Abstract
Background & Aims
Clostridium difficile (C.difficile) is the leading cause of nosocomial infectious diarrhea. Increasing incidence, antibiotic resistance and more virulent strains have dramatically increased the number of C.difficile-related deaths worldwide. The innate host response mechanisms to C.difficile are not resolved; however, we hypothesize that hypoxia-inducible factor (HIF-1) plays an innate protective role in C.difficile colitis. Thus, we assessed the impact of C.difficile toxins on the regulation of HIF-1 and evaluated the role of HIF-1α in C.difficile-mediated injury/inflammation.
Methods
In vitro studies assessed HIF-1α mRNA, protein levels and DNA binding events in human mucosal biopsies and Caco-2 cells exposed to C.difficile toxins. In vivo studies employed the murine ileal loop model of C.difficile toxin-induced intestinal injury. Mice with targeted deletion of HIF-1α in the intestinal epithelium were used to assess the impact of HIF-1α signaling in response to C.difficile toxin.
Results
Mucosal biopsies and Caco-2 cells exposed to C.difficile toxin displayed a significant increase in HIF-1α transcription and protein levels. Toxin-induced DNA binding was also observed in Caco-2 cells. Toxin-induced HIF-1α accumulation was attenuated by nitric oxide synthase inhibitors. In vivo, deletion of intestinal epithelial HIF-1α resulted in more severe toxin-induced intestinal injury and inflammation. In contrast, stabilization of HIF-1α, with dimethyloxallyl glycine, attenuated toxin-induced injury and inflammation. This was associated with an induction of HIF-1-regulated protective factors including VEGFa, CD73 and intestinal trefoil factor and down-regulation of proinflammatory molecules TNF and KC.
Conclusions
Our study is the first to describe the innate protective role for HIF-1α in response to C.difficile toxins. Harnessing the innate protective actions of HIF-1α in response to C.difficile toxins may represent a novel form of therapy for C.difficile-associated disease.
Keywords: HIF-1, Clostridium difficile, dimethyloxallyl glycine, epithelial barrier
Introduction
C.difficile is a spore-forming, toxin-producing (toxin A-TcdA; 308 kDa; toxin B-TcdB; 270 kDa) bacteria1 that is responsible for a concerning epidemic. The recent emergence of NAP-1/027 strains, that are more virulent and resistant to antibiotics, has magnified the impact of the current C.difficile crisis. Alarmingly, C.difficile-related deaths in the USA have risen by 35% per year since 19992 so that C.difficile now causes twice the number of deaths as HIV. Even with the best medical therapy patients can rapidly progress to toxic megacolon, sepsis and death.
C.difficile toxins rapidly induce intestinal injury and inflammation through disruption of the intestinal epithelial barrier and induction of proinflammatory mediators3. Both TcdA and TcdB are glucosyltransferases that irreversibly inactivate the Ras superfamily of small GTPases1. TcdA-induced changes in the actin cytoskeleton disrupts tight junctions, induces cell rounding and detachment4–6. Following detachment, intestinal epithelial cells can be driven to produce IL-8 and up-regulate ICAM-17 leading to the neutrophil chemoattraction, adhesion and subsequent mucosal inflammation8. The disruption of the mucosal barrier allows toxins to activate the immune cells within the lamina propria and to trigger the production of IL-8, IL-1β, TNF and nitric oxide (NO)7–10. These events can lead to further immune cell infiltrate and the tissue damage associated with C.difficile-associated disease (CDAD).
The hypoxia-inducible factor-1 (HIF-1) transcription factor complex responds rapidly to cellular stress, injury and inflammation and can protect the intestinal mucosa11–13. HIF-1, composed of an oxygen-labile α-subunit and a nuclear-localized β-subunit, is a master transcriptional factor and prototypical regulator of oxygen.–dependent gene transcription. The HIF-1α subunit is ubiquitously and constitutively expressed, but in normoxic conditions the protein is subject to hydroxylation by prolyl hydroxylases (PHD). This event triggers the binding of von Hippel Lindau protein which targets HIF-1α to the proteasome for degradation14. Upon stabilization during hypoxia, in the absence of hydroxylation, the HIF-1α-subunit translocates to the nucleus where it associates with its HIF-1β-subunit and initiates transcriptional events by binding to hypoxia responsive elements (HREs) present in promoter or enhancer regions of various genes. Thus, events that prevent the degradation of the HIF-1α-subunit trigger potent HIF-1-mediated transcription events14, 15.
In addition to promoting the adaptive responses to hypoxia, HIF-1 signaling has regulatory actions in a wide variety of cellular processes involved in immune13 and intestinal barrier function16–19. HIF-1 can increase the transcription of genes (e.g., VEGF, eNOS, iNOS and ITF) that are involved in wound repair, inflammation, cellular permeability and apoptosis.
The transcriptional activation of HIF-1 can also be induced in normoxia by a number of cytokines, growth factors and other circulating molecules (e.g., NO) through stabilization of the HIF-1α subunit13. Indeed, HIF-1 can attenuate several aspects of the inflammation/injury cascade and promote the expression of a suite of barrier-protective genes including intestinal trefoil factor (TFF3/ITF), mucin family members (MUC-1) and ecto-5'-nucleotidase (CD73)18. These observations led us to hypothesize that HIF-1α stabilization and downstream HIF-1 signaling events play critical innate protective roles in C.difficile-induced intestinal inflammation and injury.
To test this hypothesis, we assessed whether C.difficile toxins could elevate HIF-1α mRNA and protein levels and influence HIF-1 DNA-binding activity in a human colonic epithelial cell line. We also assessed HIF-1α expression in patients with CDAD and in healthy mucosal biopsies exposed to C.difficile toxin. Finally, we employed the mouse ileal loop model to examine the effect of C.difficile toxins on intestinal inflammation in animals with knock-down of HIF-1α targeted to the intestinal epithelium or animals treated with dimethyloxaloylglycine (DMOG), a PHD inhibitor, to inhibit the degradation of HIF-1α in vivo16.
METHODS
Human tissue sampling and HIF-1α immunohistochemistry
Colonic tissue sections obtained from patients undergoing colonic resection for CDAD were probed with HIF-1α antibody (NB100-105) using standard immunohistochemical methodology and appropriate controls. Slides were counterstained with Mayer’s hematoxylin (Sigma). All studies were approved by the Conjoint Health Research Ethics Board of the University of Calgary/Calgary Health Region.
C.difficile toxin isolation and purification
C. difficile strain NAP-1/027 (TcdA+/TcdB+; obtained from the Calgary Health Region) was grown in brain-heart infusion media under sterile anaerobic conditions. Cultures were harvested at day 5 post-inoculation by centrifugation (10000g, 60m). TcdA and TcdB were purified from concentrated and filtered culture supernatants by anion-exchange chromatography as described previously20(see supplementary material for complete method).
Assessment of mRNA expression: RNA isolation, semi-quantitative RT-PCR, and real-time RT-PCR analysis
Caco-2 or tissue biopsies were collected in Trizol and RNA isolated according to manufacturer’s protocol (Invitrogen). The total RNA was reverse transcribed by with the RT2 First-strand Kit (SABiosciences). The resulting cDNA was subjected to RT-PCR with specific primers (see supplementary materials table 1). Quantitative real-time PCR (qPCR) was performed on an ABI 7500 real-time PCR thermocycler to quantify changes in gene expression. For Caco-2 cells, human biopsies HIF-1α specific real-time primers were purchased (SABiosciences). For mouse ileum samples, specific primers for GAPDH, VEGFa, ITF, CD73, TNF and KC/CXCL1 were purchased (SABiosciences). Amplification plots were examined with Sequence Detection Software to determine the threshold cycle (CT). In all reactions endogenous control was amplified and the CT was determined.
Western blot/Protein analysis
Western immunoblots were performed on protein extracts from Caco-2 cells or tissue biopsy homogenates. We used mouse monoclonal anti-HIF-1α antibodies (NB100-105 and ab6489), rabbit polyclonal anti-β-actin antibody (Novus) and secondary HRP-conjugated antibody to mouse or rabbit (GE Healthcare). Where indicated, HIF-1α protein levels were determined in colonic biopsies exposed to media or C.difficile toxin. Biopsies were homogenized in lysis buffer (150mM NaCl, 20mM Tris pH 7.5, 1mM EDTA, 1mM EGTA, 0.1% SDS and Complete™ protease inhibitor cocktail (Roche)) at 4°C. Samples were cleared by centrifugation and supernatants were assayed for HIF-1α by chemiluminescence-based sandwich immunoassay (Meso-Scale Diagnostics) according to manufacturer’s protocols. All samples were normalized to total protein content.
Luciferase reporter and electrophoretic mobility shift assays (EMSA)
The Dual Luciferase Reporter Assay System (Promega) was used to monitor HIF-1 reporter gene activation by C.difficile toxin as per the manufacturer’s protocol (see supplementary material for complete method). EMSA was performed in the standard fashion with 32P-labelled, EPO gene-derived W18 oligonucleotide sequence containing a HIF-binding sequence (HRE) (see supplementary material for complete method).
Mouse ileal loop model
Mice (I29 epithelial HIF-1α(Ep−/−) and wild-type littermates) were maintained in a conventional single-barrier housing unit and removed from food 8h prior to surgery. I29 epithelial HIF-1α−/− (HIF-1α(Ep−/−)) mice were characterized previously by Karhausen et al. (2004)18. Mice treated with DMOG received I.P. injections (8mg in 0.3cc sterile saline) once daily for 2d prior to the surgery and an additional injection immediately following surgery, a regimen reported to upregulate HIF-1α in vivo16. Non-treatment animals were injected under the same regimen with 0.3cc sterile saline. For ileal loop surgeries, mice were anaesthetized with isoflurane and maintained under anaesthetic. The abdomen was opened and the small intestine removed from the cavity at the ileal-cecal junction. A ligature was loosely generated with suture approximately 1cm proximal to the ileal-cecal junction. C.difficile toxin (100µg total protein diluted in sterile PBS) or vehicle (matched volume of sterile PBS) was injected into the ileal lumen and the ligature closed. Another ligature distal to the injection site was applied, and the intestine was returned to the abdominal cavity. Animals were sacrificed 4h post-surgical recovery and tissue and blood were removed for analysis. Protocols for experiments involving animals were approved by the University of Calgary Animal Care Committee.
Measurement of NO, tissue myeloperoxidase and intestinal tissue damage scoring
Systemic NO was assessed with the Bioxytech NO Assay Kit (OxisResearch) and per the manufacturer’s instructions. Myeloperoxidase (MPO) activity in intestinal mucosal segments was determined with a standard protocol21. For damage scoring, fixed, embedded and sectioned specimens were stained with hematoxylin and eosin (H&E). Slides were assessed in a blind fashion and damage scores generated. Sections were assessed using the following parameters: Architectural Score–0=normal, 1=villous vacuolation/bleeding, 2=loss of epithelium, 3=complete loss of crypt/villous architecture; Inflammation Score–0=normal, 1=increased inflammatory cells in lamina propria (LP), 2=increased inflammatory cells in submucosa, 3=dense inflammatory cell mass, 4=transmural inflammation; Epithelial Damage–0=nil, 1=destruction of villous tips, 2=destruction of 1/2 of villous, 3=complete destruction of villous; Neutrophil Score– 0=nil, 1=rare PMNs mainly marginated, 2=marginated and extravasated PMNs (up to 5 near vessels), 3=marginated and extravasated through LP and epithelium.
Assessment of Transepithelial Resistance
Caco-2 cells were seeded at a density of 6×105 cells/mL onto semipermeable filters (Costar) and cultured in Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum and penicillin-streptomycin (100µg/mL; 1nM, Invitrogen) and incubated in humidified 37°C room air with 5% CO2 until transepithelial resistance (TER) was greater than 1000ohms/cm2. Caco-2 cells with a stable knock-down of HIF-1α were generated with RNA interference as described previously22. In all experiments control monolayers were treated with the appropriate vehicles for toxin (matched volume of sterile media) and pharmacological inhibitors (matched volume of DMSO).
Statistical Analysis
Comparisons between two groups were made with the student’s T-test with an alpha value of 0.05. One-way ANOVA was utilized for comparisons of multiple groups followed by Neumann-Keuls post-hoc analysis.
Results
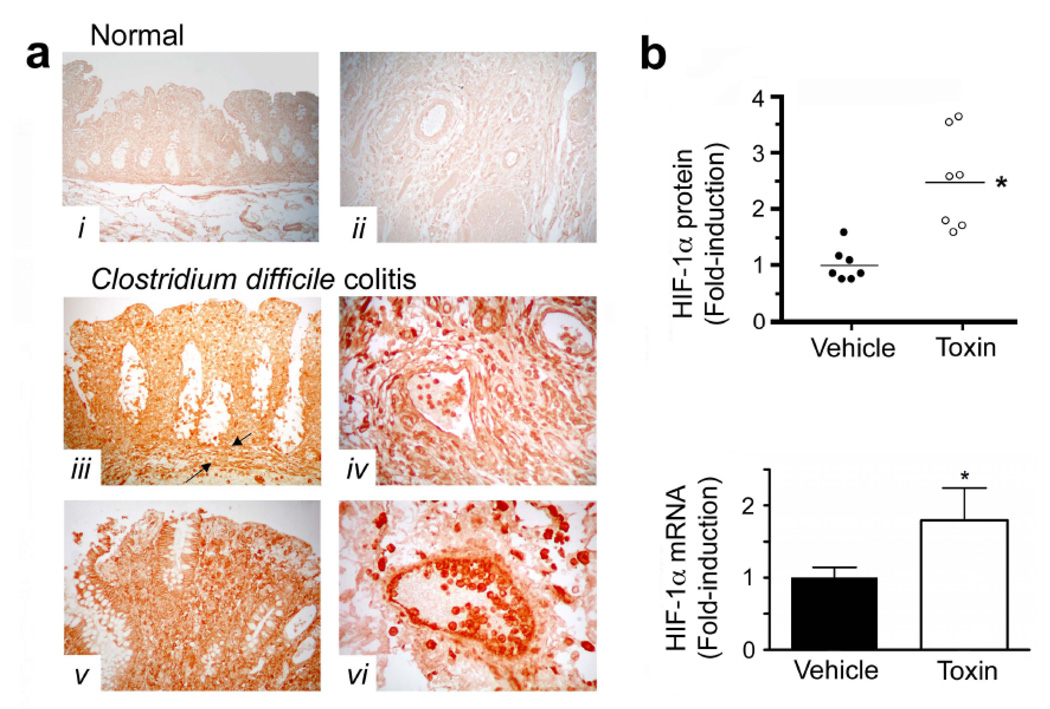
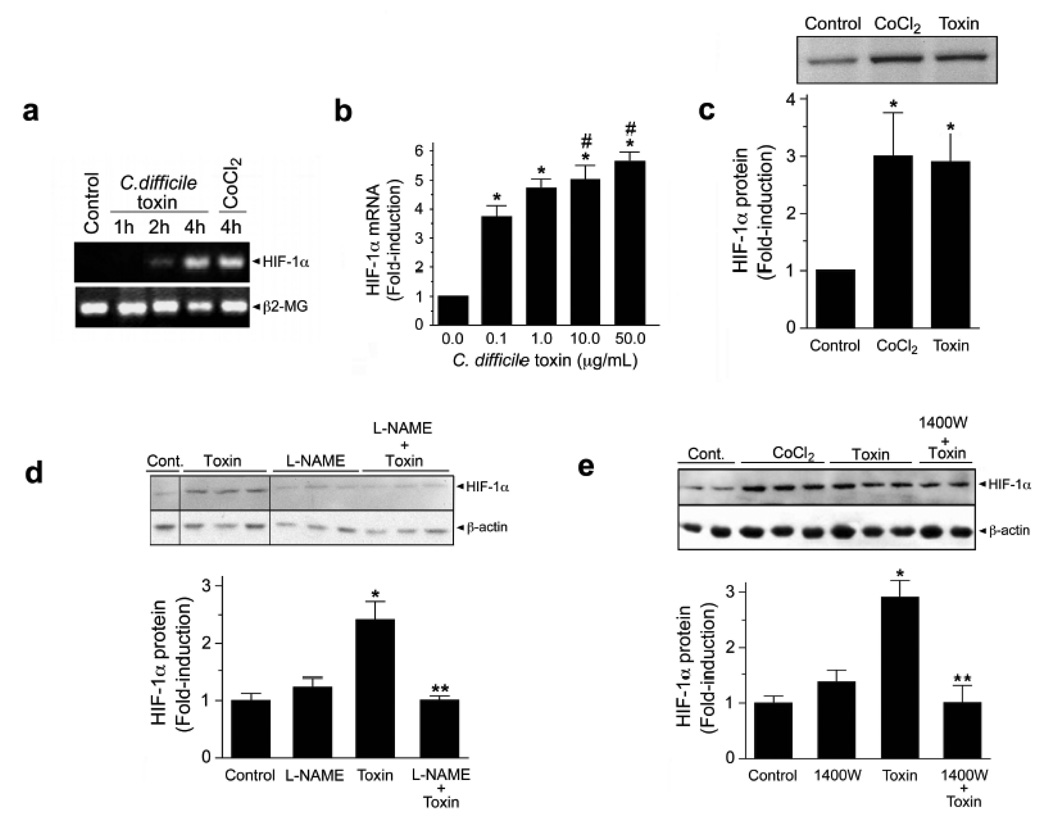
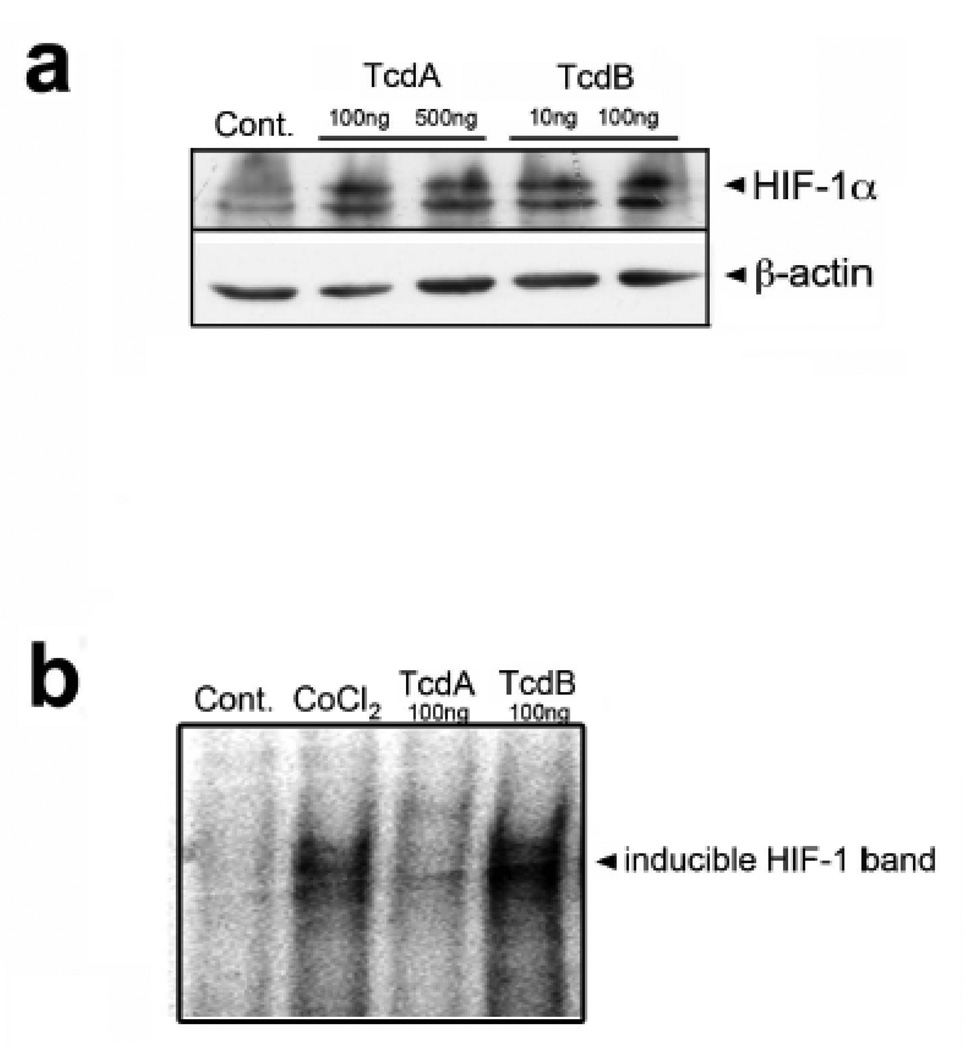
To identify a functional link between C.difficile toxin exposure and HIF-1 activation, we first analyzed the mucosal response to C.difficile toxin. Patients with C.difficile colitis displayed increased immunohistochemical staining for epithelial HIF-1α protein (Fig.1a, tissue sections iii–vi). To assess early responses to C.difficile toxins, we exposed freshly-isolated normal, human colonic mucosal biopsies to vehicle or toxin (containing both TcdA and TcdB, purified from a human NAP-1/027 isolate, see Suppl. Fig.1). Not only did the exposure to toxin significantly increase protein levels but it also enhanced HIF-1α transcription (Fig.1b). Furthermore, in vitro application of C.difficile toxin directly up-regulated HIF-1α mRNA (Fig.2a–b) and protein levels (Fig.2c–e) in Caco-2 cells in a time- and concentration-dependent fashion. Purified TcdA and TcdB could each independently increase HIF-1α protein (Fig.4a).
Figure 1.
C. difficile colitis is associated with increased HIF-1α expression. (a) Tissue sections were stained for HIF-1α protein using a mouse anti-HIF-1α monoclonal antibody (NB100-105). Immunolocalization of HIF-1α was determined in human colonic tissue obtained from patients (surgical resections): (i– ii), HIF-1α staining was absent from normal human colonic tissue obtained from biopsy during colon cancer screening or normal resection margins for colon cancer; (iii–vi), intense HIF-1α staining was noted in colonic tissues with C. difficile-associated pathology. The mucosa, submucosa and muscularis with discrete staining identified in inflammatory cells, epithelial cells, endothelial cells, as well as smooth muscle cells of the muscularis mucosa (between arrows), the intimal vasculature and the muscularis. Standard immunohistochemical controls (e.g. omission of mouse anti-HIF-1α antibody) were negative. (b) HIF-1α protein and mRNA are induced in non-inflamed, human colonic biopsies following exposure to C.difficile toxin (50µg/mL; 3h). Two biopsies (taken side by side) were collected from healthy individuals and immediately incubated with either toxin or vehicle solution (matched volume of sterile BHI media). All values are mean ± S.E.M., (n=14). * denotes p<0.05.
Figure 2.
HIF-1α mRNA and protein levels are increased following treatment of Caco-2 cells under normoxic conditions with C.difficile toxin. (a) Caco-2 cells were exposed to toxin (0.1µg/mL) or CoCl2 (100µM) for 1, 2 and 4h. PBS/BHI was added to the cells as a control. Total RNA was isolated, and the expression of HIF-1α and β2-microglobin (β2-MG; internal control) was determined by semi-quantitative RT-PCR. (b) Exposure of Caco-2 cells for 4h with increasing concentrations of C.difficile toxin increases HIF-1α gene expression as assessed with qPCR; * denotes p<0.05 compared to 0 µg/mL, # denotes p<0.05 compared to 0.1 and 0 µg/mL, n=5. (c) HIF-1α protein expression is increased in Caco-2 cells following 4h treatment with C.difficile toxin (100µg/mL) or CoCl2 (100µM). Nuclear extracts (50µg total protein) were analyzed for HIF-1α protein levels by western blotting (NB100-105). All values (mean ± S.E.M., n=4). *p<0.05. Inhibition of NO synthases attenuates toxin-induced increases in HIF-1α protein. Caco-2 cells were pretreated with 100µM L-NAME (d) or 1400W (e) for 30min prior to toxin exposure (100µg/mL; 12h). Cytosolic extracts were assessed by western blotting (NB100-105). All values are mean±S.E.M., (n=4). *p<0.05 compared to control; **p<0.05 compared to toxin treated cells.
Figure 4.
Purified TcdA and TcdB increase HIF-1α protein and DNA binding. (a) Caco-2 cells were exposed to purified TcdA (100ng/mL, 500ng/mL) and TcdB (10ng/mL, 100ng/mL) for 4h. Western blot is representative of four experiments (ab6489). (b) DNA binding of HIF-1α to the EPO HRE in Caco-2 cells exposed to CoCl2 (100µM) or purified TcdA (100ng/mL) and TcdB (100ng/mL).
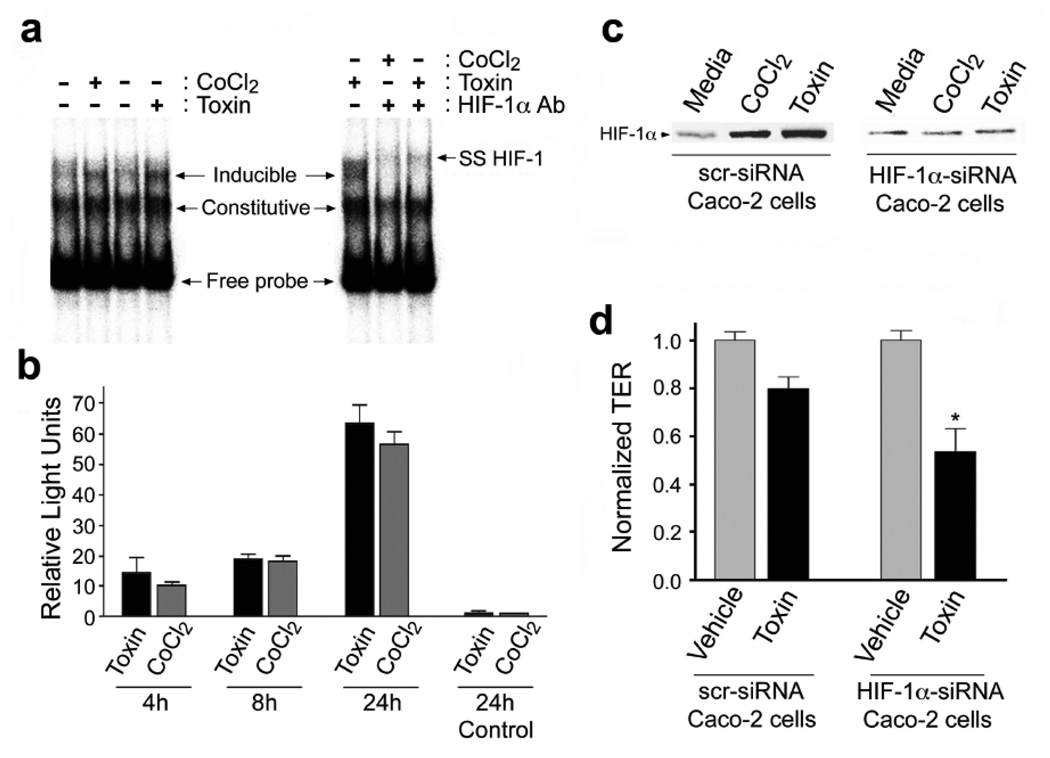
To elucidate the mechanisms involved in C.difficile toxin-mediated induction of HIF-1α we assessed the role of NO since in certain settings NO can stabilize HIF-1α23. HIF-1α protein induction in the presence of C.difficile toxin was NO-dependent since it was blocked by either L-NAME (100µM, pan-NOS inhibitor; Fig.2d) or 1400W (100µM, selective iNOS inhibitor; Fig.2e). Toxin-induced upregulation of HIF-1α mRNA was not affected by pretreatment with L-NAME (100µM), suggesting NO-dependent increases in HIF-1α protein levels involve a post-translational step(s) (Suppl. Fig.2a). Assessment of HIF-1 specific DNA-binding activity by electrophoretic mobility shift assay (EMSA) (Fig.3a) revealed increased HIF-1 binding activity in nuclear extracts isolated from cells treated with C.difficile toxins or CoCl2, (hypoxia mimetic). This finding was further supported by ‘supershift’ EMSA assays performed in the presence of a monoclonal antibody to HIF-1α and confirmed the specificity of the interaction between HIF-1 and the hypoxia response element (HRE, Fig.3a). Furthermore, purified TcdA and TcdB (Suppl. Fig.1a–b) induced HIF-1-dependent DNA binding (Fig.4b). Finally, C.difficile toxins enhanced HIF-1 transcriptional activity as assessed with a HIF-1-dependent luciferase assay (Fig.3b). To assess the impact of altered HIF-1α activity on IECs, siRNA knockdown approaches were employed24. Caco-2 cells stably transfected with siRNA targeting HIF-1α displayed attenuated HIF-1α protein increases in response to toxin treatment (Fig.3c), an effect that rendered cells more susceptible to toxin-induced reductions in TER (Fig.3d).
Figure 3.
C.difficile toxin exposure promotes HIF-1 dependent promoter binding activity and transcriptional activity in Caco-2 cells. (a) DNA binding of HIF-1α to the EPO hypoxia response element (HRE) was assessed by electrophoretic mobility shift assay (EMSA). Nuclear extracts were prepared from Caco-2 cells that had been exposed to C.difficile toxin (100µg/mL), or CoCl2 (100µM). In some cases, EMSA “supershifts” were generated with addition of anti-HIF-1α monoclonal antibody to the DNA binding reactions. (b) Toxin exposure promotes HIF-1α transcriptional activity in Caco-2 cells as measured by a luciferase reporter assay. Caco-2 cells were transiently co-transfected with firefly luciferase reporter and Renilla luciferase control plasmids. Cells were incubated for 4, 18 or 24h under normoxic conditions with 10µg/mL C.difficile toxin or 100µM CoCl2. The transfection efficiency was corrected by normalizing firefly luciferase activity to the Renilla luciferase activity. Control cells were transfected with empty pGL3 vector and treated for 24h with C.difficile toxin or CoCl2. All values (mean±S.E.M., n=4) are expressed as fold-induction relative to the controls that were arbitrarily defined as 1. (c–d) Caco-2 cells deficient in HIF-1α are more susceptible to C.difficile toxin-induced injury. (c) HIF-1α protein levels in Caco-2 cells stably transfected with HIF-1α siRNA (Caco-2 HIF-1α−/−) were not affected by C.difficile toxin or CoCl2. (d) Caco-2 HIF-1α−/− cells display increased susceptibility to C.difficile toxin, as assessed by transepithelial resistance (TER) following a 2h incubation with toxin (60µg/mL). All values are mean ± S.E.M., (n=4), * denotes p<0.05.
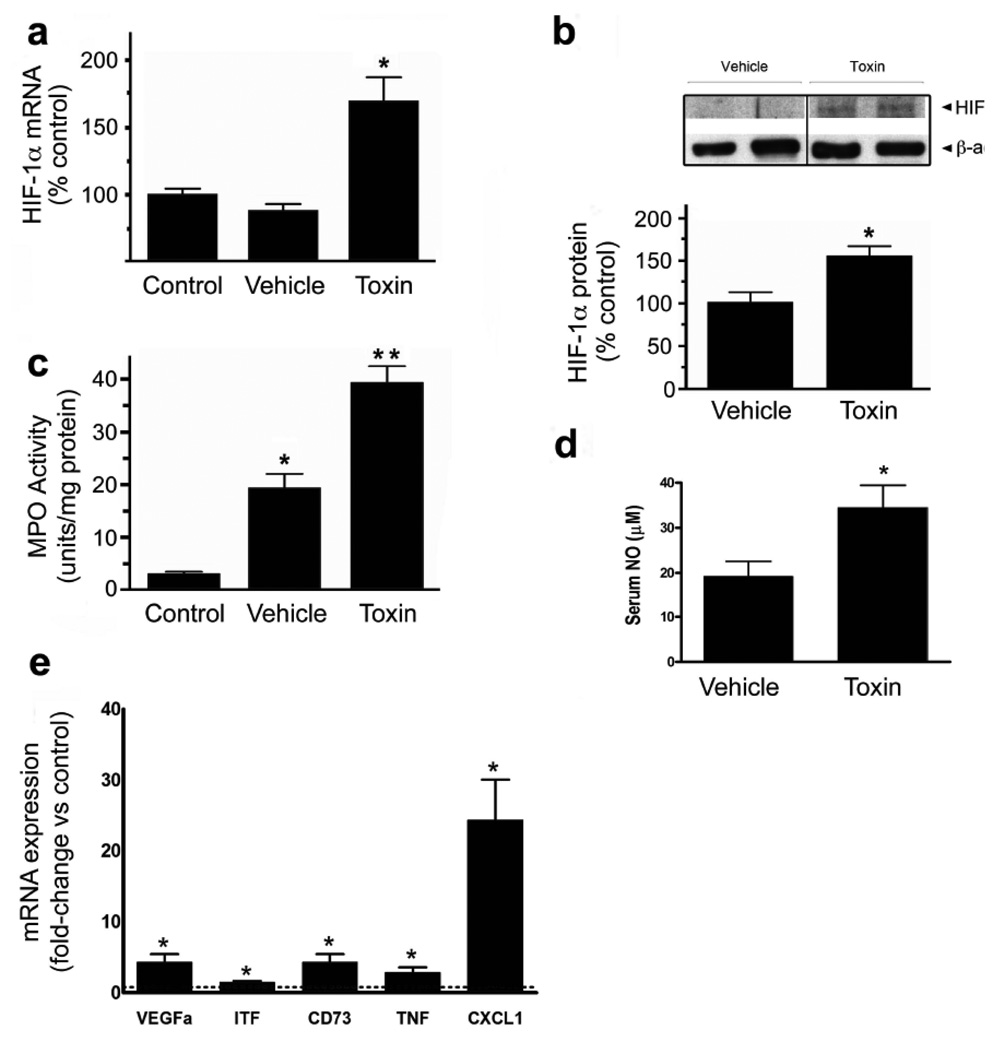
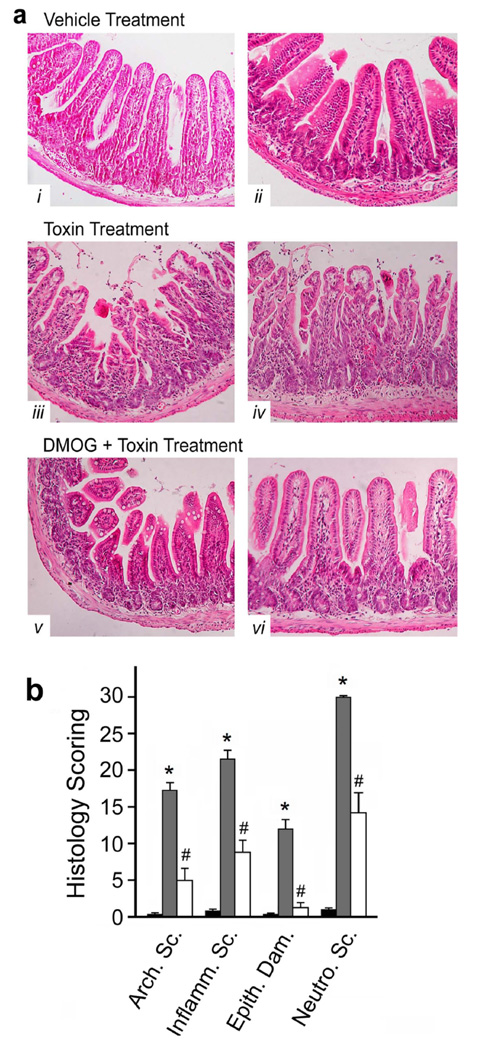
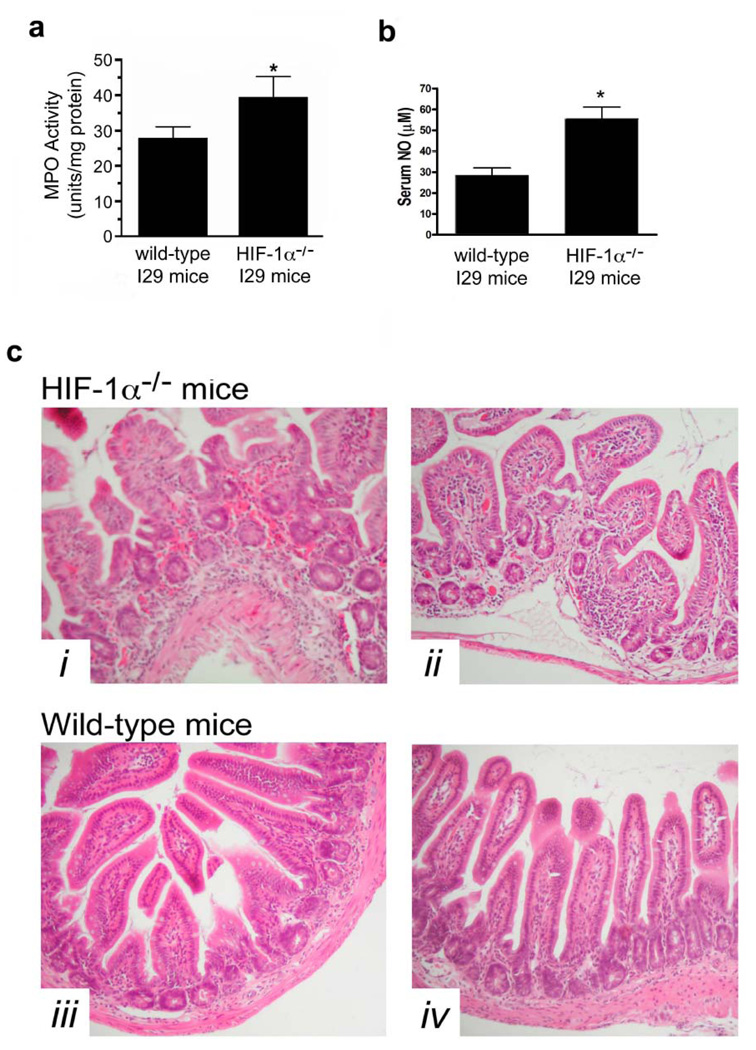
A murine ileal loop model was employed to examine these processes in vivo. The injection of C.difficile toxin into ileal loops of wild-type mice upregulated HIF-1α mRNA (Fig.5a) and protein levels (Fig.5b). This was associated with increases in HIF-1-regulated gene products (VEGFa, ITF and CD73; Fig.5e) and inflammatory mediators (TNFα and CXCL1/KC; Fig.5e) as assessed with qPCR. As described previously25, C.difficile toxins induced inflammation as evidenced by increased colonic MPO (Fig.5c) and serum NO levels (Fig.5d), as well as increased numbers of tissue neutrophils (Fig.7b). The inflammation was accompanied by severe intestinal injury, including epithelial barrier disruption, vacuolation and crypt-villus architectural changes (Fig.7a–b), all of which are hallmark characteristics of CDAD. In keeping with a putative protective role for HIF-1α, C.difficile toxin induced more severe inflammation (Fig.6a), increased systemic NO levels (Fig.6b) and exacerbated intestinal injury (Fig.6c) in mice deficient in intestinal epithelial HIF-1α.
Figure 5.
C.difficile toxin increases HIF-1α expression and induces inflammation in an in vivo model of CDAD. (a) HIF-1α mRNA levels were measured with qPCR in ileum isolated from control animals (no surgery), mice with ileal loop treated with vehicle or exposed to 100µg of C.difficile toxin in the ileal loop (*p<0.05 compared to control and vehicle, n=5). (b) HIF-1α protein was assessed in ileal tissue isolated from mice treated with vehicle or C.difficile toxin (100µg), *p<0.05 compared to vehicle, n=4 (NB100-105). (c) Tissue inflammation (MPO levels) was assessed in ileum isolated from control animals (no surgery) and mice treated with vehicle or C.difficile toxin (100µg), *p<0.05 compared to control and vehicle, **p<0.05 compared to vehicle, n=9. (d) Serum NO levels were assessed from mice treated with either vehicle or C.difficile toxin (100µg), *p<0.05 compared to vehicle, n=9. (e) qPCR was performed on RNA isolated from vehicle and toxin-treated ileal loops. Data is expressed as fold-change relative to control where 1 is equal to expression in the control group (dashed line). *p<0.05 compared to control, n=3.
Figure 7.
Animals pretreated with dimethyloxallyl glycine (DMOG, 8mg/day for 2d prior to surgery) display significantly less C.difficile toxin-induced tissue damage. (a) Representative sections of ileum stained with H&E from mice treated with vehicle (i & ii), C.difficile toxin (iii & iv), and DMOG pretreatment followed by C.difficile toxin exposure (v & vi). (b) Histological analysis of ileal sections obtained from mice treated with vehicle (sterile PBS, black bars), C.difficile toxin (100 µg, grey bars) or DMOG with C.difficile toxin (white bars): Arch. Sc.–Architectural Score; Inflamm. Sc.–Inflammation Score; Epith. Dam.–Epithelial Damage; Neutro. Sc.–Neutrophil Score, *p<0.05 compared to media; #p<0.05 compared to C.difficile toxin; n=8–12.
Figure 6.
Targeted deletion of HIF-1α in intestinal epithelium augments C.difficile toxin-induced inflammation. (a) Tissue inflammation as assessed by MPO levels in ileum isolated from wild-type I29 and HIF-1α epithelial-targeted knockout (HIF-1α−/−) mice treated with C.difficile toxin (100µg), *p<0.05 compared to wild-type I29, n=6. (b) Serum NO levels were assessed from wild-type I29 and HIF-1α epithelial-targeted knockout (HIF-1α−/−) mice treated with C.difficile toxin (100µg), *p<0.05 compared to wild-type I29, n=6. (c) H&E stained ileal sections from HIF-1α−/− (top row) and wild-type I29 (bottom row) following 4h exposure to C.difficile toxin (100µg).
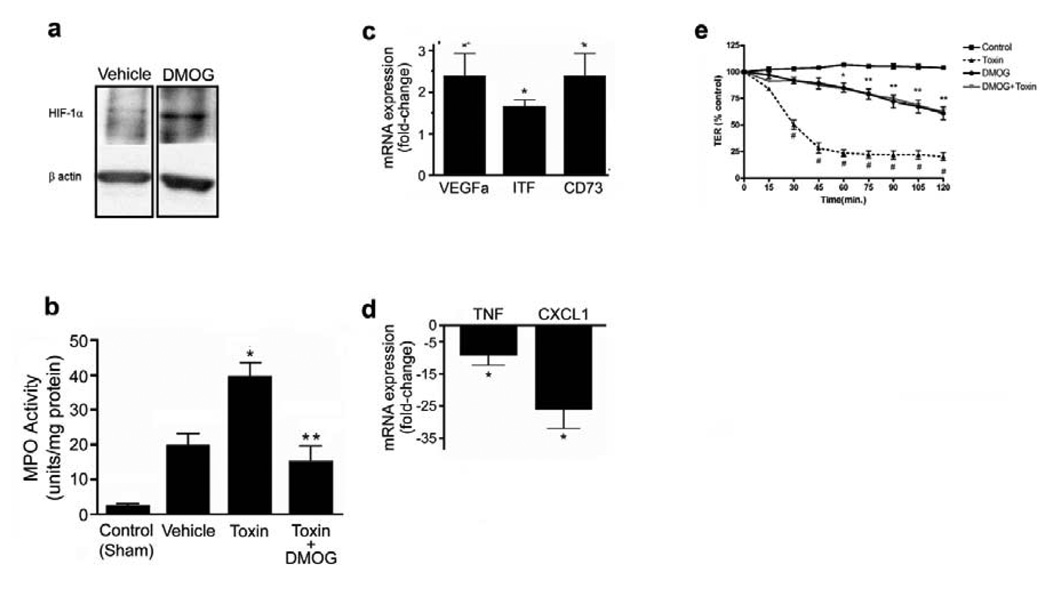
To assess the impact of upregulating HIF-1α activity, mice were treated with a PHD inhibitor, dimethyloxaloylglycine26 (DMOG; 8mg/day for 2 days before surgery), prior to C.difficile toxin exposure. DMOG attenuated toxin-induced injury (Fig.7a–b) and inflammation (Fig.8b), and improved overall histological scoring (Fig.7b). This was associated with a significant increase in expression of VEGFa and the barrier protective molecules; ITF and CD73 (Fig.8c) and significantly lower expression of TNFα and CXCL1/KC (Fig.8d) compared to vehicle-treated animals. We hypothesized that the attenuation of C.difficile toxin-induced injury afforded by the DMOG treatment may, in part, be due to enhancement of barrier function since DMOG markedly reduced toxin-induced decreases in TER in Caco-2 monolayers (Fig.8e). Furthermore, DMOG-treated (500µM, 2 hours) Caco-2 monolayers displayed a marked upregulation of HIF-1α protein (Suppl. Fig.3).
Figure 8.
Mice treated with dimethyloxallyl glycine (DMOG, 8mg/day for 2d prior to surgery) display increased levels of HIF-1α (a) in protein extracts isolated from full-thickness ileal segments as compared to mice injected with PBS vehicle and reduced (b) intestinal inflammation as assessed with MPO levels. Control indicates levels measured in animals not undergoing surgery, *p<0.05 compared to control and vehicle; **p<0.05 compared to C.difficile toxin treatment, n=6–8. Pretreatment with DMOG prior to C.difficile toxin exposure is associated with elevation of factors associated with intestinal barrier function and protection (c) and reduced levels of inflammatory mediators (d) as measured with qPCR. Data are fold-change from C.difficile toxin treatment where 1 is equal to the expression in the toxin group. *p < 0.05 compared to toxin, n=3. (e) DMOG treatment protects against C.difficile toxin-induced breakdown of epithelial barrier function. Caco-2 cells were exposed to vehicle (control), C.difficile toxin (60µg/mL), DMOG (500µM) with C.difficile toxin (60µg/mL) or DMOG (500µM) alone. Time (in minutes) indicates the time elapsed following addition of toxin to monolayer, data are expressed as a percentage of the control TER, *p<0.05 compared to control and toxin; **p<0.005 compared to toxin; #p<0.005 compared to control, n=4.
Discussion
Current therapies for C.difficile colitis that focus on eradicating the pathogen are failing. Furthermore, vaccination strategies have failed and previous exposure is not protective. Clinically, there is marked patient-to-patient variability in disease severity, even in those with identical risk factors who are infected with the same C.difficile strain, suggesting that an innate pathway may exist to protect some and not others from CDAD. It is apparent that the disruption of the mucosal barrier is a key step in the pathogenesis of CDAD; thus, therapies aimed at maintaining barrier function may prove valuable in the treatment of CDAD. A novel therapeutic paradigm would be to harness the innate protective responses of the intestinal mucosa to reduce the progression of CDAD.
In the present study we have reported that HIF-1α is upregulated in intestinal epithelial cells and mucosal biopsies exposed to C.difficile toxin under normoxic conditions. This upregulation appears to confer protection since deletion of HIF-1α rendered epithelial cells more susceptible to toxin-induced damage both in vitro and in vivo. Conversely, activation of this pathway, through pharmacological stabilization of HIF-1α, significantly reduced toxin-induced damage in vitro and in vivo. HIF-1α is known to be a critical mediator of intestinal protection during the hypoxia and inflammation that accompanies mucosal insult16–19. Thus, the C.difficile toxin-dependent induction of HIF-1α in epithelial cells may represent a novel protective mechanism that guards the intestinal tract during exposure to pathogenic luminal bacteria and toxins during episodes of CDAD.
Although we initially hypothesized that NO-dependent stabilization of HIF-1α may account for the C.difficile toxin-dependent increases in protein levels, it is apparent that various mechanisms may contribute to C.difficile toxin-dependent increases in HIF-1 signaling, including post-translational stabilization as well as de novo increases in HIF-1α transcript levels. The latter finding was puzzling given that HIF-1α abundance is predominantly regulated at the level of translation rather than transcription27. In our studies, pretreatment of cells with L-NAME had no effect on toxin-induced upregulation of HIF-1α, suggesting NO-independent mechanisms are driving this transcriptional response. Others have implicated mechanisms of HIF-1 signaling activation that were independent of HIF-1α protein stabilization28, 29, however the exact mechanisms that govern transcriptional regulation of HIF-1α have yet to be fully elucidated.
In our experimental models, upregulation of HIF-1α activity significantly reduced C.difficile toxin-induced epithelial injury, barrier disruption and neutrophil infiltration, findings that were paralleled by increases in barrier protective genes and decreases in pro-inflammatory molecules. Clearly, genetic deletion of epithelial HIF-1α resulted in more severe toxin-induced injury and inflammation. Interestingly, the increased damage observed in HIF-1α(Ep−/−) mice exposed to toxin was associated with an increase in systemic NO, as compared to the wild-type mice. Although seemingly paradoxical, the importance of this observation is two-fold. First, the elevated serum NO levels observed in HIF-1α(Ep−/−) mice suggests an enhanced activation of NOS-expressing cells, potentially those in the mucosal space, during toxin exposure. Second, despite the role of NO in the normoxic stabilization of HIF-1α, HIF-1α(Ep−/−) mice exhibit enhanced toxin-induced damage and inflammation, suggesting that a disconnect between NO and HIF-1α induction may be, in part, responsible for the increased susceptibility observed in these mice.
In stark contrast to the HIF-1α(Ep−/−) mice, wild-type mice treated with DMOG exhibited substantially less damage and inflammation when exposed to C.difficile toxin. Stabilization of HIF-1α with DMOG led to enhanced expression of molecules with potent roles in maintenance of intestinal epithelial barrier function. Our in vitro data indicates that HIF-1 signaling contributes to maintain barrier function during C.difficile toxin exposure; however, it is difficult to delineate the pro-barrier effects of HIF-1 signaling from the immunomodulatory effects in vivo that have been reported elsewhere30. The mechanism by which HIF-1 signaling is protective in animal models of colitis appears to be predominately mediated by epithelial HIF-1 expression since the PHD inhibitors were ineffective in HIF-1α(Ep−/−) mice19. In our study, the upregulation of protective genes ITF and CD73 in mice treated with DMOG was associated with improved maintenance of the intestinal ultra-structure following C.difficile toxin exposure. This was paralleled by our in vitro studies where DMOG treatment attenuated toxin-induced loss of barrier function. However, inhibition of PHDs may also effect NFκB signaling. In vitro, DMOG treatment has been reported to activate both HIF-1- and NFκB-dependent gene transcription31. Thus, inhibition of PHDs may confer gastrointestinal protection through the synergistic activity of HIF-1α and NFκB signaling pathways. This synergy would favor epithelial cell survival and act to maintain barrier function during an insult, thereby limiting the toxin-induced activation of the mucosal immune system.
CDAD is associated with a profound inflammatory cell infiltrate that is mediated in part by the ability of the C.difficile toxins to induce a potent chemokine and cytokine response. In the context of C.difficile infection, activation of HIF-1 signaling and subsequent enhancement of barrier function would limit the migration of TcdA and TcdB into the submucosal space where they would typically interact with LP monocytes. Indeed, pharmacological stabilization of HIF-1α in our in vivo studies significantly reduced the transcript levels of murine KC and TNF, two potent regulators of neutrophil chemotaxis. Furthermore, mucosal integrity was enhanced, as assessed by histology. These data suggest that the upregulation of HIF-1α and the enhancement of barrier function may be sufficient to lessen the likelihood of toxin-induced activation of the mucosal immune system, ultimately reducing the production of pro-inflammatory cytokines and immune cell infiltrate.
As noted previously, some patients develop severe CDAD resulting in sepsis and death, while others with the same risk factors and infected with the same strain of C.difficile, can have minimal disease. Based on the findings of the present study, we hypothesize that those that develop more severe disease may have a reduced ability to adequately induce HIF-1α activity and/or have altered downstream signaling. Alternatively, the severity of insult may overwhelm the protective actions of HIF-1. Such patient-to-patient differences were indeed noted in our human colonic biopsies where there was marked variably in both toxin-mediated induction of HIF-1α transcription and protein levels. These findings suggest that not only does HIF-1 offer innate protection in C.difficile-mediated disease, but it may also be responsible for the variability in clinical disease severity. However, testing this hypothesis will be exceedingly difficult since mucosal biopsies are rarely, if ever, isolated from patients with mild CDAD symptoms. Nevertheless, further study of HIF-1α and downstream molecules may give rise to important tools that could identify those patients at risk of a more severe clinical course. Furthermore, enhancing HIF-1α activity and/or targeting pathways downstream of HIF-1α may represent novel therapeutic approaches in this devastating disease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a Canadian Association of Gastroenterology/Canadian Institutes of Health Research (CIHR) postdoctoral fellowship (S.H.), AHFMR Clinical Investigatorship (P.L.B.), Canada Research Chair and Alberta Heritage Foundation for Medical Research (AHFMR) Senior Scholar award (J.A.M.), as well as grants from the National Institutes of Health DK50189, DE13499 and HL60569 (S.P.C.), Crohn’s and Colitis Foundation of America (S.P.C.) and CIHR (P.L.B. & J.A.M). Special thanks to study subjects, Ida Rabbani (IBD Translational Research Tissue Bank) and colleagues within the Gastrointestinal Research Group, especially W. MacNaughton and K. Sharkey for their helpful discussions.
Abbreviations
- HIF-1
hypoxia-inducible factor-1
- TcdA
toxin A
- TcdB
toxin B
- HRE
hypoxia responsive element
- CDAD
C. difficile-associated disease
- ITF/TFF3
intestinal trefoil factor
- DMOG
dimethyloxaloylglycine
- HIF-1α(Ep−/−)
targeted epithelial HIF-1α−/− knockout
Footnotes
Author contributions: S.A.H. and K.F. designed and performed the main experiments, analyzed the data and wrote the manuscript. D.T., J.L., J.N., E.I. and Y.L. performed western blotting and assisted with animal surgeries. W.G.W. cloned and prepared plasmids for transfection experiments. D.C. provided HIF-1α siRNA knock-down Caco-2 cells. S.P.C. and M.M.S. provided epithelial-targeted HIF-1α−/− mice, performed HIF human biopsy protein assessment and discussed the manuscript. T.L. and G.A. provided C.difficile isolates. S.M. coordinated C.difficile colitis tissue acquisition and prepared pathology slides. M.L. and K.C. contributed to the DMOG in vitro TER studies. P.L.B., J.A.M. and D.A.M. made the initial observations, supervised and coordinated the project.
Disclosures: no conflicts of interest exist
REFERENCES
- 1.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerging Infectious Diseases. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johal SS, Solomon K, Dodson S, Borriello SP, Mahida YR. Differential effects of varying concentrations of clostridium difficile toxin A on epithelial barrier function and expression of cytokines. J Infect Dis. 2004;189:2110–2119. doi: 10.1086/386287. [DOI] [PubMed] [Google Scholar]
- 4.Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 5.Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahida YR, Makh S, Hyde S, Gray T, Borriello SP. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canny G, Drudy D, Macmathuna P, O'Farrelly C, Baird AW. Toxigenic C. difficile induced inflammatory marker expression by human intestinal epithelial cells is asymmetrical. Life Sci. 2006;78:920–925. doi: 10.1016/j.lfs.2005.05.102. [DOI] [PubMed] [Google Scholar]
- 9.Linevsky JK, Pothoulakis C, Keates S, Warny M, Keates AC, Lamont JT, Kelly CP. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol. 1997;273:G1333–G1340. doi: 10.1152/ajpgi.1997.273.6.G1333. [DOI] [PubMed] [Google Scholar]
- 10.Melo Filho AA, Souza MH, Lyerly DM, Cunha FQ, Lima AA, Ribeiro RA. Role of tumor necrosis factor and nitric oxide in the cytotoxic effects of Clostridium difficile toxin A and toxin B on macrophages. Toxicon. 1997;35:743–752. doi: 10.1016/s0041-0101(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 11.Hirota SA, Beck PL, MacDonald JA. Targeting hypoxia-inducible factor-1 (HIF-1) signaling in therapeutics: implications for the treatment of inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2009;3:1–16. doi: 10.2174/187221309787158434. [DOI] [PubMed] [Google Scholar]
- 12.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- 13.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 14.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan NM, Pellett S, Wilkins TD. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Granados N, Howe K, Lu J, McKay DM. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol. 2000;156:2169–2177. doi: 10.1016/S0002-9440(10)65087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64:1765–1772. doi: 10.1158/0008-5472.can-03-3017. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;13:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 25.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009 doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Seo HW, Kim EJ, Na H, Lee MO. Transcriptional activation of hypoxia-inducible factor-1alpha by HDAC4 and HDAC5 involves differential recruitment of p300 and FIH-1. FEBS Lett. 2009;583:55–60. doi: 10.1016/j.febslet.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 29.Young RM, Wang SJ, Gordan JD, Ji X, Liebhaber SA, Simon MC. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J Biol Chem. 2008;283:16309–16319. doi: 10.1074/jbc.M710079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan R, Salloum FN, Fisher BJ, Ownby ED, Kukreja RC, Fowler AA., 3rd Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–H1580. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 31.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.