Abstract
The functions of the phosphodiesterase 8B (PDE8) family of phosphodiesterases have been largely unexplored because of the unavailability of selective pharmacological inhibitors. Here, we report a novel function of PDE8B as a major regulator of adrenal steroidogenesis using a genetically ablated PDE8B mouse model as well as cell lines treated with either a new PDE8-selective inhibitor or a short hairpin RNA (shRNA) construct against PDE8B. We demonstrate that PDE8B is highly enriched in mouse adrenal fasciculata cells, and show that PDE8B knockout mice have elevated urinary corticosterone as a result of adrenal hypersensitivity toward adrenocorticotropin. Likewise, ablation of PDE8B mRNA transcripts by an shRNA construct potentiates steroidogenesis in the commonly used Y-1 adrenal cell line. We also observed that the PDE8-selective inhibitor (PF-04957325) potentiates adrenocorticotropin stimulation of steroidogenesis by increasing cAMP-dependent protein kinase activity in both primary isolated adrenocortical cells and Y-1 cells. It is noteworthy that PDE8s have their greatest control under low adrenocorticotropin-stimulated conditions, whereas other higher Km PDE(s) modulate steroidogenesis more effectively when cells are fully stimulated. Finally, both genetic ablation of PDE8B and long-term pharmacological inhibition of PDE8s cause increased expression of steroidogenic enzymes. We conclude that PDE8B is a major regulator of one or more pools of cAMP that promote steroidogenesis via both short- and long-term mechanisms. These findings further suggest PDE8B as a potential therapeutic target for the treatment of several different adrenal diseases.
Introduction
Glucocorticoids serve a number of important roles in mammalian physiology as they regulate glucose and fat metabolism, mediate stress responses, and influence the immune system, among other functions (McCann et al., 2000; Aguilera et al., 2007; Macfarlane et al., 2008; Vinson, 2009). The major murine glucocorticoid is corticosterone. Corticosterone is synthesized by adrenal zona fasciculata (AZF) cells, found in the thickest layer of the adrenal cortex. Steroidogenesis by these cells is regulated by the pituitary hormone adrenocorticotropic hormone (adrenocorticotropin) (Vinson, 2003). Among the many functions of corticosterone, high circulating levels can inhibit corticotropin-releasing factor release from the hypothalamus and adrenocorticotropin production from the pituitary gland. These effects on the hypothalamus and pituitary form an efficient feedback regulatory loop known as the hypothalamic-pituitary-adrenal (HPA) axis, which maintains glucocorticoid homeostasis (Jacobson, 2005).
Adrenocorticotropin binding to its receptor, the melanocortin 2 receptor (MC2R), stimulates production of the second-messenger cyclic adenosine monophosphate (cAMP) in AZF cells. cAMP then activates cAMP-dependent protein kinase (PKA), which in turn promotes steroidogenesis by at least three mechanisms (Stocco et al., 2005; Manna et al., 2009). First, PKA can regulate the availability of free cholesterol, the initial substrate for corticosterone biosynthesis. This occurs by phosphorylation and activation of hormone-sensitive lipase (HSL) (also known as cholesterol ester hydrolase), which catalyzes the hydrolysis of stored cholesterol esters into free cholesterol and a fatty acid (Arakane et al., 1997; Kraemer and Shen, 2002). Second, PKA can phosphorylate and activate the steroidogenic acute regulatory protein (StAR), a key regulator of free cholesterol transfer from stores to the mitochondrial membrane (Stocco, 2001). This process then allows a steroidogenic cytochrome p450 enzyme, p450scc, to convert cholesterol into pregnenolone, initiating corticosterone synthesis. This synthetic pathway includes the enzymes 3βHSD, p450c21, and p450c11 that catalyze a cascade of reactions, ultimately leading to the production of corticosterone. It is important to mention that the transport of cholesterol to mitochondria by StAR protein is usually regarded as the rate-limiting step of this pathway. However, under some conditions, HSL activity can acutely limit synthesis. Finally, PKA activation also has a long-term influence on steroidogenesis. In the long-term phase of steroid production, mRNA transcripts of several of the key steroidogenic genes increase because of cAMP/PKA-mediated activation of transcription factors, including SF-1 and DAX-1 (Simpson and Waterman, 1988; Davis and Lau, 1994; Sewer and Waterman, 2003). All of these regulatory processes are controlled by cAMP, although possibly by different pools.
The level of cAMP in each of these pools is determined by its rate of synthesis by adenylyl cyclases and rate of degradation by phosphodiesterases (PDEs) (Conti and Beavo, 2007). Of these PDEs, the PDE8 family is one of the more recently discovered. The PDE8 family consists of two distinct genes: Pde8a and Pde8b. Both PDE8A and 8B hydrolyze cAMP with a very high affinity (Km ∼0.15 μM). Unlike other cAMP-specific PDEs, PDE8s are insensitive to a common nonselective PDE inhibitor, 3-isobutyl-1-methylxanthine (IBMX), but can be inhibited by a high concentration of dipyridamole (Soderling et al., 1998). Until recently, no truly selective PDE8 inhibitor has been available. However, Pfizer has reported on a small molecule that selectively inhibits PDE8 (Vang et al., 2010). This new selective PDE8 inhibitor, PF-04957325 (structure in Supplemental Fig. 5), has a reported in vitro IC50 of 0.7 nM against PDE8A, 0.2 nM against PDE8B, and >1.5 μM against all other PDE isoforms (Vang et al., 2010). In this study, we have used this pharmacological tool with isolated primary adrenal cells as well as with a commonly used adrenocortical cell line, Y-1 cells, to demonstrate that PDE8 inhibition potentiates steroid production under subsaturating levels of adrenocorticotropin stimulation.
In addition, we have taken a genetic approach by using a global PDE8B knockout (KO) mouse model to investigate the long-term consequence of PDE8B ablation on steroidogenesis. In 2006, our laboratory reported the modulation of testosterone secretion by PDE8A in Leydig cells using a similar PDE8A KO (Vasta et al., 2006). These studies showed that PDE8A plays an important role in regulating a pool of cAMP that promotes testicular steroidogenesis. Here, we report that the other member of the PDE8 family, PDE8B, regulates adrenocorticotropin-stimulated AZF steroidogenesis by both short- and long-term mechanisms. Finally, a mutation in PDE8B has also been implicated in a single severe case of adrenal hyperplasia in humans (Horvath et al., 2008a). Together, these data suggest the general importance of PDE8s in cAMP regulation of steroid production.
Materials and Methods
β-Gal Activity Staining.
Adrenal glands were fixed in 4% (w/v) paraformaldehyde on ice for 6 h. The fixed glands were sequentially rinsed with 10% sucrose, soaked in 20% sucrose for 8 h, and then soaked in 30% sucrose overnight to remove paraformaldehyde. The tissue was embedded in Tissue-Tek O.C.T. compound and sectioned on a cryostat into 20-μm thick floating slices in phosphate-buffered saline (PBS), pH 7.4. β-Galactosidase activity was evaluated by staining the sections with 5-bromo-4-chloro-3-hydroxyindole (X-gal), as adapted from a protocol described previously (Duffield et al., 2005). In brief, after mounting the floating sections on slides, the slides were placed in an X-gal staining mixture [2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, 5 mM EGTA, 20 mM K3Fe(CN)6, 20 mM K4Fe(CN)6 · H2O, and 1 mg/ml X-gal in PBS, pH 7.4] at 37°C overnight. After the incubation with X-gal, the slides were washed three times with PBS, pH 7.4, once with water, and then counterstained with eosin and mounted with Permount (Thermo Fisher Scientific, Waltham, MA).
Real-Time PCR.
Whole mouse adrenal glands were disrupted with a Dounce homogenizer in RTL buffer from an RNeasy Mini Kit (QIAGEN, Valencia, CA). Total RNA was isolated using Qiashredder columns and the RNeasy Mini Kit according to the manufacturer's protocol. Then cDNA samples were generated with QIAGEN Omniscript RT kits using 2 μg of total RNA for each reaction. Relative gene expression was determined by performing real-time PCR on a MX3000P QPCR system (Stratagene, La Jolla, CA) and analyzed with Mx-Pro software. The primers for adrenal cytochromes P450 have been reported previously and verified (Otawa et al., 2007; Cooray et al., 2008). Reverse-transcriptase PCRs were run with iTaq SYBR Supermix (Bio-Rad Laboratories, Hercules, CA) with the following thermal profile: denaturing at 95°C for 15 s, annealing at 58°C for 1 min, and extension at 72°C for 1 min for 40 cycles.
Immunoprecipitation and PDE Activity Assay.
Immunoprecipitation of PDE8B was performed with goat polyclonal antibodies to PDE8B [PDE8B (I-16) from Santa Cruz Biotechnology, Santa Cruz, CA] in tissue lysates made in 0.5% Triton X-100, 1 mM EDTA, and 1:100 diluted protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) in PBS, pH 7.4. The immunopellet was resuspended and washed three times with lysis buffer before PDE activity was measured. PDE activity was measured as described previously (Hansen and Beavo, 1982; Soderling et al., 1998). In brief, the activity assay was carried out at very low substrate conditions (10–20 nM [3H]cAMP, ∼100,000 cpm/reaction) in 40 mM MOPS, pH 7.5, 15 mM magnesium acetate, 2 mM EGTA, and 0.2 mg/ml bovine serum albumin (BSA) in a final volume of 0.25 ml. The reaction time and amount of lysate were maintained so that less than 30% of the substrate was hydrolyzed.
Animals.
The PDE8B KO mice used in these studies were generated on a 129 genetic background by Deltagen, Inc. (San Carlos, CA) under contract to Pfizer, Inc. (Sandwich, United Kingdom). The animals were then backcrossed with C57BL/6 mice obtained from Charles River (Margate, Kent, UK) or The Jackson Laboratory (Bar Harbor, ME) for 12 to 15 generations. All animal usage and procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington (Seattle, WA) in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All experiments were performed on PDE8B KO animals between the ages of 4 and 12 months, using their WT littermates as controls.
The double PDE8A/8B knockout mice were established in the laboratory by crossing the PDE8B KO with the PDE8A KO described previously (Vasta et al., 2006). Deltagen generated the PDE8A KO with a knockout strategy similar to that of the PDE8B KO.
Urinary Corticosterone Measurements.
All corticosterone measurements were carried out between 6 PM and 12 AM during the dark cycle when the animals were most active. First, animals were provided with 4 mM saccharin water ad libitum in their home cages to increase their water consumption 2 h before an experiment or any handling (at 4 PM). Urine from an individual mouse reflecting the basal, nonstressed, state was collected at 6 PM. In general, the animals urinated spontaneously during handling. Occasionally, gentle pressure was applied to the bladder to encourage urination. Animals were then injected intraperitoneally with 1 ml of isotonic saline to increase frequency of urination. The combination of handling and saline injection provided a mild stress. Mice were then placed individually in urine collection cages (constructed with a disposable 96-well plate and a 96-well strip well frame, with 1-mm mesh above the wells to separate feces from urine; transparent plastic sheets were used as walls). All mice were handled and injected for 2 days before the actual urine sample collections. On the third day, their urine samples were collected at 3 and 6 h after intraperitoneal injection, and these were regarded as poststimulated state samples.
Primary Mouse Adrenal Cell Culture and Pregnenolone Measurement.
A primary adrenal cell isolation protocol was adapted as described previously (Enríquez de Salamanca and García, 2003; Supornsilchai et al., 2005). In brief, mouse adrenal glands were removed, and the attached fat tissue was trimmed off. Whole adrenal glands were minced with a pair of scissors and digested in a buffer (90 mM HEPES, 120 mM NaCl, 5 mM KCl, 1 mM CaCl2, 4.5 mM glucose, and 15 mg/ml BSA) containing freshly prepared collagenase type IV (2 mg/ml) and DNase (0.1 mg/ml) (Worthington Biochemical Corporation, Lakewood, NJ). The minced adrenals were incubated with digestion buffer for 1 h at 37°C with agitation (∼150 rpm, orbital). The digested tissue was then triturated 15 to 20 times with a 1 ml of Eppendorf pipette and filtered once into a polystyrene tube fitted with a cell-strainer cap (Falcon; BD Biosciences Discovery Labware, Bedford, MA). Cells were collected by brief centrifugation (∼300g, 10 min) and washed twice with oxygenated Hanks' balanced salt solution plus 1 mg/ml BSA (further purified fraction V and γ-globulin free; Sigma-Aldrich). Cells were then resuspended in Dulbecco's modified Eagle's medium/F-12 (1:1) media with 1 mg/ml BSA and plated in 96-well plates. The cells then were allowed to recover for 3 h in the incubator at 37°C. After recovery, the cells were preincubated with 10 μM trilostane [(4α,5α,17β)-4,5-epoxy-3,17-hydroxyandrost-2-ene-2-carbonitrile)] (sanofi-aventis, Bridgewater, NJ) for 30 min to inhibit the conversion of pregnenolone to progesterone. Synthetic full-length adrenocorticotropin peptide (1–39) (AnaSpec, San Jose, CA) was used on some cells to stimulate steroidogenesis. The amount of pregnenolone in the media was determined by pregnenolone enzyme-linked immunosorbent assay (Diagnostics Biochem Canada, Inc., London, ON, Canada).
Y-1 Cell Culturing and Reagents.
Y-1 cells were cultured in F12K media with 20% fetal bovine serum. Stocks of Y-1 cells were frozen in media with 5% DMSO according to the American Type Culture Collection (Manassas, VA) guidelines. The nonselective PDE inhibitors used in the cell culturing models, dipyridamole and IBMX, were purchased from Sigma-Aldrich. A low PDE2A Y-1 expression clone was established in our laboratory by clonal selection and used in all reported studies. Y-1 cells were originally obtained from the American Type Culture Collection.
shRNA Ablation of PDE8B.
Y-1 cells were transfected with either an shRNA construct (3′-aatcctcatcaaacgcatgat-5′) or control shRNA plasmid (SureSilencing shRNA plasmids; SA Biosciences, Frederick, MD) using the Nucleofector technology (Lonza Walkersville Inc., Walkersville, MD). The control shRNA plasmid had all the same elements as shRNA plasmid such as a green fluorescent protein marker and a 21-nucleotide shRNA sequence (3′-ggaatctcattcgatgcatac-5′) with no known target. The Y-1 cells were transfected according to the manufacturer's instruction. In brief, Y-1 cells (2.5 million cells per transfection) were resuspended in buffers provided with the Cell Line Nucleofector Kit V along with 2 μg of plasmid DNA. Y-1 cells were electroporated with a Nucleofector Device (program T-32). The transfected Y-1 cells were plated and allowed to recover for 48 h before testing. Transfection efficiency was monitored by determining the percentage of cells expressing measurable green fluorescent protein plasmid marker. The knockdown efficiency was measured by real-time PCR probing for PDE8B mRNA (detecting exon 8–9). Steroid secretion of Y-1 cells containing shRNA plasmids was determined using the same pregnenolone kit described earlier.
Results
PDE8B Is Highly Expressed In AZF Cells.
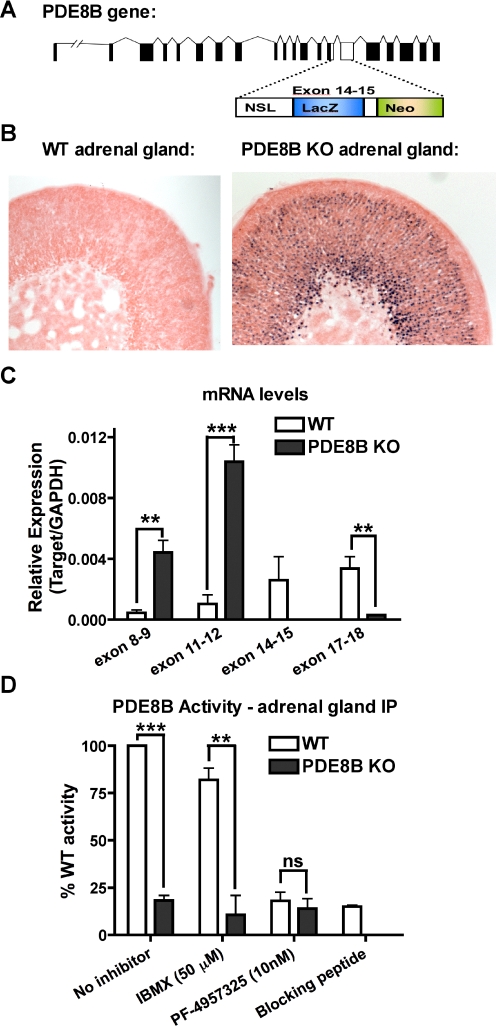
In the PDE8B KO animals used in these studies, a critical region in the catalytic domain (exon 14–15) was replaced with DNA sequence encoding a lacZ reporter gene and a neomycin resistance gene followed by a stop codon (Fig. 1A). The exogenous lacZ gene also contained a nuclear targeting sequence that directs the β-galactosidase activity to the nucleus of cells having active endogenous PDE8B promoter. The enzymatic activity of the gene product (β-galactosidase) therefore can be used as an indirect measurement of PDE8B mRNA expression. Using this method, we found that the PDE8B promoter was highly active in the adrenal gland. Moreover, we observed that more than 80% of the adrenocortical cells contained blue (X-gal-stained) nuclei (Fig. 1B). These results indicate that the PDE8B gene is transcribed in most of the adrenocortical cells.
Fig. 1.
PDE8B is highly expressed in AZF cells. A, the catalytic domain (exon 14–15) of PDE8B gene was disrupted by a construct containing a lacZ reporter gene, a neomycin resistance gene, and premature stop codons. B, X-gal staining of PDE8B KO adrenal gland showed that the promoter of PDE8B gene was active, and PDE8B was expressed in the AZF cells. C, the full-length PDE8B mRNA was not transcribed in the PDE8B KO adrenal. However, the 5′ mRNA region of PDE8B was up-regulated perhaps due to the absence of functional PDE8B (n = 3). D, the absence of functional PDE8B enzyme in PDE8B KO adrenals was shown by immunoprecipitating PDE8B from the PDE8B KO in comparison to the WT control (n = 3). The data are reported as means ± S.E.M., and the data were analyzed by Student's t test (unpaired, two-tailed): ns, no significance; **, p < 0.01; ***, p < 0.001.
As shown in Fig. 1C, disruption of PDE8B gene was verified by real-time PCR. We observed that the amplicons of the deleted exon 14 to 15 region and the 3′-region downstream of this region were either very low or undetectable in the PDE8B KO. However, all of the 5′-PDE8B mRNA amplicons were increased in relation to WT, perhaps because of a lack of functional PDE8B enzymes. Using Western blotting with commercially available antibodies (with either C- or N-terminal epitope), we did not detect any truncated PDE8B proteins in the PDE8B KO adrenal glands. To further demonstrate that no active PDE8B protein was produced in the PDE8B KO, we performed immunoprecipitation with an antibody [Santa Cruz Biotechnology, PDE8B (I-16)] against an epitope in PDE8B that is upstream of the disrupted exon 14 to 15. The results show that IBMX-insensitive PDE8B activity can be precipitated with this antibody from the WT but not PDE8B KO adrenal lysate (Fig. 1D). In addition, we also demonstrated that the new selective PDE8 inhibitor PF-04957325 blocked the immunoprecipitated PDE activity at 10 nM. Finally, a lack of PDE activity in the peptide blocking control indicated antibody specificity for PDE8B. Overall, these data verify the absence of functional PDE8B activity in the PDE8B KO mice used in these studies.
PDE8B KO Mice Exhibit Adrenal Hypersensitivity In Vivo.
The general importance of cAMP/PKA signaling in steroidogenesis plus the high expression of PDE8B in the adrenal cortex led us to hypothesize that PDE8B is a modulator of one or more pools of cAMP stimulated by adrenocorticotropin in AZF cells. In addition, because PDE8A modulates testosterone production in Leydig cells (Vasta et al., 2006), we speculated that PDE8B might play a similar role in AZF cells. Up to now, specific functions of PDE8B have remained relatively unexplored because of the unavailability of a selective inhibitor. Therefore, the PDE8B KO mice provided us with a good tool to test for possible role(s) of PDE8B in adrenal steroidogenesis.
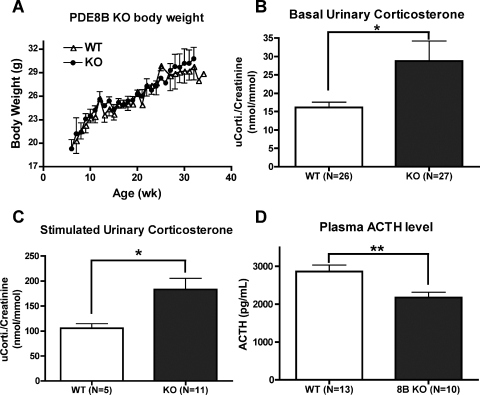
These PDE8B KO mice did not exhibit any obvious reproductive or developmental defects. They seemed healthy and maintained a normal weight under regular chow diet (Fig. 2A). However, we were interested to determine whether complete ablation of the PDE8B gene in the mouse could induce an adrenal hyperplasia phenotype as reported for one patient who had a point mutation in the PDE8B gene (Horvath et al., 2008a). However, by comparing the adrenal weight and morphology of serial cross-sections of the adrenal glands, we did not observe any obvious increase in the size of the PDE8B KO adrenal glands (Supplemental Fig. 1).
Fig. 2.
PDE8B KO mice exhibit adrenal hypersensitivity in vivo. PDE8B KO mice had no gross developmental defects compared with their WT littermates. A, the PDE8B KO mice maintained normal body weight under standard lab chow diet (n = 3–7). B, PDE8B KO mice had elevated basal urinary corticosterone (n = 26–27). C, PDE8B KO mice also exhibited increased stimulated corticosterone levels, when mice were mildly stressed via an intraperitoneal saline injection (n = 5–11). D, the circulating adrenocorticotropin (ACTH) level of the PDE8B KO was not higher than WT control (n = 10–13). The data are reported as means ± S.E.M., and the data were analyzed by Student's t test (unpaired, two-tailed): *, p < 0.05; **, p < 0.01.
We then tried to determine whether the PDE8B KO mice had any adrenal hypersensitivity to adrenocorticotropin in vivo. As one test of this idea, we determined urinary corticosterone under basal and mild stress conditions as a measure of adrenal steroid production. As shown in Fig. 2, B and C, we found that PDE8B KO mice had elevated levels of urinary corticosterone in both basal and stressed conditions compared with their littermate WT controls. These data suggest that PDE8B KO mice exhibit adrenal hypersensitivity toward adrenocorticotropin. However, it also was possible that the loss of PDE8B might be causing an increase in adrenocorticotropin. Therefore, we measured serum adrenocorticotropin levels by enzyme-linked immunosorbent assay and found that the PDE8B KO mice had a lower, not higher, circulating adrenocorticotropin level compared with the WT (Fig. 2D). This result is consistent with the idea that the suppressed adrenocorticotropin level is due to a long-term elevation of corticosterone negatively feeding back to the HPA axis. Overall, these findings suggest that PDE8B is an important regulator of adrenal steroidogenesis in vivo. Therefore, ablation of the PDE8B gene causes adrenal hypersensitivity toward adrenocorticotropin and an altered HPA axis as a result of abnormal corticosterone production.
PDE8B Gene Ablation Increases mRNA Expressions of StAR Protein and MC2R.
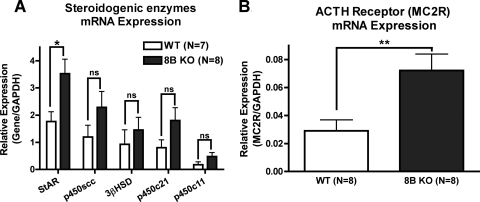
We then investigated the molecular mechanisms by which PDE8B ablation might increase adrenal steroid production. Because the expression of several key steroidogenic enzymes are known to be transcriptionally regulated by cAMP, we hypothesized that the ablation of PDE8B might increase the expression of these genes, namely StAR protein, several cytochromes P450, and MC2R (Mountjoy et al., 1994; Sewer and Waterman, 2003; Hammer et al., 2005; Manna et al., 2009). As shown in Fig. 3, A and B, PDE8B KO adrenal glands expressed mRNA for these cAMP-regulated steroidogenic genes at a higher level than WT. There was an approximately 2-fold increase in mRNA levels of StAR protein and MC2R in the KO adrenal glands. However, the mRNA levels of several of the other cAMP-regulated enzymes (P450scc, P45021, P450c11b, and 3βHSD) showed no statistically significant increase in the PDE8B KO. Therefore, it seems that an increase in mRNA levels of StAR protein and MC2R may explain at least part of the adrenal adrenocorticotropin hypersensitivity seen in the PDE8B KO animals in vivo.
Fig. 3.
PDE8B gene ablation increases mRNA expressions of StAR protein and MC2R. The long-term phase of steroidogenesis elicits cAMP-dependent transcriptional activation to increase steroid production. A, PDE8B KO adrenals had an increase of mRNA level of steroidogenic enzyme, StAR protein (n = 7–8). B, the mRNA of the adrenocorticotropin (ACTH) receptor (MC2R), which is a known cAMP-activated transcript, was also elevated in PDE8B KO adrenals (n = 8). The data are reported as means ± S.E.M., and the data were analyzed by Student's t test (unpaired, two-tailed): ns, no significance; *, p > 0.05; **, p < 0.01.
PDE8 Inhibition with PDE8-Selective Inhibitors (Dipyridamole and PF-04957325) Increases Short-Term Adrenal Steroid Production in Y-1 Cells.
In addition to the long-term effects on gene transcription mentioned above, it seemed possible that loss/inhibition of PDE8B activity also might have short-term effects on corticosterone production. To test this idea, we used two adrenal cell culture models, the mouse adrenocortical Y-1 cell line and primary isolated mouse adrenal cells, to study the effects of PDE8 inhibition on more short-term phases of steroidogenesis. In both cases, we measured pregnenolone secreted into the media as the readout of steroid production because it is an early immediate product of the rate-limiting step of steroidogenesis. In all experiments, cells were pretreated with a 3βHSD inhibitor (10 μM trilostane) (Bhattacharyya et al., 1995; Chaffin et al., 2000). At this concentration, trilostane effectively prevents most conversion of pregnenolone to corticosterone. Moreover, the Y-1 cells still respond to adrenocorticotropin-stimulation in the presence of trilostane, and their activation was reflected by a dose-dependent increase in pregnenolone production (Supplemental Fig. 2).
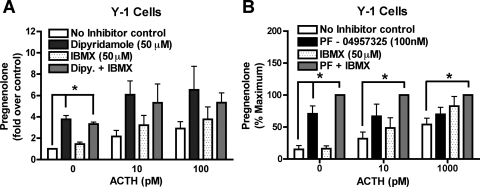
We first showed that a semiselective PDE8 inhibitor, dipyridamole (IC50 ∼20 μM), potentiated adrenocorticotropin-induced pregnenolone production in Y-1 cells. It is noteworthy that dipyridamole seemed to be more effective than the nonselective PDE inhibitor IBMX, which does not inhibit PDE8 (Fig. 4A). However, because dipyridamole can also inhibit several other PDEs, we also tested a new selective PDE8 inhibitor, PF-04957325, to determine whether it had a similar potentiating effect on pregnenolone production in Y-1 cells. As expected, treatment with the selective inhibitor PF-04957325 potentiated pregnenolone production in Y-1 cells, whereas IBMX only slightly increased steroid production (Fig. 4B). Together, these findings suggest that one or more PDE8s are important modulators of the pool(s) of cAMP that promote steroidogenesis in Y-1 cells. Because these cells contain both PDE8A and 8B, and because neither inhibitor distinguishes between these two gene products, it was still unclear from these experiments whether one or both PDE8 isoforms are important regulators of steroidogenesis in the Y-1 cells.
Fig. 4.
PDE8 inhibition with inhibitors increases short-term adrenal steroid production in Y-1 cells. The commonly used Y-1 adrenal cell line was used for short-term steroid measurements. A, treatment with the semiselective PDE8 inhibitor, dipyridamole, potentiated basal steroid production in Y-1 cells, whereas IBMX only slightly increased steroid production (n = 3). Pregnenolone secreted from Y-1 no inhibitor control cells averaged 0.824 ng/100,000 cells/h. B, the more PDE8 selective inhibitor PF-04957325 showed similar results (n = 3 or 4). Maximum pregnenolone secreted (in ng/100,000 cells/h) from Y-1 cells averaged 1.37 under no adrenocorticotropin (ACTH) stimulation, and 0.782 at 10 pM adrenocorticotropin, and 1.37 at 1000 pM adrenocorticotropin. The data are reported as means ± S.E.M., and data were analyzed with one-way ANOVA with Dunnett post hoc test: *, p < 0.05.
shRNA against PDE8B also Potentiates Steroidogenesis in Y-1 Cells.
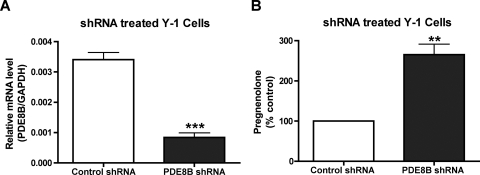
To address the issue of Y-1 cells containing both PDE8 isoforms, we used shRNA-induced RNA interference against PDE8B to convincingly demonstrate the effect of PDE8B in steroidogenesis. Using an electroporation system, we were able to transfect the majority of Y-1 cells with the shRNA containing plasmids shown in Supplemental Fig. 3. As presented in Fig. 5A, transfection with this shRNA construct significantly reduced the amount of PDE8B mRNA transcripts. More interestingly, this short-term ablation of PDE8B caused an increase in basal steroid production (Fig. 5B) similar to the effect observed when Y-1 cells were treated with the PDE8-selective inhibitor PF-04957325 in Fig. 4B. We interpret this result to support the notion that the potentiation in steroid production is caused by the ablation of PDE8B and not because of unintended non–sequence-specific effect because the control shRNA plasmid containing shRNA sequence lacking a specific target had no effect on steroidogenesis in Y-1 cells. Second, it is highly unlikely that any toxic effect of introducing an exogenous plasmid would elicit a gain of function such as an increase in steroid production. These data further support the idea that PDE8B is a major modulator of adrenal steroidogenesis.
Fig. 5.
shRNA against PDE8B also increases short-term adrenal steroid production in Y-1 cells. shRNA induced RNA interference was used to verify the modulation of PDE8B in adrenal steroidogenesis. A, PDE8B mRNA transcripts were greatly attenuated by the shRNA construct (n = 3). B, furthermore, this reduction of PDE8B mRNA expression elicited an increase in basal steroid production in Y-1 cells similar to that seen in the PDE8 inhibitor treated Y-1 cells shown in Fig. 4A (n = 3). Pregnenolone secreted from Y-1 cells transfected with control shRNA averaged 0.2 ng/million cells/h. The data are reported as means ± S.E.M., and the data were analyzed by Student's t test (unpaired, two-tailed): **, p > 0.01; ***, p < 0.001.
PDE8 Inhibitor also Potentiates Steroidogenesis in Primary Isolated Adrenal Cells.
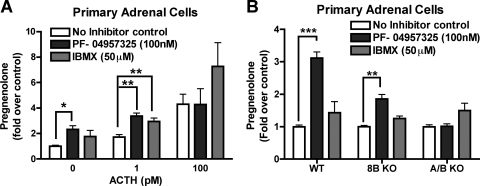
To demonstrate that the Y-1 cell line was a representative model for adrenocortical cells, we also used primary isolated adrenal cells from wild-type animals to determine whether PDE8B can modulate short-term steroid synthesis. Under a relatively short adrenocorticotropin-stimulating condition (1 h), WT primary adrenal cells seemed to be more responsive to adrenocorticotropin when PDE8s were inhibited. As presented in Fig. 6A, IBMX, a nonselective PDE inhibitor that does not inhibit PDE8s, potentiated pregnenolone production most effectively under the condition of saturating adrenocorticotropin concentration, whereas the PDE8 inhibitor PF-04957325 did not increase pregnenolone production under maximum stimulation. However, PF-04957325 did potentiate pregnenolone production of both basal and low doses of adrenocorticotropin. These in vitro experiments strongly suggest that one or more PDE8s are also effective modulators of acute steroidogenesis, in addition to regulating cAMP-dependent gene expression in the long-term phase (Fig. 3). Second, other PDEs are probably controlling some additional aspects of the cAMP-dependent pathway, because the nonselective PDE inhibitor IBMX causes an increase in steroidogenic capacity (higher maximum effect), which is distinct from the increased in responsiveness (lower EC50) observed because of PDE8 inhibition.
Fig. 6.
Treatment with PDE8 inhibitor increases short-term adrenal steroid production in primary isolated adrenal cells. Primary isolated adrenal cells were also used for acute steroid measurements. A, these cells responded to 100 nM selective PDE8 inhibitor PF-04957325 treatment with an elevated basal steroid production. It is noteworthy that these primary adrenal cells became insensitive to PDE8 inhibitor treatment when the cells were fully stimulated with adrenocorticotropin (ACTH). IBMX-sensitive PDEs became the predominate PDEs regulating the pool(s) of cAMP generated upon adrenocorticotropin stimulation (n = 3–5). B, the effect of the PDE8 inhibitor on steroidogenesis in the 8B and 8A/8B double KO cells was also tested. The effect of PF-04957325 was partially but not completely abrogated in adrenal cells from PDE8B KO. However, the effect of the PDE8 inhibitor was entirely abolished in isolated adrenal cells from double PDE8A/B KO adrenals. This strongly suggests that the drug inhibits only PDE8s at 100 nM in WT adrenal cells (n = 5). Basal pregnenolone secreted (in ng/10,000 cells/h) by primary isolated adrenal cells averaged 0.65 for WT cells, 0.84 for PDE8B KO cells, and 1.28 for PDE8A/B KO cells. The data are reported as means ± S.E.M., and data were analyzed with one-way ANOVA with Dunnett post hoc test: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The Effect of the PDE8 Inhibitor PF-04957325 Is Due Only to Blocking of PDE8 Activity.
To verify the specificity of PF-04957325 in the cell, we tested the selectivity of this compound using primary adrenal cells from the PDE8B KO and PDE8A/8B double KO animals. As presented in Fig. 6B, this PDE8 inhibitor had only a partial effect to potentiate basal steroid production in PDE8B KO adrenal cells. More importantly, this compound had no effect in the PDE8A/B double KO cells. Because PF-04957325 fully blocks the activity of both PDE8A and 8B at 100 nM in vitro, the partial effect observed from the PDE8B KO is probably due to PDE8A inhibition. This finding also provides evidence in intact cells that PF-04957325 is working by selectively inhibiting only PDE8A and 8B, and not other PDEs, in these cells. This observation also confirms the hypothesis that both PDE8s are important modulators of pool(s) of cAMP promoting adrenal steroidogenesis. However, it is still unclear if PDE8A and 8B are regulating the same or distinct pools of cAMP in these cells. PDE8 inhibition increases basal PKA activity and increases mRNAs of steroidogenic enzymes in Y-1 cells.
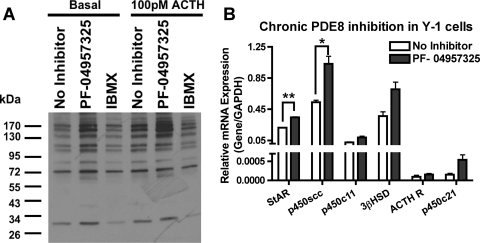
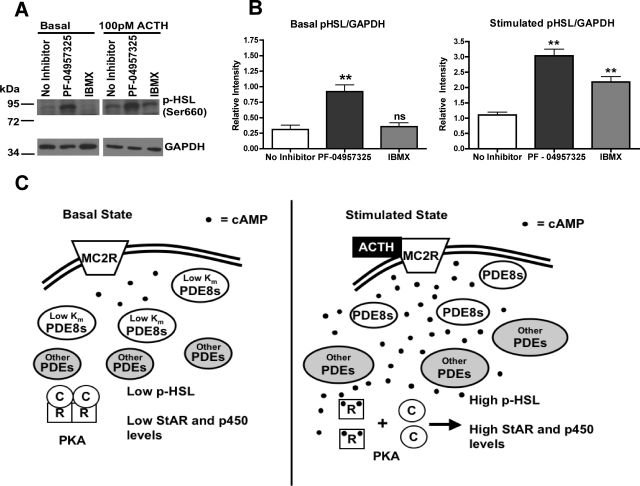
Next, we tried to determine the mechanisms by which PDE8B modulates acute steroidogenesis. We found that PF-04957325 increased basal PKA activity in Y-1 cells, whereas IBMX treatment did not (Fig. 7A). For instance, treatment with the PDE8 inhibitor increased the phosphorylation state of a number of proteins under basal and submaximal stimulated condition, as shown by Western blot analysis with a phospho-PKA substrate (RRXS/T) antibody (Cell Signaling Technology Inc., Danvers, MA). In addition, we identified one of the proteins to be HSL by probing with a phospho-HSL antibody (Ser660) (Cell Signaling Technology Inc.). We observed that PF-04957325 treatment potentiated the basal phosphorylation state of HSL, whereas IBMX treatment did not (Fig. 8, A and B). Furthermore, PF-04957325 treatment and IBMX treatment potentiated the phosphorylation state of HSL when cells were stimulated with a submaximal dose of adrenocorticotropin (Fig. 8, A and B). This observation is consistent with the previous finding that IBMX does potentiate steroid production but only under the stimulated condition in Fig. 7A. These results suggest that one role for PDE8(s) is to control substrate availability by lowering basal cAMP levels and keeping PKA inactive near stored cholesterol esters (the substrate for HSL/CEH). This finding is also consistent with the observation that PDE8 inhibition increases the basal steroid level, whereas IBMX has little to no effect in the Y-1 cells.
Fig. 7.
PDE8 inhibition increases basal PKA activity and also mRNAs of steroidogenic enzymes. Here, we tested two mechanisms by which PDE8 might regulate steroidogenesis. A, short-term treatment with PF-04957325 increased the phosphorylation state of multiple proteins in Y-1 cells, as shown by Western blotting with a general phospho-PKA substrate selective (RRXS/T) antibody (n = 4). B, long-term treatment with the selective PDE8B inhibitor elicited an increase in mRNA levels of steroidogenic enzymes in Y-1 cells (n = 3). The data are reported as means ± S.E.M., and the data were analyzed by Student's t test (unpaired, two-tailed): *, p < 0.05; **, p < 0.01.
Fig. 8.
PDE8 inhibition increases the phosphorylation state of HSL. A, short-term treatment with PF-04957325 increased the phosphorylation state of HSL under both basal and submaximal adrenocorticotropin (ACTH) stimulation, as shown by Western blot analysis with a phospho-HSL antibody (Ser660). B, the phospho-HSL bands were quantified by ImageJ and analyzed by one-way ANOVA analysis and p values obtained with Dunnett post hoc test (n = 3): ns, no significance; **, p < 0.01. C, a diagram demonstrates the current model for modulation of adrenal steroidogenesis by PDEs. The low Km and Vmax values of the PDE8s are depicted as modulating the basal state of cAMP thereby keeping PKA. However, under higher adrenocorticotropin stimulation, PDE8s are overwhelmed by the higher level of cAMP and PKA becomes activated. Under this elevated cAMP condition, other higher Km PDEs become predominate in modulation of steroidogenesis as shown by the experiment in Fig. 6A.
To determine whether the PDE8 inhibitor also produced an increase in cAMP-dependent transcription similar to that produced by the global ablation of PDE8B seen in the KO mice, we treated Y-1 cells with PF-04957325 continuously. After Y-1 cells were pretreated with PDE8 inhibitor for 16 h, and then stimulated with 100 pM adrenocorticotropin for 2 h, we observed a significant increase in mRNA expression of StAR protein and p450scc (Fig. 7B). This finding further verifies the observation in the PDE8B KO that PDE8B modulates steroidogenesis in part via increases in cAMP-dependent transcription.
Discussion
Because of the unavailability of selective pharmacological inhibitors and the lack of knowledge about regulating partners, functional studies for the PDE8 family of phosphodiesterases have been difficult and, until recently, have remained largely unexplored. We adopted a genetic approach that used a global PDE8B KO mouse model and a new PDE8-selective inhibitor (PF-04957325) to determine what role if any PDE8B might play in adrenal steroidogenesis. Using an X-gal staining method based on the insertion of β-galactosidase into the PDE8B knockout cassette (Duffield et al., 2005), we were able to show that PDE8B transcripts are highly enriched in a majority of the adrenocortical zona fasciculata cells.
In general, the PDE8B knockout mice develop normally as evidenced by a normal body weight and fertility. However, when examined more closely for an adrenal-related phenotype substantial differences from WT animals can be seen. PDE8B KO mice have elevated urinary corticosterone levels in the face of suppressed levels of circulating adrenocorticotropin. This long-term elevation of glucocorticoid levels is probably due to an adrenal hypersensitivity to adrenocorticotropin, which would be expected if PDE8B has an important role in modulating steroidogenesis. It is noteworthy that we observed no detectable increase in adrenal size as reported by Horvath et al. (2008b) for one patient with severe adrenal hyperplasia. This difference may be due to either species variation or perhaps more likely to an additional mutation present in the patient that allows for hyperplasia of the adrenal gland. Nevertheless, the ablation of functional PDE8B enzyme in the mouse is sufficient to sensitize the adrenal AZF cells to adrenocorticotropin and cause an increase in corticosterone production in vivo. This observation is consistent with the idea that the activity of PDE8B normally acts as a negative modulator of adrenal steroidogenesis. To our knowledge, this is the first report of a functional phenotype for a PDE8B KO mouse.
We then became interested in the mechanisms by which ablation of functional PDE8B can cause an increase in corticosterone production. We found that PDE8B KO mice had increased the expression of several key steroidogenic genes in the adrenal compared with their littermates, including transcripts for StAR protein and MC2R. Likewise, when PDE8 was continuously inhibited in Y-1 cells, we also observed that cAMP-dependent transcription was activated. Therefore, we suggest that ablation of functional PDE8B leads to a long-term increase in basal cAMP levels in specific compartments that control cAMP-dependent gene regulation. This cAMP elevation is capable of increasing the transcript levels of several key steroidogenic enzymes and therefore increasing urinary corticosterone in the PDE8B KO mice.
In addition to adrenocorticotropin sensitization in the whole-animal model, we also observed a similar sensitization effect on steroid production in cell culture models of both Y-1 cells and isolated primary adrenal cells. Using a semiselective PDE8 inhibitor (dipyridamole) and the selective PDE8 inhibitor PF-04957325, we showed that short-term PDE8 inhibition potentiates basal as well as submaximal adrenocorticotropin-stimulated pregnenolone. Moreover, PF-04957325 had no additive effect under the maximal adrenocorticotropin stimulation. In other words, PDE8 inhibition by PF-04957325 increased the responsiveness of adrenal cells toward adrenocorticotropin by shifting the EC50 for adrenocorticotropin to the left without increasing the maximum effect. We further demonstrated that this potentiation in pregnenolone production by PDE8 blockade is due at least in part to an increase in substrate availability. Treatment with PF-04957325 increased PKA activity and the phosphorylation state of HSL in Y-1 cells. As shown in Fig. 7A, PDE8A/B inhibition substantially increased the phosphorylation state of several other proteins, whereas a high-dose of IBMX did not. These results suggest that PDE8A/B control the pool of cAMP/PKA stimulated by adrenocorticotropin in Y-1 cells.
Because of the fact that the new PDE8-selective inhibitor does not distinguish between PDE8A and 8B, it remains unclear whether PDE8A and PDE8B have distinct or overlapping roles in Y-1 cells. However, the ability of PDE8B to modulate acute steroidogenesis was demonstrated by 1) an increase in steroid production elicited by short-term ablation of PDE8B mRNA transcript via an shRNA induced RNA interference as shown in Fig. 5B, and 2) a significant decrease in the potentiating effect of the PDE8 inhibitor on steroidogenesis in PDE8B KO adrenal cells (Fig. 6B). Furthermore, the PDE8A/8B double KO adrenal cells were completely insensitive toward the PDE8 inhibitor, which further validates the selectivity of this compound. From X-gal staining experiments, we also observed that PDE8A transcripts are expressed at low levels in a subpopulation of AZF. Therefore, we conclude that both PDE8A and PDE8B inhibition can modulate acute steroidogenesis. However, it is still not clear whether PDE8A and 8B regulate steroid production by the same mechanisms or even in the same cell types.
The ability of PDE8B to modulate steroidogenesis is not surprising because of the general importance of cyclic nucleotide signaling in this process and the relatively high expression levels of PDE8B mRNAs in the adrenal cortex. However, it is known that other PDEs also can modulate this process. For example PDE2A is known to regulate the effect of atrial natriuretic peptide on aldosterone production from adrenal glomerulosa cells (MacFarland et al., 1991). Here, we report a seemingly distinct mechanism by which PDE8B modulates short-term steroid production as shown in Fig. 8C. PDE8s seem to be regulating basal steroidogenesis, whereas other IBMX-sensitive PDEs modulate steroidogenesis more effectively when cells are stimulated. The low Km (∼0.15 μM) of the PDE8s would seem to make them ideal modulators of the low cAMP level present in the basal state, whereas higher Km PDEs are better suited to regulate cAMP in a fully stimulated state. Furthermore, we also observed an additive effect of PF-04957325 and IBMX on basal pregnenolone production from Y-1 cells. Taken together, these findings suggest that both PDE8s and at least one additional IBMX-sensitive PDE, such as PDE2A, can regulate one or more pools of cAMP that in turn control steroidogenesis. Furthermore, pharmacological inhibition of all of these PDEs is needed to achieve a maximum potentiating effect on adrenal steroid production. Many questions still remain with regard to the roles of different PDEs in modulating adrenal steroidogenesis. However, PDE8B gene ablation clearly elicits increased corticosterone production, which is at least partially due to an increased number of steroidogenic enzymes in the adrenal glands. In addition, short-term inhibition of PDE8s can increase basal PKA activity, thereby promoting short-term steroid production.
In addition to adrenal glands, we also screened for other peripheral tissues that expresses PDE8B gene using the X-gal staining technique and found that PDE8B expression is rather limited. Second, we demonstrate that PDE8B is a effective modulator of adrenal steroid production. Therefore, we believe that PDE8B might be a good therapeutic target to treat various adrenal diseases. For instance, a PDE8-selective inhibitor might be used to correct adrenal insufficiency, and a PDE8 activator might be used to treat Cushing's syndrome.
Supplementary Material
Acknowledgments
We thank Pfizer, Inc. for generously providing the PDE8 inhibitor PF-04957325 for this study. We thank Stephen Kraynik for critical reading of this manuscript and all members of our laboratory for discussions.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants R01-GM083926, R01-GM083926-02S1]; and Pharmacological Sciences Training.
This work was partially presented at the Experimental Biology meetings: Tsai LL, Shimizu-Albergine M, and Beavo JA (2008) Modulation of adrenal steroidogenesis by c-AMP specific phosphodiesterase 8. FASEB J 22:828.2; and Tsai LL, Shimizu-Albergine M, and Beavo JA (2009) Disruption of the phosphodiesterase 8B (PDE8B) gene alters the hypothalamic-pituitary-adrenal (HPA) axis. FASEB J 23:582.4
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/mol.110.069104.
ABBREVIATIONS:
- AZF
- adrenal zona fasciculate
- HPA
- hypothalamic-pituitary-adrenal
- MC2R
- melanocortin 2 receptor
- PKA
- cAMP-dependent protein kinase
- HSL
- hormone sensitive lipase
- StAR protein
- steroidogenic acute regulatory protein
- PDE
- phosphodiesterase
- IBMX
- 3-isobutyl-1-methylxanthine
- shRNA
- short hairpin RNA
- ANP
- atrial natriuretic peptide
- PBS
- phosphate-buffered saline
- KO
- knockout
- WT
- wild type
- PCR
- polymerase chain reaction
- BSA
- bovine serum albumin
- ANOVA
- analysis of variance
- MOPS
- 3-(N-morpholino)propanesulfonic acid
- X-gal
- 5-bromo-4-chloro-3-hydroxyindole.
Authorship Contributions
Participated in research design: Tsai, Shimizu-Albergine, and Beavo.
Conducted experiments: Tsai and Shimizu-Albergine.
Performed data analysis: Tsai, Shimizu-Albergine, and Beavo.
Wrote or contributed to the writing of the manuscript: Tsai, Shimizu-Albergine, and Beavo.
References
- Aguilera G, Kiss A, Liu Y, Kamitakahara A. (2007) Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress 10:153–161 [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd (1997) Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. (1995) Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem 270:11595–11602 [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Dissen GA, Stouffer RL. (2000) Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the peri-ovulatory interval in monkeys. Mol Hum Reprod 6:11–18 [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 [DOI] [PubMed] [Google Scholar]
- Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertová M, Cheetham ME, Elphick MR, Clark AJ. (2008) The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology 149:1935–1941 [DOI] [PubMed] [Google Scholar]
- Davis IJ, Lau LF. (1994) Endocrine and neurogenic regulation of the orphan nuclear receptors Nur77 and Nurr-1 in the adrenal glands. Mol Cell Biol 14:3469–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. (2005) Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115:1743–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enríquez de Salamanca A, García R. (2003) Rat glomerulosa cells in primary culture and E. coli lipopolysaccharide action. J Steroid Biochem Mol Biol 85:81–88 [DOI] [PubMed] [Google Scholar]
- Hammer GD, Parker KL, Schimmer BP. (2005) Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- Hansen RS, Beavo JA. (1982) Purification of two calcium/calmodulin-dependent forms of cyclic nucleotide phosphodiesterase by using conformation-specific monoclonal antibody chromatography. Proc Natl Acad Sci USA 79:2788–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libè R, Patronas Y, Robinson-White A, Remmers E, et al. (2008a) A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet 16:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Mericq V, Stratakis CA. (2008b) Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med 358:750–752 [DOI] [PubMed] [Google Scholar]
- Jacobson L. (2005) Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am 34:271–292 [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ. (2002) Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43:1585–1594 [DOI] [PubMed] [Google Scholar]
- MacFarland RT, Zelus BD, Beavo JA. (1991) High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem 266:136–142 [PubMed] [Google Scholar]
- Macfarlane DP, Forbes S, Walker BR. (2008) Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol 197:189–204 [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Stocco DM. (2009) Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SM, Antunes-Rodrigues J, Franci CR, Anselmo-Franci JA, Karanth S, Rettori V. (2000) Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz J Med Biol Res 33:1121–1131 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Bird IM, Rainey WE, Cone RD. (1994) ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol 99:R17–R20 [DOI] [PubMed] [Google Scholar]
- Otawa M, Arai H, Atomi Y. (2007) Molecular aspects of adrenal regulation for circadian glucocorticoid synthesis by chronic voluntary exercise. Life Sci 80:725–731 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. (2003) ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech 61:300–307 [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR. (1988) Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol 50:427–440 [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. (1998) Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA 95:8991–8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM. (2001) Tracking the role of a star in the sky of the new millennium. Mol Endocrinol 15:1245–1254 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. (2005) Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- Supornsilchai V, Svechnikov K, Seidlova-Wuttke D, Wuttke W, Söder O. (2005) Phytoestrogen resveratrol suppresses steroidogenesis by rat adrenocortical cells by inhibiting cytochrome P450 c21-hydroxylase. Horm Res 64:280–286 [DOI] [PubMed] [Google Scholar]
- Vang AG, Ben-Sasson SZ, Dong H, Kream B, DeNinno MP, Claffey MM, Housley W, Clark RB, Epstein PM, Brocke S. (2010) PDE8 regulates rapid Teff cell adhesion and proliferation independent of ICER. PLoS One 5:e12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta V, Shimizu-Albergine M, Beavo JA. (2006) Modulation of Leydig cell function by cyclic nucleotide phosphodiesterase 8A. Proc Natl Acad Sci USA 103:19925–19930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson GP. (2003) Adrenocortical zonation and ACTH. Microsc Res Tech 61:227–239 [DOI] [PubMed] [Google Scholar]
- Vinson GP. (2009) The adrenal cortex and life. Mol Cell Endocrinol 300:2–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.