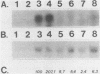
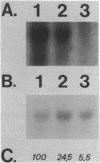
Abstract
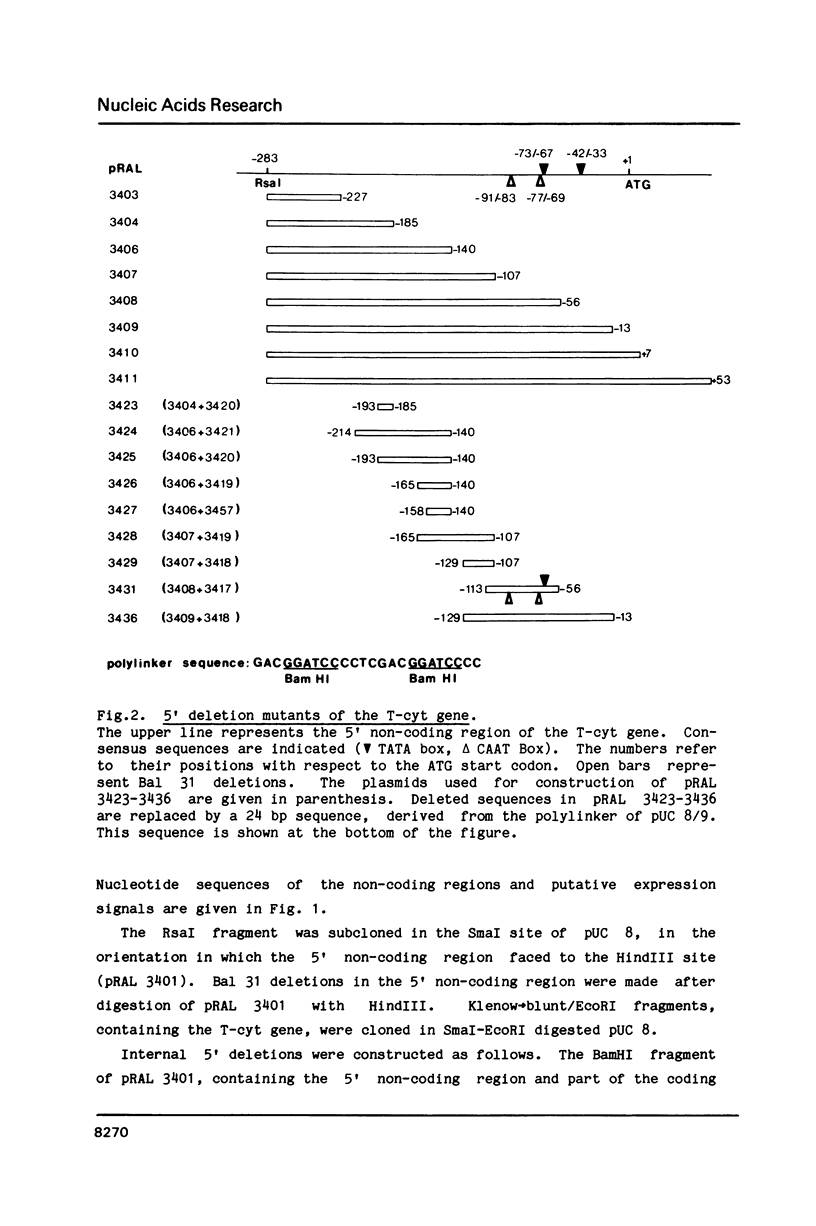
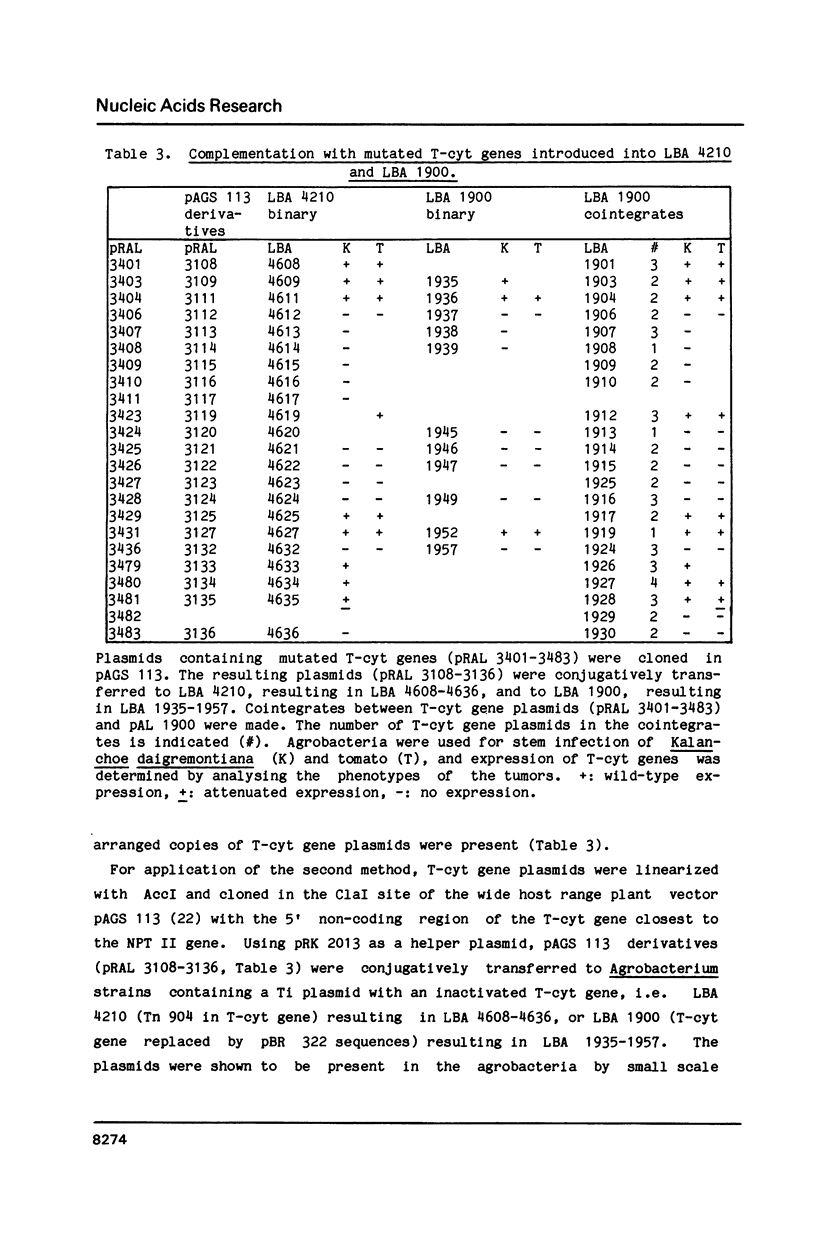
Within the 5' and 3' non-coding regions of the T-cyt gene from the octopine T-DNA of Agrobacterium tumefaciens sequences required for expression of this gene in plant cells were identified by deletion mutagenesis. The results show that 184 bp of the 5' non-coding region and 270 bp of the 3' non-coding region are sufficient for wild-type expression. Within the 5' non-coding region two essential expression signals were identified: (1.) an activator element located between -185 and -129 with respect to the ATG start codon and (2.) one out of two TATA boxes. Deletions of the activator element or the two TATA boxes resulted in nonfunctional genes. Deletion of the upstream TATA box and both putative CAAT boxes did not significantly affect expression. Within the 3' non-coding region, the polyadenylation box most distal to the stop codon was not essential for expression, but sequences more upstream, including a second polyadenylation box were found to be required for wild-type expression.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bruce W. B., Gurley W. B. Functional domains of a T-DNA promoter active in crown gall tumors. Mol Cell Biol. 1987 Jan;7(1):59–67. doi: 10.1128/mcb.7.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann I., Marner F. J., Schröder G., Waffenschmidt S., Schröder J. Tumour genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J. 1985 Apr;4(4):853–859. doi: 10.1002/j.1460-2075.1985.tb03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Caput D., Williams S. R., Martin D. W., Jr Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4281–4285. doi: 10.1073/pnas.80.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. B., Flick J. S., Rogers S. G. Nucleotide sequence of the tmr locus of Agrobacterium tumefaciens pTi T37 T-DNA. Nucleic Acids Res. 1984 Jun 11;12(11):4665–4677. doi: 10.1093/nar/12.11.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidekamp F., Dirkse W. G., Hille J., van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983 Sep 24;11(18):6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Schilperoort R. A. The molecular genetics of crown gall tumorigenesis. Adv Genet. 1984;22:209–283. doi: 10.1016/s0065-2660(08)60041-3. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Ooms G., Schilperoort R. A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980 Sep;143(3):1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Klee H., Montoya A., Garfinkel D., Fuller S., Flores C., Nester E., Gordon M. Nucleotide sequence and transcript mapping of the tmr gene of the pTiA6NC octopine Ti-plasmid: a bacterial gene involved in plant tumorigenesis. J Mol Appl Genet. 1984;2(4):354–362. [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Simpson J., Timko M. P., Cashmore A. R., Schell J., Montagu M. V., Herrera-Estrella L. Light-inducible and tissue-specific expression of a chimaeric gene under control of the 5'-flanking sequence of a pea chlorophyll a/b-binding protein gene. EMBO J. 1985 Nov;4(11):2723–2729. doi: 10.1002/j.1460-2075.1985.tb03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]