse and dendrite maintenance mechanisms are compromised in psychiatric and neurodegenerative disorders.
Keywords: neurodegeneration, dendritic spine, actin cytoskeleton, psychiatric disease
PREFACE: SYNAPSES STABILIZE DENDRITIC ARBORS
Excitatory glutamatergic synapses form between presynaptic axon specializations and specialized protrusions, called dendritic spines, that extend from the dendritic shaft. Dendritic spines initiate as thin filopodial structures that emerge from the dendritic shaft (Fiala et al. 1998, Holtmaat et al. 2005, Ziv & Smith 1996, Zuo et al. 2005). These dendritic filopodia contain small clusters of adhesion and scaffolding proteins that serve as the building blocks of the postsynaptic specialization (Niell et al. 2004). Contact of a filopodium with a presynaptic compartment promotes the stabilization and enlargement of the filopodium tip into a dendritic spine (Ziv & Smith 1996, Zuo et al. 2005).
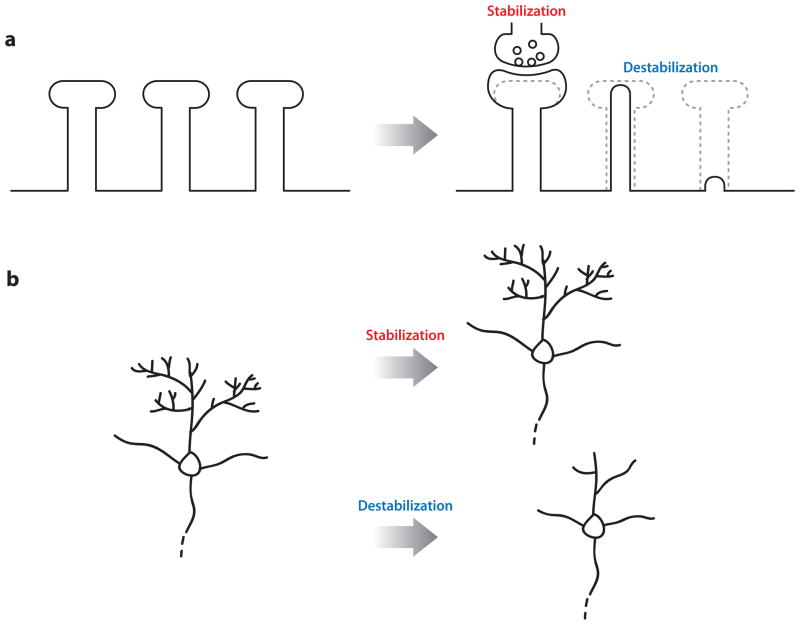
Dendritic arbors are highly dynamic, exhibiting frequent branch additions and retractions, with only a subset stabilized and maintained (Cline 2001, Dailey & Smith 1996, Wong et al. 2000). Productive synapse formation and the accompanying activation of post-synaptic signaling mechanisms promote arbor stability (Niell et al. 2004, Rajan et al. 1999, Wu & Cline 1998). These stabilizing influences likely explain the age-dependent slowing of dendritic spine and branch dynamics that follows the peak of synapse formation and refinement (Holtmaat et al. 2005, Trachtenberg et al. 2002, Wu et al. 1999). Conversely, a loss of, or reduction in, synaptic inputs leads to dendritic loss in vivo (Figure 1), which indicates that the maintenance of synaptic input is critical for dendritic stability (Coleman & Riesen 1968, Jones & Thomas 1962, Le Gros Clark 1957, Matthews & Powell 1962, Sfakianos et al. 2007).
Figure 1.
Stabilization of dendritic spines. (a) Synaptic activity, including adhesive contact between pre- and postsynaptic compartments and trophic signaling between the two compartments, enhances dendritic spine stability. (b) Synaptic support maintains the integrity of dendritic architecture over time. The loss of this support results in destabilization of dendritic spines followed by dendritic simplification.
INTRODUCTION: SYNAPSES AND DENDRITES MUST BE MAINTAINED FOR LONG PERIODS
The vast majority of stable dendritic spines and filopodia-like protrusions on mature dendrites have synapses (Arellano et al. 2007, Harris 1999). Long-term confocal microscopy imaging in adult rodents indicates that a large fraction of dendritic spines as well as complete dendritic arbors are stable for extended time periods of several months, and possibly years (Holtmaat et al. 2005, Majewska et al. 2006, Trachtenberg et al. 2002, Zuo et al. 2005). Together, these findings argue that individual synapses may last for the majority of an organism’s lifetime, possibly decades in the case of humans.
Synapse and dendritic spine loss and dendritic atrophy are observed in the aging human brain (Uylings et al. 2000). Reductions in synapse number and dendritic arbor size are also associated with psychiatric illnesses, such as schizophrenia and major depressive disorder (MDD), as well as neurodegenerative diseases, such as Alzheimer’s disease (AD) (Anderton et al. 1998; Broadbelt et al. 2002; Cotter et al. 2001, 2002; Flood 1991; Flood et al. 1987a, 1987b; Glantz & Lewis 1997, 2000; Kalus et al. 2000; Rajkowska et al. 1999; Woo et al. 1998; Hanks & Flood 1991; Law et al. 2004; Stockmeier et al. 2004). These reductions in synaptic connectivity are believed to be a major contributor to the altered mood and impaired perception and cognition that characterize these conditions.
Given the importance of synapse stability for human brain health, a major unmet goal in neuroscience is to understand how neurons achieve long-term synaptic stability. Elucidating these mechanisms is an essential prerequisite to developing therapies to prevent synapse loss in aging and disease. Here, we review the cellular and biochemical mechanisms that support long-term synaptic maintenance. We also discuss how disease pathology may undermine the mechanisms that maintain long-term synapse and dendrite stability.
EXPERIMENTAL APPROACHES TO STUDY SYNAPSE AND DENDRITE MAINTENANCE
Synapse and dendrite maintenance can be studied in long-term, established neuronal cultures, which are maintained long enough to allow synapses to form; in brain slices; and in whole animals. Each of these systems has both benefits and limitations. Cultured neuron and slice models offer more ease of manipulation, but may lack essential in vivo determinants of synapse and dendrite maintenance. For example, neuronal cultures grown on two-dimensional plastic dishes do not faithfully mimic the soft tissue properties or supportive (e.g., glia) or nutrient supply systems of the brain. Cultured neurons also do not establish normal neuronal wiring patterns. For these reasons, synapses in cultured neurons may be inherently less stable than synapses and dendrites in vivo. Although whole animal systems are more difficult to manipulate, they offer the only opportunity to investigate the importance of synapse and dendrite maintenance regulators in the physiological context. Ideally, investigations of synapse and dendrite maintenance would employ complementary techniques/approaches, using cultured systems to pose specific mechanistic questions and animal models to explore how manipulation of these mechanisms affects synapse and dendrite stability in vivo. Indeed, for most of the regulatory molecules we discuss below, loss of function leads to similar effects in both in vitro and in vivo systems.
Many key regulators of synapse and dendrite maintenance also have essential functions in neuronal migration, axon or dendrite targeting, or synapse formation. Thus, the general approach to study roles in synapse maintenance involves using genetic (e.g., RNA interference or gene knockout) or pharmacological tactics to inhibit a potential regulator after synapses and dendrites are properly formed. Excitatory synapse dissolution requires dendritic spine shrinkage or retraction. The effects of these manipulations on dendritic spine stability are assessed by monitoring both spine density and morphology, including spine length, head shape, and width or volume. Defects in spine maintenance can be reflected by a decrease in spine number and size, as well as a proportional increase in the smaller, thinner spines or filopodia-like protrusions. Dendrite arbor structure can be assessed by labeling the neurons via dye injection or fluorescent protein expression, and quantified using computer-assisted camera lucida tracing systems.
PROTEINS THAT PROVIDE SUPPORT FOR SYNAPSE MAINTENANCE
Long-term synapse and dendrite maintenance relies heavily on structural proteins that support the overall synaptic framework and organize the synaptic signaling machinery. We review here how the actin cytoskeleton, adhesion receptors, and scaffolding proteins provide structural support for long-term synapse and dendrite maintenance.
F-Actin Provides Long-Term Structural Support for the Presynaptic Compartment and Dendritic Spines
The actin cytoskeleton provides essential structural support for long-term synapse maintenance. A presynaptic filamentous (F−) actin meshwork acts as a scaffold to organize the neurotransmitter release machinery, tether regulators near sites of release, and facilitate vesicle trafficking and endocytosis (Dillon & Goda 2005, Halpain 2003, Zhang & Benson 2001). Postsynaptically, F-actin provides integrity for dendritic spines and organizes signaling machinery at the postsynaptic density (Dillon & Goda 2005, Schubert & Dotti 2007, Sekino et al. 2007). We highlight here features of the F-actin cytoskeleton and its regulators that are especially critical for long-term synapse maintenance. For a more comprehensive treatment of F-actin and its regulators at the synapse, we refer readers to an excellent recent review in this series (Dillon & Goda 2005).
Dendritic spines have diverse morphologies (e.g., thin, stubby, and mushroom shapes), which can be altered in response to activity or injury (Harris 1999, Sorra & Harris 2000). F-actin is highly enriched in dendritic spines compared with other parts of the neuron (Cohen et al. 1985, Fifkova & Delay 1982, Matus et al. 1982). The dendritic spine neck is composed of bundled actin similar to that found in filopodia and microvilli. The spine head contains a branched F-actin network analogous to that found in lamellipodia (Cheng et al. 2000, Landis & Reese 1983). The visualization of GFP-labeled actin in spines reveals that spines can undergo significant changes in shape (Fischer et al. 1998, Star et al. 2002). This dynamic behavior results from cycles of continuous actin polymerization/depolymerization involving up to 95% of the total spine actin (Honkura et al. 2008, Okamoto et al. 2004, Star et al. 2002, Zhang & Benson 2001). These observations reveal the importance of F-actin dynamics in supporting spine shape and its dynamic remodeling. This structural plasticity likely contributes to the ability of synapses to alter their synaptic signaling strength based on experience, a phenomenon known as synaptic plasticity.
Although the precise configuration of the actin cytoskeleton determines dendritic spine morphology, long-term spine maintenance requires perfectly balanced cycles of actin polymerization and depolymerization within the spine. Treatment with jasplakinolide, which induces actin polymerization, results in spine enlargement, whereas latrunculin-A or cytochalasin-D, which shift the actin equilibrium toward increased G-actin, reduce spine motility and cause spine shrinkage or loss (Allison et al. 1998, Fischer et al. 1998, Honkura et al. 2008, Okamoto et al. 2004, Star et al. 2002). Together, these experiments reveal that F-actin structure is critical for dendritic spine maintenance.
F-ACTIN REGULATORS CONTRIBUTE TO SYNAPSE AND DENDRITIC SPINE MAINTENANCE
F-actin structure is controlled by a large set of regulatory proteins, a growing number of which have been implicated in long-term synapse and dendritic spine maintenance (Figure 2). In many cases, disruption or altered activity of these regulators contributes to psychiatric or neurodegenerative disorders associated with defects in dendritic spine or synapse maintenance (Table 1). Here, we highlight key actin regulators that play essential roles in synapse and dendritic spine maintenance.
Figure 2.
Molecular mechanisms that regulate dendritic spine stability. The actin cytoskeleton and its regulators, adhesion receptors, and scaffolding proteins provide physical support for long-term synaptic maintenance. Most signaling pathways regulate spine stability by either 1. directly or 2. indirectly (via RhoGTPase signaling pathways) regulating actin dynamics or its interactions with adhesion and scaffolding molecules. 3. Adhesion molecules mediate signaling from the presynaptic or extracellular compartments into dendritic spines to evoke changes in Rho GTPase and other signaling cascades that control F-actin structure and stability. 4. Scaffolding proteins both interact directly with F-actin to support cytoskeletal structure and organize signaling molecules that impinge on these cytoskeletal control mechanisms.
Table 1.
Molecules associated with neurological disorders with reduced synapse or destabilized dendritic spine pathology. The identified molecules are known to regulate the synapse and/or dendritic spine stability, and their up- or downregulation, deletion, or loss-of-function (LOF) mutations have been implicated in psychiatric, neurological, or neurodegenerative disordersa
| Molecule | Alteration | Related neurological disorders | References |
|---|---|---|---|
| Actin-related proteins | |||
| Cofilin | ↑ | AD | Zhao et al. 2006 |
| LIMK | +/− | Williams syndrome | Bellugi et al. 1999, Frangiskakis et al. 1996 |
| Drebrin | ↓ | AD | Lacor et al. 2007 |
| Rho-GTPase regulators | |||
| Oligophrenin-1 | LOF mutation | X-linked mental retardation | Billuart et al. 1998, Zanni et al. 2005 |
| Kalirin-7 | ↓ | AD, schizophrenia | Hill et al. 2006, Youn et al. 2007 |
| Scaffolding protein | |||
| Shank3 | +/− | ASD | Durand et al. 2007 |
| Adhesion molecule | |||
| Neurexin:Neuroligin | −/−, LOF mutation | ASD, mental retardation, schizophrenia | Reviewed by Sudhof, 2008 |
| Trophic factors | |||
| BDNF | ↓ | MDD, Rett syndrome | Chang et al. 2006 |
| Neuregulin-1:ErbB4 | LOF mutation | Schizophrenia | Reviewed by Mei & Xiong, 2008 |
| Corticosteroids | ↑ | MDD, chronic stress, Cushing’s disease | Tata et al. 2006, Woolley et al. 1990, Patil et al. 2007 |
Abbreviations: AD, Alzheimer disease; ASD, autism spectrum disorders; MDD, major depressive disorder.
Rho Family GTPases: Rho and Rac
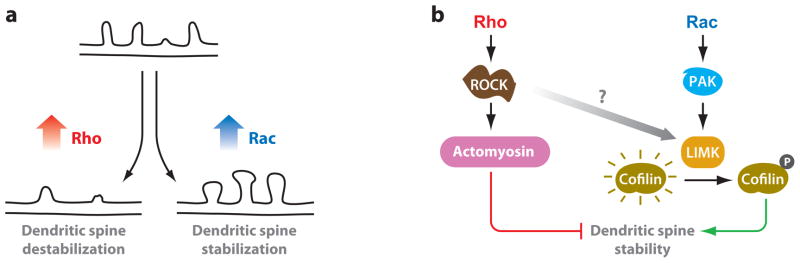
Rho family GTPases are a class of small GTPases that control actin dynamics and rearrangement to regulate numerous cellular functions, including spine morphogenesis and stability (Govek et al. 2004, Nadif Kasri & Van Aelst 2008). In particular, Rac1 (Rac) and RhoA (Rho) play important roles in spine maintenance (Figure 3) (Nakayama et al. 2000, Tashiro & Yuste 2004). In general, Rac activation stimulates F-actin polymer-ization and dendritic spine stability, whereas Rho activation in mature neurons leads to synapse and dendritic spine loss and dendritic regression (Govek et al. 2004, Lee et al. 2000, Ruchhoeft et al. 1999, Tashiro et al. 2000), although localized reductions in Rho activity are associated with spine collapse under some conditions (Schubert et al. 2006).
Figure 3.
Rho family GTPases have opposite effects on dendritic spine stability. (a) Rho and Rac have opposite effects on dendritic spine maintenance. Rho activation causes dendritic spine destabilization characterized by spine retraction and collapse, resulting in dendritic segments that lack spines. Rac activation promotes dendritic spine enlargement and stabilization. (b) Activation of RhoA stimulates Rho-associated protein kinase (ROCK) and actomyosin contractility, which causes spine collapse and synapse loss. Conversely, Rac activates Pak1 and LIMK to phosphorylate and inhibit cofilin, thereby stabilizing F-actin and promoting dendritic spine stability. Rho signaling through ROCK can also activate LIMK, but whether this contributes to spine stability is unclear.
Rac and Rho regulate spine structure and maintenance by controlling actin cytoskeletal dynamics. Downstream effectors of Rac1 include p21-activated kinase 1 (Pak1) and insulin receptor substrate p53 (IRSp53) (Ethell & Pasquale 2005). Activation of Pak1 regulates spine stability by activating LIM kinase-mediated cofilin inhibition (Meng et al. 2002, Penzes et al. 2003). Activation of IRSp53 by Rac induces WASP family verprolin-homologous protein (WAVE) and Arp2/3-mediated actin polymerization, resulting in spine head enlargement (Ethell & Pasquale 2005, Miki et al. 2000). Rho activation stimulates Rho-associated protein kinase (ROCK), a major Rho effector, which stimulates actomyosin contractility, resulting in spine shortening and retraction (Ethell & Pasquale 2005, Fukata et al. 2001). Interestingly, Rac and Rho share some downstream effectors, including myosin light chain kinase and LIMK (Maekawa et al. 1999). Exactly how they engage similar molecules to achieve opposite effects on spine stability needs to be further investigated. Finally, Rac and Rho are activated by guanine nucleotide-exchange factors (GEFs) and turned off by GTPase-activating proteins (GAPs), and both classes of regulators have been implicated in the regulation of synapse and dendritic spine stability.
Regulators of Rho Family GTPases
Oligophrenin-1
Mutations in oligophrenin-1 (OPHN1), a RhoGAP, are associated with X-linked mental retardation (Billuart et al. 1998, Zanni et al. 2005). OPHN1 is predominantly expressed in the brain and colocalizes with PSD-95 and F-actin in dendritic spines (Govek et al. 2004). OPHN1 overexpression in established neuronal cultures has no effect on spine density, but it enlarges spine head size, which is known to correlate with increased spine stability (Nadif Kasri et al. 2009). Conversely, OPHN1 knockdown in vitro increases RhoA activity and destabilizes dendritic spines by reducing spine density and length (Govek et al. 2004, Nadif Kasri et al. 2009). The OPHN1 knockdown spine phenotype mimics the phenotype resulting from RhoA activation, whereas inhibiting ROCK blocks the spine destabilization that accompanies OPHN1 knockdown in mature neurons (Govek et al. 2004).
Arg and p190RhoGAP
Our laboratory has identified a Rho inhibitory pathway that regulates synapse and dendrite stability. In non-neuronal cells, integrin-mediated adhesion activates the Abl-related-gene (Arg) nonreceptor tyrosine kinase to phosphorylate and activate the Rho inhibitor p190RhoGAP (Bradley et al. 2006, Hernandez et al. 2004, Peacock et al. 2007). p190RhoGAP is concentrated in the postsynaptic compartment and aids in maintaining dendritic spine stability (Moresco & Koleske 2003, Sfakianos et al. 2007). p190RhoGAP phosphorylation is significantly reduced in the arg−/− mouse hippocampus, and this is accompanied by elevated RhoA activity (Hernandez et al. 2004, Sfakianos et al. 2007). Axons, dendrites, and synapses develop normally in the arg−/− mouse hippocampus and cortex and reach their fully mature size and number by postnatal day 21. However, hippocampal synapses then destabilize, leading to 30% loss of synapses by postnatal day 31, followed by dendritic atrophy by early adulthood (Sfakianos et al. 2007). Biochemical studies suggest that the peak of Arg-mediated p190RhoGAP activation occurs just after the peak of synaptogenesis (Hernandez et al. 2004, Sfakianos et al. 2007), suggesting Arg and p190RhoGAP signaling is critical for long-term stabilization and maintenance of a subset of nascent synapses.
Kalirin-7 RacGEF
Kalirins (Kal) are RacGEFs, and Kal-7 is the predominant isoform in the adult rat brain (Johnson et al. 2000; Penzes et al. 2000, 2001). Kal-7 interacts and colocalizes with PSD-95 and F-actin in dendritic spines (Ma et al. 2003, Penzes et al. 2001). Knockdown or genetic ablation of Kal-7 in established neuronal cultures leads to dendritic spine simplification, leading to longer and thinner dendritic filopodia, and remaining synapses contain thinner postsynaptic densities (Ackermann & Matus 2003, Ma et al. 2008b). Several synaptic adhesion pathways act through Kal-7 to regulate F-actin rearrangement and spine stability. For example, ephrinB-EphB2 signaling across the synapse activates Kal-7 signaling through Rac and Pak1 to maintain dendritic spine stability (Penzes et al. 2003). Moreover, N-cadherin engagement and NMDA receptor activity stimulate Kal-7-dependent Rac1 activation, resulting in F-actin polymerization and enlargement of existing spines (Xie et al. 2008, 2007). Kal-7 expression is reduced in the brains of patients with schizophrenia and AD, consistent with the reduction of synapses and dendritic spines in these diseases (Hill et al. 2006, Youn et al. 2007).
ADF/Cofilin and LIM Kinase 1
The closely related actin depolymerizing factors (ADFs) and cofilin catalyze the severing of actin filaments. Depending on local cellular conditions (G- and F-actin concentration, ADP- versus ATP-bound F-actin, and other factors), cofilin can either disassemble actin filaments or sever filaments to provide new barbed ends to support F-actin assembly (Carlier et al. 1997, dos Remedios et al. 2003). Thus, cofilin controls the balance of G-actin versus F-actin locally. Cofilin localizes to the spine head in both developing and mature synapses where it associates with the spine periphery and with the postsynaptic density (Hotulainen et al. 2009, Racz & Weinberg 2006). Cofilin knockdown in established neuronal cultures reduces F-actin turnover in spines and decreases dendritic spine density, with a more pronounced effect on less stable thin spines than on other classes (Hotulainen et al. 2009).
LIM kinases phosphorylate cofilin at Serine 3, thereby inhibiting its function. LIM kinase 1 (LIMK-1) is a neuron-specific ADF/cofilin kinase highly enriched in mature synapses (Bernard et al. 1994, Proschel et al. 1995, Wang et al. 2000, Yang et al. 1998). Cultured hippocampal neurons from limk-1−/− mice exhibit reduced ADF/cofilin phosphorylation and altered F-actin accumulation in spines, resulting in abnormal spines with thicker necks and smaller heads (Meng et al. 2003, 2002). Synaptic GTPase activating protein (synGAP), a Rac inhibitor, attenuates this pathway to regulate cofilin activity in spines (Carlisle et al. 2008). Cultured hippocampal neurons from syngap+/− and syngap−/− mice exhibit increased phospho-cofilin and increased actin polymerization, leading to spine head enlargement, which demonstrates that disruption of cofilin regulation alters F-actin structure within spines.
LIMK-1 also has an important role in the presynaptic compartment to regulate Drosophila neuromuscular junction stability (Eaton & Davis 2005). In this context, LIMK-1 acts downstream of the Wit (wishful thinking) type II bone morphogenetic protein (BMP) receptor. Loss of LIMK-1 function causes presynaptic motor neuron terminal regression, leaving behind unpaired postsynaptic membrane specializations. Overexpression of the ADF/cofilin phosphatase slingshot, which, like the limk-1 mutant, should reduce ADF/cofilin phosphorylation, does not cause neuromuscular junction instability at the synapse. This finding suggests that LIMK-1 targets a presynaptic substrate other than ADF/cofilin to regulate synapse stability.
In humans, the LIMK-1 gene is among a microcluster of genes located on chromosome 7q11.23 and haploinsufficient in Williams syndrome, a developmental disorder associated with hypersociability and impairments in some cognitive tasks. These observations suggest that the altered synaptic cytoskeletal control may underlie some of these behavioral deficits, but more biochemical and cytoarchitectural work must be done to clearly establish this relationship (Bellugi et al. 1999, Frangiskakis et al. 1996).
Myosin IIB
Actomyosin contractility is an essential contributor to long-term spine maintenance. Class II myosins are cytoskeletal motor proteins that promote actomyosin contractility in nonmuscle cells. Myosin IIB localizes to dendritic spines, where it is enriched in the postsynaptic density (PSD) (Ryu et al. 2006). Inhibition of myosin IIB by siRNA or the myosin IIB inhibitor blebbistatin causes dendritic spine heads to elongate and become more filopodial in mature cultured neurons; coincident with this morphological transition, myosin IIB inhibition leads to a reduction in mini-excitatory postsynaptic currents, which reflects a loss of synapses (Ryu et al. 2006, Webb et al. 2007). These observations indicate that myosin IIB–based contractility is essential for synapse maintenance.
Drebrin
Drebrin is an F-actin binding protein expressed in neurons predominantly during early post-natal brain development (Ishikawa et al. 1994, Shirao et al. 1988). Drebrin colocalizes with F-actin in dendritic spine heads in mature cultured hippocampal neurons (Biou et al. 2008, Takahashi et al. 2003) and the amount of endogenous drebrin in dendritic spines positively correlates with the spine head size in the adult mouse cerebral cortex (Kobayashi et al. 2007). Overexpression of drebrin in immature neurons induces formation of long F-actin bundled filopodia and accumulation of synaptic proteins such as PSD-95 (Mizui et al. 2005). After synapses are properly formed, overexpression of drebrin causes destabilization of globular spines to polymorphic and very thin filopodia-like spines (Biou et al. 2008, Hayashi & Shirao 1999, Ivanov et al. 2009). These alterations of spine structure are dependent on the F-actin binding of drebrin (Biou et al. 2008, Hayashi & Shirao 1999, Ivanov et al. 2009). Together, these data suggest that drebrin levels must be perfectly balanced in the dendritic spine for long-term synapse stability, but drebrin-mediated regulation of spine stability is poorly understood. Drebrin may interact with profilin to facilitate actin polymerization and elongate dendritic spines (Mammoto et al. 1998). In support of this hypothesis, profilin IIa can regulate activity-dependent spine stabilization (Ackermann & Matus 2003).
CaMKII
CaMKII is a calcium-calmodulin protein kinase implicated in synaptic plasticity (Colbran & Brown 2004, Fink & Meyer 2002, Hudmon & Schulman 2002). CaMKII consists of two predominant isoforms, α and β, in the brain. CaMKIIβ is expressed from embryonic stages to adulthood, but CaMKIIα is only expressed after birth (Fink et al. 2003, Lin & Redmond 2008). Both isoforms are implicated in long-term dendritic spine stability. For example, CaMKIIα levels in spines correlate with overall spine size (Asrican et al. 2007), and blocking CaMKIIα activity in mature neuronal cultures can inhibit activity-induced spine enlargement and lead to spine destabilization (Yamagata et al. 2009, Zha et al. 2009). In addition to its enzymatic role in dendritic spine signaling, CaMKIIβ uniquely contains an F-actin-binding domain that directly regulates F-actin stability (Fink et al. 2003, Lin & Redmond 2008, Okamoto et al. 2007, Shen et al. 1998). CaMKIIβ knockdown in hippocampal slices leads to a significant loss of mature spines, transforming them into immature dendritic filopodia (Okamoto et al. 2007). Intriguingly, CaMKIIβ ’s spine maintenance function does not require its kinase activity. In fact, CaMKIIβ activation dislocates it from the postsynaptic area, leading to dendritic spine destabilization (Okamoto et al. 2007).
NMDA Receptor
The actin cytoskeleton interacts functionally with NMDA receptors to support synaptic activity, which is essential for long-term synapse activity. Both depolymerization of the actin cytoskeleton and actomyosin inhibitors diminish NMDA receptor activity, indicating that that NMDA receptor activity is tightly coupled to the configuration of the F-actin cytoskeleton (Lei et al. 2001, Rosenmund & Westbrook 1993). The NR1A, -1C, and -2B receptor subunits bind directly to the F-actin-crosslinking protein α-actinin, an interaction that can be blocked by calmodulin binding to the tails (Wyszynski et al. 1997). Calmodulin binds the NR1A tail and mediates Ca2+-dependent inactivation of NMDA receptor currents (Ehlers et al. 1996, Zhang et al. 1998). Thus, α-actinin binding may protect NMDA receptors from this mode of inhibition. NMDA receptors also cluster at the postsynaptic density via interactions between their tails and postsynaptic scaffolding proteins (Feng & Zhang 2009), many of which couple directly or indirectly to the cytoskeleton. This may explain why synaptic NMDA receptor clustering can be maintained in established neuronal cultures even when F-actin or α-actinin is removed (Allison et al. 1998, Halpain et al. 1998).
RNAi-mediated knockdown of the NMDA receptor in vitro causes spine destabilization and loss of synapses, but NMDA channel blockers do not destabilize spines or synapses (Alvarez et al. 2007). Instead, structure/function studies indicate that specific NMDA C-terminal sequences are essential to mediating long-term synapse and dendrite maintenance. These findings indicate that, in addition to their activity-based roles, NMDA receptors have distinct structural roles in maintaining spines and synapses.
SYNAPTIC SCAFFOLDING PROTEINS PROVIDE STRUCTURAL SUPPORT FOR LONG-TERM MAINTENANCE
The PSD is an electron-dense submembranous region that directly apposes the presynaptic compartment. PSD scaffolding proteins organize and concentrate receptors and signaling molecules in this small area to optimize responsivity to presynaptic activity. PSD size contributes to the size of the spine head, which positively correlates with spine maintenance (Harris & Stevens 1989, Holtmaat et al. 2006, Kasai et al. 2003, Trachtenberg et al. 2002). It is therefore not surprising that scaffolding proteins have critical roles in synapse and dendritic spine stabilization (Figure 2).
PSD-95
PSD-95 is a major PSD scaffolding protein that belongs to the membrane-associated guany-late kinase (MAGUK) protein family (Cho et al. 1992). PSD-95 contains several PDZ and protein-protein interaction domains that stabilize interactions between neurotransmitter receptors, synaptic adhesion receptors, and actin-associated scaffolding molecules (Ethell & Pasquale 2005). Knockdown of PSD-95 in cultured neurons results in reductions in spine density and size (Ehrlich et al. 2007). PSD-95 appears to be particularly important for activity-dependent synapse stabilization. Stimuli that normally promote long-term enhancement of synaptic efficacy in hippocampal slices instead lead to spine destabilization in PSD-95 knockdown neurons (Ehrlich et al. 2007).
Shank/Homer
The Shank and Homer proteins are associated with PSD-95 and colocalize in the PSD (Naisbitt et al. 1999, Tu et al. 1999, Xiao et al. 2000). In addition to providing stabilizing support to PSD-95, Shank/Homer complexes recruit IP3 receptors and F-actin to synapses, and therefore enlarge spine heads to stabilize dendritic spines (Sala et al. 2001, Xiao et al. 2000). Shank1B overexpression in mature neurons selectively enlarges the spine head, consistent with a primary function in spine stabilization (Sala et al. 2001). Accordingly, spine density is not affected in neurons from shank1−/− mice, although they have smaller dendritic spines and thinner PSDs (Hung et al. 2008). By contrast, knockdown of Shank3 in cultured hippocampal neurons results in the increase of dendritic filopodia, indicating destabilized dendritic spines (Roussignol et al. 2005). Mutations of shank3 are linked to Autism spectrum disorders, suggesting that the failure of Shank/Homer-induced synapse stabilization may have significant consequences during brain development in humans (Durand et al. 2007).
STABILIZING INTERACTIONS AT THE SYNAPSE VIA ADHESION SYSTEMS
Once formed, synapses are maintained by adhesion systems that mediate stabilizing interactions between the pre- and postsynaptic compartments and between the synapse and supporting glia and extracellular matrix (ECM) (Figure 2).
Cadherins and Catenins
Dimerization of both pre- and postsynaptic cadherins mediates stable adhesion between the compartments. Cadherins also employ intracellular binding partners called catenins to regulate the activity of RhoA and Rac1 GTPases to coordinate F-actin remodeling within the spine and promote cadherin clustering at adhesion sites (Arikkath & Reichardt 2008, Kwiatkowski et al. 2007, Nelson 2008). Cadherin-catenin complexes couple functionally to the actin cytoskeleton, thereby providing additional stabilization of the actin cytoskeleton. Blocking N-cadherin function in cultured hippocampal neurons alters the normal distribution of post-synaptic proteins and leads to increased numbers of filopodia, suggesting that N-cadherin helps scaffold the postsynaptic proteins that mediate spine stability (Togashi et al. 2002). Here, it is important to remember that diverse cadherin isoforms present at the synapse may play redundant roles in spine stabilization (Manabe et al. 2000).
Overexpression of αN-catenin increases spine head width and reduces dendritic spine turnover. αN-catenin-deficient neurons have more motile and destabilized spines (Abe et al. 2004). Deleting β-catenin after synaptogenesis destabilizes mushroom spines and increases the number of thin filopodia-like spines (Okuda et al. 2007). Cultured hippocampal neurons from β-catenin knockout mice exhibit greater spine density with reduced head width and length, suggesting that β-catenin also plays a stabilizing role in spines (Arikkath 2009). Interestingly, p120ctn deletion in mature neurons also causes a significant reduction in N-cadherin levels, decreased Rac1 activity, and increased RhoA activity. These alterations result in a significant loss of dendritic spines and synapses in vitro and in vivo (Elia et al. 2006), highlighting the role of catenins in maintaining dendritic spine stability.
Neurexin/Neuroligin Complexes
Neuroligins are postsynaptic transmembrane proteins that bind to presynaptic neurexins to stabilize synaptic contacts. Neuroligins form complexes with scaffolding proteins via their intracellular domain, thereby regulating synaptic function and stability (Craig & Kang 2007). Overexpression of Neuroligin-1 (NL-1) in cultured hippocampal neurons results in altered spine morphology, with an increase in spine-like protrusions and excitatory synaptic puncta (Chih et al. 2005). Knockdown of either neuroligin isoform reduces spine density and suppresses excitatory synapse formation (Chih et al. 2005). Although knockdown of neuroligins in cultured neurons shows reduced synapse formation, cultured NL1-3 triple knockout neurons have synapses that are indistinguishable from wild-type neurons (Chih et al. 2005, Varoqueaux et al. 2006). Although synapse numbers are not changed in NL1-3 knockout neurons, glutamatergic and GABAergic synaptic activity is significantly reduced. These studies suggest that neuroligins support long-term synaptic maintenance by recruiting receptors to the synapse and allowing proper synaptic function in vivo. In humans, deletions or mutations in neuroligins are associated with several cognitive disorders, including Autism spectrum disorders, mental retardation, and schizophrenia, but the precise genetic and biochemical mechanisms by which these mutations contribute to disease are still unknown (Sudhof 2008).
Ephrins and Eph Receptors
Eph receptors, including both EphA and EphB receptors, are receptor tyrosine kinases located on the postsynaptic compartment. EphAs (including EphA1-8 and A10) and EphBs (EphB1-4, and B6) interact with their presynaptic ligands, ephrinAs 1–5 and ephrinBs 1–3, respectively, to form bidirectional adhesion and signaling complexes that regulate synapse formation and maintenance (Kayser et al. 2008, Klein 2009). EphB1-3 knockout neurons exhibit an increased number of dendritic filopodia, whereas knockdown of EphB receptors reduces spine density (Kayser et al. 2008). Besides interacting with its ligand, the EphB receptor also interacts in cis with syndecan-2, a heparin sulfate proteoglycan enriched at post-synaptic sites (Ethell & Yamaguchi 1999, Irie & Yamaguchi 2004, Rapraeger & Ott 1998). Syndecan-2 regulates dendritic spine morphology via the actin cytoskeleton by signaling through EphB2 receptors and the downstream Rho GTPase (Ethell et al. 2001, Irie & Yamaguchi 2004, Penzes et al. 2003, Shi et al. 2009, Tolias et al. 2007). Knockdown of syndecan-2 in mature hippocampal neurons results in reduced spine numbers (Lin et al. 2007).
Ephrin-A3 is the predominant ephrin-A isoform in the adult hippocampus. EphA4, the ephrin-A3 receptor, localizes on dendritic spines while ephrin-A3 localizes on astrocytes, together forming a bridge between the dendritic spine and surrounding glia cell. EphA4 activation by ephrin-A3 inhibits integrin adhesion pathways and therefore destabilizes dendritic spines. However, disrupting EphA4-ephrin interaction or deletion of EphA4 also results in aberrant spine morphology, although the overall dendritic structure is normal (Bourgin et al. 2007, Murai et al. 2003). These studies suggest that glial cells provide negative cues via ephrin-A3 and EphA4 signaling to regulate spine stability (Ethell & Pasquale 2005). EphA4 activation by ephrin-A1 recruits and activates cyclin-dependent kinase 5, resulting in activation of the RhoGEF ephexin1, leading to increased Rho activity and spine destabilization (Fu et al. 2007). In other contexts, EphA4 activation can contribute to Rac1-mediated spine stabilization (Inoue et al. 2009). A recent study indicates that EphA4 is processed by γ-secretase to enhance the formation and maintenance of dendritic spines (Inoue et al. 2009). Mutation of presenilin1 (PS1), a subunit of γ-secretase, impairs EphA4 processing, and this may contribute to the pathology of the familial AD resulting from PS1 mutations. Thus, EphA4 activation may generate opposite effects on spine maintenance that are dependent on the stimuli and downstream signaling pathways.
Integrins
Integrins are the major mediators of cell-ECM interactions. Functional integrin receptors are formed by heterodimers of α and β subunits (Juliano 2002). Integrins bind to ECM and relay intracellular signaling events that promote actin assembly via the recruitment of actin polymerization regulators or structural proteins (e.g., actin crosslinker proteins) that couple directly to the actin cytoskeleton (Blystone 2004, DeMali et al. 2003).
Inhibiting β 1-integrin signaling using ligand-blocking peptides results in the transformation of existing dendritic spines to elongated dendritic filopodia (Bourgin et al. 2007, Shi & Ethell 2006). This destabilization of dendritic spines is mediated by NMDA receptor and CaMKII activation; blocking NMDAR and CaMKII activation reverts the destabilization phenotype. One major consequence of blocking β 1-integrin signaling is dephosphorylation of Crk-associated substrate (Cas). Correspondingly, Cas knockdown decreases dendritic spine density and length, phenotypes similar to those observed when β 1-integrin signaling is blocked (Bourgin et al. 2007). Blocking β 1-integrin signaling with disrupting peptides leads to dramatic and rapid retraction of dendritic arbors of chick retinal ganglion cells, a phenotype that may result from loss of stabilizing synaptic contacts (Marrs et al. 2006).
Although it is diffusely expressed in neurons, integrin α5, which can pair with β 1, localizes to synapses after synaptic stimulation, where it regulates spine stability via Src and Rac activation. This process depends on the activation of GIT1, a signaling adaptor that localizes Rac (Webb et al. 2007). Integrin α5 knockdown in cultured hippocampal neurons leads to an 80% decrease of synapse numbers and a reduced number of spines and dendritic protrusions (Webb et al. 2007). Several integrin ligands, such as laminin and reelin, have also been shown to affect dendritic spine stability (Liu et al. 2001, Seil 1998). Mutations or altered expression of these ligands have been linked to neurological disorders, including schizophrenia and AD, suggesting that altering integrin signaling may be involved in disease pathology (Costa et al. 2001, Huang et al. 1995, Liu et al. 2001, Rodriguez et al. 2000, Zhan et al. 1995).
SYNAPTIC ACTIVITY PROMOTES LONG-TERM SYNAPSE AND DENDRITE MAINTENANCE: THE IMPORTANCE OF TROPHIC SUPPORT PATHWAYS
Classic experiments demonstrated that destruction of afferent axons leads to the atrophy of target dendrites (Coleman & Riesen 1968, Jones & Thomas 1962, Le Gros Clark 1957, Matthews & Powell 1962). Postsynaptic degeneration could result simply from the physical loss of the presynaptic pairing partner; however, manipulations that reduce synaptic activity (e.g., odor or light deprivation) also compromise synapse and dendritic spine number and overall dendrite maintenance (Benson et al. 1984, Valverde 1967). These findings provided the first hints that synaptic activity supports long-term synapse maintenance. Subsequent work has revealed that the pairing of pre- and postsynaptic compartments is reinforced by the transmission of stabilizing signals between the two compartments. In this section, we review how trophic signaling pathways act to promote long-term synapse and dendrite stability.
BDNF and TrkB Mediate Bidirectional Stabilizing Cross-Talk Across the Synapse
Brain-derived neurotrophic factor (BDNF) was first identified as a factor that allowed survival of neurons that were unresponsive to nerve growth factor (NGF) (Barde et al. 1982). Subsequent work has demonstrated that BDNF is a member of the NGF family of neurotrophins, which includes NGF, NT-3, and NT-4/5 (Carvalho et al. 2008, Reichardt 2006). BDNF binds to both the P75NTR receptor and the tyrosine receptor kinase B (TrkB) receptor, but we focus on TrkB binding and its role in promoting long-term synapse and dendrite maintenance (Carvalho et al. 2008).
The loss of BDNF or TrkB in the mouse brain leads to dramatic and widespread loss of dendritic spines and dendritic arbors in diverse neuronal populations (Baquet et al. 2004, Gorski et al. 2003, Schober et al. 1998, Xu et al. 2000). These effects are especially prominent in the cerebral cortex, which is significantly smaller in both BDNF- and TrkB-deficient brains (Gorski et al. 2003, Xu et al. 2000). Both knockout animals also exhibit an increased neuronal packing density that results from a gross reduction in cortical neuron dendrite arbor size. Reductions in hippocampal spine density are reported in aged trkB+/− animals, suggesting that aging and reduced BDNF signaling may confer vulnerability to synapse loss (von Bohlen und Halbach et al. 2003).
Both TrkB and BDNF are expressed by pre-and postsynaptic neurons. BDNF is stored in vesicles in the pre- and postsynaptic compartments and can be released by depolarization and/or glutamatergic stimulation (Hartmann et al. 2001, Kohara et al. 2001, Kojima et al. 2001). Once released, BDNF exerts a stabilizing influence on both the pre- and postsynaptic compartments (Figure 4a). BDNF signaling through TrkB is essential to stabilize presynaptic release sites in Xenopus optic neuron axons (Hu et al. 2005, Marshak et al. 2007). TrkB is also expressed postsynaptically at the neuromuscular junction where it colocalizes in muscle with acetylcholine receptors (AChRs) (Gonzalez et al. 1999). A kinase-deficient splice TrkB isoform (TrkB.t1) can block AChR clustering and destabilize neuromuscular post-synaptic specializations (Gonzalez et al. 1999). Similarly, TrkB.t1 can also induce postsynaptic dendritic degeneration when expressed in facial motor neurons (De Wit et al. 2006).
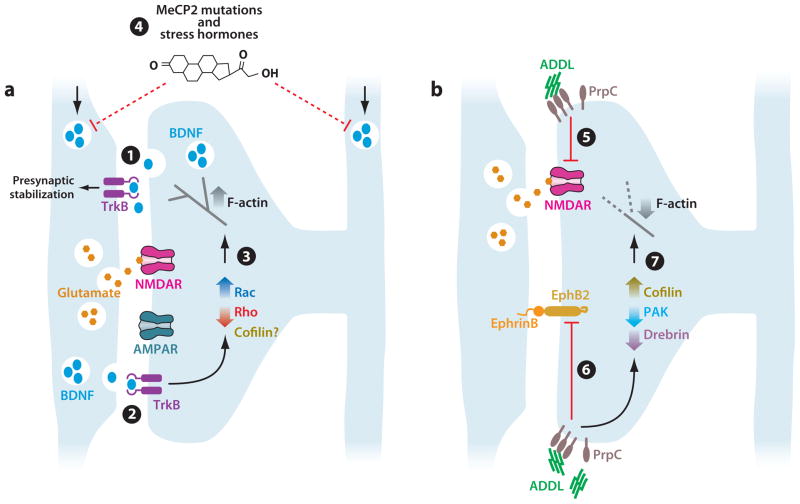
Figure 4.
Targeting of synaptic stabilization mechanisms in disease. (a) Model for synapse stabilization by BDNF signaling through TrkB. 1. Synaptic activity stimulates BDNF release from the postsynaptic compartment, which binds to presynaptic TrkB receptors. This leads to stabilization of the presynaptic compartment. 2. Activity-based BDNF release from the presynaptic compartment activates postsynaptic TrkB. 3. Postsynaptic TrkB may activate Rac, inhibit Rho, and regulate cofilin to stimulate increased F-actin assembly to stabilize synapses. 4. Mutations in the MeCP2 gene, as in Rett syndrome, and elevated stress hormones likely destabilize synapses by reducing BDNF levels. (b) Model for synapse destabilization by Aβ-derived diffusible ligands. 5, 6. Binding of ADDL (Aβ-derived diffusible ligands) to its receptor PrpC on the dendritic spine leads to reduced amounts of both NMDA receptor (5) and EphB2 (6), both of which help stabilize synapses. 7. ADDL stimulation increases cofilin activity, decreases PAK activity, and reduces drebrin levels leading to reduced F-actin levels and spine shrinkage.
BDNF acts through TrkB to stimulate several well-characterized downstream signaling cascades, including activation of Ras GTPase, Erk1/2 MAP kinase, phospholipase C gamma, and phosphatidylinositol-3-kinase (Carvalho et al. 2008, Reichardt 2006). Ongoing work should clarify which, if any, of these pathways mediate the stabilizing influence of BDNF on synapses. In addition, BDNF stimulates several cytoskeletal effector pathways that may be especially relevant for these processes. For example, BDNF stimulation promotes cofilin dephosphorylation in developing neuron growth cones, leading to increased growth cone filopodia (Fass et al. 2004, Gehler et al. 2004). Similar activation of BDNF signaling may promote cofilin-dependent F-actin network assembly at synapses. In support of this, bath application of BDNF to hippocampal slices synergizes with electrical stimulation to produce robust postsynaptic F-actin assembly (Rex et al. 2007). However, BDNF treatment also increases cofilin phosphorylation under these conditions. Determining the precise role of cofilin will require testing how nonphosphorylatable “activated” or phosphomimetic “inactive” cofilin affects synaptic F-actin assembly in response to activity.
BDNF signaling through TrkB also acts on the Tiam1, a GTPase exchange factor, to activate the Rac1 GTPase, stimulating local F-actin assembly (Miyamoto et al. 2006). Consistent with Rac1 as a downstream target, BDNF stimulation activates the PAK in hippocampal brain slices (Rex et al. 2007). In addition, the kinase-deficient TrkB.t1 isoform has a unique intracellular sequence that can bind RhoGDI, a Rho inhibitor. BDNF activation stimulates release of RhoGDI from TrkB.t1 to attenuate Rho signaling (Ohira et al. 2006). Thus, BDNF may promote spine and synapse stability via TrkB-mediated activation of Rac and inhibition of Rho.
Loss of BDNF signaling in neurons may contribute to developmental and neurodegenerative disorders in humans. The majority of Rett syndrome (RS) cases are caused by mutations in the MeCP2 DNA binding protein (Van den Veyver & Zoghbi 2000), and BDNF expression is diminished in Mecp2-null mice (Chang et al. 2006). These mutants exhibit similar pathology to brain region-specific BDNF-knockout mice, and mice deficient for both proteins exhibit greatly reduced viability compared with either single knockout. BDNF overexpression in Mecp2-null mice increases survival rates and spontaneous synaptic activity. These observations suggest deficiencies in BDNF may contribute to the pathology of Rett syndrome. Furthermore, TrkB mRNA levels are known to decrease in the aging rat and human hippocampus and may contribute to reductions in synapses, dendritic spines, and dendritic complexity that occur in the aging brain (Silhol et al. 2005, Uylings et al. 2000, Webster et al. 2006).
Neuregulin-1 Stimulates ErbB4 to Enhance Excitement (and Stability) at the Synapse
Neuregulin-1 (Nrg1) is a growth factor that binds and activates the ErbB family of receptor tyrosine kinases. ErbB4 is enriched in the postsynaptic density, and its activity can be increased or decreased by treatments that elevate or inhibit synaptic activity, respectively (Li et al. 2007). Overexpression of ErbB4 in hippocampal slices leads to dendritic spine enlargement, whereas ErbB4 knockdown leads to spine shrinkage and loss. ErbB4 overexpression increases AMPA currents, an effect that requires ErbB4 kinase activity and activity of the NMDA receptors, prominent tyrosine kinase substrates. Knockdown of ErbB4 leads to relative reductions in both AMPA and NMDA currents, but this effect is blocked by Nrg1 knockdown in the presynaptic neuron. Exogenous Nrg1 can also promote increased numbers of spines and spine enlargement in cultured hippocampal neurons. Together, these data suggest that presynaptic Nrg1 signaling to post-synaptic ErbB4 supports long-term synapse maintenance by promoting synaptic activity and dendritic spine size. In support of this model, conditional knockout of both ErbB2 and ErbB4 in mice leads to a reduction in hippocampal and cortical dendritic spine density, despite the apparent absence of other gross neuronal defects (Barros et al. 2009).
SYNAPSE LOSS AND DENDRITIC ATROPHY ARE COMMON IN PSYCHIATRIC AND NEURODEGENERATIVE DISORDERS
Reduced synaptic connectivity and dendritic atrophy are hallmarks of several psychiatric disorders and neurodegenerative diseases, where they are associated with impairments in perception, affect, and cognition. Although defects in initial circuit development may contribute to these conditions, the impairments of brain function often only become manifest in late adolescence or adulthood. An emerging theme is that the pathophysiological changes responsible for these diseases act by specifically targeting mechanisms of synapse and dendrite maintenance. In this section, we review these pathological conditions, with a specific emphasis on the current view of how disease-specific mechanisms are believed to destabilize synapses and dendrites.
Major Depression, Chronic Stress, and Cushing’s Disease: Synapse Destabilization by Corticosteroids
MDD is a debilitating disorder characterized by pervasive sadness; feelings of guilt or worthlessness; reduced engagement in pleasurable activities; and alterations in sleep, eating, and work patterns. Several anatomical features consistent with a reduction in synapses and dendritic arbor extent have been observed in the hippocampi and anterior cingulate and orbitofrontal cortices of individuals with MDD, including reduced tissue volume, decreased cell soma size, increased neuronal packing density, and decreased staining for dendritic spine markers (Cotter et al. 2002, 2001; Law et al. 2004; Rajkowska et al. 1999; Stockmeier et al. 2004). These observations strongly suggest that reduced synaptic connectivity may contribute to MDD symptomology.
Chronic stress is a significant risk factor for MDD (Kendler et al. 1999, Pittenger & Duman 2008). In rats, chronic restraint stress leads to significant dendritic atrophy in the hippocampus and prefrontal cortex, which are areas affected in humans with MDD (Cook & Wellman 2004, Radley et al. 2008, Watanabe et al. 1992). Stress leads to an elevation of the corticosteroid stress hormones, which has been causally linked to synapse loss and dendritic regression. For example, daily injection of corticosteroids over three weeks leads to reduced synapse density in the rat hippocampal CA3 region (Tata et al. 2006), accompanied by regression of dendrite arbors (Woolley et al. 1990), and similar effects have been observed in the prefrontal cortex (Wellman 2001).
Elevated circulating corticosteroids are also found in patients with Cushing’s disease, a multisymptom disease caused by pituitary tumors that result in increased levels of adreno-corticotropic hormone, which stimulates elevated cortisol production. A similar disorder, Cushing’s syndrome, is sometimes observed in individuals undergoing excessive long-term glucocorticoid treatment for inflammatory and autoimmune diseases (Patil et al. 2007). Imaging studies reveal a significant loss of cortical and subcortical brain volume in these patients, likely reflecting a significant loss of synapses and dendrite arbors (Patil et al. 2007).
Long-term elevation of corticosteroids causes synapse loss and dendritic regression by interfering directly with synapse and dendrite maintenance mechanisms. Steroids act through their receptors, which are DNA-binding transcriptional regulators. Significant efforts have focused on identifying the relevant target genes regulated by corticosteroids. Both chronic stress and chronic corticosteroid exposure decrease hippocampal BDNF mRNA and protein (Gourley et al. 2009, Nibuya et al. 1999, Smith et al. 1995), and BDNF levels are also reduced in MDD patients (Schmidt & Duman 2007) (Figure 4a). This finding suggests that corticosteroids cause synapse loss and dendritic atrophy by attenuating trophic support provided by BDNF:TrkB signaling. In support of this hypothesis, direct infusion of BDNF into the brain can ameliorate the depressive symptoms that accompany synapse loss and dendritic atrophy in animal models of chronic stress or depression (Gourley et al. 2008, Shirayama et al. 2002). Also, antidepressant treatments lead to an elevation of BDNF levels in MDD patients and appear to block the inhibition of BDNF levels caused by chronic stress or corticosteroids in animal models (Schmidt & Duman 2007). Moreover, genetic disruption or blockade of BDNF:TrkB signaling can prevent the efficacy of antidepressant treatment in chronic stress animal models (Schmidt & Duman 2007).
Although these studies implicate reduced BDNF:TrkB signaling as a major contributor to the behavioral symptoms of chronic stress disorders and MDD, the cellular and molecular mechanisms by which antidepressants or BDNF infusion reduce depression-like symptoms are not yet known. It will be particularly interesting to examine whether these treatments act by restoring synapse loss and reducing dendritic atrophy associated with these disorders.
Alterations in Nrg1:ErbB4 Signaling: A Link to Schizophrenia
Schizophrenia is a brain disorder characterized by significant changes in perception, including hallucinations and delusions, but also by flat affect, reduced socialization, and cognitive impairment. Increased neuronal packing density in the prefrontal cortex of the schizophrenic brain provided the first hints that reduced synaptic connectivity may contribute to its pathophysiology (Selemon & Goldman-Rakic 1999). Reduced immunostaining for presynaptic markers (Glantz & Lewis 1997, Woo et al. 1998), decreased dendritic spine density, and reductions in dendritic arbor size have been noted in schizophrenia patients (Broadbelt et al. 2002, Glantz & Lewis 2000, Kalus et al. 2000).
Recent studies revealed an association between polymorphisms in nrg1 and erbb4 and increased risk for schizophrenia (Mei & Xiong 2008). These mutations may compromise Nrg1:ErbB4-mediated trophic synapse support, thereby leading to reductions in synaptic connectivity. Such an erosion of synaptic connectivity would be consistent with the decrease in glutamatergic function observed in schizophrenia (Mei & Xiong 2008). However, Nrg1 stimulation increases ErbB4 signaling and heightens the attenuation of NMDA receptor phosphorylation in tissue isolated from schizophrenia patients (Hahn et al. 2006), suggesting ErbB4 signaling pathways may be hypersensitized in schizophrenia. Thus, a major unresolved issue is exactly how synaptic Nrg1:ErbB4 signaling is disrupted in schizophrenia and if this contributes to schizophrenia pathophysiology.
Aβ-Derived Diffusible Ligands in Alzheimer’s Disease: Synaptic Superdestroyers?
AD is the most common form of human dementia and it leads to progressive impairments in memory, cognition, and behavior. The brains of affected individuals exhibit significant synapse loss and dendritic arbor regression, followed by neuronal death (Anderton et al. 1998; Flood 1991; Flood et al. 1987a,b; Hanks & Flood 1991). The degree of cognitive and memory impairment in AD patients correlates with the extent of synapse and dendritic arbor loss, and not with neuronal death (Falke et al. 2003, Terry et al. 1991). Thus, preventing synapse loss and dendritic regression represents a major therapeutic target for AD treatment.
One hallmark of AD that distinguishes it from the normal aging brain is the presence of elevated levels of amyloid beta (Aβ) peptide fragments derived from the amyloid precursor protein. Aβ peptides form soluble aggregates, termed Aβ-derived diffusible ligands (ADDLs), and insoluble aggregates, termed amyloid plaques (APs), in the AD brain. APs are often found in contact with dystrophic neurites, neuronal processes with abnormal trajectories that may reflect axon or dendrite degeneration adjacent to the plaque, but AP burden does not correlate well with the extent of disease symptomology (Haass & Selkoe 2007). ADDL levels correlate better than APs with the cognitive impairment in AD patients, and injection of patient-derived ADDLs into animals can induce acute memory loss, suggesting a direct link between ADDLs and AD symptomology (Haass & Selkoe 2007, Shankar et al. 2008).
Several recent studies characterized the potent ability of ADDLs to induce synapse destabilization (Figure 4b). Soluble Aβ oligomers can bind directly and preferentially to dendritic spines and promote spine destabilization and functional synapse loss (Lacor et al. 2007, Lauren et al. 2009, Ma et al. 2008a, Shankar et al. 2007). Aβ oligomer application leads to reductions in the EphB2 and NMDA receptors (Lacor et al. 2007). In addition, ADDL application leads to reductions in PAK levels and activity (Zhao et al. 2006). These reductions correlate with increased levels of the actin-severing protein cofilin, and decreased levels of the F-actin-stabilizing spine protein drebrin (Lacor et al. 2007, Zhao et al. 2006). These changes appear to have functional relevance as parallel changes in PAK, cofilin, and drebrin are observed in AD patient samples (Zhao et al. 2006). Moreover, cofilin activity is essential for the spine-destabilizing effects of ADDLs (Shankar et al. 2007).
In addition to their effects on cytoskeletal signaling pathways, ADDLs induce cofilin aggregation (Zhao et al. 2006). Similar cofilin-rich insoluble aggregates, termed Hirano bodies, are found in AD brains. Cofilin aggregates disrupt the microtubular network within neurites, and may “choke off” synapses by preventing delivery of materials via the microtubule highway (Minamide et al. 2000). Together, these data suggest that ADDLs may be “synaptic superdestroyers” that target synapses for destabilization by interfering simultaneously with synaptic adhesive systems (EphB2), activity-based maintenance mechanisms (NMDA receptor), and cytoskeletal stabilization mechanisms (PAK, drebrin, and cofilin). The recent identification of prion precursor protein (PrP) as a functional ADDL receptor should greatly promote efforts to understand how ADDL triggers the chain of events that result in synapse destabilization (Lauren et al. 2009).
Alterations in Rho GTPase signaling may also contribute to synapse destabilization in AD. One recent study demonstrated an increase in Rho activity and a decrease in Rac activity in an APP transgenic AD mouse model (Petratos et al. 2008). The decreased Rac1 activity may underlie the reductions in PAK levels or activity noted in AD brains (Zhao et al. 2006). These pathways may represent important targets for therapies to block AD progression.
Acknowledgments
We apologize to our colleagues whose research could not be appropriately cited owing to space limitations. We thank Thomas Biederer, Ron Duman, Shannon Gourley, Charles Greer, Michael Koelle, Susumu Tomita, and Sloan Warren for thoughtful discussions and critical feedback on the review. Our research is supported by NIH Grants NS39475 and CA133346, an American Heart Association Established Investigator Award, awards from the Yale Interdisciplinary Research Consortium on Stress, Self-Control and Addiction, and an anonymous donor.
- Filopodia
thin, finger-like structures with long, parallel bundled actin filaments that protrude from the cell surface
- Schizophrenia
a psychiatric disorder characterized by disturbed cognition, perception, and behavior
- MDD
major depressive disorder
- AD
Alzheimer’s disease
- Arp2/3 complex
the actin-related protein 2/3 complex that binds to the sides of actin filaments and nucleates new actin filament branches
- LIMK
LIM domain (lin-11, Isl-1, and Mec-3)-containing kinase
- PSD-95
post-synaptic density protein of 95 KD
- AMPA/NMDA receptors
Two glutamate-gated ion channels: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and NMDA (N-methyl D-aspartic acid) receptors. AMPA receptors mediate Na+ influx to allow membrane depolarization. This depolarization, together with glutamate binding, activates NMDA receptors to mediate Ca2+ influx
- ADF
actin depolymerizing factor
- Profilin
small actin-binding protein that exchanges ATP for ADP, allowing G-actin: ATP to be added to the barbed end of a growing actin filament
- Autism spectrum disorders
a class of developmental disorders characterized by deficits in social interaction and communication ability associated with restricted and stereotyped behavior
- Extracellular matrix (ECM)
extracellular protein meshwork consisting of proteins and proteoglycans that provides a substrate for cell adhesion and migration
- BDNF
brain-derived neurotrophic factor
- TrkB
tyrosine receptor kinase B
- Rett syndrome (RS)
a genetic disorder, primarily affecting female toddlers, characterized by a developmental regression: the loss of acquired language and motor skills, increased stereotyped behaviors, and mental retardation
- Amyloid beta (Aβ)
a peptide derived from cleavage of the amyloid precursor proteins. Soluble Aβ oligomers can lead to dendritic spine atrophy. Insoluble Aβ aggregates form amyloid plaques in the brains of individuals with Alzheimer’s disease
- ADDLs
Aβ-derived diffusible ligands
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Yu-Chih Lin, Email: yu-chih.lin@yale.edu.
Anthony J. Koleske, Email: anthony.koleske@yale.edu.
LITERATURE CITED
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci. 2004;7:357–63. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Ridenour DA, Sabatini BL. Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J Neurosci. 2007;27:7365–76. doi: 10.1523/JNEUROSCI.0956-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH, Callahan L, Coleman P, Davies P, Flood D, et al. Dendritic changes in Alzheimer’s disease and factors that may underlie these changes. Prog Neurobiol. 1998;55:595–609. doi: 10.1016/s0301-0082(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Espinosa A, Fairen A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–69. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Arikkath J. Regulation of dendrite and spine morphogenesis and plasticity by catenins. Mol Neurobiol. 2009;40:46–54. doi: 10.1007/s12035-009-8068-x. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–94. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrican B, Lisman J, Otmakhov N. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27:14007–11. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–58. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–53. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA. 2009;106:4507–12. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: evidence from Williams syndrome. Trends Neurosci. 1999;22:197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- Benson TE, Ryugo DK, Hinds JW. Effects of sensory deprivation on the developing mouse olfactory system: a light and electron microscopic, morphometric analysis. J Neurosci. 1984;4:638–53. doi: 10.1523/JNEUROSCI.04-03-00638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O, Ganiatsas S, Kannourakis G, Dringen R. Kiz-1, a protein with LIM zinc finger and kinase domains, is expressed mainly in neurons. Cell Growth Differ. 1994;5:1159–71. [PubMed] [Google Scholar]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–26. doi: 10.1038/31940. [DOI] [PubMed] [Google Scholar]
- Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur J Neurosci. 2008;27:2847–59. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- Blystone SD. Integrating an integrin: a direct route to actin. Biochim Biophys Acta. 2004;1692:47–54. doi: 10.1016/j.bbamcr.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007;178:1295–307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–36. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, et al. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–22. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28:13673–83. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–24. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–48. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Cheng XT, Hayashi K, Shirao T. Non-muscle myosin IIB-like immunoreactivity is present at the drebrin-binding cytoskeleton in neurons. Neurosci Res. 2000;36:167–73. doi: 10.1016/s0168-0102(99)00123-6. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–28. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–42. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–26. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Cohen RS, Chung SK, Pfaff DW. Immunocytochemical localization of actin in dendritic spines of the cerebral cortex using colloidal gold as a probe. Cell Mol Neurobiol. 1985;5:271–84. doi: 10.1007/BF00711012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–27. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Riesen AH. Evironmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968;102:363–74. [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis. 2001;8:723–42. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–53. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–94. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit J, Eggers R, Evers R, Castren E, Verhaagen J. Long-term adeno-associated viral vector-mediated expression of truncated TrkB in the adult rat facial nucleus results in motor neuron degeneration. J Neurosci. 2006;26:1516–30. doi: 10.1523/JNEUROSCI.4543-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–82. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–73. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–55. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–81. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB, Yamaguchi Y. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31:1001–13. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol. 1999;144:575–86. doi: 10.1083/jcb.144.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke E, Nissanov J, Mitchell TW, Bennett DA, Trojanowski JQ, Arnold SE. Subicular dendritic arborization in Alzheimer’s disease correlates with neurofibrillary tangle density. Am J Pathol. 2003;163:1615–21. doi: 10.1016/S0002-9440(10)63518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass J, Gehler S, Sarmiere P, Letourneau P, Bamburg JR. Regulating filopodial dynamics through actin-depolymerizing factor/cofilin. Anat Sci Int. 2004;79:173–83. doi: 10.1111/j.1447-073x.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–11. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–50. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–97. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–99. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–54. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. II. Subiculum. Brain Res. 1991;540:83–95. doi: 10.1016/0006-8993(91)90494-g. [DOI] [PubMed] [Google Scholar]
- Flood DG, Buell SJ, Horwitz GJ, Coleman PD. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987a;402:205–16. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Flood DG, Guarnaccia M, Coleman PD. Dendritic extent in human CA2–3 hippocampal pyramidal neurons in normal aging and senile dementia. Brain Res. 1987b;409:88–96. doi: 10.1016/0006-8993(87)90744-x. [DOI] [PubMed] [Google Scholar]
- Frangiskakis JM, Ewart AK, Morris CA, Mervis CB, Bertrand J, et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86:59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of nonmuscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Gehler S, Shaw AE, Sarmiere PD, Bamburg JR, Letourneau PC. Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin. J Neurosci. 2004;24:10741–49. doi: 10.1523/JNEUROSCI.2836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:660–69. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, et al. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–83. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–65. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–16. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008;64:884–90. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–72. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–28. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Halpain S. Actin in a supporting role. Nat Neurosci. 2003;6:101–2. doi: 10.1038/nn0203-101. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–44. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res. 1991;540:63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–48. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–97. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–97. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999;19:3918–25. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–66. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–83. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–91. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–29. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpaa K, Faix J, et al. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol. 2009;185:323–39. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–98. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Huang DY, Weisgraber KH, Strittmatter WJ, Matthew WD. Interaction of apolipoprotein E with laminin increases neuronal adhesion and alters neurite morphology. Exp Neurol. 1995;136:251–57. doi: 10.1006/exnr.1995.1101. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, et al. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–64. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EPHB receptor signaling in dendritic spine development. Front Biosci. 2004;9:1365–73. doi: 10.2741/1325. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, et al. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem. 1994;269:29928–33. [PubMed] [Google Scholar]
- Ivanov A, Esclapez M, Pellegrino C, Shirao T, Ferhat L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J Cell Sci. 2009;122:524–34. doi: 10.1242/jcs.033464. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275:19324–33. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- Jones WH, Thomas DB. Changes in the dendritic organization of neurons in the cerebral cortex following deafferentation. J Anat. 1962;96:375–81. [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–65. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–68. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. Drebrin A content correlates with spine head size in the adult mouse cerebral cortex. J Comp Neurol. 2007;503:618–26. doi: 10.1002/cne.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–23. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, et al. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Weis WI, Nelson WJ. Catenins: playing both sides of the synapse. Curr Opin Cell Biol. 2007;19:551–56. doi: 10.1016/j.ceb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis DM, Reese TS. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol. 1983;97:1169–78. doi: 10.1083/jcb.97.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–32. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161:1848–55. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark W. Inquiries into the anatomical basis of olfactory discrimination. Proc R Soc Lond. 1957;146:299–319. doi: 10.1098/rspb.1957.0013. [DOI] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–16. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Lei S, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. J Neurosci. 2001;21:8464–72. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–97. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA. 2008;105:15791–96. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Lei YT, Hong CJ, Hsueh YP. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–41. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, et al. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA. 2001;98:3477–82. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, et al. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008a;283:14132–43. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang Y, Eipper BA, Mains RE. Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci. 2003;23:10593–603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, et al. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008b;28:12368–82. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–98. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–29. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Sasaki T, Asakura T, Hotta I, Imamura H, et al. Interactions of drebrin and gephyrin with profilin. Biochem Biophys Res Commun. 1998;243:86–89. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, et al. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci. 2000;15:534–46. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Honda T, Fuller L, Thangavel R, Balsamo J, et al. Dendritic arbors of developing retinal ganglion cells are stabilized by beta 1-integrins. Mol Cell Neurosci. 2006;32:230–41. doi: 10.1016/j.mcn.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–56. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Powell TP. Some observations on transneuronal cell degeneration in the olfactory bulb of the rabbit. J Anat. 1962;96:89–102. [PMC free article] [PubMed] [Google Scholar]
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci USA. 1982;79:7590–94. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci. 2003;14:233–40. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–33. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamaguchi H, Suetsugu S, Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–35. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–36. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci USA. 2006;103:10444–49. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizui T, Takahashi H, Sekino Y, Shirao T. Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol Cell Neurosci. 2005;30:149–57. doi: 10.1016/j.mcn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Moresco EM, Koleske AJ. Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr Opin Neurobiol. 2003;13:535–44. doi: 10.1016/j.conb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–60. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–302. doi: 10.1101/gad.1783809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Van Aelst L. Rho-linked genes and neurological disorders. Pflugers Arch. 2008;455:787–97. doi: 10.1007/s00424-007-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–82. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–38. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–55. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–60. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Ohira K, Homma KJ, Hirai H, Nakamura S, Hayashi M. TrkB-T1 regulates the RhoA signaling and actin cytoskeleton in glioma cells. Biochem Biophys Res Commun. 2006;342:867–74. doi: 10.1016/j.bbrc.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–23. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y. Beta-catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci USA. 2007;104:13479–84. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil CG, Lad SP, Katznelson L, Laws ER., Jr Brain atrophy and cognitive deficits in Cushing’s disease. Neurosurg Focus. 2007;23:E11. doi: 10.3171/foc.2007.23.3.13. [DOI] [PubMed] [Google Scholar]
- Peacock JG, Miller AL, Bradley WD, Rodriguez OC, Webb DJ, Koleske AJ. The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol Biol Cell. 2007;18:3860–72. doi: 10.1091/mbc.E07-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–74. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275:6395–403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, et al. The neuronal Rho-GEF kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–42. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Petratos S, Li QX, George AJ, Hou X, Kerr ML, et al. The beta-amyloid protein of Alzheimer’s disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain. 2008;131:90–108. doi: 10.1093/brain/awm260. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Proschel C, Blouin MJ, Gutowski NJ, Ludwig R, Noble M. Limk1 is predominantly expressed in neural tissues and phosphorylates serine, threonine and tyrosine residues in vitro. Oncogene. 1995;11:1271–81. [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–56. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–50. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38:357–68. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rapraeger AC, Ott VL. Molecular interactions of the syndecan core proteins. Curr Opin Cell Biol. 1998;10:620–28. doi: 10.1016/s0955-0674(98)80038-0. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signaling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–29. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A, et al. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Natl Acad Sci USA. 2000;97:3550–55. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–14. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–70. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19:8454–63. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J, Liu L, Wong TP, Wu DC, Burette A, et al. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–82. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–30. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antide-pressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schober A, Wolf N, Huber K, Hertel R, Krieglstein K, et al. TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla. J Neurosci. 1998;18:7272–84. doi: 10.1523/JNEUROSCI.18-18-07272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Da Silva JS, Dotti CG. Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J Cell Biol. 2006;172:453–67. doi: 10.1083/jcb.200506136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–12. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- Seil FJ. The extracellular matrix molecule, laminin, induces purkinje cell dendritic spine proliferation in granule cell depleted cerebellar cultures. Brain Res. 1998;795:112–20. doi: 10.1016/s0006-8993(98)00265-0. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27:10982–92. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–22. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29:8129–42. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao T, Kojima N, Kato Y, Obata K. Molecular cloning of a cDNA for the developmentally regulated brain protein, drebrin. Brain Res. 1988;464:71–74. doi: 10.1016/0169-328x(88)90020-4. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–24. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–11. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–46. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–11. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 2003;23:6586–95. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the Rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–38. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–40. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–74. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci USA. 2007;104:7265–70. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–94. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, West MJ, Coleman PD, De Brabander JM, Flood DG. Neuronal and cellular changes in the aging brain. In: Clark CM, Trojanowski JQ, editors. Neurodegenerative Dementias: Clinical Features and Pathological Mechanisms. New York: McGraw-Hill; 2000. pp. 61–76. [Google Scholar]
- Valverde F. Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res. 1967;3:337–52. doi: 10.1007/BF00237559. [DOI] [PubMed] [Google Scholar]
- Van den Veyver IB, Zoghbi HY. Methyl-CpG-binding protein 2 mutations in Rett syndrome. Curr Opin Genet Dev. 2000;10:275–79. doi: 10.1016/s0959-437x(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–54. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency in trkB and/or trkC neurotrophin receptors causes structural alterations in the aged hippocampus and amygdala. Eur J Neurosci. 2003;18:2319–25. doi: 10.1046/j.1460-9568.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wigston DJ, Rees HD, Levey AI, Falls DL. LIM kinase 1 accumulates in presynaptic terminals during synapse maturation. J Comp Neurol. 2000;416:319–34. doi: 10.1002/(sici)1096-9861(20000117)416:3<319::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–45. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Zhang H, Majumdar D, Horwitz AF. Alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem. 2007;282:6929–35. doi: 10.1074/jbc.M610981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–51. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–36. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–46. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–26. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci. 1999;19:4472–83. doi: 10.1523/JNEUROSCI.19-11-04472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, et al. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–42. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–74. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–91. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–56. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, et al. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–45. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Kobayashi S, Umeda T, Inoue A, Sakagami H, et al. Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning. J Neurosci. 2009;29:7607–18. doi: 10.1523/JNEUROSCI.0707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Youn H, Jeoung M, Koo Y, Ji H, Markesbery WR, et al. Kalirin is underexpressed in Alzheimer’s disease hippocampus. J Alzheimers Dis. 2007;11:385–97. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- Zanni G, Saillour Y, Nagara M, Billuart P, Castelnau L, et al. Oligophrenin 1 mutations frequently cause X-linked mental retardation with cerebellar hypoplasia. Neurology. 2005;65:1364–9. doi: 10.1212/01.wnl.0000182813.94713.ee. [DOI] [PubMed] [Google Scholar]
- Zha XM, Dailey ME, Green SH. Role of Ca2+/calmodulin-dependent protein kinase II in dendritic spine remodeling during epileptiform activity in vitro. J Neurosci Res. 2009;87:1969–79. doi: 10.1002/jnr.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan SS, Kamphorst W, Van Nostrand WE, Eikelenboom P. Distribution of neuronal growth-promoting factors and cytoskeletal proteins in altered neurites in Alzheimer’s disease and nondemented elderly. Acta Neuropathol. 1995;89:356–62. doi: 10.1007/BF00309629. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–53. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–81. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–42. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–89. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]