Abstract
Whilst the pathophysiology and genetics of mitochondrial disease are slowly being unraveled, currently no effective remedy for mitochondrial disorders is available. One particular strategy in mitochondrial medicine presently under study is metabolic manipulation. This approach is aimed at counteracting the deranged cell biological homeostasis caused by mitochondrial dysfunction, using dietary modifications or small molecule therapy. Cell biological alterations caused by mitochondrial dysfunction include increased reactive oxygen species production, enhanced lipid peroxidation and altered cellular calcium homeostasis. This review covers the five principles of metabolic manipulation: (1) prevention of oxidative damage by reactive oxygen species, (2) amelioration of lipid peroxidation, (3) correction of altered membrane potential, (4) restoration of calcium homeostasis, and (5) transcription regulation interference. We hypothesize that a combination of compounds targeting different metabolic pathways will abolish cellular disturbance arising as a consequences of mitochondrial dysfunction, and thereby improve or stabilize clinical features. However, only a handful of compounds have reached efficacy testing in mammals, and it remains unknown to what extent metabolic manipulation will affect the whole organism. Until a potent remedy is found, patients will remain dependent on supportive, not curative, interventions.
Introduction
Despite progress in our current understanding of the pathophysiology and genetics of mitochondrial disease, no effective cure for mitochondrial disorders has been found (Smeitink et al. 2006). Supportive therapy is the only treatment approach we can offer our patients to date (Chinnery et al. 2006). Due to the increased knowledge of metabolism and pathophysiology, new therapeutic approaches are being discovered. Current treatment strategies applied in mitochondrial treatment development include (1) gene therapy (replacement or repair), (2) controlled regulation of specific transcriptional regulators, (3) metabolic manipulation, and (4) altering the balance between wild-type and mutated mtDNA (e.g., by exercise training) in the case of oxidative phosphorylation (OXPHOS) defects with a mitochondrial DNA (mtDNA) origin (Koene and Smeitink 2009). The effect of some of these interventions has already been explored in humans; however, most research in this field is still at the level of single cell research (Koene and Smeitink 2009).
Many in vitro experiments have been done using the metabolic manipulation strategy (Koene and Smeitink 2009). In the context of mitochondrial disease, this is defined as ‘reversing the consequences of mitochondrial dysfunction using dietary modification or small molecule therapy to compensate for a deranged biological process’. Strategies used to correct the deranged cell biological processes in mitochondrial dysfunction include, for example, the prevention of reactive oxygen species damage using scavenging enzymes and compounds restoring disturbed mitochondrial calcium metabolism. Compounds altering these disturbed processes can, for example, be nutraceuticals, a contraction of “nutrition” and “pharmaceutical” used for a group of food components (such as vitamins, polyhenols, benzoquinones, etc.) claimed to have a beneficial effect on health or medical conditions.
Here, we review the current status of research in mitochondrial medicine regarding the application of metabolic manipulators in oxidative phosphorylation dysfunction.
Metabolic manipulators: compounds to repair mitochondrial dysfunction
Mitochondrial dysfunction leads not only to a reduced ATP production but also influences a variety of up- and downstream processes, including an altered cellular redox state (Distelmaier et al. 2009a), increased production of superoxide (Balaban et al. 2005), changes in membrane potential (Distelmaier et al. 2009a) and the mitochondrial morphology (Koopman et al. 2005b; Smeitink et al. 2006) (Fig. 1). We hypothesize that the metabolic and cellular alterations seen as a consequence of mitochondrial dysfunction work together to hamper cellular function resulting in the variety of clinical symptoms present in patients. Therefore, we propose that repairing the problems arising as a consequence of disturbed mitochondrial function is a well-founded way of developing further treatment for mitochondrial disease.
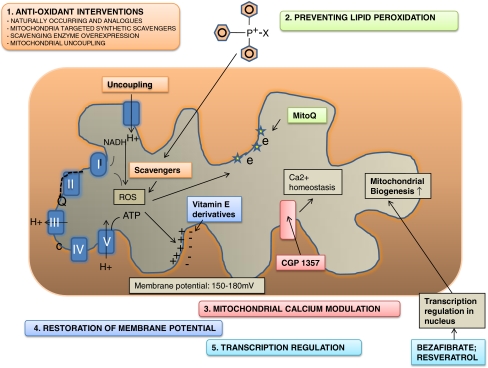
Fig. 1.
Metabolic manipulation strategies. The mitochondrial oxidative phosphorylation system consists of five complexes (I–V; blue). Electrons are transported (broken line) through complex I and II to complex III via Co-enzyme Q10 (Q) and to complex IV via cytochrome oxidase (c), creating a proton gradient (for schematic purposes only proton transport at complex III is depicted). This gradient is the driving force behind the production of ATP by complex V. When gene mutations or secondary dysfunction causes failure in the electron transport chain, increased oxidative stress is thought to be one of the consequences. We describe five approaches which may correct the proposed cell biological consequences. Prevention of oxidative damage (1; orange) can be achieved by either stimulating or over expressing naturally occurring antioxidants, or by scavenger supplementation. To facilitate membrane transport several triphenylphosphonium-based compounds such as TTP-vitE have been generated. Uncoupling of the respiratory chain leads to reduced oxidative damage, but also to a reduced membrane potential. Since oxidative damage is thought to cause lipid peroxidation substances preventing lipid peroxidation were designed (2; green), e.g., MitoQ. Restoring the disturbed calcium homeostasis (3; pink) has been achieved on a cellular level by CGP 1357, a benzothiazepine drug inhibiting the mitochondrial sodium/calcium (Na+/Ca2+) exchanger. The mitochondrial membrane potential (4; blue) a key indicator of mitochondrial health, can be restored by the vitamin E derivates. Finally, transcription up-regulation (5; turquoise) of genes involved in cellular energy metabolism and subsequent mitochondrial biogenesis is achieved by over expressing the transcription factor PGC1A. NADH Nicotinamide adenine dinucleotide (reduced form); Q/COQ co-enzyme Q10; c cytochrome oxidase; SOD superoxide dismutase; vit E vitamin E; CGP 1357 a benzothiazepine drug inhibiting the mitochondrial sodium/calcium (Na+/Ca2+) exchanger; PGC-1A peroxisome proliferator-activated receptor γ (PPAR-Γ) coactivator 1α; MitoQ TTP with co-enzyme Q10 attached; ATP adenosine triphospate; Ca2+ calcium; e electron
In the next paragraphs, we will summarize the consequences of mitochondrial dysfunction and subsequently discuss how metabolic manipulation might counteract these cell biological alterations (see also Table 1).
Table 1.
A summary of therapeutic strategies in metabolic manipulation
| 1. Preventing oxygen damage |
| i) Supplementation of naturally occurring antioxidants |
| ii) Mitochondria-targeted scavenging compounds |
| iii) Uncoupling of the mitochondrial respiratory chain |
| 2. Preventing lipid peroxidation |
| 3. Restoring the mitochondrial membrane potential |
| 4. Modulation of mitochondrial calcium homeostasis |
| 5. Transcription regulation |
Preventing oxidative damage: introduction
The superoxide anion, hydrogen peroxide and the hydroxyl radical are collectively referred to as reactive oxygen species (ROS). ROS plays an important role in the expression of many proteins like transcription factors, kinases, ion channels and phosphatases (Koopman et al. 2009). However, ROS can lead to harmful effects such as DNA damage and lipid peroxidation, resulting in subsequent membrane damage and to changes in the mitochondrial network. Cells are equipped with their own anti-oxidant mechanisms to counteract these potentially harmful effects of ROS (Balaban et al. 2005; Murphy 2009). If the production of reactive oxygen species (ROS) becomes too great to be counterbalanced by its antioxidant system, damage to proteins, lipids and DNA might occur (Balaban et al. 2005; Smeitink et al. 2006), which is thought to have harmful effects on cellular and mitochondrial (ultra)structure, activity and matrix protein diffusion (Koopman et al. 2007, 2008a).
To what extent ROS are indeed involved in disease pathophysiology is largely dependent on model systems and experimental conditions used. For example, their role in mitochondrial disease is still under debate (Balaban et al. 2005; Murphy 2009). Chemical inhibition of complex I by rotenone, a frequently used model to study complex I deficiency, shows all of the above stated consequences (Fato et al. 2009). However, no mtDNA abnormalities or increased lipid peroxidation has been observed in genetically defective complex I human cell lines, although these cell lines do show increased ROS production (Distelmaier et al. 2009a; Koopman et al. 2009). Despite the ongoing debate regarding the role of ROS in mitochondrial disease, much effort is devoted to studying the effects of an increased scavenger potential in mitochondrial dysfunction. Experiments to reduce ROS have explored the following strategies: (1) supplementing naturally occurring antioxidants and analogues (Chen et al. 1997; Quinzii et al. 2007), (2) treatment with synthetic scavenging compounds (Koopman et al. 2005a; Murphy 2008; Murphy and Smith 2007; Szeto 2006b), and (3) increased uncoupling of the mitochondrial respiratory chain (Sluse et al. 2006).
The following experiments have to be interpreted with great caution, since it is still uncertain whether ROS has a major role in the pathophysiology of mitochondrial disease. Furthermore, ROS is not only harmful to cells but also has a regulatory function in protein expression of, for example, transcription factors (Semenza 2004). Therefore, influencing its concentration might disturb cell viability.
Supplementation of naturally occurring antioxidants and analogues
The most well-studied anti-oxidant, Co-enzyme Q10 (CoQ10) (ubiquinone), acts as an electron transporter in the mitochondrial respiratory chain where it transports electrons from complex I and II to complex III (Lenaz and Genova 2009). In the rare case of CoQ10 biosynthesis defects (OMIM #606426), high doses of CoQ10 have proven to lead to clinical improvement (Quinzii et al. 2007; Di Giovanni et al. 2001). CoQ10 is frequently prescribed in patients suffering from other mitochondrial diseases with many case reports of beneficial effects (Barak et al. 1995; Wang et al. 2008). We reviewed two trials that studied the effects of CoQ10 in patients with mitochondrial encephalopathy and chronic progressive external ophthalmoplegia (CPEO) (Chen et al. 1997; Chinnery et al. 2006). One reports subjective improvement and globally a significant increase in muscle strength in 8 patients with different types of mitochondrial encephalopathy (Chen et al. 1997), but the other trial in 17 patients with CPEO (mutations not known) did not show any benefit (Chinnery et al. 2006). So, to date, there is no evidence that CoS10 should be structurally prescribed to patients with mitochondrial diseases other than CoQ10 biosynthesis defects. However, if a co-enzyme Q deficiency has not been ruled out, a clinical trial (n = 1) should be considered.
Idebenone (hydroxydecyl benzoquinone), a synthetic CoQ10 compound, is thought to have two modes of action: (1) it acts as an electron carrier, shuttling electrons from complex I and II to complex III (Esposti et al. 1996; James et al. 2005), and (2) it acts as an antioxidant, for which the benzoquinone undergoes reversible redox reactions (Meier and Buyse 2009).
It has been suggested that Idebenone might be beneficial in Leber hereditary optic neuropathy (LHON) (OMIM #535000) (Mashima et al. 1992, 2000), a mitochondrial genetic disease often associated with a mitochondrial complex I gene mutation (Man et al. 2002), that preferentially causes blindness in young adult males. Results from a retrospective study with idebenone, vitamin B2, and vitamin C suggest a faster recovery of vision in LHON patients with m.11778G>A, m.3460G>A and m.14484T>C mutations (Mashima et al. 2000). It is difficult to interpret these data, since the natural disease progression of LHON differs significantly (Man et al. 2002). Besides, no prospective studies have been described.
Idebenone has been effectively used in Friedreich ataxia (FA) since 1999 (Meier and Buyse 2009). Friedreich ataxia (OMIM #229300) is a progressive, multisystem, degenerative disorder caused by a reduction in frataxin, resulting in mitochondrial dysfunction and oxidative damage. Treatment with idebenone is generally well tolerated and associated with improvement in neurological function and activities of daily life in about 200 patients with FA (Di Prospero et al. 2007; Meier and Buyse 2009). Whilst there are some similarities between FA patients and mitochondrial disease patients, no conclusions can be drawn from the observations in FA patients.
Oral supplementation, as is the case with CoQ10 and its variants, is the easiest way to administer antioxidants. Not all oral supplementations, however, are properly absorbed. Glutathione, for example, a major endogenous antioxidant produced by all aerobic cells (Lash 2006; Witschi et al. 1992), will be, following oral ingestion, hydrolyzed by intestinal and hepatic gamma-glutamyltransferase (Witschi et al. 1992). Increasing circulating glutathione to a proposed clinically beneficial concentration by oral administration of single doses is not possible (Witschi et al. 1992).
Several naturally occurring groups of compounds are known for their ability to manipulate the intracellular environment. These derivatives, also known as nutraceuticals, include the quinones, vitamins and polyphenols, the latter including flavenoids. Presently, numerous in vitro studies are being performed with these complex natural molecules; for example, the assessment of the interaction of quercetin derivatives, other than their antioxidant ability, with mitochondria, (Biasutto et al. 2009). The vitamin E analogue Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a phenolic antioxidant with a chromane structure similar to vitamin E but without the hydrophobic polyisoprenoid tail of the latter (Tafazoli et al. 2005), has been shown to quench cellular reactive oxygen species in genetically defective OXPHOS fibroblasts (Koopman et al. 2008b). The vitamin E remaining after its dehydrogenation is a relatively unreactive free radical (Distelmaier et al. 2009b; Koopman et al. 2008b). An increased restoration of ROS to lower levels and restoration of both the amount and activity of fully assembled complex I was observed in complex I-deficient fibroblasts treated with Trolox (Koopman et al. 2008b). Trolox had its largest effect on complex I protein levels and activity in patient cells with a mild complex I deficiency (Koopman et al. 2008b). All of these experiments are still only on a cellular level.
Mitochondria-targeted synthetic scavenging compounds
For a more efficient delivery of scavengers to the source of the ROS production, special transporters have been designed to bring the compounds to the mitochondrial inner membrane (IMM) (Hoye et al. 2008; Szeto 2006b). To move molecules into the mitochondrial matrix, both the cell membrane and the two mitochondrial membranes have to be passed, of these the IMM is impermeable to uncharged and water-soluble molecules. Targeted mitochondrial delivery can be obtained by (1) endogenous mitochondrial import, using the natural import system of the cell (Neupert 1997), (2) fusion of closed compartments with the mitochondria (Muratovska et al. 2001), or by (3) facilitated transport across the lipid bilayer (Macias et al. 2007). We will only discuss the latter, including constructed shuttles like the lipophilic cation shuttles Triphenylphosphonium (Murphy and Smith 2007), Szeto-Schiller tetrapeptides (Szeto 2006a), Gramicidin S analogs (Macias et al. 2007), mitochondria penetrating peptides (Horton et al. 2008) and the SkQ cations (Skulachev 2009).
Lipophilic cation shuttles have a large hydrophobic surface area which, together with the negatively charged electron gradient that facilitates cation passage, enables these molecules to pass through the two lipid bilayers of the mitochondria (Hoye et al. 2008; Murphy and Smith 2007). These components and their cargo accumulate within the mitochondria up to 1,000-fold, in comparison to those in untargeted analogues (Murphy and Smith 2007). Triphenylphosphonium (TPP), one of such lipophilic cation shuttles is designed to shuttle anti-oxidants to the mitochondrial matrix (Murphy and Smith 2007). TPP-conjugated derivates of ubiquinone (James et al. 2005, 2007; Kelso et al. 2001), tocopherol (Smith et al. 1999), lipoic acid (Brown et al. 2007), spin traps (Murphy et al. 2003) and the peroxidase mimetic Ebselen (Filipovska et al. 2005) can successfully import anti-oxidants into the mitochondria in vitro (Murphy 2008) and might be able to decrease ROS production (Murphy 2008). Although these experiments seem promising, these shuttles depend on the electrical polarization of the mitochondrial membrane for their uptake and may, therefore, not be applicable in all cases of mitochondrial dysfunction (Szeto 2006b). Furthermore, some of these agents may be toxic to the cell, as is the case with high concentrations of the spin trap alpha-phenyl N-tertiary-butyl nitrone (PBN) (Albano et al. 1986).
In addition, the mode of action of some of these compounds might be other than predicted. This is for example the case for MitoQ (Antipodean Company), a TTP shuttle with CoQ10 as cargo. In a complex I rotenone inhibition experiment, MitoQ did not scavenge the increased ROS as measured by HEt oxidation (Koopman et al. 2005a), but did decrease lipid peroxidation and normalized mitochondrial morphology (Koopman et al. 2007; Murphy 2009). High doses of MitoQ were administered orally to young healthy mice for up to 28 weeks to study the effects on whole-body physiology, metabolism, and gene expression (Rodriguez-Cuenca et al. 2010). There were no changes in the expression of mitochondrial or antioxidant genes as assessed by DNA microarray analysis. There was also no increase in oxidative damage to mitochondrial protein, DNA, or cardiolipin, and the activities of mitochondrial enzymes were unchanged. No adverse effects were reported in the mice. Whether MitoQ will be beneficial in patients with mitochondrial disorders has to be studied in randomized clinical trials.
The Szeto-Schiller (SS) tetrapeptide is a vehicle for mitochondrial scavenger targeting, using a independent potential delivery (Adlam et al. 2005; Magwere et al. 2006; Murphy and Smith 2007; Smith et al. 1999, 2008; Szeto 2006a, b). The water-soluble SS tetrapeptides can have alternating aromatic residues and basic amino acids (aromatic-cationic peptides), which are designed to reduce ROS in mitochondria and protect against lipid peroxidation and cell death in neuronal and cardiac cells (Zhao et al. 2004). Chemically induced complex I deficiency by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a mouse model for Parkinson, showed the neuroprotective effects of the administration of SS peptides (Yang et al. 2009). Surprisingly, a SS peptide which did not have a scavenging capacity also demonstrated significant neuroprotective effects on dopaminergic neurons of chemically induced Parkinson mice (Yang et al. 2009). SS peptides are also claimed to have an inhibitory effect on lipid peroxidation (Szeto 2006a); however, there is no evidence to confirm this statement. Studies with isolated mitochondria showed that both SS-31 and SS-20 prevented MPP+-induced inhibition of oxygen consumption, ATP production and mitochondrial swelling. It is not clear what the biochemical or pharmacological mechanism is underlying these observations. It might be either a ROS reducing effect of the SS peptide alone, or another, unknown pathophysiological mechanism underlying the neurodegeneration observed in this Parkinson model.
Mitochondria penetrating peptides (MPP), cationic, but also lipophilic synthetic peptides, were also reported to exhibit efficient cellular uptake with a targeted mitochondrial localization (Horton et al. 2008). For example, the Gramicidin S analogue 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) peptides were designed to transport the TEMPO cargo into mitochondria (Macias et al. 2007). Gramicidin was used because of its high affinity to bacterial membranes (Sholtz et al. 1975) and demonstrates an ability to manipulate mitochondrial membrane potential. TEMPO peptides can be used to deliver antioxidants and radical scavengers to mitochondria and were shown to prevent ROS-induced apoptosis in a rat model for ischemia-reperfusion injury (Macias et al. 2007).
Finally, a penetrating cation (Sk) with plastoquinone (Q), a quinone acting as a electron carrier in the electron transport chain has been extensively studied by Skulachev and co-workers (Antonenko et al. 2008; Skulachev 2009; Skulachev et al. 2009). SkQ, of which several variants have been synthesized, are rechargeable antioxidants with a high affinity for the inner leaflet of the mitochondrial inner membrane. The antioxidant properties of these compounds have been studied extensively both in vitro and in vivo, and there is evidence for results reaching from recovering blindness in mammals to geroprotection (Skulachev et al. 2009). The claimed success of SkQ claims to prevent lipid peroxidation, senescence and ischemia reperfusion injury (Skulachev 1998). Independent studies are awaited to confirm the observed results.
Mitochondrial uncoupling regulation
Mitochondrial uncoupling proteins (UCPs) increase proton leakage in the IMM, thereby reducing oxygen stress by lowering the electrochemical gradient (Cannon and Nedergaard 2004; Sullivan et al. 2004). The net effect of uncoupling is (1) a reduction of ROS production, (2) dissipation of heat, (3) reduction of ATP synthesis, and (4) reduction of calcium influx to the mitochondrial matrix. Uncoupling can be stimulated by either high levels of cellular/mitochondrial ROS (Sluse et al. 2006) or by circulating free fatty acids (FFA) (Jarmuszkiewicz et al. 2004).
The ketogenic diet, a high-fat, low-protein, low-carbohydrate diet, provides an increased free fatty acid (FFA) concentration in plasma, which stimulates uncoupling by the mitochondrial UCPs in mice (Sullivan et al. 2004; Davis et al. 2008). Also, a decreased ROS production was found in the hippocampus of these mice, without decreasing mitochondrial ATP production (Sullivan et al. 2004).
Ketogenic diet is not only known for its uncoupling activity, but is also thought to provide an alternative source of acetyl Co-A by inducing fatty acid beta-oxidation as well as changing the heteroplasmy state in favor of the wild-type mtDNA copy number (Santra et al. 2004). In children with pyruvate dehydrogenase complex (PDHC) deficiency and a variety of isolated and combined OXPHOS system deficiencies, the ketogenic diet was reported to improve epilepsy (Kang et al. 2007; Wexler et al. 1997) and mental development in PDHC deficiency (Wexler et al. 1997). Although these results in PDHC and mitochondrial diseases complicated by epilepsy seem promising, ketogenic diets are often complicated by metabolic disturbances, such as hypoglycemias and hypercholesterolemia as well as by gastrointestinal symptoms and lethargy (Duchowny 2005; Toshima et al. 1982; Wexler et al. 1997; Wijburg et al. 1992). We think it is worthwhile investigating the effect of a ketogenic diet in patients with mitochondrial disease. However, this may also have major side effects because the defective mitochondria are not able to process the excess of lipids in the ketogenic diet.
Preventing lipid peroxidation and mitochondrial network
Mitochondria are highly dynamic organelles, forming an extensive network with constant fusion and fission (Detmer and Chan 2007). Lipid peroxidation as a consequence of an increased ROS production changes the structural architecture of the mitochondrial network (Bach et al. 2003; Hood 2001), and is generally accompanied by changes in mitochondrial content, ultrastructure, and enzyme levels (Rossignol et al. 2004).
Treating chemically inhibited Complex I-deficient cells with the antioxidant MitoQ abolished lipid peroxidation and normalized mitochondrial shape (Koopman et al. 2005a). Fibroblasts of Complex I-deficient patients, showing increased ROS formation, displayed no signs of increased lipid peroxidation (Verkaart et al. 2007) and seem to have adapted to increased levels of ROS. Unraveling these adaptive mechanisms may provide new targets for therapeutic intervention.
Modulation of mitochondrial calcium homeostasis
Mitochondria play a central role in ensuring the homeostasis of ionic calcium (Ca2+), an all round intracellular signaling molecule that regulates a large variety of cellular processes (Berridge et al. 2003). During cell stimulation, Ca2+ is released from the endoplasmic reticulum to the cytosol and taken up by mitochondria, where it stimulates mitochondrial dehydrogenases giving rise to increased ATP production (Brini et al. 1999; Korzeniewski 2007; Pinton et al. 2008; Valsecchi et al. 2009).
Cells harboring mutations in nuclear or mitochondrial DNA often show disturbances in Ca2+ homeostasis (Brini et al. 1999; Distelmaier et al. 2009a). Complex I-deficient cells have an almost normal resting Ca2+ balance in mitochondria and cytosol, but a lower endoplasmic reticulum Ca2+ concentration (Visch et al. 2004). However, the peak in mitochondrial Ca2+ concentration seen after hormonal stimulation with bradykinin was lower than expected (Visch et al. 2004). Moreover, Ca2+ was removed from the cytosol at a slower rate (Visch et al. 2004). In a heart-specific knockout of the mitochondrial transcription factor A (Tfam) mouse model, a clear association of a disturbed Ca2+ homeostasis with cathecholamine-induced arrhythmias was found (Tavi et al. 2005).
This suggests that Ca2+ homeostasis is inevitable for cell functioning, and above all that disturbances in cellular Ca2+ homeostasis lead to clinical abnormalities (Brini et al. 1999; Tavi et al. 2005; Visch et al. 2004).
CGP37157 is a benzothiazepine drug which inhibits the mitochondrial sodium/calcium (Na+/Ca2+) exchanger. CGP37157 restores Ca2+ handling and ATP production in cells with a hampered energy metabolism. In Complex I-deficient cells harboring a NDUFS7 or NDUFS4 mutation, treatment with a CGP37157 resulted in an normalization of stimulated mitochondrial Ca2+ concentration and ATP production, as well as a normal cytosolic Ca2+ removal (Visch et al. 2004, 2006). Treatment of cybrid cells expressing the m.8356T>C (MERFF) or the m.8993T>G (NARP) mutation with CGP37157 mostly restored both the Ca2+ uptake and the stimulation of ATP production (Brini et al. 1999). We are not aware of clinical studies aimed at correcting cellular calcium homeostasis.
Transcription regulation
Peroxisome proliferator-activated receptor γ (PPAR-γ) co-activator 1α (PGC-1α) is a transcriptional factor regulator that stimulates transcription of genes involved in cellular energy metabolism (Wenz 2009). The study of PGC-1α has received much attention for its possible roles in the treatment of the metabolic syndrome (Canto and Auwerx 2009), its potential neuroprotective capacity (St-Pierre et al. 2006), and its roles in various other pathologies (Baur and Sinclair 2006).
PGC-1α is not only known for its role in the transcription of proteins involved in energy metabolism but is also under interest because of its ROS-mediated cell death prevention (St-Pierre et al. 2006), and the stimulation of mitochondrial biogenesis (Wenz 2009). PPARS also affects mtDNA levels by modulating transcription of the mtDNA transcription factor A (Tfam) gene (Handschin and Spiegelman 2006). PGC-1α is physiologically regulated by for example exercise (Taivassalo and Haller 2005) and calorie intake (Corton and Brown-Borg 2005) but can also be manipulated by pharmacologic agents, for example, bezafibrate (Bastin et al. 2008) and resveratrol (Oliva et al. 2008) (Fig. 2). The latter is known as a stimulator of silent information regulator two (Sir2) proteins (Sirtuins), which catalyze NAD+-dependent deactetylation within proteins such as PGC-1α (Hallows et al. 2009). NAD+, also known as a biochemical electron carrier shuttling electrons from the Krebs cycle to complex I, is therefore considered the direct link between cellular stress and an increased metabolism (Houtkooper et al. 2009) (Fig. 2).
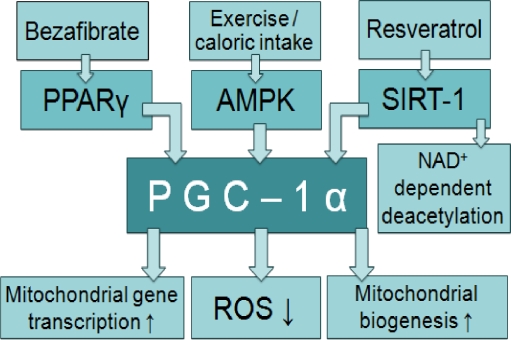
Fig. 2.
Stimulation of transcription of mitochondrial genes. Peroxisome proliferator-activated receptor γ (PPAR-γ) co-activator 1α (PGC-1α) stimulates transcription of genes involved in mitochondrial energy metabolism, by increasing their nuclear transcription and expression. Stimulation of PGC-1α also causes a reduction in reactive oxygen species, an increase in mitochondrial biogenesis, and a beneficial shift in heteroplasmy. All this leads to an increased cellular energy production; however, the long-term effects of an increased mitochondrial biogenesis are unknown. PGC-1α is stimulated via adenosine monophosphate (AMP), activated protein kinase (AMPK), by physiological processes such as exercise, as well as by pharmacological agents for example bezafibrate and resveratrol. Bezafibrate directly stimulates PPARγ, the transcription factor working together with PGC-1α. Resveratrol stimulates silent information regulator two proteins (Sirtuins) which catalyze NAD+-dependent deactetylation within PGC-1α. Since NAD+ reflects the cells energy metabolism, this is described as the direct link between external physiological stimuli and the regulation of mitochondrial biogenesis. PGC-1α peroxisome proliferator-activated receptor γ (PPAR-γ) coactivator 1α; PPAR-γ peroxisome proliferator-activated receptor γ; ROS reactive oxygen species; AMPK adenosine monophosphate (AMP), activated protein kinase; SIRT-1 silent information regulator 1 protein; NAD nicotinamide adenine dinucleotide
The stimulation of the PPAR/PGC-1α pathway also has consequences for mitochondrial biogenesis (Wenz 2009). Increased mitochondrial biogenesis is a natural compensation mechanism commonly seen in skeletal muscle of patients with OXPHOS deficiencies (DiMauro and Schon 2008), and has been described as be a maladaptive effect in an attempt to increase ATP supply (Murdock et al. 1999). Since an increase in the biogenesis of healthy mitochondria shifts the balance (heteroplasmy) and thereby increases cell metabolism, this strategy has been studied to treat patients with mtDNA mutations. PGC-1α stimulation through exercise is known to increase mitochondrial mass and oxidative capacity (Taivassalo and Haller 2005). To verify the role of PGC-1α in mitochondrial biogenesis, a mouse model for cytochrome c oxidase (COX) deficiency with transgenic expression of PGC-1α was used (Wenz et al. 2008). These PGC-1α-expressing mice have a delayed onset of myopathy, increased mitochondrial biogenesis, increased ATP levels and increased health and lifespan compared to COX deficient littermates (Wenz et al. 2008). PGC-1α stimulation thus positively affects mitochondrial function in COX-deficient cells.
Resveratrol (3,5,4′-trihydroxystilbene), a polyphenolic phytoalexin, stimulates sirtuin activity and thereby transcription of the nuclear genes involved in energy metabolism (Almeida et al 2009). Also, a spectacular increase in the cells’ own anti-oxidant machinery, such as mitochondrial superoxide dismutase MnSOD2 and glutathione, was observed (Das et al. 2008; Kode et al. 2008; Robb et al. 2008).
Healthy mice were fed with either a normal (SD) or a high calorie diet (HC), either with resveratrol (HCR) or without (HC). The HCR mice tend to have an increased survival rate and rotarod performance compared to HC mice and do not significantly differ from the SD mice (Baur et al. 2006). Also, their cardiovascular risk profile was improved compared to the mice having no resveratrol. The livers of the HCR mice had considerably more mitochondria than those of HC controls and were not significantly different compared to those of the SD group (Baur et al. 2006). In another experiment with 1-year-old mice on the HC diet that had been treated with resveratrol for 6 weeks, the acetylation status of PGC-1α in the resveratrol-fed mice was threefold lower than the diet-matched controls. This enhanced enzyme activity can be either stimulated by resveratrol or SIRT1. Since the latter was not detectably increased, it can be concluded that resveratrol stimulates PCG-1α expression in mice on a HC diet (Baur et al. 2006; Milne and Denu 2008).
A double-blind, randomized, placebo-controlled study with resveratrol in healthy volunteers showed that frequent administration of resveratrol was well tolerated, but low plasma concentrations were achieved despite a 4-hourly oral administration regime (Almeida et al. 2009), possibly due to a rapid and extensive first pass metabolism (Walle et al. 2004). There are no data on the side effects of continual resveratrol administration.
Studies in healthy volunteers focusing on the effects of resveratrol on cardiovascular parameters show a stimulation of human platelet nitric oxide production, increased HDL cholesterol and inhibited platelet aggregation, and oxidation of low-density lipoproteins (Bhat et al. 2001; Gresele et al. 2008). From a mitochondrial disease point of view, resveratrol theoretically has a stimulating effect on mitochondria and has proven to increase mitochondrial biogenesis in the liver of mice on continual resveratrol treatment. In addition, administration seems well tolerated. However, we are not aware of any studies assessing mitochondrial enzyme complex activity in animals or humans treated with resveratrol, or resveratrol administration in animal models for mitochondrial disease or human mitochondrial disease patients.
Another pharmacologic PPAR pan-agonist called bezafibrate, was administered in COX-deficient mice shortly before disease onset. Bezafibrate was shown to lead to mitochondrial proliferation and an enhanced OXPHOS capacity per muscle mass as well as a reduction of the myopathy and prolongation of the lifespan (Wenz et al. 2008). The increase in ATP production per muscle mass was thought to play the key role in the clinical improvement of the myopathic mice.
Bezafibrate was also studied in a clinical trial in six adults with mild carnitine palmitoyltransferase II (CPT2) deficiency (Bonnefont et al. 2009). CPT2 (OMIM #255110) is a translocation protein shuttling long-chain fatty acyl-CoAs over the inner mitochondrial membrane (McGarry and Brown 1997). Patients with CPT2 deficiency may present either in infancy or as adults; the latter presenting with recurrent attacks of myalgia and muscle stiffness or weakness, occasionally associated with myoglobinuria. The frequency of these attacks is highly variable and, inbetween attacks, patients have no clinical symptoms (Bonnefont et al. 2004). Administration of bezafibrate in patients with CPT2 deficiency fully restored fatty acid oxidation capacity in muscle cells, probably caused by stimulating the expression of the mutated gene (Bonnefont et al. 2009; Djouadi et al. 2005). Also, patients had fewer periods of rhabdomyolysis, and quality of life parameters reached the control ranges on all domains (Bonnefont et al. 2009). No adverse effects were reported.
Although transcription regulation seems a very promising strategy in treating mtDNA defects, the long-term complications of mitochondrial proliferation should be studied, since both healthy and hampered mitochondria will proliferate (Wenz 2009). Excessive mitochondrial proliferation in PPAR stimulation may result in cardiomyopathy (Lehman et al. 2000).
In addition to that, a recent study showed that the activation of sirtuins by resveratrol and other synthetic SIRT1 agonists was not caused by a direct interaction between sirtuins and its agonists (Pacholec et al. 2010). NMR and calorimetry techniques demonstrated that the reported effect was caused by the interaction of the SIRT1 with the fluorophore (Pacholec et al. 2010). In vivo experiments did not confirm the beneficial effects of SRT1720 on plasma glucose and mitochondrial capacity in mice on a high fat diet (Pacholec et al. 2010).These results show that the effect of small molecules on sirtuin expression is most uncertain.
Conclusion
Metabolic manipulation is one way in which to develop a well-founded and effective therapy for mitochondrial disorders. Some examples of metabolic manipulation intervention seem very promising at the cellular level, though very few compounds have been tested in vivo. The real challenge in mitochondrial medicine is to design an effective clinical trial to definitively address the question of whether or not such therapeutic compounds are beneficial. This has proven to be very difficult in this heterogeneous population with an unpredictable natural history.
Acknowledgements
Part of this work was supported by the European Community’s sixth Framework Program for Research, Priority 1 “Life sciences, genomics and biotechnology for health, contract number LSHM-CT-2004-503116, the Ministry of Economic Affairs of the Netherlands (IOP-Genomics), the Prinses Beatrix Fonds, the Energy4All Foundation and the ZonMW AGIKO Grant. We thank Lovice Sutherland for carefully reviewing our manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Ca2+
Calcium
- CoQ10
Co-enzyme Q10
- CPEO
Chronic progressive external ophthalmoplegia
- CPT2
Carnitine palmitoyltransferase II
- COX
Cytochrome c oxidase
- ER
Endoplasmic reticulum
- FA
Friedreich ataxia
- IMM
Inner mitochondrial membrane
- LHON
Leber hereditary optic neuropathy
- MitoQ
Mitoquinone
- MMP
Mitochondrial membrane potential
- MnSOD2
Manganese–super oxide dismutase 2
- MPP
Mitochondria penetrating peptide
- mtDNA
Mitochondrial DNA
- NAD+
Nicotinamide adenine dinucleotide
- NADH
Nicotinamide adenine dinucleotide reduced form
- NARP
neuropathy, ataxia and retinitis pigmentosa
- OXPHOS
Oxidative phosphorylation
- PBN
Alpha-phenyl N-tertiary-butyl nitrone
- PDHC
Pyruvate dehydrogenase complex
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator-1 alpha
- PPARγ
Peroxisome proliferator-activated receptor gamma
- rAAV-SOD2
Recombinant adeno-associated virus (rAAV) containing the SOD2 gene
- ROS
Reactive oxygen species
- Sir 2
Silent information regulator two
- SIRT
Sirtuin
- Sirtuins
Silent information regulator two (Sir2) proteins
- SOD
Superoxide dismutase
- SS
Szeto-Schiller
- TIM
Translocase of the inner membrane
- TOM
Translocase of the outer membrane
- TPP
Triphenylphosphonium
Footnotes
References to electronic databases: e.g., OMIM disorder/gene accession number(s)
OMIM
#606426 Co-enzyme Q biosynthesis defects;
#535000 Leber Hereditary Optic Neuropathy;
#229300 Friedreich Ataxia;
#255110 carnitine palmitoyltransferase II deficiency (adult onset)
Competing interest: None declared.
References
- Adlam VJ, Harrison JC, Porteous CM, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Albano E, Cheeseman KH, Tomasi A et al (1986) Effect of spin traps in isolated rat hepatocytes and liver microsomes. Biochem Pharmacol 22: 3955–60. [DOI] [PubMed]
- Almeida L, Vaz-da-Silva M, Falcao A, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Avetisyan AV, Bakeeva LE, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 2008;73:1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barak Y, Arnon S, Wolach B, et al. MELAS syndrome: peripheral neuropathy and cytochrome C-oxidase deficiency: a case report and review of the literature. Isr J Med Sci. 1995;31:224–229. [PubMed] [Google Scholar]
- Bastin J, Aubey F, Rotig A, et al. Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab. 2008;93:1433–1441. doi: 10.1210/jc.2007-1701. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bhat KPL, Kosmeder JW, 2nd, Pezzuto JM. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3:1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- Biasutto L, Sassi N, Mattarei A et al (2009) Impact of mitochondriotropic quercetin derivatives on mitochondria. Biochim Biophys Acta (in press) [DOI] [PubMed]
- Bonnefont JP, Djouadi F, Prip-Buus C, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bonnefont JP, Bastin J, Behin A, et al. Bezafibrate for an inborn mitochondrial beta-oxidation defect. N Engl J Med. 2009;360:838–840. doi: 10.1056/NEJMc0806334. [DOI] [PubMed] [Google Scholar]
- Brini M, Pinton P, King MP, et al. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nat Med. 1999;5:951–954. doi: 10.1038/11396. [DOI] [PubMed] [Google Scholar]
- Brown SE, Ross MF, Sanjuan-Pla A, et al. Targeting lipoic acid to mitochondria: synthesis and characterization of a triphenylphosphonium-conjugated alpha-lipoyl derivative. Free Radic Biol Med. 2007;42:1766–1780. doi: 10.1016/j.freeradbiomed.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RS, Huang CC, Chu NS. Coenzyme Q10 treatment in mitochondrial encephalomyopathies. Short-term double-blind, crossover study. Eur Neurol. 1997;37:212–218. doi: 10.1159/000117445. [DOI] [PubMed] [Google Scholar]
- Chinnery P, Majamaa K, Turnbull D et al (2006) Treatment for mitochondrial disorders. Cochrane Database Syst Rev CD004426. [DOI] [PubMed]
- Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- Das S, Khan N, Mukherjee S, et al. Redox regulation of resveratrol-mediated switching of death signal into survival signal. Free Radic Biol Med. 2008;44:82–90. doi: 10.1016/j.freeradbiomed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Davis LM, Rho JM, Sullivan PG. UCP-mediated free fatty acid uncoupling of isolated cortical mitochondria from fasted animals: correlations to dietary modulations. Epilepsia. 2008;49(Suppl 8):117–119. doi: 10.1111/j.1528-1167.2008.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Mirabella M, Spinazzola A, et al. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57:515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- Di Prospero NA, Baker A, Jeffries N, et al. Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Distelmaier F, Koopman WJ, van den Heuvel LP, et al. Mitochondrial complex I deficiency: from organelle dysfunction to clinical disease. Brain. 2009;132:833–842. doi: 10.1093/brain/awp058. [DOI] [PubMed] [Google Scholar]
- Distelmaier F, Visch HJ, Smeitink JA, et al. The antioxidant Trolox restores mitochondrial membrane potential and Ca2+ -stimulated ATP production in human complex I deficiency. J Mol Med. 2009;87:515–522. doi: 10.1007/s00109-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouadi F, Aubey F, Schlemmer D, et al. Peroxisome proliferator activated receptor delta (PPARdelta) agonist but not PPARalpha corrects carnitine palmitoyl transferase 2 deficiency in human muscle cells PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity Sirt1 is involved in energy metabolism: the role of chronic ethanol feeding and resveratrol High absorption but very low bioavailability of oral resveratrol in humans Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. J Clin Endocrinol Metab. 2005;90:1791–1797. doi: 10.1210/jc.2004-1936. [DOI] [PubMed] [Google Scholar]
- Duchowny MS. Food for thought: the ketogenic diet and adverse effects in children. Epilepsy Curr. 2005;5:152–154. doi: 10.1111/j.1535-7511.2005.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposti MD, Ngo A, Ghelli A, et al. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996;330:395–400. doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- Fato R, Bergamini C, Bortolus M, et al. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovska A, Kelso GF, Brown SE, et al. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J Biol Chem. 2005;280:24113–24126. doi: 10.1074/jbc.M501148200. [DOI] [PubMed] [Google Scholar]
- Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Smith BC, Lee S, et al. Ure(k)a! Sirtuins regulate mitochondria. Cell. 2009;137:404–406. doi: 10.1016/j.cell.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Horton KL, Stewart KM, Fonseca SB, et al. Mitochondria-penetrating peptides. Chem Biol. 2008;15:375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Canto C, Wanders RJ et al (2009) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev (in press) [DOI] [PMC free article] [PubMed]
- Hoye AT, Davoren JE, Wipf P, et al. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- James AM, Cocheme HM, Smith RA, et al. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- James AM, Sharpley MS, Manas AR, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- Jarmuszkiewicz W, Navet R, Alberici LC, et al. Redox state of endogenous coenzyme q modulates the inhibition of linoleic acid-induced uncoupling by guanosine triphosphate in isolated skeletal muscle mitochondria. J Bioenerg Biomembr. 2004;36:493–502. doi: 10.1023/B:JOBB.0000047331.25248.7a. [DOI] [PubMed] [Google Scholar]
- Kang HC, Lee YM, Kim HD, et al. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–88. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, et al. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Koene S, Smeitink J. Mitochondrial medicine: entering the era of treatment. J Intern Med. 2009;265:193–209. doi: 10.1111/j.1365-2796.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Verkaart S, Visch HJ, et al. Inhibition of complex I of the electron transport chain causes O2-. -mediated mitochondrial outgrowth. Am J Physiol Cell Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Visch HJ, Verkaart S, et al. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol. 2005;289:C881–C890. doi: 10.1152/ajpcell.00104.2005. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Verkaart S, Visch HJ, et al. Human NADH:ubiquinone oxidoreductase deficiency: radical changes in mitochondrial morphology? Am J Physiol Cell Physiol. 2007;293:C22–C29. doi: 10.1152/ajpcell.00194.2006. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Distelmaier F, Hink MA, et al. Inherited complex I deficiency is associated with faster protein diffusion in the matrix of moving mitochondria. Am J Physiol Cell Physiol. 2008;294:C1124–C1132. doi: 10.1152/ajpcell.00079.2008. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Verkaart S, van Emst-de Vries SE, et al. Mitigation of NADH: ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim Biophys Acta. 2008;1777:853–859. doi: 10.1016/j.bbabio.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Nijtmans LG, Dieteren CE et al (2009) Mammalian mitochondrial complex I: biogenesis, regulation and reactive oxygen species generation. Antioxid Redox Signal (in press) [DOI] [PubMed]
- Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys Chem. 2007;129:93–110. doi: 10.1016/j.bpc.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–573. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Macias CA, Chiao JW, Xiao J, et al. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245:305–314. doi: 10.1097/01.sla.0000236626.57752.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, et al. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Man PY, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet. 2002;39:162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima Y, Hiida Y, Oguchi Y. Remission of Leber’s hereditary optic neuropathy with idebenone. Lancet. 1992;340:368–369. doi: 10.1016/0140-6736(92)91442-b. [DOI] [PubMed] [Google Scholar]
- Mashima Y, Kigasawa K, Wakakura M, et al. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J Neuroophthalmol. 2000;20:166–170. doi: 10.1097/00041327-200020030-00006. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Meier T, Buyse G. Idebenone: an emerging therapy for Friedreich ataxia. J Neurol. 2009;256(Suppl 1):25–30. doi: 10.1007/s00415-009-1005-0. [DOI] [PubMed] [Google Scholar]
- Milne JC, Denu JM. The sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Lightowlers RN, Taylor RW, et al. Targeting large molecules to mitochondria. Adv Drug Deliv Rev. 2001;49:189–198. doi: 10.1016/s0169-409x(01)00134-x. [DOI] [PubMed] [Google Scholar]
- Murdock DG, Boone BE, Esposito LA, et al. Up-regulation of nuclear and mitochondrial genes in the skeletal muscle of mice lacking the heart/muscle isoform of the adenine nucleotide translocator. J Biol Chem. 1999;274:14429–14433. doi: 10.1074/jbc.274.20.14429. [DOI] [PubMed] [Google Scholar]
- Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Echtay KS, Blaikie FH, et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- Oliva J, French BA, Li J, et al. Sirt1 is involved in energy metabolism: the role of chronic ethanol feeding and resveratrol. Exp Mol Pathol. 2008;85:155–159. doi: 10.1016/j.yexmp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Blasedale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, et al. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, DiMauro S, Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb EL, Page MM, Wiens BE, et al. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Cocheme HM, Logan A, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010;48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Gilkerson R, Aggeler R, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- Santra S, Gilkerson RW, Davidson M, et al. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol. 2004;56:662–669. doi: 10.1002/ana.20240. [DOI] [PubMed] [Google Scholar]
- Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- Sholtz KF, Solovjeva NA, Kotelnikova AV, et al. Effect of gramicidin S and its derivatives on the mitochondrial membrane. FEBS Lett. 1975;58:140–144. doi: 10.1016/0014-5793(75)80244-4. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Functions of mitochondria: from intracellular power stations to mediators of a senescence program. Cell Mol Life Sci. 2009;66:1785–1793. doi: 10.1007/s00018-009-9183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP, Anisimov VN, Antonenko YN, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Sluse FE, Jarmuszkiewicz W, Navet R, et al. Mitochondrial UCPs: new insights into regulation and impact. Biochim Biophys Acta. 2006;1757:480–485. doi: 10.1016/j.bbabio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Smeitink JA, Zeviani M, Turnbull DM, et al. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Smith RA, Porteous CM, Coulter CV, et al. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Smith RA, Adlam VJ, Blaikie FH, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafazoli S, Wright JS, O’Brien PJ. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem Res Toxicol. 2005;18:1567–1574. doi: 10.1021/tx0500575. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Haller RG. Exercise and training in mitochondrial myopathies. Med Sci Sports Exerc. 2005;37:2094–2101. doi: 10.1249/01.mss.0000177446.97671.2a. [DOI] [PubMed] [Google Scholar]
- Tavi P, Hansson A, Zhang SJ, et al. Abnormal Ca(2+) release and catecholamine-induced arrhythmias in mitochondrial cardiomyopathy. Hum Mol Genet. 2005;14:1069–1076. doi: 10.1093/hmg/ddi119. [DOI] [PubMed] [Google Scholar]
- Toshima K, Kuroda Y, Hashimoto T, et al. Enzymologic studies and therapy of Leigh’s disease associated with pyruvate decarboxylase deficiency. Pediatr Res. 1982;16:430–435. doi: 10.1203/00006450-198206000-00006. [DOI] [PubMed] [Google Scholar]
- Valsecchi F, Esseling JJ, Koopman WJ et al (2009) Calcium and ATP handling in human NADH:Ubiquinone oxidoreductase deficiency. Biochim Biophys Acta (in press) [DOI] [PubMed]
- Verkaart S, Koopman WJ, Cheek J, et al. Mitochondrial and cytosolic thiol redox state are not detectably altered in isolated human NADH:ubiquinone oxidoreductase deficiency. Biochim Biophys Acta. 2007;1772:1041–1051. doi: 10.1016/j.bbadis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Visch HJ, Rutter GA, Koopman WJ, et al. Inhibition of mitochondrial Na+-Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J Biol Chem. 2004;279:40328–40336. doi: 10.1074/jbc.M408068200. [DOI] [PubMed] [Google Scholar]
- Visch HJ, Koopman WJ, Zeegers D, et al. Ca2+-mobilizing agonists increase mitochondrial ATP production to accelerate cytosolic Ca2+ removal: aberrations in human complex I deficiency. Am J Physiol Cell Physiol. 2006;291:C308–C316. doi: 10.1152/ajpcell.00561.2005. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Wang SB, Weng WC, Lee NC, et al. Mutation of mitochondrial DNA G13513A presenting with Leigh syndrome, Wolff-Parkinson-White syndrome and cardiomyopathy. Pediatr Neonatol. 2008;49:145–149. doi: 10.1016/S1875-9572(08)60030-3. [DOI] [PubMed] [Google Scholar]
- Wenz T. PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life. 2009;61:1051–1062. doi: 10.1002/iub.261. [DOI] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, et al. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wexler ID, Hemalatha SG, McConnell J, et al. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- Wijburg FA, Barth PG, Bindoff LA, et al. Leigh syndrome associated with a deficiency of the pyruvate dehydrogenase complex: results of treatment with a ketogenic diet. Neuropediatrics. 1992;23:147–152. doi: 10.1055/s-2008-1071331. [DOI] [PubMed] [Google Scholar]
- Witschi A, Reddy S, Stofer B, et al. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhao K, Calingasan NY et al (2009) Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal (in press) [DOI] [PMC free article] [PubMed]
- Zhao K, Zhao GM, Wu D, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]