Abstract
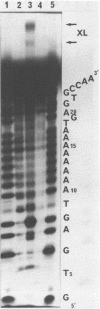
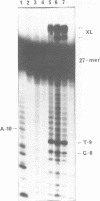
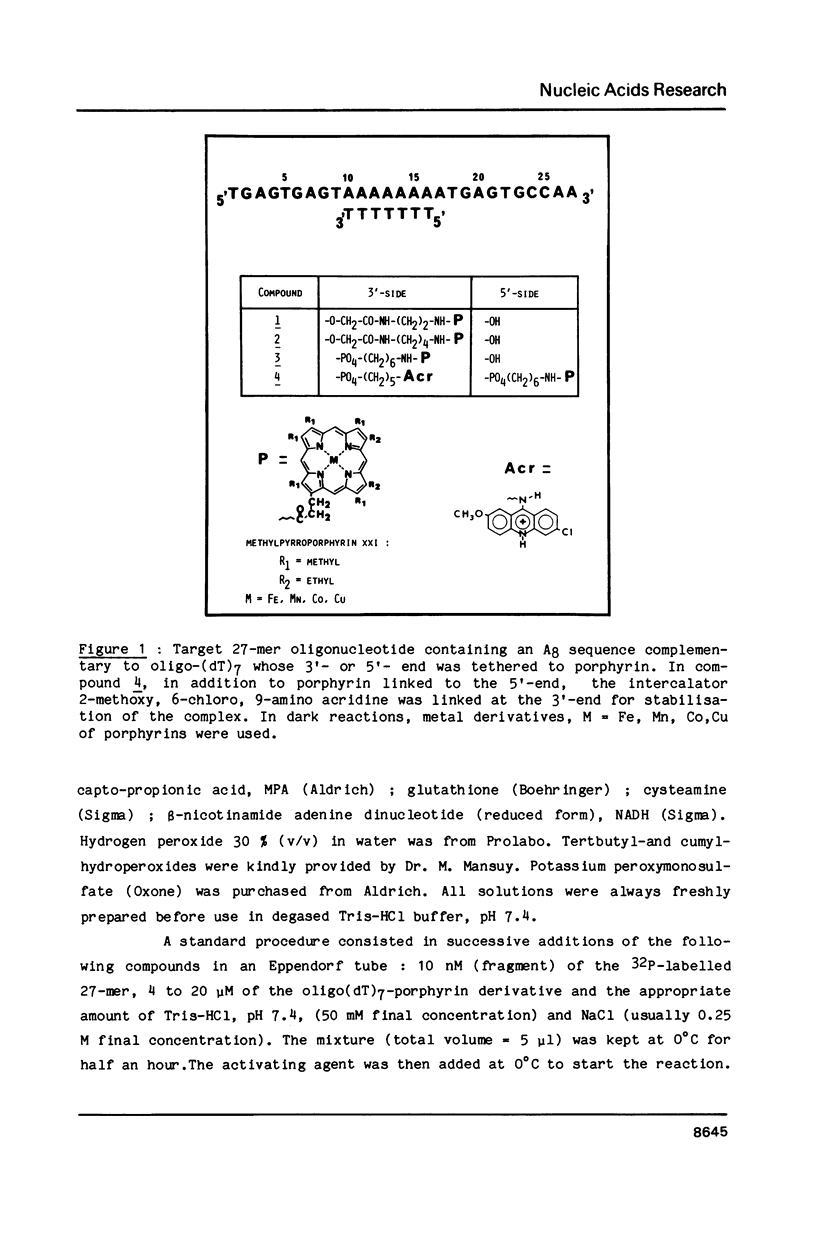
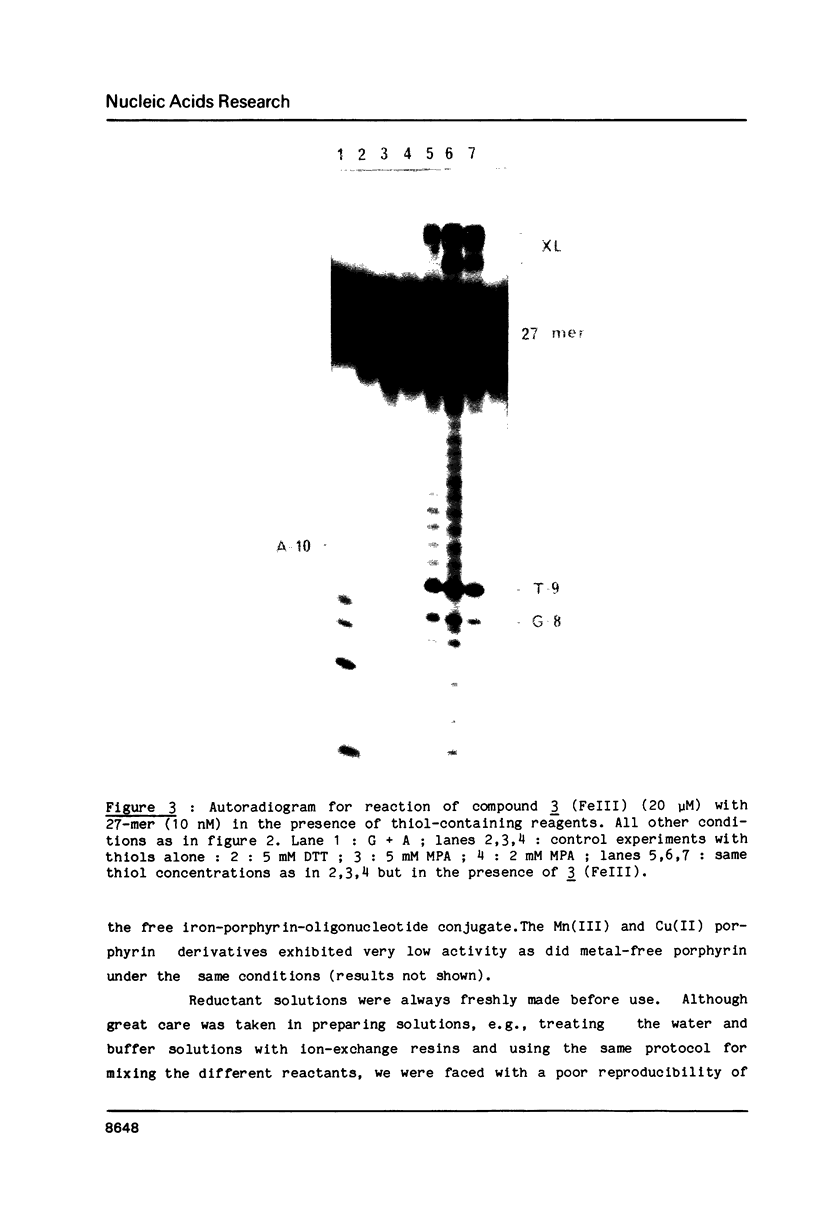
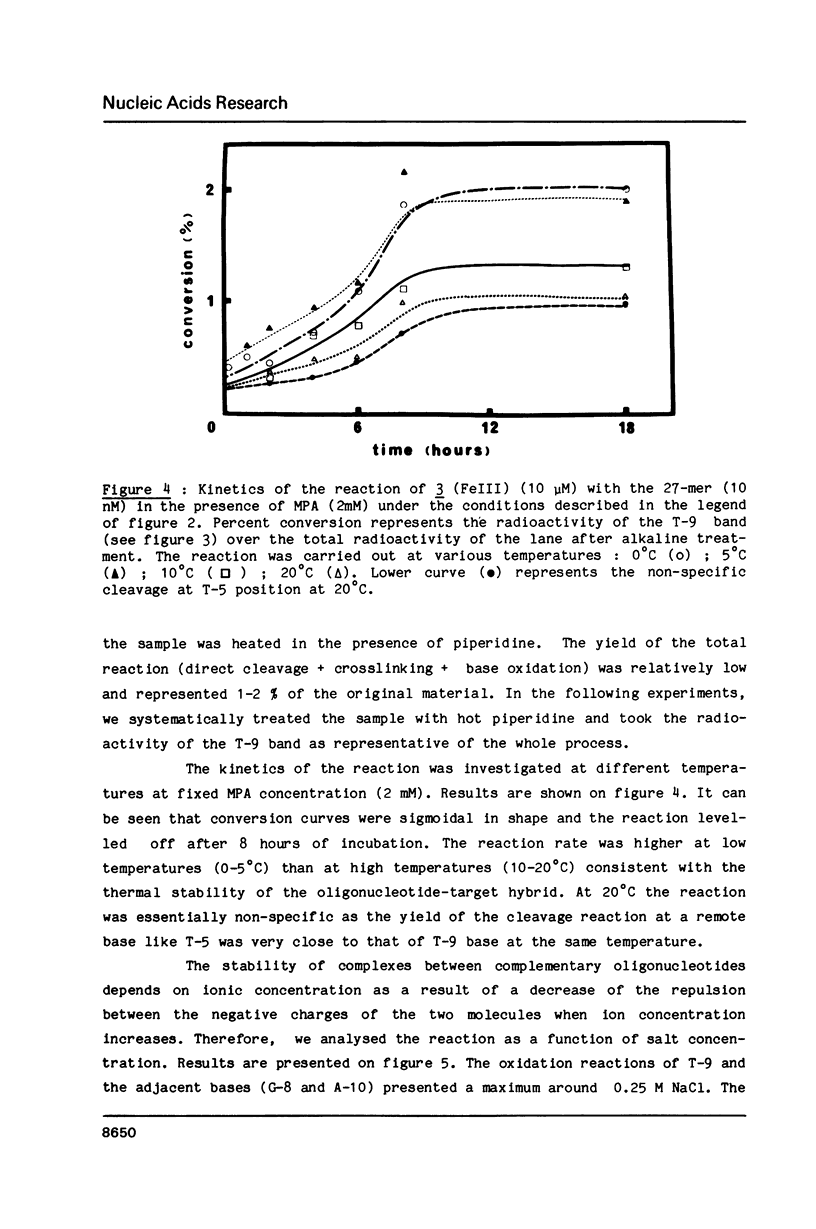
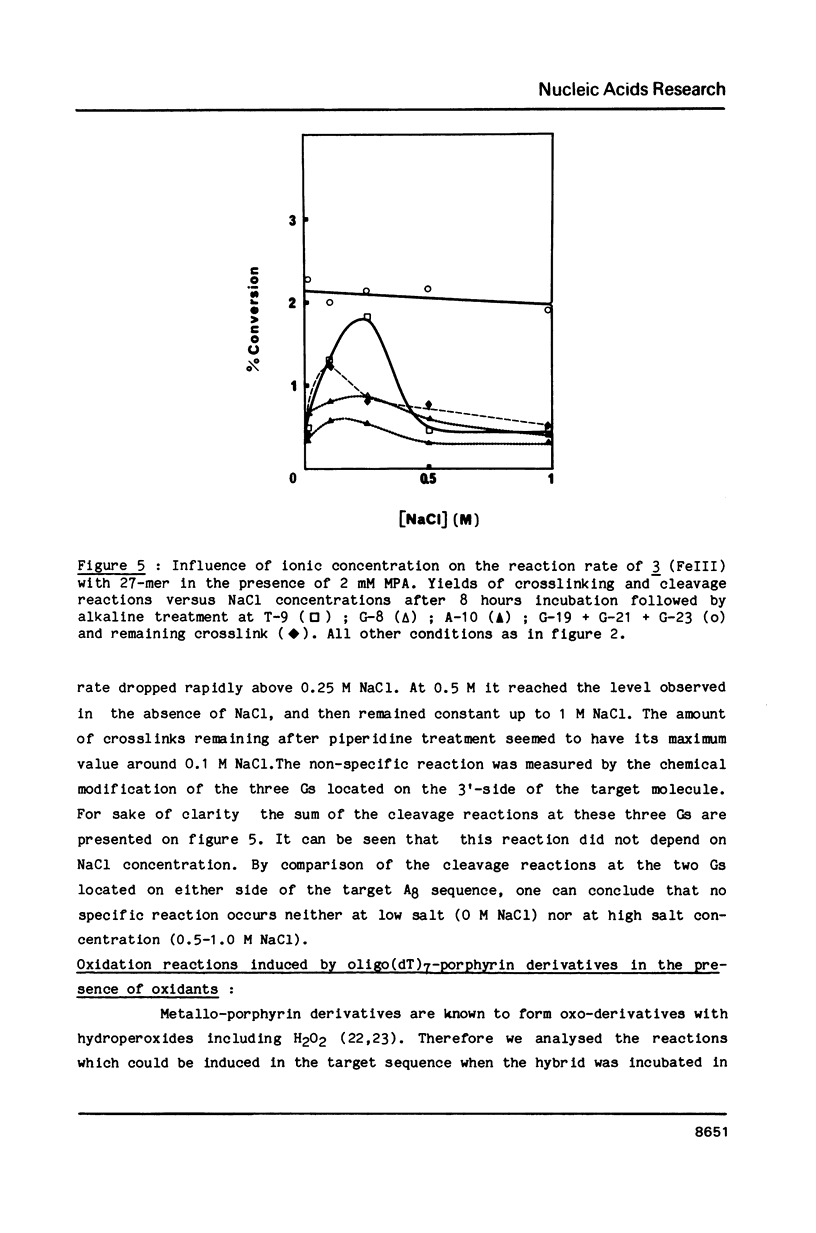
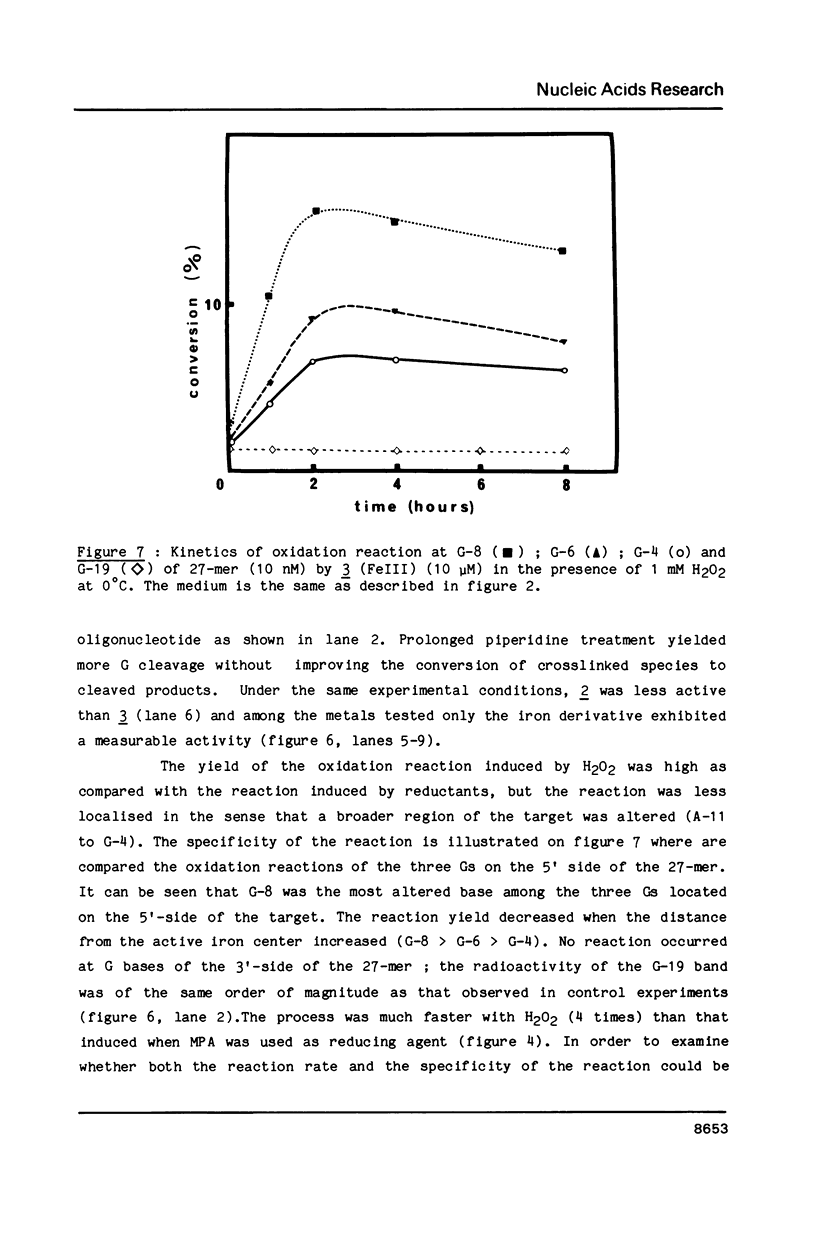
Oligo-heptathymidylates covalently linked to porphyrins bind to complementary sequences and can induce local damages on the target molecule. In dark reactions, iron porphyrin derivatives exhibited various chemical reactivities resulting in base oxidation, crosslinking and chain scission reactions. Reactions induced by reductants, such as ascorbic acid, dithiothreitol or mercapto-propionic acid, led to very localised reactions. A single base was the target for more than 50% of the damages. Oxidising agents such as H2O2 and its alkyl derivatives induced reactions that extended to a wider range of altered bases. The specificity of the chemical modifications observed in these systems is discussed from a mechanistic point of view.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aft R. L., Mueller G. C. Hemin-mediated DNA strand scission. J Biol Chem. 1983 Oct 10;258(19):12069–12072. [PubMed] [Google Scholar]
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou S. H., Chang W. C., Jou Y. S., Chung H. M., Lo T. B. Specific cleavages of DNA by ascorbate in the presence of copper ion or copper chelates. J Biochem. 1985 Dec;98(6):1723–1726. doi: 10.1093/oxfordjournals.jbchem.a135445. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiel R. J., Beerman T. A., Mark E. H., Datta-Gupta N. DNA strand scission activity of metalloporphyrins. Biochem Biophys Res Commun. 1982 Aug;107(3):1067–1074. doi: 10.1016/0006-291x(82)90630-1. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Datta-Gupta N., Mark E. H., Howard J. C. Induction of DNA damage by porphyrin photosensitizers. Cancer Res. 1981 Sep;41(9 Pt 1):3543–3545. [PubMed] [Google Scholar]
- Hashimoto Y., Iijima H., Nozaki Y., Shudo K. Functional analogues of bleomycin: DNA cleavage by bleomycin and hemin-intercalators. Biochemistry. 1986 Sep 9;25(18):5103–5110. doi: 10.1021/bi00366a019. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Iijima H., Shudo K. Functional analogs of bleomycin: sequence-specific DNA cleavage with porphyrin (Fe) intercalators. Gan. 1984 Jul;75(7):567–570. [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot G., Asseline U., Thuong N. T., Hélène C. 31P NMR studies of the binding of the oligonucleotide (Ap)3A to an oligodeoxythymidylate covalently linked to an acridine derivative. J Biomol Struct Dyn. 1986 Apr;3(5):913–921. doi: 10.1080/07391102.1986.10508473. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Helene C., Chassignol M., Thuong N. T. Targeted cleavage of polynucleotides by complementary oligonucleotides covalently linked to iron-porphyrins. Biochemistry. 1986 Nov 4;25(22):6736–6739. doi: 10.1021/bi00370a002. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sondhi S. M., Ong C. W., Skorobogaty A., Kishikawa H., Dabrowiak J. C. Deoxyribonucleic acid cleavage specificity of a series of acridine- and acodazole-iron porphyrins as functional bleomycin models. Biochemistry. 1986 Sep 9;25(18):5111–5117. doi: 10.1021/bi00366a020. [DOI] [PubMed] [Google Scholar]
- Nieuwint A. W., Aubry J. M., Arwert F., Kortbeek H., Herzberg S., Joenje H. Inability of chemically generated singlet oxygen to break the DNA backbone. Free Radic Res Commun. 1985;1(1):1–9. doi: 10.3109/10715768509056532. [DOI] [PubMed] [Google Scholar]
- Piette J., Merville-Louis M. P., Decuyper J. Damages induced in nucleic acids by photosensitization. Photochem Photobiol. 1986 Dec;44(6):793–802. doi: 10.1111/j.1751-1097.1986.tb05539.x. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Gaudemer A., Verlhac J. B., Kraljic I., Sissoëff I., Guillé E. Photocleavage of DNA in the presence of synthetic water-soluble porphyrins. Photochem Photobiol. 1986 Dec;44(6):717–724. doi: 10.1111/j.1751-1097.1986.tb05529.x. [DOI] [PubMed] [Google Scholar]
- Ward B., Skorobogaty A., Dabrowiak J. C. DNA cleavage specificity of a group of cationic metalloporphyrins. Biochemistry. 1986 Nov 4;25(22):6875–6883. doi: 10.1021/bi00370a021. [DOI] [PubMed] [Google Scholar]