Abstract
Schistosomiasis is an important public health concern in more than 76 developing countries. Advent of an anti-schistosome vaccine would undoubtedly add to the existing control measures and may eventually help in the elimination of this disease. In the present study we have attempted to dissect the role(s) of antibodies in Sm-p80 mediated protection by intravenously transferring pooled sera from mice immunized with Sm-p80-pcDNA3 or purified IgG from baboons immunized with Sm-p80-pcDNA3, into naïve C57BL/6 mice, respectively, prior to challenge with cercariae. The passive transfer of antibodies from protected mice (homologous transfers) as well as transfer of total IgG from baboons (heterologous transfers), into naïve mice showed statistically significant reductions in worm burden and the number of eggs in the tissues. Immunizations of antibody knockout mice (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) with recombinant Sm-p80 in the presence of CpG-motif oligodeoxynucleotides as an adjuvant, resulted in substantial reduction of Sm-p80-mediated protection, compared to C57BL/6 (normal) control group of mice. Down regulation of cytokines that have important effects on B cell proliferation as well as the recovery of higher number of parasites in antibody knockout indicated a significant role(s) of antibodies in Sm-p80-mediated protection against Schistosoma mansoni in mice. In toto, these studies appear to suggest that antibodies play a significant role in Sm-p80 mediated protection.
Keywords: Schistosoma mansoni, Sm-p80, passive transfer, antibody knockout mice
Introduction
In spite of the ongoing efforts to control human schistosomiasis, the morbidity of the disease is still on the rise. Currently there are 210 million estimated infections[1;2] and up to 779 million people are at risk of getting the infection[3;4]. It is obvious that the current control programs to reduce morbidity and mortality have been inadequate and many commonly employed measures have been ineffective especially in the settings of the developing world[3–7]. It would therefore be sagacious to also concentrate on other potential control strategies, for examples, an appropriate protective vaccine that could be integrated into ongoing control programs[6–8]. To this effect, application of contemporary progress to move towards testing and evaluating candidate vaccines with respect to formulation, adjuvant systems and spectrum of immune responses would be an important step forward.
Efforts to develop a so far elusive vaccine has led to the description of more than one hundred schistosome antigens that have shown varying degrees of protection against challenge infection in animal models, predominately in mice[6;9;10]. In this regard, one notable antigen, Sm-p80 has shown significant and consistent protective efficacy that has been mostly characterized by cellular effectors related to important mediator cytokines especially IL-2, IL-12 and IFN-γ in mice and nonhuman primate models[11–15]. Sm-p80 has been implicated in schistosome immune evasion process through its role(s) in surface membrane turnover[15;16]. Even though a large repertoire of Sm-p80-based immunization strategies have been shown to greatly reduce both adult worms and egg production in vaccinated animals[12;17–22]; the role(s) of antibodies in Sm-p80-mediated protections has not yet been fully elucidated. Therefore the objective of the present study was to decipher the role(s) of antibodies in Sm-p80-mediated protection via the transfer of sera obtained from mice vaccinated with Sm-p80-pcDNA3[19] and transfer of purified total IgG obtained from the baboons vaccinated with Sm-p80-pcDNA3[18] into naïve C57BL/6 mice and then determine the levels of protection following an experimental challenge. To further dissect the role(s) of antibodies and to corroborate the results of passive transfer experiments, using a previously optimized Sm-p80-based vaccination strategy[18], antibody deficient mice (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) of the genetic backbone of the C57BL/6 [23] were immunized and the protection was determined. Results of both passive transfer and immunizations using knockout mice revealed an important role for antibodies in Sm-p80-mediated protection against experimental challenge infection in mice.
Materials and Methods
Parasites
Biomphalaria glabrata snails, infected with Schistosoma mansoni were provided by the Schistosomiasis Resource Center, Biomedical Research Institute, Rockville, MD.
Source of Serum and purified IgG for Passive Transfer Experiments
The sera used in homologous transfer experiments were obtained from C57BL/6 mice which were used in our previously published studies[19]. These mice were immunized with either pcDNA3 alone or with Sm-p80-pcDNA3[19]. For the heterologous experiments, IgG was purified from sera of individual baboons, which were used in our previously published studies[18]. Individual baboons were immunized with either pcDNA3 or Sm-p80-pcDNA3[18].
Animals, Passive Transfer and Immunization
This study comprised of three separate components: (i) passive transfer of sera obtained from previously immunized mice[19], into naïve mice, i.e., homologous transfer (Ho); (ii) passive transfer of purified IgG obtained from previously immunized baboons[18] into naïve mice, i.e., heterologous transfer (He); and (iii) immunization of normal C57BL/6 and antibody knockout mice with previously optimized Sm-p80-based immunization scheme[19] Briefly, 107 naïve C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA) and 20 antibody deficient mice (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) were purchased from Jackson Laboratories (Bar Harbor, ME).
For the homologous transfer experiments, 20 C57BL/6 mice were randomly divided into two groups [control (HoC) and experimental (HoE)]. The control group of mice received 100µl pooled sera obtained from mice previously immunized with pcDNA3[19]; whereas the animals in the experimental group were given 100µl pooled sera obtained from mice previously vaccinated with Sm-p80-pcDNA3[19]. Intravenous injections were given at days −6 and −2 before challenge with schistosome cercariae. This schedule was selected based on the average in vivo half life of heterologous antibodies, in particular mouse IgG which is 6–8 days for the first half life [24] however, antibodies persist for at least 10 additional days after the first half life in the murine system. The first half life of most nonhuman primates IgG is at least 2 weeks[25]. This schedule thus allows for appreciable amounts of antibodies to be present when the mice were experimentally challenged and reach lung stage which peaks at day 5–6 for S. mansoni [26].
For the heterologous transfer experiments, 57 C57BL/6 naïve mice were divided into two groups [20 mice in the control group (HeC) and 37 mice in the experimental group (HeE)]. In this case, Melon TM Gel IgG spin purification kits were used to purify IgG from sera sample of each baboon a[18]s per the manufacturer’s protocol (Thermo Fisher Scientific Inc., Rockford, IL). One hundred µg of purified total IgG from individual baboons (IT08, IT09, and IT12; see [18]for further details) immunized with pcDNA3, was given intravenously to mice in the control group. Similarly, mice in the experimental group were given 100µg of purified total IgG obtained from individual baboons (IT94, IN07, IG29, IP15, IP17, and IG97; see [18] for further details) vaccinated with Sm-p80-pcDNA3. Intravenous injections were given at days −6 and −2 before challenge with cercariae.
For the rSm-p80 immunization experiment, 20 antibody knockout mice (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) were divided into two groups (10 animals each for control and experimental groups, respectively). In parallel, 20 normal C57BL/6 mice (10 for the control group and 10 for the experimental group) were also used in this experiment for comparison purposes. Each animal in the control groups of both the antibody knockout mice and C57BL/6 group received 50µg of synthetic CpG-motif ODN (Coley Pharmaceutical Group, Wellesley, MA) followed by two successive monthly immunizations for two months; whereas the experimental groups of knockout mice and normal C57BL/6, received 25µg of rSm-p80 plus 50µg of CpG-motif ODN[17] Mice from all of the groups were boosted with the same formulation, a month apart, for two months, as previously outlined[17] All the above described experiments were repeated at least twice.
Parasite challenge, worms and egg count determination
Two days after the last serum or IgG transfer (day zero); all of the mice from both passive transfer groups were exposed to 150 cercariae. The animals which were immunized with ODN or rSm-p80 plus ODN were exposed to 150 cercariae one month after the last booster. Six weeks post-challenge, all of the animals were sacrificed and adult parasites were recovered by perfusion of the hepatic portal vein. Reduction in worm burden and egg production was determined as previously outlined[17–19;21;22;27]
Enzyme-linked immunosorbent assay (ELISA)
Sera samples were collected from mice of both homologous and heterologous passive transfer experiments, immediately before sacrifice. Using ELISA, antibody titers for IgA, IgG (including all subtypes IgG1, IgG2a, IgG2b, IgG3, and IgG4) and IgM were determined, as described previously[18;19]
Splenocyte proliferation assays and cytokine production by proliferating splenocytes
After sacrificing each animal from both homologous and heterologous passive transfer experiments as well as the rSm-p80 immunization of antibody knockout experiments, the spleen of each animal was minced and washed in RPMI 1640 complete media. For in vitro splenocyte proliferation assays, the concentration of rSm-p80 was optimized to develop a standard assay; a 96-well flat-bottom plates seeded with 5×105 pooled splenocytes of each respective group in total volume of 200µl, then stimulated with 0.5µg nonspecific mitogen (ConA) or with 1.2µg rSm-p80. Subsequently, the cells were incubated at 37°C with 5% CO2, for 24 h and after removing the supernatant of each sample for cytokine assessment, proliferation was determined by MTT as described previously[18] ELISA Panel Kit (eBioscience, San Diego, CA) was used to estimate the production of IL-2, IL-4, IL-10 and IFN-γ in the supernatant of the proliferating splenocytes as described previously[17;19;21;27].
In vitro expression of cytokines in Sm-p80 stimulated splenocytes by RT-PCR
The splenocyte samples from each animal of both homologous and heterologous passive transfer experiments as well as the rSm-p80 immunization of antibody knockout and normal C57BL/6 groups were pooled in their respective groups. The cells were then cultured for 24h in six-well plates at 370C with 5% CO2. Triplicate wells were treated with rSm-p80 (12µg/ml); and the other three wells without any antigen stimulation as described previously[17]. The RNA from each group was extracted by TRIzol reagent. Established standard procedures were used for reverse transcription reactions of cDNA synthesis and for the estimation of relative expression of major cytokines[17;21;22;27].The relative concentration of each cytokine was determined using the Quantity One Program (Version 4.6.2., Bio-Rad, Hercules, CA). Relative fold difference in cytokine mRNA expression was calculated after rSm-p80 stimulated control and experimental groups minus 1.
Flow Cytometric Analysis
In order to track Th1 and Th2 profile changes after immunization of the antibody knockout and C57BL/6 mice, the harvested splenocytes were seeded into 24 well plate (2×106 cells each well) for flow cytometry assay. The cells were incubated with or without rSm-p80 (10µg/ml) overnight; and 4h before the end of incubation, Brefeldin A at a dilution of 1:1000 was added. Direct staining was performed to indentify CD3 and CD8 positive cells. Intracellular staining was performed to detect IFN-γ or IL-4 secreting cells. The data obtained were analyzed using BD CellQuset™ Prosoftware (BD Biosciences, San Jose, CA).
Statistical analysis
For the analysis of these data, MINTAB 15.1 (Kruskal-Wallis Test) was applied to make comparison of control and experimental groups involving non-normal distributions. SigmaPlot version 11.0 (One-way ANOVA followed by Dunnetts procedure) was used to compare experimental and control groups; minimum type I error of α = 0.05 was considered for statistically significant differences.
Results
Protection at the level of worm and egg reduction
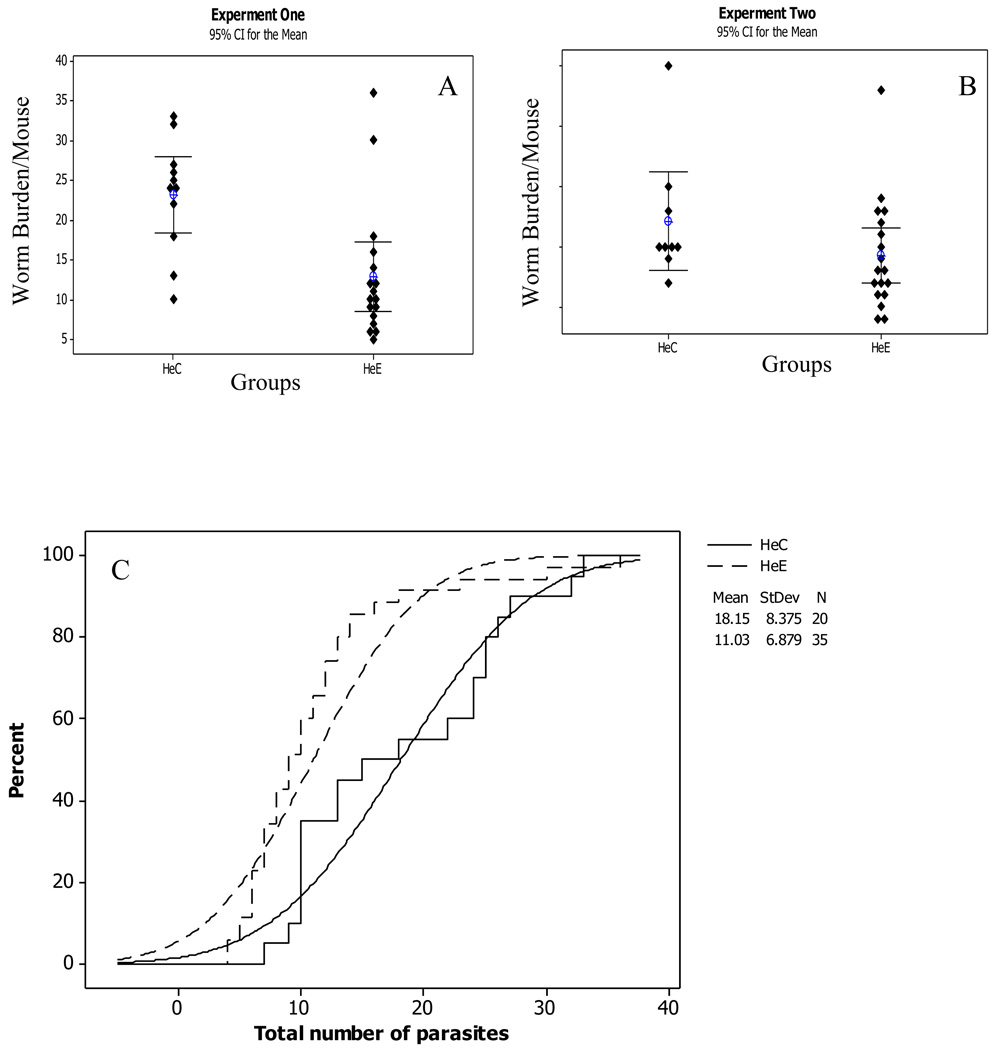
The homologous serum transfer and heterologous purified IgG transfer from mice [19] and baboons[18] immunized with Sm-p80 were repeated twice as shown in (Figs 1 and 2). The results of both independent experiments were combined to determine the mean number of adult parasites per individual mouse and the corresponding percent recovery (Fig 1C and Fig 2C). The empirical cumulative distribution function curves indicate the relative effect of the Sm-p80 specific serum and IgG transfer. The parasite recovery in the homologous experimental animals (HoE) was significantly reduced (p < 0.01) with a relative protection of 31%. Similarly in the purified IgG transfer experiment (HeE), the number of worms recovered was reduced in the experimental animals as compared to their respective controls. The reduction in the number of parasites in the heterologous experimental test group was 45% (p < 0.01). The recovery of parasites in the knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) experimental group was lower compared to its respective control; the relative protection in this case was 18% (p<0.05). However, the corresponding parasite reduction in the normal C57BL/6 experimental group was 63% (p< 0.01).
Figure 1.
Protection in homologous (Ho) serum transfer from Sm-p80 immunized mouse. This experiment was done twice; (A) and (B) are two similar experiments; the result of both experiments are combined in (C); the percentage of worm recovery in the control (HoC) animals indicated by solid lines and in experimental (HoE) animals by broken lines; from the empirical cumulative distribution function curves, number of worms in the HoE group has significantly reduced relative to the controls (95% CI for the Mean, p < 0.01).
Figure 2.
Protection in heterologous (He) purified IgG transfer from Sm-p80 immunized baboons. This experiment was repeated twice independently (A) and (B) and results of both are combined in (C). The percentage of worm recovery in the control (HeC) was indicated by solid lines, while broken lines represent the experimental (HeE). The curves show the relative effect of purified IgG transfer. The number of worms in HeE group has significantly reduced (95% CI for the Mean, p = 0.001).
The number of eggs per gram of the liver and intestine was also quantified and is summarized in Table 1. In both tissues the numbers of eggs were significantly lower in homologous and heterologous experimental groups. The decrease in egg production in mice of the homologous transfer experiment was 59%, whereas the reduction in egg production of the heterologous transfer group of mice was 90%. Both of these values were statistically significant (p < 0.01). Additionally, the egg reduction was 47% in the normal C57BL/6 experimental immunization group. The corresponding reduction in egg production in the antibody knockout animals was lower (36%), however egg reduction in both groups was significant (p < 0.05).
Table 1.
Worm load, egg output in liver and intestine (homologous or heterologous IgG transfer) and in liver and intestine of (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) knockout and normal C57BL/6 mice).
| Groups (Transfer/Immunization) |
n | Worms Mean (P value) |
Eggs/g of liver Mean (P value) |
Eggs/g of intestine Mean (P value) |
% protection¥ (Worm/Eggs) |
|---|---|---|---|---|---|
| HoC (Sera) | 10 | 25.6 | 12886.21 | 3991.90 | |
| HoE (Sera) | 10 | 16.0(p<0.01) | 5324.87(P<0.01) | 1644.61(p<0.01) | 31.14/58.71 |
| HeC (IgG) | 20 | 17.8 | 11983.12 | 1662.23 | |
| HeE (IgG) | 35 | 11.0(p<0.01) | 998.82(P<0.01) | 362.12(p<0.001) | 45.0/90.02 |
| KoC (ODN) | 9 | 29.0 | 4929.6 | 2288.3 | |
| KoE (ODN + rSm-p80) | 9 | 23.8(p<0.05) | 3466.0(P<0.05) | 1152.9(p<0.01) | 18.0/36.0 |
| NC (ODN) | 10 | 28.2 | 1411.1 | 339.7 | |
| NE (ODN + rSm-p80) | 10 | 10.4 (p<0.01) | 758.2 (P<0.01) | 169.1 (p<0.01) | 63.12/47.0 |
The experiments were repeated twice; the codes: HoC, Homologous Control; HoE Homologous Experimental; HeC, Heterologous Control; HeE, Heterologous Experimental; NC, Normal C57BL/6 control; NE, Normal Experimental; KoC and KoE are Knockout control and Knockout experimental C57BL/6 mice respectively. KoC and NC were immunized with ODN, but KoE and NE were immunized with rSm-p80 plus ODN.
Total worm/or eggs (1-E/C) X 100.
Antibodies Profiles
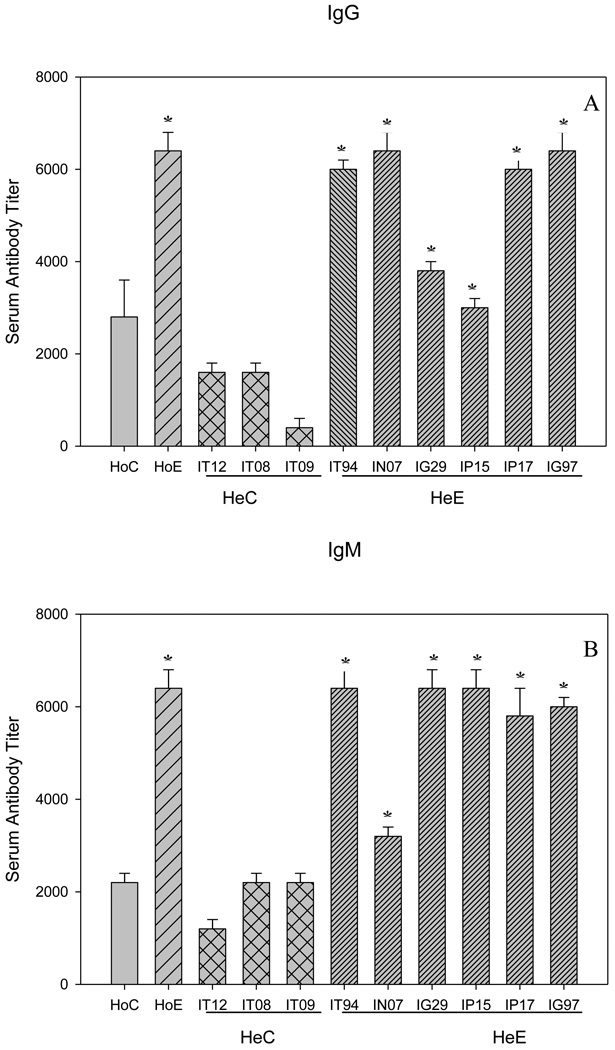
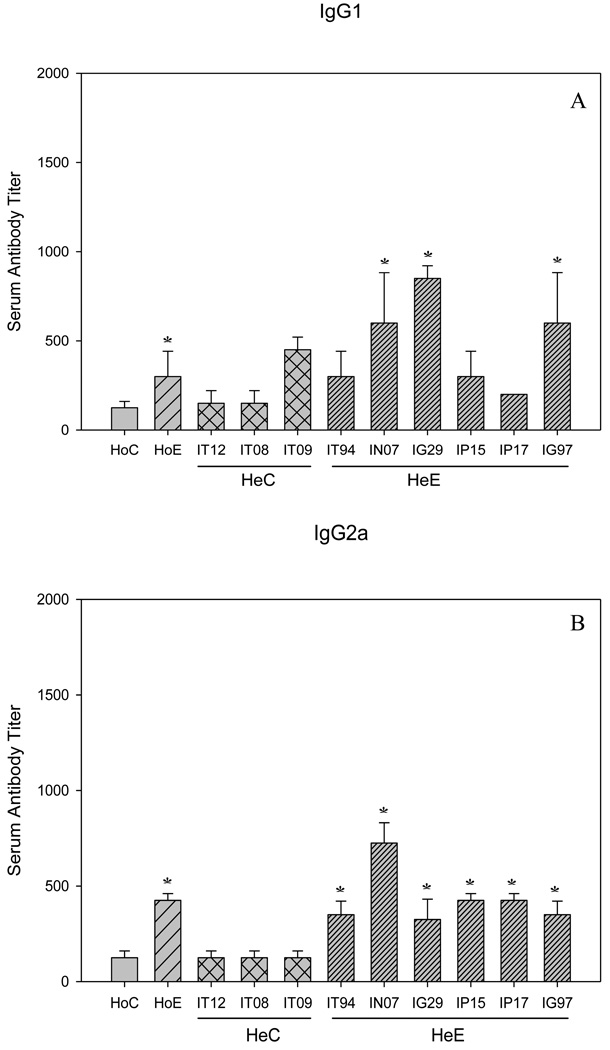
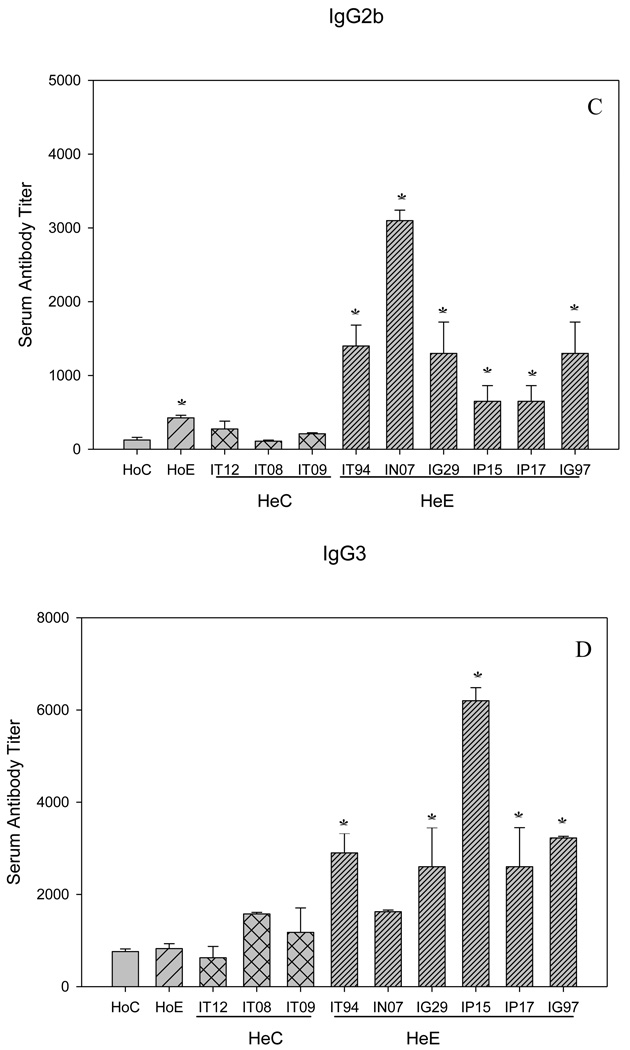
In addition to the passively transferred antibodies, in both Sm-p80 specific serum and IgG transfer experiments, the considerable protection as a function of adult parasite reduction and decrease in egg production may be due to the induction of antibody responses in the test groups. The titers of both IgG and IgM ranged from 3200 to 6400 (Fig 3, A and B). The end titers of IgG2a and IgG2b are 800 and 3200 respectively; that is relatively lower nevertheless the differences were statistically significant as compared to controls (Fig 4, B and C). The titers of IgG1 and IgG3 tend to be higher, 800 and 6400, respectively; but not in all groups of mice that received serum or purified IgG (Fig 4, A and D). IgA was not detectable in both transfer experiments (data not shown). In summary, IgM, IgG and some of the IgG subtypes (Fig 4, A–D) were found to be significantly induced in Sm-p80 specific serum (HoE) and purified IgG (HeE) groups of mice.
Figure 3.
Sm-p80 specific antibody titers in immunized mice. Total IgG (A), and IgM (B), the given titer values represent the mean of triplicate tests (±SEM). Significance (*P) is considered at p < 0.01; 95 % confidence interval. IT12, IT08, IT09, IT94, IN07, IG29, IP15, IP17, IG97 are the identifiers of baboons from which the IgG was purified. Details of vaccination experiment has been given previously[18].
Figure 4.
Sm-p80 specific antibody titers of IgG subclasses in both homologous (Ho) and heterologous (He) experiments. Shown in panels (A) IgG1; (B) IgG2a; (C) IgG2b and (D), IgG3. The given titer values represent the mean of triplicate tests, standard error of the mean (SEM). Significance (*p) is considered at P < 0.01; 95 % confidence interval. IT12, IT08, IT09, IT94, IN07, IG29, IP15, IP17, IG97 are the identifiers of baboons from which the IgG was purified. Details of vaccination experiment has been given previously[18].
In vitro splenocytes proliferation and cytokines
The in vitro proliferation of splenocytes from transfer groups showed marked differences between the Sm-p80 specific antibody transfer groups and its corresponding controls (Table 2). Proliferations of splenocyte resulted in a significantly higher stimulation as compared to the controls without rSm-p80 stimulation. Higher IFN-γ production was observed in the homologous experimental group (228.6 pg/ml) and the change was significant in the heterologous experimental animals (125.3 pg/ml) (Table 3). The level of IL-2 production was relatively higher but insignificant in response to Sm-p80. However, the profile of these cytokines was very different in knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) experimental animals (KoE) as well as in its corresponding normal experimental (NE) animals which received a combination of rSm-p80 plus ODN immunizations (Table 3). Both IL-2 and IFN-γ production in the supernatant of proliferating splenocytes were quite high, in both of these groups. The difference of IFN-γ was more than double which was statistically significant (p<0.01). However, the changes in the level of IL-4 and IL-10 were not statistically significant (p>0.05).
Table 2.
Proliferation of splenocytes of homologous and heterologous mice as well as the (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) knockout and normal C57BL/6 mice after in vitro rSm-p80 stimulation
| Groups (Transfer/Immunization) |
Stimulation™ |
|||
|---|---|---|---|---|
| n | Mock (media) | Con A | Sm-p80 | |
| HoC (Sera) | 10 | 11.4 | 57.7 | 15.4 |
| HoE (Sera) | 10 | 10.2 | 46.6 | 25.9* |
| HeC (IgG) | 20 | 4.5 | 28.4 | 12.3 |
| HeE (IgG) | 35 | 10.2 | 48.0 | 21.8* |
| KoC (ODN) | 9 | 5.0 | 46.1 | 31.9 |
| KoE (ODN + rSm-p80) | 9 | 32.0 | 74.4 | 58.0** |
| NC (ODN) | 10 | 47.6 | 76.3 | 50.3 |
| NE (ODN + rSm-p80) | 10 | 36.2 | 114.1 | 71.3** |
The given codes: HoC, Homologous Control; HoE, Homologous Experimental; HeC, Heterologous Control; HeE, Heterologous Experimental; NC, Normal C57BL/6 control; NE, Normal Experimental; KoC and KoE are Knockout control and experimental C57BL/6 mice respectively. KoC and NC were immunized with ODN, but KoE and NE were immunized with rSm-p80 plus ODN; ODN, oligodeoxynucleotides; ™Proliferation of splenocytes in percent
p < 0.01,
p < 0.001
Table 3.
The level of cytokine production of Sm-p80 stimulated splenocytes of (homologous and heterologous transfer mice) and (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) knockout and normal C57BL/6 mice after rSm-p80 stimulation in vitro for 24 hours; values are given in pg/ml (Mean±SD).
| Groups (Transfer/Immunization) |
n | IL-2 (pg/ml) | IL-4 (pg/ml) | IL-10 (pg/ml) | INF-γ (pg/ml) |
|---|---|---|---|---|---|
| HoC (Sera) | 10 | 4.4±0.8 | 2.1±0.0 | 3.1±0.8 | 98.2±1.0 |
| HoE (Sera) | 10 | 4.7±0.2 | 2.3±0.1 | 3.3±1.2 | 228.6±1.1** |
| HeC (IgG) | 20 | 4.2±0.0 | 2.2±0.0 | 3.2±1.2 | 98.2±1.1 |
| HeE (IgG) | 35 | 4.5±0.0 | 2.2±0.1 | 3.3±0.8 | 125.3±4.4* |
| KoC (ODN) | 9 | 5.94±0.73 | 9.81±2.59 | 38.96±6.31 | 111.1±23.57 |
| KoE (ODN + rSm-p80) | 9 | 23.25±5.89* | 11.66±0.93€ | 51.10±0.88€ | 258.13±7.39* |
| NC (ODN) | 10 | 12.71±3.58 | 8.30±0.47 | 39.46±1.17 | 103.70±15.71 |
| NE (ODN + rSm-p80) | 10 | 19.40±5.67* | 12.00±0.47€ | 58.00±1.75€ | 278.14±8.32* |
The given codes: HoC, Homologous Control; HoE, Homologous Experimental; HeC, Heterologous Control; HeE, Heterologous Experimental; NC, Normal C57BL/6 control; NE, Normal Experimental; KoC and KoE are Knockout control and experimental C57BL/6 mice respectively. KoC and NC were immunized with ODN, but KoE and NE were immunized with rSm-p80 plus ODN; ODN, oligodeoxynucleotides;
p < 0.001;
p < 0.01;
p > 0.05
mRNA cytokine expression via RT-PCR
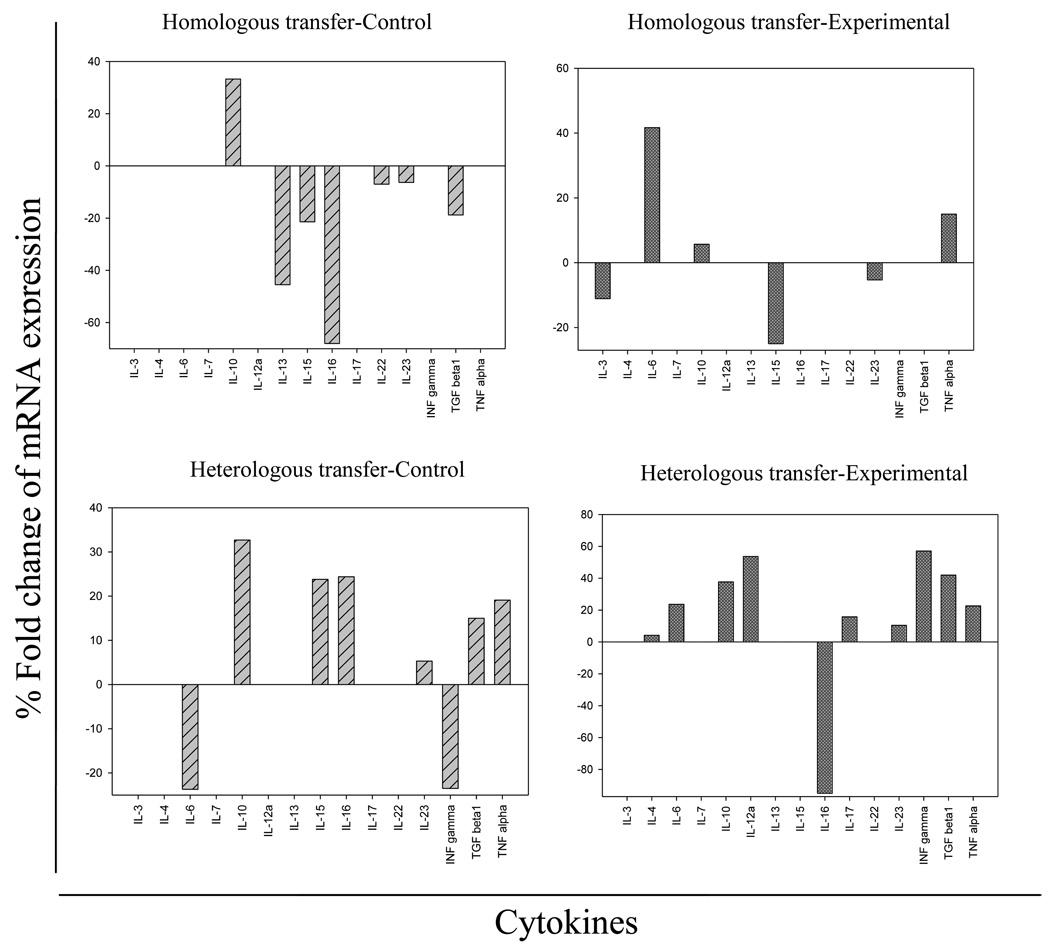
Additionally the mRNA cytokine expression of 27 cytokines was estimated after in vitro stimulation of splenocytes with Sm-p80 by RT-PCR. The expressions of several important cytokines were found to be changed when stimulated with the antigen. The cytokines which were upregulated or down-regulated are summarized for all transfer groups in Fig 5. The expression of cytokines (IL-6 and TNF-α) was induced in homologous experimental group (Fig.5, upper right panel), while IL-3 was down regulated; the remaining cytokines were not affected by the in vitro stimulation with rSm-p80. The cytokine expression in heterologous transfer groups also displayed a unique pattern. Cytokines including IL-6, IL-12α, IL-17, INF-γ and TGFβ1 were induced in the splenocytes of heterologous experimental animals (Fig. 5, lower right panel).
Figure 5.
mRNA expression of different cytokines after Sm-p80 stimulation of splenocytes from homologous (Ho) and hetrologous (He) transfer animals. The cytokines which either induced or inhibited after Sm-p80 stimulation are included in the figure; the left panels (A and C) are homologous control (HoC) and heterologous control (HeC) groups respectively; similarly the right panels (B and D) represent experimental groups of the homologous (HoE) and the heterologous (HeE) transfer experiments.
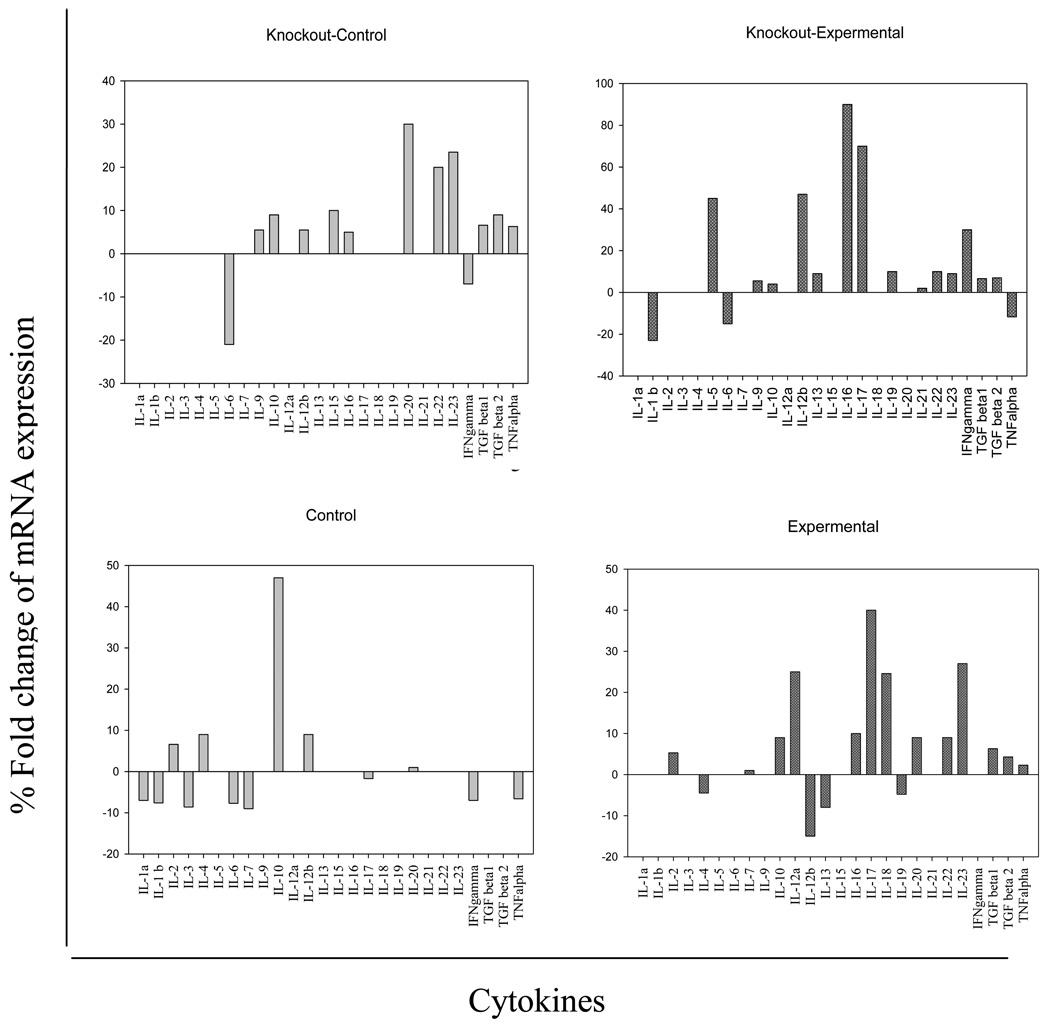
Similarly, in the rSm-p80 stimulated splenocytes of antibody knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) mice and matching normal C57BL/6 experiments (rSm-p80 and ODN) immunized groups, a number of the cytokines including IFN-γ, IL-5, IL-12β, IL-16, and IL-17 were upregulated (Fig.6, right upper panel). The number of upregulated cytokines in the normal C57BL/6 rSm-p80 plus ODN immunization group (Fig.6, right lower panel) was notable due to the increased expression of TGFβ1, TGFβ2, TNF-α, IL-2, IL-12α, IL-16, IL-17, IL-18, IL-20, IL-22 and IL-23. The comparison in all cases was based on the percent fold change as the level of quantified expressions on gels, as described previously[17;21;22]
Figure 6.
mRNA expression of different cytokines after Sm-p80 stimulation in antibody knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) and normal C57BL/6 mice. The cytokines which either upregulated or down-regulated after Sm-p80 stimulation are included in the figure; the upper panels (left and right) are knockout control (KoC) and knockout experimental (KoE); and similarly lower panels (left and right) represent control (NC) and experimental (NE) normal C57BL/6 mice respectively.
Flow Cytometery
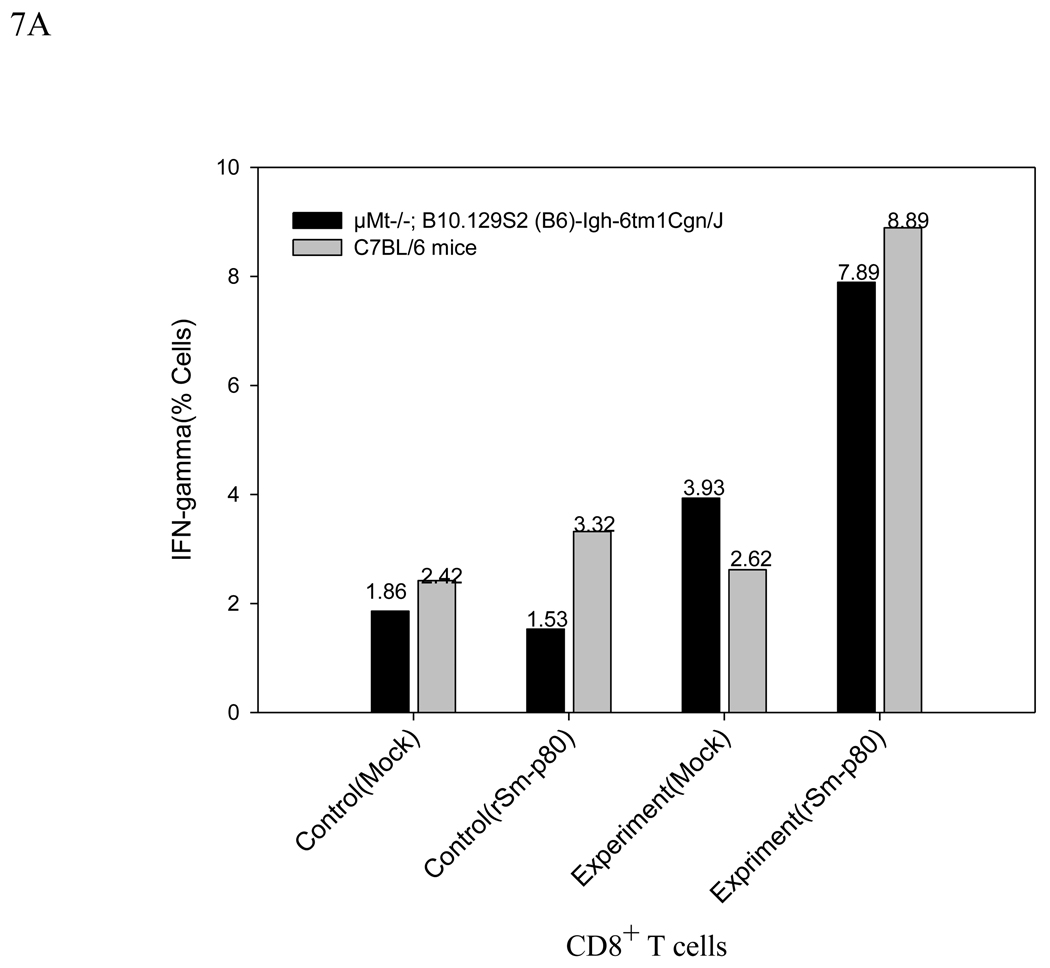
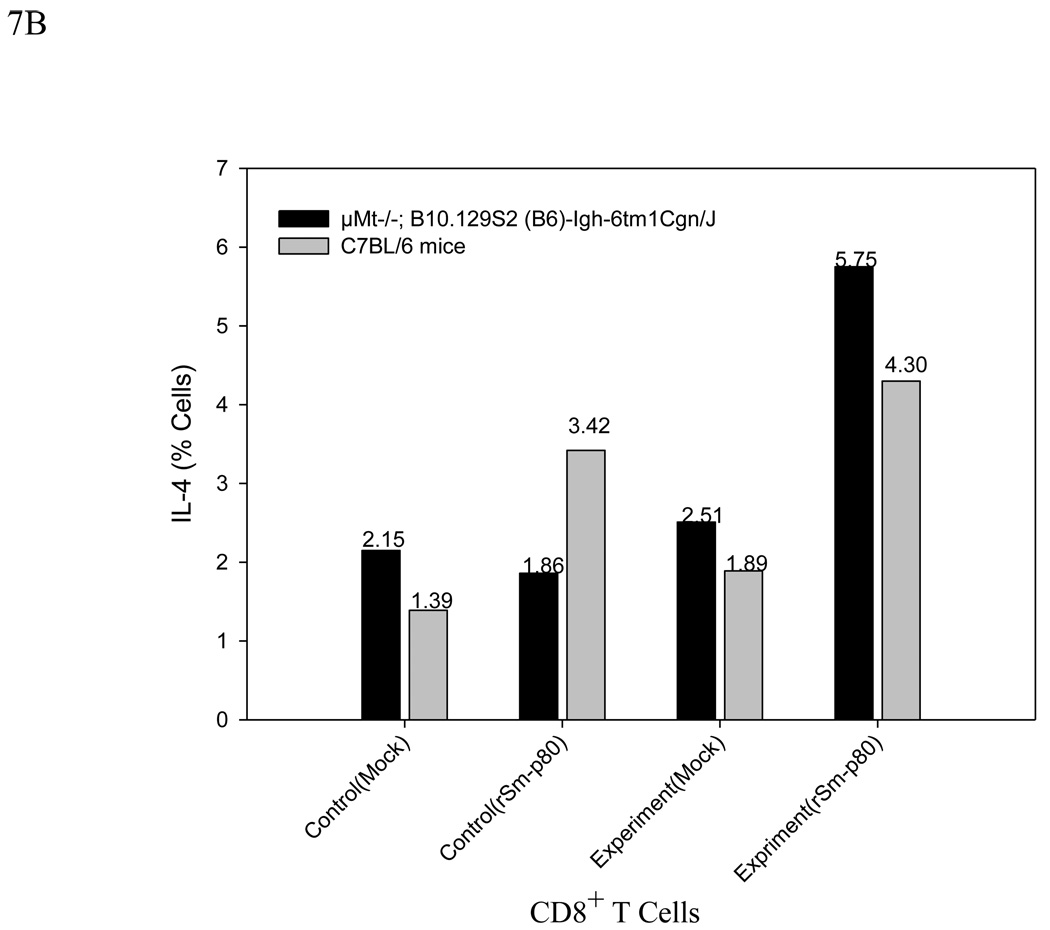
The in vitro rSm-p80-stimulated splenocytes of both ODN immunization and rSm-p80 plus ODN immunization groups of knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) and the normal animals were analyzed by flow cytometry for intracellular synthesis of IFN-γ and IL-4 by CD3 positive CD8 T cells. In the rSm-p80 plus ODN immunization groups, significant populations of CD8 T cells were stained for intracellular IFN-γ; specifically, 7.89%, and 8.89% for the knockout and the normal animals, respectively. Considerable populations of 5.75% (knockout) and 4.3% (normal) were stained for intracellular IL-4 as well. On the other hand, only ODN immunization of the antibody knockout mice induced 1.86% IL-4 and 1.53% IFN-γ producing CD8 T cells, and the same immunization in the normal animals induced 3.42% IL-4 and 3.32% IFN-γ producing CD8 T cells. In this case, the percentage of IFN-γ producing cell populations of the knockout control with rSm-p80 stimulation was significantly different from its corresponding negative control in which only 1.53 % of the CD8 T cells were IFN-γ producing. The flow cytometry results are summarized in Fig.7, A and B.
Figure 7.
A Flow cytometric analysis after 24h incubation without rSm-p80 (Mock) or with rSm-p80 stimulation. CD3 membrane marker gating is used to see IFN-γ production from CD3 positive CD8 T cells. Intracellular secretion of IFN-γ in splenocytes of control (ODN immunized) and experimental (rSm-p80 + ODN immunized) groups of both knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) (KoC) and normal C57BL/6 (NC) mice.
B Flow cytometric analysis after 24h incubation without rSm-p80 (Mock) or with rSm-p80 stimulation. CD3 membrane marker gating is used to see IL-4 production from CD3 positive CD8 T cells. Intracellular secretion of IL-4 in splenocytes of control (ODN immunized) and experimental (rSm-p80 + ODN immunized) groups of both knockout (µMt−/−; B10.129S2 (B6)-Igh-6tm1Cgn/J) (KoE) and normal C57BL/6 (NE) mice.
Discussion
Previous studies from our laboratory have demonstrated significant levels of Sm-p80 mediated protection against S. mansoni challenge; mostly the roles of signature cytokines IL-2, IL-12 and IFN-γ have been consistently demonstrated as the essential players of the antigen (Sm-p80) induced protection against the parasite[12;13;17–19;21;22;27]. This can be correlated to the production of toxic nitrogen oxide metabolites that have been implicated to be key mechanism of microbicidal activity of IFN-γ dependent effectors such as macrophages in killing the schistosomula of S. mansoni[28–32]. This is the first study that has demonstrated that upon transfer of Sm-p80 specific serum and purified IgG from Sm-p80-pcDNA vaccinated mice and baboons into naïve mice, confers protection against challenge infection. Simultaneous study of rSm-p80 immunization of the antibody knockout mice on the other hand, showed decreased but not completely abolished the Sm-p80-mediated protection as compared to higher protection in normal C7BL/6 mice. This indicates the importance of both antibodies and cellular effectors in the rSm-p80-mediated protective effects.
In our previously published studies, Sm-p80-pcDNA vaccination of C57BL/6 resulted in 59% and 84% reduction of worms and eggs respectively[19], and simultaneous direct Sm-p80-pcDNA immunization in baboons resulted in 38% and 32 % reduction of worms and eggs[18]. Similarly, in the present study, protection in the experimental transfer groups from the mice and baboons appeared to be significant; for example in the Sm-p80 specific serum transfer (homologous), the protection was 31.14% and 58.71% as the level of parasites and egg per gram of tissues; transfer of purified IgG from baboons similarly reduced worms by 45% and eggs by 90%, which is significant.
The notable drop in rSm-p80-mediated protection from 63% (in normal C57BL/6) to 18% in the antibody knockout group of mice has demonstrated that antibodies are one of the essential elements of Sm-p80-mediated protective components against S. mansoni challenge. This is reinforced by the consistent induction of antibodies, as described in our previously published studies on Sm-p80-based vaccination strategies[17–19;21;22] and by others[33–36]. The high reduction in egg production in the heterologous transfer is noteworthy and this effect may be a consequence of the parasite specific antibodies which may mediate the effector function of macrophages on the normal maturation[31] and coupling of female worms. This is consistent with our prior observations in which we have shown pronounced reductions in egg production using Sm-p80 vaccine formulations[18].
Our data clearly indicate that antibodies play a role in conferring Sm-p80-mediated protection; how is this achieved is still speculative. One possible mechanism may be via the antibody-dependent cellular cytotoxicity (ADCC)[37;38]. However, the broader concept of ADCC in schistosome immunity is being debated in literature[39]. Nonetheless, as it relates to this study, the parasite specific antibodies may bind to transforming immature parasites to target for immune effectors such as natural killer (NK) cells, monocytes [31] and other effectors through interactions with Fc region of the bound antibodies[40]. As noted previously, the killing of schistosomula has shown to be mediated by macrophages and endothelial cells through the key functions involving IFN-γ[41;42]. Parasite specific antibody transfer from irradiated cercariae induced similarly displayed protection from S. mansoni challenge[35]. The situation of ADCC and/or complement activation therefore could be one of the possible components of protection in both homologous and heterologous transfer experiments. The antibody responses and simultaneous induction of IFN-γ showed the significance of IFN-γ in proliferation of antibody producing B- cells[43]. This could be linked to the INF-γ and IL-2 in the Sm-p80 based vaccinations of mice[19] and baboons[18]. The considerable population of IFN-γ producing CD3+ CD8 T cells in the antibody knockout and normal animals (rSm-p80 and ODN immunization) gives the measure of cellular responses as well. Nevertheless, the reduced protection in the rSm-p80 immunized knockout mice is indicative that the production of antibodies is one of the important effectors induced by Sm-p80 based immunizations. Consequently, these results contradicted the notions that underestimated the roles of B cells in protecting schistosome infections[44]. The induction of IL-4 and INF-γ in the heterologous experimental group, are in fact associated with B-cell development and formation of plasma cells[43;45]. Moreover, the significance of IL-17 in mediating the crucial cross-talk between immune system and tissues can also be contemplated in the experimental transfer animals and in the rSm-p80 plus ODN immunizations. A role for IL-17 has already been implicated in tissue immunity[46–48]. From the mixed effectors responses we reported earlier[21;22], IL-17 seems to be a key linker of different arms of immune responses[17–19;32]. Possible regulatory events that involve IL-17 as well as IL-6 and IL-10 have also been suggested.[49]
The rationale for stimulating the recipient spleen cells with rSm-p80 for proliferation and cytokine analysis was that the migrating schistosome larvae release excretory and secretory proteins including Sm-p80 [50] as well as Sm-p80 released following surface membrane turnover by the adult parasite (and from dead parasites) is capable of priming host immune system. Since we believe that exposure to cercariae under natural conditions will provide the necessary boosters to the vaccine, we wanted to gather data from this experiment also, though this aspect is not directly related to the passive transfer of immunity. The stimulation of the essential cytokines and the intracellular staining of IFN-γ producing T cells confirm growth factor functions of the cytokines; that means as proliferation facilitating agent of antibody producing cells and networking with cell-mediated immune effectors[9;11–14;14;15;17–19;27]. The results of both mice and nonhuman primates have consistently confirmed that Sm-p80 vaccine based protections involve recruitment of antibodies as well as characteristic Th1 type responses. Additionally, this study has demonstrated that transfer of homologous or heterologous Sm-p80 specific antibodies or IgG can confer significant protection against S. mansoni challenge. Similarly, the protection in the antibody knockouts, mRNA expression of signature cytokines and flow analysis explicitly show the essential role of normal functioning B cells in amplifying protective effectors in Sm-p80 mediated immunity.
Acknowledgments
This work was supported in part by grants from the Thrasher Research Fund (Award No. 02824-5) and the National Institute of Allergy and Infectious Diseases (R01AI71223) to Afzal A. Siddiqui.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Berriman M, Haas BJ, LoVerde PT, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009 Jul 16;460(7253):352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007 Sep 6;357(10):1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines. 2008 Aug;7(6):745–752. doi: 10.1586/14760584.7.6.745. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006 Jul;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JL, Schleinitz MD, Carabin H, McGarvey ST. Decision-model estimation of the age-specific disability weight for schistosomiasis japonica: a systematic review of the literature. PLoS Negl Trop Dis. 2008;2(3):e158. doi: 10.1371/journal.pntd.0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008 Jan;21(1):225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturrock RF. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem Inst Oswaldo Cruz. 2001;96 Suppl:17–27. doi: 10.1590/s0074-02762001000900003. [DOI] [PubMed] [Google Scholar]
- 8.Hotez PJ, Ferris MT. The antipoverty vaccines. Vaccine. 2006 Jul 26;24(31–32):5787–5799. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008 Apr;102(5):825–833. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RA, Coulson PS. Why don't we have a schistosomiasis vaccine? Parasitol Today. 1998 Mar;14(3):97–99. doi: 10.1016/s0169-4758(97)01198-8. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui AA, Phillips T, Charest H, et al. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003 Jul;71(7):3844–3851. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui AA, Phillips T, Charest H, et al. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003 Jun 20;21(21–22):2882–2889. doi: 10.1016/s0264-410x(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005 Mar;12(1):3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005 Feb 10;23(12):1451–1456. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui AA, Zhou Y, Podesta RB, et al. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993 Mar 24;1181(1):37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 16.Young BW, Podesta RB. Complement and 5-HT increase phosphatidylcholine incorporation into the outer bilayers of Schistosoma mansoni. J Parasitol. 1986 Oct;72(5):802–803. [PubMed] [Google Scholar]
- 17.Ahmad G, Zhang W, Torben W, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009 Nov;105(6):1767–1777. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad G, Zhang W, Torben W, et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine. 2009 May 11;27(21):2830–2837. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009 Mar;31(3):156–161. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997 Oct;15(15):1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Ahmad G, Torben W, Siddiqui AA. Sm-p80-based DNA vaccine made in a human use approved vector VR1020 protects against challenge infection with Schistosoma mansoni in mouse. Parasite Immunol. 2010;32:252–258. doi: 10.1111/j.1365-3024.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Ahmad G, Torben W, et al. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis. 2010 Apr 1;201(7):1105–1112. doi: 10.1086/651147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.guilar-Delfin I, Wettstein PJ, Persing DH. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect Immun. 2003 Apr;71(4):2002–2008. doi: 10.1128/IAI.71.4.2002-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988 Feb;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 25.Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006 Jan 1;176(1):346–356. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 26.Gobert GN, Chai M, McManus DP. Biology of the schistosome lung-stage schistosomulum. Parasitology. 2007 Apr;134(Pt 4):453–460. doi: 10.1017/S0031182006001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad G, Zhang W, Torben W, Noor Z, Siddiqui AA. Protective effects of Sm-p80 in the presence of resiquimod as an adjuvant against challenge infection with Schistosoma mansoni in mice. Int J Infect Dis. 2010 Sep;14(9):e781–e787. doi: 10.1016/j.ijid.2010.02.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994 Sep 15;153(6):2533–2543. [PubMed] [Google Scholar]
- 30.James SL, Hibbs JB., Jr The role of nitrogen oxides as effector molecules of parasite killing. Parasitol Today. 1990 Sep;6(9):303–305. doi: 10.1016/0169-4758(90)90261-2. [DOI] [PubMed] [Google Scholar]
- 31.Pearce EJ, James SL. Post lung-stage schistosomula of Schistosoma mansoni exhibit transient susceptibility to macrophage-mediated cytotoxicity in vitro that may relate to late phase killing in vivo. Parasite Immunol. 1986 Sep;8(5):513–527. doi: 10.1111/j.1365-3024.1986.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 32.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007 Dec;19(6):353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Harn DA, Gu W, Oligino LD, Mitsuyama M, Gebremichael A, Richter D. A protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J Immunol. 1992 Jan 15;148(2):562–567. [PubMed] [Google Scholar]
- 34.Jankovic D, Aslund L, Oswald IP, et al. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996 Jul 15;157(2):806–814. [PubMed] [Google Scholar]
- 35.Mangold BL, Dean DA. Passive transfer with serum and IgG antibodies of irradiated cercaria-induced resistance against Schistosoma mansoni in mice. J Immunol. 1986 Apr 1;136(7):2644–2648. [PubMed] [Google Scholar]
- 36.Roberts SM, Boot C, Wilson RA. Antibody responses of rodents to a tegument membrane preparation from adult Schistosoma mansoni. Parasitology. 1988 Dec 97;(Pt 3):425–435. doi: 10.1017/s0031182000058832. [DOI] [PubMed] [Google Scholar]
- 37.Capron A, Dessaint JP, Capron M, Pierce RJ. Schistosomiasis: from effector and regulation mechanisms in rodents to vaccine strategies in humans. Immunol Invest. 1992 Aug;21(5):409–422. doi: 10.3109/08820139209069382. [DOI] [PubMed] [Google Scholar]
- 38.Capron M, Capron A. Rats, mice and men - models for immune effector mechanisms against schistosomiasis. Parasitol Today. 1986 Mar;2(3):69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- 39.Tallima H, Montash M, Veprek P, Velek J, Jezek J, El RR. Differences in immunogenicity and vaccine potential of peptides from Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase. Vaccine. 2003 Jul 4;21(23):3290–3300. doi: 10.1016/s0264-410x(03)00180-4. [DOI] [PubMed] [Google Scholar]
- 40.Keating JH, Wilson RA, Skelly PJ. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006 Dec;92(6):1365–1369. doi: 10.1645/GE-864R.1. [DOI] [PubMed] [Google Scholar]
- 41.Coulson PS. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 42.James SL. Experimental models of immunization against schistosomes: lessons for vaccine development. Immunol Invest. 1992 Aug;21(5):477–493. doi: 10.3109/08820139209069385. [DOI] [PubMed] [Google Scholar]
- 43.Defrance T, Aubry JP, Vanbervliet B, Banchereau J. Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol. 1986 Dec 15;137(12):3861–3867. [PubMed] [Google Scholar]
- 44.Anderson S, Coulson PS, Ljubojevic S, Mountford AP, Wilson RA. The radiation-attenuated schistosome vaccine induces high levels of protective immunity in the absence of B cells. Immunology. 1999 Jan;96(1):22–28. doi: 10.1046/j.1365-2567.1999.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Defrance T, Vanbervliet B, Aubry JP, et al. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- 46.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008 Apr;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouyang W, Filvaroff E, Hu Y, Grogan J. Novel therapeutic targets along the Th17 pathway. Eur J Immunol. 2009 Mar;39(3):670–675. doi: 10.1002/eji.200839105. [DOI] [PubMed] [Google Scholar]
- 49.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007 Dec;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 50.El Ridi R, Tallima H, Mahana N, Dalton JP. Innate immunogenicity and in vitro protective potential of Schistosoma mansoni lung schistosomula excretory-secretory candidate vaccine antigens. Microbes Infect. 2010;12:700–709. doi: 10.1016/j.micinf.2010.04.012. [DOI] [PubMed] [Google Scholar]