Abstract
AIM: To explore the role of prostaglandin F2α (PGF2α)) on pacemaker activity in interstitial cells of Cajal (ICC) from mouse small intestine.
METHODS: In this study, effects of PGF2α in the cultured ICC cells were investigated with patch clamp technology combined with Ca2+ image analysis.
RESULTS: Externally applied PGF2α (10 μmol/L) produced membrane depolarization in current-clamp mode and increased tonic inward pacemaker currents in voltage-clamp mode. The application of flufenamic acid (a non-selective cation channel inhibitor) or niflumic acid (a Cl- channel inhibitor) abolished the generation of pacemaker currents but only flufenamic acid inhibited the PGF2α-induced tonic inward currents. In addition, the tonic inward currents induced by PGF2α were not inhibited by intracellular application of 5’-[-thio]diphosphate trilithium salt. Pretreatment with Ca2+ free solution, U-73122, an active phospholipase C inhibitor, and thapsigargin, a Ca2+-ATPase inhibitor in endoplasmic reticulum, abolished the generation of pacemaker currents and suppressed the PGF2α-induced tonic inward currents. However, chelerythrine or calphostin C, protein kinase C inhibitors, did not block the PGF2α-induced effects on pacemaker currents. When recording intracellular Ca2+ ([Ca2+]i) concentration using fluo-3/AM, PGF2α broadly increased the spontaneous [Ca2+]i oscillations.
CONCLUSION: These results suggest that PGF2α can modulate pacemaker activity of ICC by acting non-selective action channels through phospholipase C-dependent pathway via [Ca2+]i regulation
Keywords: Prostaglandin F2α, Interstitial cells of Cajal, Tonic inward currents, Intestinal motility
INTRODUCTION
Prostaglandins (PGs) of the E, F, and I series are widely distributed in all body tissues, including the gastrointestinal (GI) tract and have been shown to affect water and electrolyte transport, mucous secretion and blood flow[1-3]. Also, there is much evidence that PGs may be involved in the control of the contractions of intestinal smooth muscle. Inhibition of endogenous PG synthesis by indomethacin appears to enhance intestinal motility by inducing a fed-like pattern[4-6]. These reports suggest that endogenous PGs may play an important role in the modulation of intestinal motility.
In general, PGE2 is known to contract longitudinal muscle and to relax circular muscle, whereas PGF2α induces contractions of both muscular layers[6-9]. PGF2α causes contractions of both colonic muscle layers in the guinea pig and in humans and rats[10,11]. Endogenous PGF2α is suggested to modify contractile activity of antral smooth muscle and intestinal muscle[12,13]. Therefore, exogenous PGF2α and PGF2α synthesized in the intestinal smooth muscle cells may affect motor activities.
The interstitial cells of Cajal (ICC) have functions of pacemaker cells and neuromediator cells in the tunica muscularis of the GI tract[14]. The ICC generate the rhythmic oscillations in membrane potential known as slow waves and this generation of slow waves is due to spontaneous inward currents called pacemaker currents[15,16]. Although the exact mechanisms regarding these events are still unclear, many reports have suggested that the activation of non-selective cation channels, Cl- channels, and spontaneous intracellular Ca2+([Ca2+]i) activities in ICC are involved in the production of pacemaker activity[15-17]. Also, it is well known that many endogenous agents such as neurotransmitters, hormones and paracrine substances modulate GI tract motility by influencing ICC.
There are many reports that PGF2α has a role in GI motility by acting on smooth muscles but no studies have been performed to determine the effects of PGF2α on electrical events in ICC. Therefore, the purpose of our study was to investigate the signal transduction effects of PGF2α on pacemaker activity in cultured ICC.
MATERIALS AND METHODS
Ethics
All experiments were carried out according to the guiding principles for the care and use of animals approved by the ethics committee of Chosun University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize both the number of animals used and their suffering.
Solutions and drugs
The cells were bathed in a solution containing: 5 mmol/L KCl, 135 mmol/L NaCl, 2 mmol/L CaCl2, 10 mmol/L glucose, 1.2 mmol/L MgCl2, and 10 mmol/L HEPES, adjusted to pH 7.2 with Tris. The pipette solution contained 140 mmol/L KCl, 5 mmol/L MgCl2, 2.7 mmol/L K2ATP, 0.1 mmol/L Na2GTP, 2.5 mmol/L creatine phosphate disodium, 5 mmol/L HEPES, 0.1 mmol/L EGTA, adjusted to pH 7.2 with Tris.
Drugs used were: PGF2α, guanosine 5’-[-thio]diphosphate trilithium salt (GDP-β-S), U-73122, Calphostin C, Chelethrine, and Thapsigargin. All drugs were purchased from the Sigma Chemical Co (St. Louis, MO, USA). Flufenamic acid and niflumic acid were purchased from Calbiochem (San Diego, CA, USA).
All drugs were dissolved in DW or DMSO to prepare stock solutions (10 or 100 mmol/L), and were either added to the bath solution or applied to the whole-cell preparations by superfusion. The final concentration of DMSO was less than 0.05%.
Preparation of cells and tissues
Balb/C mice (3- to 7-d-old) of either sex were anesthetized with ether and sacrificed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the cecum were removed and opened along the mesenteric border. The luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection. Small strips of intestinal muscle were equilibrated in Ca2+-free Hank’s solution for 30 min and the cells were dispersed with an enzyme solution containing collagenase (Worthington Biochemical Co, Lakewood, NJ, USA), 1.3 mg/mL; bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA), 2 mg/mL; trypsin inhibitor (Sigma), 2 mg/mL; and ATP, 0.27 mg/mL. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg/mL, Falcon/BD) in 35 mm culture dishes. The cells were then cultured at 37°C in a 95% O2-5% CO2 incubator in smooth muscle growth medium (SMGM, Clonetics Co., San Diego, CA, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (SCF, 5 ng/mL, Sigma). Interstitial cells of Cajal (ICC) were identified immunologically with a monoclonal antibody for Kit protein (ACK2) labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA).
Patch clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from cultured ICC. Currents or potentials were amplified by use of an Axopatch 1-D (Axon Instruments, Foster, CA, USA). Command pulse was applied using an IBM-compatible personal computer and pClamp software (version 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on an oscilloscope, a computer monitor and a pen recorder (Gould 2200, Gould, Valley view, OH, USA).
Results were analyzed using pClamp and Sigma plot (version 9.0) software. All experiments were performed at 30°C.
Measurement of the [Ca2+]i concentration
Changes in the [Ca2+]i concentration were monitored by using fluo-3/AM, which was initially dissolved in dimethyl sulfoxide and stored at -20°C. The cultured ICC on coverslips (25 mm) were rinsed twice with a bath solution (5 mmol/L KCl, 135 mmol/L NaCl, 2 mmol/L CaCl2, 10 mmol/L glucose, 1.2 mmol/L MgCl2 and 10 mmol/L HEPES, adjusted to pH 7.4 with Tris). The coverslips were then incubated in the bath solution containing 5 μmol/L fluo-3 with 5% CO2 at 37°C for 5 min, rinsed two more times with the bath solution, mounted on a perfusion chamber, and scanned every 0.4 s with a Nikon Eclipse TE200 inverted microscope equipped with a Perkin-Elmer Ultraview confocal scanner and a Hamamatsu Orca ER 12-bit CCD camera (× 400). Fluorescence was determined with an excitation wavelength of 488 nm, and emitted light was observed at 515 nm. During scanning of the Ca2+ imaging, the temperature of the perfusion chamber containing the cultured ICC was kept at 30°C. The variations of [Ca2+]i fluorescence emission intensity were expressed as F1/F0 where F0 is the intensity of the first imaging.
Statistical analysis
Data are expressed as the mean ± SE. Differences in the data were evaluated by Student’s t-test. P < 0.05 was taken as a statistically significant difference. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
RESULTS
Effect of PGF2α on pacemaker potentials and currents in cultured ICC
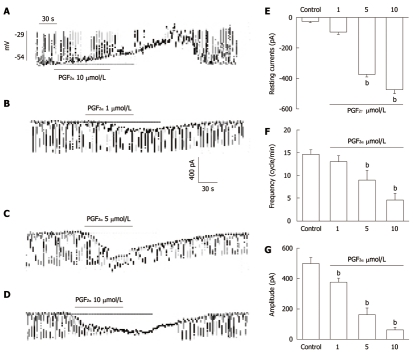
ICC, identified by Kit immunofluorescence, had a distinctive morphology that was easily recognized in cultures. We thus performed the electrophysiological recording from cultured ICC under current (I = 0) and voltage clamp mode. Under current clamp mode, ICC showed spontaneous pacemaker potentials. The resting membrane potential was -53 ± 4 mV and amplitude was 27 ± 2 mV. In the presence of PGF2α (10 μmol/L), membrane potentials were depolarized to -29 ± 3.4 mV and the amplitude of pacemaker potentials was decreased to 3.9 ± 1.6 mV (n = 5, Figure 1A, bar graph not shown). These results are in agreement with previous studies showing that ICC have spontaneous pacemaker activity and we also found PGF2α to have action on this electrical activity of ICC. Under a voltage clamp at a holding potential of -70 mV, the ICC generated spontaneous inward currents. Treatment with various concentrations of PGF2α in cultured ICC produced tonic inward currents and decreased the frequency and the amplitude of pacemaker currents in a dose-dependent manner (Figure 1B-D). As shown in Figure 1E-G, the values of frequency, amplitude and resting currents with regard to pacemaker currents under control conditions were significantly different from those obtained in the presence of PGF2α.
Figure 1.
The effects of Prostaglandin F2α on pacemaker potentials and pacemaker currents recorded in cultured interstitial cells of Cajal from mouse small intestine. A: Pacemaker potentials of interstitial cells of Cajal (ICC) exposed to Prostaglandin F2α (PGF2α) (10 μmol/L) in the current-clamping mode (I = 0). Vertical solid line scales denote amplitude of pacemaker potential and horizontal solid line scales denote duration of recording (s) pacemaker potentials; B-D: Pacemaker currents of ICC recorded at a holding potential of -70 mV exposed to various concentrations of PGF2α (1, 5, and 10 μmol/L). The dotted lines indicate zero current levels. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker currents. The responses to PGF2α are summarized in E-G. The bars represent mean ± SE. bP < 0.01 vs the untreated control.
Effects of non-selective cation channel blocker or Cl- channel blocker on PGF2α-induced responses in cultured ICC
In order to characterize the tonic inward currents induced by PGF2α, we used flufenamic acid, a non-selective cation channel blocker, or niflumic acid, a Cl- channel blocker. Figure 2A shows that treatment with flufenamic acid (10 μmol/L) abolished the generation of pacemaker currents and blocked the PGF2α-induced tonic currents in ICC. The summarized bar graph (Figure 2B) indicates that the resting currents produced by PGF2α were -21 ± 9 pA in the presence of flufenamic acid and that this value was significantly different when compared with control values obtained in the absence of flufenamic acid (n = 4). In the presence of niflumic acid (10 μmol/L), the pacemaker currents were abolished. Under this condition, PGF2α still produced the tonic inward currents (Figure 2C). In the presence of niflumic acid, the resting currents produced by PGF2α were -98 ± 12 pA; this value was not significantly different when compared with control values obtained in the absence of niflumic acid (n = 5, Figure 2D).
Figure 2.
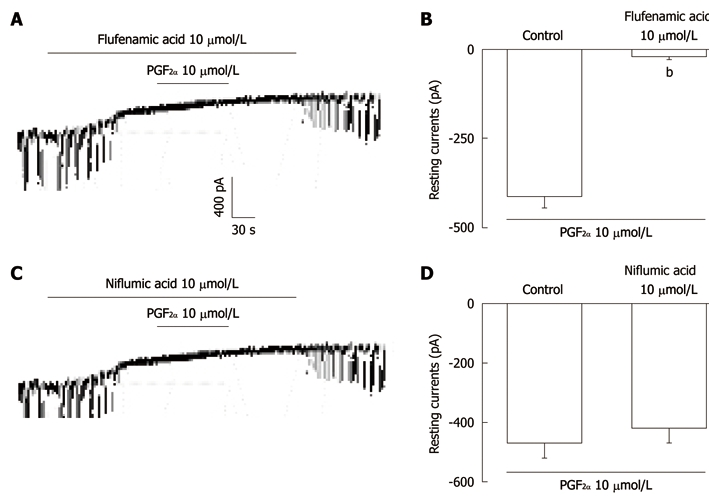
The effects of flufenamic acid or niflumic acid on prostaglandin F2α-induced responses in pacemaker currents in cultured interstitial cells of Cajal from mouse small intestine. A: Application of flufenamic acid (10 μmol/L) abolished the generation of pacemaker currents. Under these conditions, the prostaglandin F2α (PGF2α) (10 μmol/L) did not produce tonic inward currents; C: Niflumic acid (10 μmol/L) also abolished the generation of pacemaker currents. However, niflumic acid did not block the PGF2α (10 μmol/L)-induced tonic inward currents. The dotted lines indicate zero current levels. Responses to the PGF2α in the presence of flufenamic acid or niflumic acid are summarized in B and D. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker currents. The bars represent mean ± SE. bP < 0.01 vs the untreated control.
No involvement of G proteins in the PGF2α-induced tonic inward currents in cultured ICC
The effects of GDP-β-S, a nonhydrolysable guanosine 5’-diphosphate analogue which permanently inactivates GTP binding proteins, were examined to determine whether the G-protein is involved in the effects of PGF2α in ICC. When GDP-β-S (1 mmol/L) was in the pipette, PGF2α (10 μmol/L) still showed the tonic inward currents (Figure 3A). In the presence of GDP-β-S in the pipette, the resting currents in the control were -23 ± 9 pA. The resting currents during treatment with PGF2α in the presence of GDP-β-S were -159.9 ± 36 pA (n = 4, Figure 3B), which were not significantly different from those obtained by treatment with PGF2α in the absence of GDP-β-S.
Figure 3.
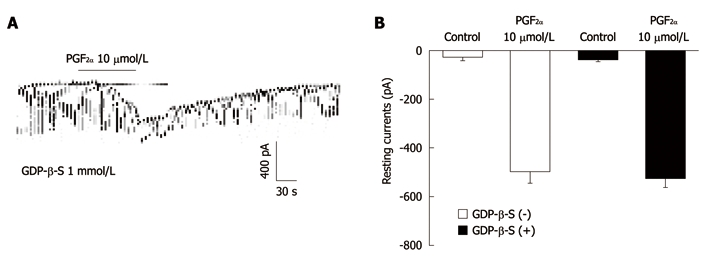
The effects of 5’-[-thio]diphosphate trilithium salt on response to prostaglandin F2α-induced pacemaker currents from interstitial cells of Cajal from mouse small intestine. A: Pacemaker currents from interstitial cells of Cajal exposed to prostaglandin F2α (PGF2α) (10 μmol/L) in the presence of 5’-[-thio]diphosphate trilithium salt (GDP-β-S) (1 mmol/L) in the pipette. The tonic inward currents with suppressed amplitude and frequency induced by PGF2α remained unchanged during internally applied GDP-β-S (1 mmol/L). The dotted lines indicate the zero current levels. The effects of PGF2α in the presence of GDP-β-S are summarized in B. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker currents. Bars represent mean ± SE. The effects of GDP-β-S on PGF2α-induced pacemaker currents were not significantly different from the PGF2α-induced pacemaker currents in the absence of GDP-β-S.
External Ca2+-free solution and Ca2+-ATPase inhibitor of endoplasmic reticulum suppress PGF2α effects in cultured ICC
To investigate the role of external Ca2+ or internal Ca2+, PGF2α was tested under external Ca2+-free conditions and in the presence of thapsigargin, a Ca2+-ATPase inhibitor of endoplasmic reticulum. The application of external Ca2+-free solution completely inhibited the pacemaker currents in voltage clamp mode at a holding potential of -70 mV and in this condition, PGF2α (10 μmol/L)-induced effects on pacemaker currents were blocked (n = 5, Figure 4A). The value of resting currents with PGF2α (10 μmol/L) in Ca2+-free solution was significantly different when compared with control values obtained in normal solution (Figure 4B). In addition, the treatment with thapsigargin (5 μmol/L) inhibited the pacemaker currents in ICC and blocked the PGF2α-induced tonic inward currents (Figure 4C). In the presence of thapsigargin, the value of resting currents during treatment with PGF2α was significantly different from those obtained by treatment with PGF2α in the absence of thapsigargin (n = 6, Figure 4D).
Figure 4.
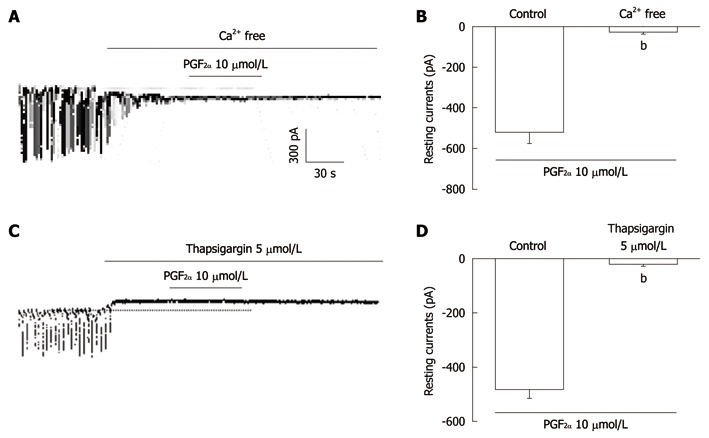
The effects of an external Ca2+-free solution or thapsigargin on the prostaglandin F2α-induced response in pacemaker currents in cultured interstitial cells of Cajal from mouse small intestine. A: External Ca2+-free solution abolished the generation of pacemaker currents. Under these conditions, the prostaglandin F2α (PGF2α) (10 μmol/L)-induced tonic inward currents were blocked; C: Thapsigargin (5 μmol/L) abolished the generation of pacemaker currents. Thapsigargin also blocked the PGF2α (10 μmol/L)-induced tonic inward currents. The dotted lines indicate the zero current levels. Responses to the PGF2α in the external Ca2+-free solution and in the presence of thapsigargin are summarized in B and D. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker current. The bars represent mean ± SE. bP < 0.01 vs the untreated control.
Effects of phospholipase C inhibitor on the PGF2α-induced tonic inward currents in cultured ICC
To investigate whether the PGF2α-induced effects on pacemaker currents are mediated by the activation of phospholipase C (PLC), we treated the ICC with U-73122, a PLC inhibitor. U-73122 (5 μmol/L) abolished the generation of pacemaker currents and blocked the PGF2α-induced tonic inward currents (Figure 5A). In the presence of U-73122, the tonic inward currents produced by PGF2α (10 μmol/L) were -21 ± 11 pA. The value of resting currents induced by PGF2α was significantly different from those obtained by treatment with PGF2α in the absence of U-73122 (n = 5, Figure 5B). These results suggest that the PGF2α-induced tonic inward currents may be a PLC-dependent mechanism.
Figure 5.
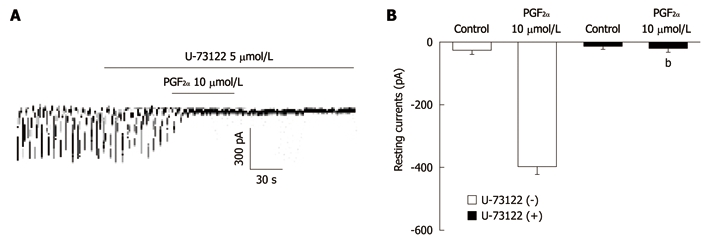
The effects of U-73122 on the prostaglandin F2α-induced response in pacemaker currents of cultured interstitial cells of Cajal from mouse small intestine. A: U-73122 (5 μmol/L) abolished the generation of pacemaker currents. U-73122 also blocked the Prostaglandin F2α (PGF2α) (10 μmol/L)-induced tonic inward currents. The dotted lines indicate the zero current levels. Responses to the PGF2α are summarized in B. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker current. The bars represent mean ± SE. The effect of U-73122 on PGF2α-induced pacemaker currents was significantly different from the PGF2α-induced pacemaker currents in the absence of U-73122 (bP < 0.01).
Effects of protein kinase C inhibitor on PGF2α-induced responses in cultured ICC
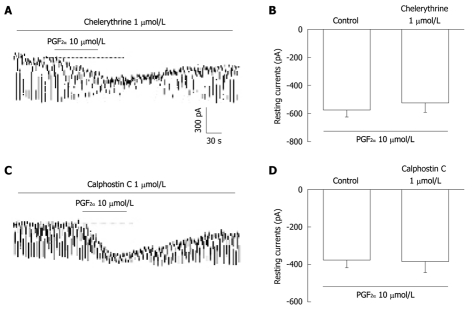
We tested the effects of chelerythrine or calphostin C, inhibitors of protein kinase C (PKC), to investigate whether PGF2α-induced responses to pacemaker currents are mediated by the activation of PKC. Chelerythrine (1 μmol/L) or calphostin C (10 μmol/L) did not have an effect on tonic inward currents induced by PGF2α (10 μmol/L) (Figure 6A and C) and the value also was not significantly different when compared with the tonic inward currents induced by PGF2α obtained in the absence of chelerythrine or calphostin C (n = 5, Figure 6B and D).
Figure 6.
The effects of chelerythrine or calphostin C on the prostaglandin F2α-induced response in pacemaker currents in cultured interstitial cells of Cajal from mouse small intestine. A and C: Pacemaker currents of interstitial cells of Cajal exposed to prostaglandin F2α (PGF2α) (10 μmol/L) in the presence of chelerythrine (1 μmol/L) or calphostin C (1 μmol/L). Under these conditions, the PGF2α caused tonic inward currents. The dotted lines indicate the zero current levels. Responses to the PGF2α in the presence of chelerythrine or calphostin C are summarized in B and D. Vertical solid line scales denote amplitude of pacemaker current and horizontal solid line scales denote duration of recording (s) pacemaker current. The bars represent mean ± SE. No significant difference from the untreated control.
Increasing of [Ca2+]i intensity by PGF2α
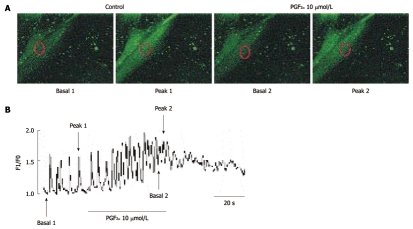
Because many reports have suggested that [Ca2+]i oscillations in ICC could be considered to be the primary mechanism for the pacemaker activity in GI activity, we examined the effect of PGF2α on [Ca2+]i oscillations in ICC. In this study, we measured spontaneous [Ca2+]i oscillations of ICC which are connected with cell clusters. Spontaneous [Ca2+]i oscillations observed in ICC which were loaded with fluo3-AM and the time series data show the spontaneous regular [Ca2+]i oscillations. In the presence of PGF2α (10 μmol/L), the basal points of [Ca2+]i oscillations were increased but the peak points of [Ca2+]i oscillations were slightly decreased (Figure 7A). The data of the time series are summarized in Figure 7B. These results suggest that the action of PGF2α on ICC may involve the regulation of spontaneous [Ca2+]i oscillations.
Figure 7.
Effects of Prostaglandin F2α on intracellular Ca2+ oscillations in cultured interstitial cells of Cajal from mouse small intestine. A: Sequential fluorescence intensity images of fluo-3-loaded cultured interstitial cells of Cajal under normal conditions. The representative frames indicate in (B) basal 1, 2, peak 1 and 2. The exposure time of each frame was 500 ms; B: Fluorescence intensity change plotted in (A) red marker in absence and presence of Prostaglandin F2α (PGF2α) (10 μmol/L). The horizontal solid line scales denote duration of fluorescence intensity change (s).
DISCUSSION
Although the actions of PGF2α have been demonstrated with regard to GI motility in tissue and smooth muscle cells, this is the first study in ICC in which an attempt has been made to determine the effects of PGF2α on electrical activity in the small intestine.
The results of the present study demonstrate that PGF2α regulates intestinal motility by modulating the pacemaker currents of ICC and that this modulation is mediated via the action on non-selective cation channels and [Ca2+]i mobilization in a PKC-independent manner.
Most regions of the GI tract generate spontaneous electrical and mechanical activity in the absence of stimulation. When electrical recordings are made from smooth muscle cells lying in the GI tract, a regular discharge of long lasting waves of depolarization, called slow waves, is detected. It has recently become apparent that slow waves are generated by a specialized population of smooth muscle cells, known as ICC[18]. ICC generate spontaneous pacemaker inward currents that depolarize membrane, this spreads to smooth muscle via gap junctions resulting in depolarization of membrane in smooth muscle leading to contraction by generating acting potentials through voltage-dependent Ca2+ channel activation[18]. From previous studies, many reports suggested that PGF2α usually showed contractile actions in in vivo and in vitro studies[19-21]. These reports indicate the possibility that PGF2α may have stimulatory functions on the electrical activity of ICC. In the present study we found that ICC produced spontaneous pacemaker inward currents under voltage clamp mode and that the application of PGF2α evoked the tonic inward currents of pacemaker currents. This result offers the new suggestion that the regulation of electrical activity in ICC may be involved in the contractile effects of PGF2α in the GI tract.
Until now, the exact mechanism of pacemaker activity generation has not been fully understood in ICC. Two suggestions exist: that pacemaker currents of ICC are mediated by the activation of voltage-independent non-selective cation channels and that inwardly rectifying Cl- channels can be generated by the rhythmic inward currents[15-17]. For checking of these suggestions, we used the blockers of non-selective cation and inwardly rectifying Cl- channels and we could find that flufenamic acid, a non-selective cation blocker, abolished pacemaker current generation and that PGF2α-induced tonic inward currents were blocked by flufenamic acid. However, although niflumic acid, a Cl- channel blocker, also abolished pacemaker generation, niflumic acid did not block the PGF2α-induced tonic inward currents. Furthermore, we have already found that substance P and bradykinin may modulate intestinal motility acting on ICC through the activation of non-selective cation channels[22,23]. Therefore, these data strongly provide support for the suggestion that, and it is likely that, both Cl- channels and non-selective cation channels are essentially needed for the generation of the spontaneous pacemaker currents in ICC. However, the agent-induced tonic inward currents in ICC may modulate the pacemaker currents by regulating non-selective cation channels in ICC.
The effects of PGs are mediated by specific receptors, classified into basic types (DP, EP, FP, IP, and TP) according to the PG ligand that each binds with the greatest affinity. They have different cell- and tissue-specific functions, as determined by selective coupling to G proteins and by the expression of splicing isoforms. In the case of PGF2α, PGF2α induces inositol (1,4,5) trisphosphate (IP3) production and increases Ca2+ levels via FP receptors, which are coupled to G proteins for functional action in various tissues. For example, PGF2α induces phosphoinositol (PI) turnover in isolated luteal cells and the effect of PGF2α in inducing Ca2+ mobilization in mouse fibroblasts occurs in conjunction with formation of IP3, and is pertussis toxin-insensitive[24,25]. These findings suggest that FP receptors can interact with a member of the G protein family to activate phopholipase C (PLC), leading ultimately to an IP3-mediated mobilization of Ca2+ from intracellular pools. However, there also appears to be a secondary phase of Ca2+ release by PGF2α-treated mouse fibroblasts that is likely due to extracellular Ca2+ entry. In this present study, when GDP-β-S was present in the pipette, the tonic inward pacemaker currents induced by PGF2α were still present. This means that the effects of PGF2α on electrical activity in ICC may be not related to G proteins. Additionally, since many proposals have been made that PGF2α may have biological activity through mobilization of [Ca2+]i and PLC activation, we used thapsigargin, a potent endoplasmic reticulum Ca2+-ATPase inhibitor and U-73122, an active PLC inhibitor, and found that these inhibitors suppressed the PGF2α-induced tonic inward currents. These results strongly support the proposition that the release of Ca2+ from internal storage and PLC activation by PGF2α are essential to produce tonic inward currents. Also, these findings have correlations with previous suggestions. In addition, it is well known that the generation of pacemaker currents is dependent upon [Ca2+]i oscillation and that the periodic release of Ca2+ from endoplasmic reticulum is essential for generating pacemaker currents. We believe that our experiments using thapsigargin and U-73122 underscore this information.
In many tissues, it is well known that the binding of PGF2α to its receptor results in not only the activation of PLC (and PLC is the initial step of the PI cascade that generates diacylglycerol (DAG), IP3, and Ca2+ release) but also in the activation of a PKC-dependent pathway. The increase in [Ca2+]i not only promotes translocation of some PKC isozymes to the plasma membrane, but, in concert with DAG, is essential in activating the conventional isoforms of PKC[22]. However, in the present study, chelerythrine or calphostin C, specific and potent PKC inhibitors, did not block PGF2α-induced effects, suggesting that PKC is not involved in the actions of PGF2α in ICC.
The periodic pacemaker activity of ICC is dependent on [Ca2+]i oscillations. The pacemaker mechanism is initiated by release of Ca2+ from the endoplasmic reticulum and is followed by reuptake of Ca2+ into the mitochondria[26]. In our results, we found spontaneous [Ca2+]i oscillations in ICC and this means that the spontaneous pacemaker activity of ICC is closely involved with [Ca2+]i oscillations in this experiment; the treatment with PGF2α in ICC increased the basal point of [Ca2+]i oscillation and decreased the peak point. However, the [Ca2+]i intensity was broadly increased as the action of tonic inward currents reversed. Our previous report suggested that PGE2 inhibited [Ca2+]i oscillations by ATP-sensitive K+ channel activation in cultured ICC[27]. The observed actions of PGE2 on [Ca2+]i oscillation in ICC support the suggestion that [Ca2+]i oscillations are important actions of pacemaker activity. Namely, PGE2 and PGF2α have opposing actions in ICC. However, both PGE2 and PGF2α have the same target for modulating the pacemaker activity of ICC; that is, the [Ca2+]i in ICC. Therefore, these results suggest that the spontaneous oscillation of [Ca2+]i is essential for pacemaker activity of ICC and that the [Ca2+]i can be the main regulatory target for various endogenous agents or neurotransmitters in ICC.
In the present study, externally applied PGF2α depolarized ICC membranes and formed spike potentials which would result in muscle contraction. As the ICC are electrically coupled with nearby smooth muscles through the gap junctions, the resulting contraction propagates around and along the gut in a coordinated manner and ultimately regulates GI motility.
In conclusion, this study describes the effects of PGF2α on ICC in the mouse small intestine. PGF2α depolarized the membrane with increased tonic inward currents, which were activated by non-selective cation channels via external Ca2+ influx, PLC, and internal Ca2+ mobilization, in a PKC-independent manner. Thus, PGF2α may play a very important role in regulating the rhythm and contraction of small intestinal smooth muscles by acting on ICC.
COMMENTS
Background
Prostaglandins (PGs) of the E, F, and I series are widely distributed in all body tissues, including the gastrointestinal (GI) tract. Many reports have suggested that PGF2α plays an important role in the modulation of intestinal motility.
Research frontiers
There are many reports that PGF2α has a function in GI motility by acting on smooth muscles but no studies have been performed to determine the effects of PGF2α on electrical events in interstitial cells of Cajal (ICC). Therefore, the purpose of our study was to investigate the signal transduction effects of PGF2α on pacemaker activity in cultured ICC.
Innovations and breakthroughs
This study showed the actions of PGF2α on ICC in the mouse small intestine. PGF2α depolarized the membrane with increased tonic inward currents, which were activated by non-selective cation channels via external Ca2+ influx, phospholipase C, and internal Ca2+ mobilization, in a protein kinase C-independent manner.
Applications
The role of PGF2α in ICC may be one theory for understanding the excitatory action of PGF2α in GI motility.
Terminology
ICC have functions of pacemaker cells and neuromediator cells in the tunica muscularis of the GI tract. The ICC generate rhythmic oscillations in membrane potential known as slow waves and this generation of slow waves is due to spontaneous inward currents called pacemaker currents.
Peer review
It is an interesting paper dealing with a demanding subject. They found that PGF2α can regulate intestinal motility through the modulation of ICC pacemaker activities.
Footnotes
Supported by Research Fund from Chosun Hospital 2008
Peer reviewer: Filip Braet, Associate Professor, Australian Key Centre for Microscopy and Microanalysis, Madsen Building (F09), The University of Sydney, Sydney NSW 2006, Australia
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
References
- 1.Bennett A, Hensby CN, Sanger GJ, Stamford IF. Metabolites of arachidonic acid formed by human gastrointestinal tissues and their actions on the muscle layers. Br J Pharmacol. 1981;74:435–444. doi: 10.1111/j.1476-5381.1981.tb09989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders KM, Northrup TE. Prostaglandin synthesis by microsomes of circular and longitudinal gastrointestinal muscles. Am J Physiol. 1983;244:G442–G448. doi: 10.1152/ajpgi.1983.244.4.G442. [DOI] [PubMed] [Google Scholar]
- 3.Robert A. Prostaglandins: effects on the gastrointestinal tract. Clin Physiol Biochem. 1984;2:61–69. [PubMed] [Google Scholar]
- 4.Botting JH, Salzmann R. The effect of indomethacin on the release of prostaglandin E2 and acetylcholine from guinea-pig isolated ileum at rest and during field stimulation. Br J Pharmacol. 1974;50:119–124. doi: 10.1111/j.1476-5381.1974.tb09598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burleigh DE. The effects of indomethacin on the tone and spontaneous activity of the human small intestine in vitro. Arch Int Pharmacodyn Ther. 1977;225:240–245. [PubMed] [Google Scholar]
- 6.Sanders KM, Ross G. Effects of endogenous prostaglandin E on intestinal motility. Am J Physiol. 1978;234:E204–E208. doi: 10.1152/ajpendo.1978.234.2.E204. [DOI] [PubMed] [Google Scholar]
- 7.Bennett A, Fleshler B. Prostaglandins and the gastrointestinal tract. Gastroenterology. 1970;59:790–800. [PubMed] [Google Scholar]
- 8.Bennett A, Fleshler B. Action of prostaglandin E 1 on the longitudinal muscle of the guinea-pig isolated colon. Br J Pharmacol. 1969;35:P351–P352. doi: 10.1111/j.1476-5381.1969.tb07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konturek SJ, Thor P, Konturek JW, Pawlik W. Role of prostaglandins in intestinal secretion, motility, and circulation. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:647–650. [PubMed] [Google Scholar]
- 10.Ishizawa M, Minowa T. Effects of PGD2 and PGF2 alpha on longitudinal and circular muscles of guinea-pig isolated proximal colon. Prostaglandins. 1982;24:843–850. doi: 10.1016/0090-6980(82)90064-8. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa M. Contractile effects of PGD2 and PGF2 alpha on mechanical and electrical activities in longitudinal and circular muscles of the guinea-pig colon. Prostaglandins. 1983;26:175–186. doi: 10.1016/0090-6980(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 12.Sanders KM, Szurszewski JH. Does endogenous prostaglandin affect gastric antral motility? Am J Physiol. 1981;241:G191–G195. doi: 10.1152/ajpgi.1981.241.2.G191. [DOI] [PubMed] [Google Scholar]
- 13.Sanders KM. Role of prostaglandins in regulating gastric motility. Am J Physiol. 1984;247:G117–G126. doi: 10.1152/ajpgi.1984.247.2.G117. [DOI] [PubMed] [Google Scholar]
- 14.Farrugia G, Szurszewski JH. Heme oxygenase, carbon monoxide, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:321–324. doi: 10.1002/(SICI)1097-0029(19991201)47:5<321::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513(Pt 1):203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 17.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 18.Sanders KM, Koh SD, Ordög T, Ward SM. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:100–105. doi: 10.1111/j.1743-3150.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 19.Bennett A, Eley KG, Stockley HL. The effects of prostaglandins on guinea-pig isolated intestine and their possible contribution to muscle activity and tone. Br J Pharmacol. 1975;54:197–204. doi: 10.1111/j.1476-5381.1975.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruch HP, Schmidt E, Laven R, Kehrer G, Wasner KH. The role of prostaglandines in peristalsis of the human colon. Acta Hepatogastroenterol (Stuttg) 1978;25:303–307. [PubMed] [Google Scholar]
- 21.Wienbeck M, Sperling T. The effects of prostaglandins F2 alpha and E2 on the motility of the cat colon in vitro. Z Gastroenterol. 1984;22:580–585. [PubMed] [Google Scholar]
- 22.Jun JY, Choi S, Yeum CH, Chang IY, You HJ, Park CK, Kim MY, Kong ID, Kim MJ, Lee KP, et al. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur J Pharmacol. 2004;495:35–42. doi: 10.1016/j.ejphar.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Choi S, Park do Y, Yeum CH, Chang IY, You HJ, Park CG, Kim MY, Kong ID, So I, Kim KW, et al. Bradykinin modulates pacemaker currents through bradykinin B2 receptors in cultured interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2006;148:918–926. doi: 10.1038/sj.bjp.0706806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujino H, Srinivasan D, Pierce KL, Regan JW. Differential regulation of prostaglandin F(2alpha) receptor isoforms by protein kinase C. Mol Pharmacol. 2000;57:353–358. [PubMed] [Google Scholar]
- 25.Sugiyama T, Sakai T, Nozawa Y, Oka N. Prostaglandin F2 alpha-stimulated phospholipase D activation in osteoblast-like MC3T3-E1 cells: involvement in sustained 1,2-diacylglycerol production. Biochem J. 1994;298(Pt 2):479–484. doi: 10.1042/bj2980479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KM, Ördög T, Koh SD, Ward SM. A Novel Pacemaker Mechanism Drives Gastrointestinal Rhythmicity. News Physiol Sci. 2000;15:291–298. doi: 10.1152/physiologyonline.2000.15.6.291. [DOI] [PubMed] [Google Scholar]
- 27.Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006;18:187–198. doi: 10.1159/000097516. [DOI] [PubMed] [Google Scholar]