Abstract
The mechanical properties of tissues and cells including renal glomeruli are important determinants of their differentiated state, function, and responses to injury but are not well characterized or understood. Understanding glomerular mechanics is important for understanding renal diseases attributable to abnormal expression or assembly of structural proteins and abnormal hemodynamics. We use atomic force microscopy (AFM) and a new technique, capillary micromechanics, to measure the elastic properties of rat glomeruli. The Young's modulus of glomeruli was 2,500 Pa, and it was reduced to 1,100 Pa by cytochalasin and latunculin, and to 1,400 Pa by blebbistatin. Cytochalasin or latrunculin reduced the F/G actin ratios of glomeruli but did not disrupt their architecture. To assess glomerular biomechanics in disease, we measured the Young's moduli of glomeruli from two mouse models of primary glomerular disease, Col4a3−/− mice (Alport model) and Tg26HIV/nl mice (HIV-associated nephropathy model), at stages where glomerular injury was minimal by histopathology. Col4a3−/− mice express abnormal glomerular basement membrane proteins, and Tg26HIV/nl mouse podocytes have multiple abnormalities in morphology, adhesion, and cytoskeletal structure. In both models, the Young's modulus of the glomeruli was reduced by 30%. We find that glomeruli have specific and quantifiable biomechanical properties that are dependent on the state of the actin cytoskeleton and nonmuscle myosins. These properties may be altered early in disease and represent an important early component of disease. This increased deformability of glomeruli could directly contribute to disease by permitting increased distension with hemodynamic force or represent a mechanically inhospitable environment for glomerular cells.
Keywords: glomerulus, biophysics, HIV-associated neuropathy, Alport syndrome, atomic force microscopy, actin, cytoskeleton
relatively little is known about the biomechanical properties of glomeruli and their cell and matrix components, but they are important for a number of reasons. Glomerular capillaries are constantly exposed to hemodynamic forces, and they can be injured in response to excessive hemodynamic stress or presumably by normal hemodynamic forces in the setting of structural or mechanical abnormalities of the capillary wall (9, 18, 19, 31, 32). In addition to opposing hemodynamic forces and maintaining the structural integrity of glomeruli, these mechanical properties of glomeruli are important because the mechanical environment cells reside in is a fundamental factor that determines cell behavior during normal development and in disease. In a number of examples, different cell types have different optimal mechanical environments in which they express proteins characteristic of their mature differentiated state, specifying a phenotype that permits normal tissue function (11, 15, 16, 20). For example, normal cells do not grow on soft agar, whereas malignant cells do. Similarly, embryonic cardiac myocytes in vitro lose striations and do not beat normally in an abnormally stiff environment such as that of a scar from a myocardial infarct (10). Therefore, the mechanical environment can have a role similar to the chemical environment in determining the differentiated characteristics of cells, and an abnormal mechanical environment can cause pathology rather than simply be a late consequence of it.
The mechanical properties of tissues and cells are commonly characterized by measuring their elasticity, their recoverable deformation in response to a force. Elasticity is defined as force per unit area divided by deformation (stress divided by strain), and the units are Pascals (Pa) (20). All methods to analyze elasticity involve measuring deformation in response to a mechanical force. Elasticity can be measured by a number of techniques including atomic force microscopy (AFM), a technique that measures local characteristics (subcellular or single cell level), rheometry, or microindentation that measure multicellular or tissue characteristics, or the newly developed capillary micromechanics that measures the complete mechanical characteristics (compressive and shear deformation) of a soft object such as a glomerulus (20, 21, 26, 41). Local and aggregate tissue mechanical properties may have different implications for tissue behavior and response to injury.
We used AFM to measure the Young's modulus or deformability of the outer surface of glomerular capillaries and the new micromechanics method that involves deformation of whole glomeruli in glass microcapillaries, capillary micromechanics, to measure the full elastic response of whole glomeruli (41, 44). To determine whether abnormal glomerular biophysical properties are associated with glomerular disease, we studied glomeruli from two different mouse models of glomerular disease Col4a3−/− mice, a model of Alport syndrome that has a primary defect in the glomerular basement membrane (GBM), and HIV-associated nephropathy (HIVAN), a disease that primarily affects podocytes (6, 30). In both models, we found that the glomeruli are significantly more compliant than controls at a stage of disease where glomerulosclerosis is minimal. These results indicate that glomeruli have defined mechanical properties that depend on cytoskeletal structure and that, surprisingly, glomeruli in two mechanistically distinct disease models are significantly more deformable than normal glomeruli at early stages of disease.
METHODS
Materials.
Sprague-Dawley rats were obtained from Harlan. The transgenic mouse models of HIVAN (Tg26HIV/nl on the FVB/N background) and Alport syndrome (Col4a3−/− and Col4a3+/− mice on the C57BL/6J background) were described previously (1, 5, 30). Chemicals, including latrunculin and blebbistatin, were obtained from Sigma unless stated otherwise. Cytochalasin D was purchased from Calbiochem. DMEM was from Meditech, and fetal bovine serum was from HyClone. The antibodies were obtained as follows, actin (Santa Cruz Biotech sc-15335), α-actinin-4 (Abcam ab59468), the EGF receptor (Santa Cruz Biotech sc-03), p125 focal adhesion kinase (Santa Cruz Biotech sc-557), filamin [our own (36)], nonmuscle myosin IIa and IIb (Sigma M8064, M7939), and talin (Sigma T3287). The secondary antibodies, goat anti-rabbit IgG and goat anti-mouse IgG horseradish peroxidase, were from Pierce.
Isolation of glomeruli.
Glomeruli were prepared using a standard differential sieving technique. The cortices of rat kidneys were minced and pushed though a screen (106 μm). The postscreen homogenate was suspended in PBS with 1 g/l glucose and 35 mg/l pyruvate (GIBCO Invitrogen) and filtered through a 100-μm nylon mesh (Falcon), and the (glomeruli) were collected on a 70-μm mesh nylon filter (Falcon). Mouse glomeruli were prepared in a similar manner, except that the sieve sizes were different (180, 90, and 45 μm, W. S. Tyler, Cleveland, OH). This preparation results in intact glomeruli with minimal contamination (<2%) by tubular cells (8, 38). Glomeruli from the Col4a3+/− and Col4a3−/− mice were prepared using a modification of the method of Takemoto et al. (40). Glomeruli were maintained in DMEM with 10% fetal bovine serum at room temperature. All procedures were in compliance with National Institutes of Health guidelines for the use and care of laboratory animals. Studies and protocols using the TG26HIV/nl and WT mice were approved by the CWRU IACUC, and studies and protocols using the Col4a3−/− and Col4a3+/− mice were approved by the Washington University IACUC.
Measurement of filamentous (F) and monomeric (G) actin ratios.
Glomeruli were homogenized in buffer (20 mM HEPES pH 7.4, 100 mM NaCl, 1 μM ATP, 1 mM NaVO4, 50 mM NaF, 1% Triton X-100, and protease inhibitors) by passage through a 25-gauge needle. The homogenate was centrifuged at 100,000 g for 1 h at 4°C. The supernatant (containing G-actin) was removed, and the pellet (containing F-actin) was resuspended in the same volume of buffer containing 15 mM HEPES, pH 7.5, 0.15 mM NaCl, 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 10 mM EDTA, 1 mM DTT, 1 mM NaVO4, and protease inhibitors. Equal volumes of the fractions containing F and G actin and ∼10 μg protein were separated on SDS gels, and the bands corresponding to actin were identified on Western blots with an anti-actin antibody (Cytoskeleton) and standard chemiluminescence (24). Films were quantified using NIH Image J software.
Immunofluorescence microscopy.
Whole isolated glomeruli were immersion fixed in 4% paraformaldehyde-based fixative and transferred to wells of a 96-well plate. The glomeruli were stained in rhodamine-phalloidin (Invitrogen) solutions, rinsed in PBS, and embedded as a whole mount preparation. Confocal images were collected with an Olympus IX81 equipped with a Disk Scanning Unit.
Capillary micromechanics.
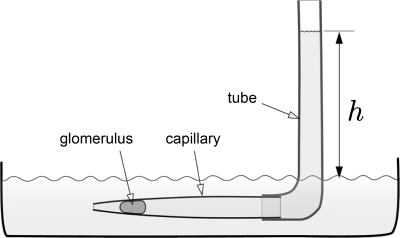
To measure the mechanical response at the scale of the entire glomerulus, we employed a newly developed technique based on deformation of single glomeruli in tapered microcapillaries, “capillary micromechanics,” shown schematically in Fig. 3 (44). The device consists of a tapered glass capillary connected to a flexible tube. To minimize friction and prevent sticking of the glomeruli to the walls of the glass capillary, the inside surfaces of the capillary are coated with bovine serum albumin. For measurements, a dilute suspension of glomeruli in phosphate-buffered saline (PBS, Dulbecco's containing calcium and magnesium) and albumin flows into the device, and a glomerulus becomes lodged in the tapered capillary. By adjusting the height (h) of fluid in the tube, the pressure difference (p) between the inlet and the outlet of the device is controlled. Deformation (shape and volume change) of glomeruli is measured directly by imaging the glomerulus using optical microscopy combined with image analysis. Since changes in both shape and volume are quantified, both the compressive (K) and shear (G) moduli are defined. Other elastic parameters including the Poisson ratio (ν) and the Young's modulus (E) can be directly expressed in terms of K and G (44).
Fig. 3.
Capillary micromechanics; schematic of experimental setup. The technique is based on deformation of glomeruli in a tapered capillary. The tip of the capillary leads into a fluid bath filled with buffer. A flexible tube is attached at the backside of the capillary; the filling height h determines the pressure difference (1 mm H2O is ∼10 Pa) and thereby the externally applied stress acting on the glomerulus. Glomeruli are imaged (shape and volume) at each pressure step after the glomerulus lodges in the capillary.
The externally applied stress on the glomerulus along the longitudinal direction is equated with p, whereas the stress along the radial direction is given by pwall, the pressure that the walls of the glass capillary exert on the glomerulus. The contact area of the glomerulus with the glass surface is a band with the shape of a tapered cylinder; the wall pressure depends on the length Lband and the average radius Rband of this contact band, as well as on the taper angle of the capillary as
where we have assumed the absence of friction on the glass walls.
By equating the pressure-induced, externally applied stress on a glomerulus with the internal elastic stresses that result from the changes in shape and volume, we derive the following expressions for K and G
where εr and εz are the radial and the longitudinal strain deformations, respectively.
The compressive modulus is related to changes in the volume of the glomerulus, and the relevant strain deformation is the volumetric strain ΔV/V or (2εr + εz), whereas the relevant stress is the average pressure acting on the glomerulus (2 pwall + p)/3. The shear modulus is related to changes in the shape of the glomerulus; the relevant strain is the difference between the radial and the longitudinal strain (εr − εz), and the relevant stress is the difference between the radial stress and the longitudinal stress (pwall − p)/2. The Young's modulus E, which characterizes a mixture of compressive and shear deformation modes, can be expressed as a function of K and G as E = 9KG/(3K + G).
Atomic force microscopy.
Glomeruli were placed in PBS and allowed to settle to the bottom of a 35-mm Petri dish. AFM measurements were conducted at room temperature using a DAFM-2X Bioscope (Veeco, Woodbury, NY) mounted on an Axiovert 100 microscope (Zeiss, Thornwood, NY). A silicon nitride cantilever (196 μm long, 23 μm wide, 0.6 μm thick, 0.06 N/m spring constant) with a bead tip (1 μm in diameter) was used for indentation. To calculate the Young's modulus, the first 500–800 nm of tip deflection are fit to the Hertz model for a sphere
where fbead is the force on the bead, dcantilever is the deflection of the cantilever measured by the AFM, E is the Young's modulus, v is the Poisson ratio, R is the radius of the bead, and δ is the vertical displacement of the cantilever (41). As the external surface of glomeruli is composed primarily of exposed capillaries, the measurements will most likely probe the properties of the external capillary wall. At least 10 measurements were performed on different areas on each glomerulus, and at least 10 glomeruli were studied in each group. These measurements assume that the glomerulus conserves volume during the measurement and that therefore the value of v is near 0.5.
RESULTS
Effects of cytoskeleton-active agents on glomeruli.
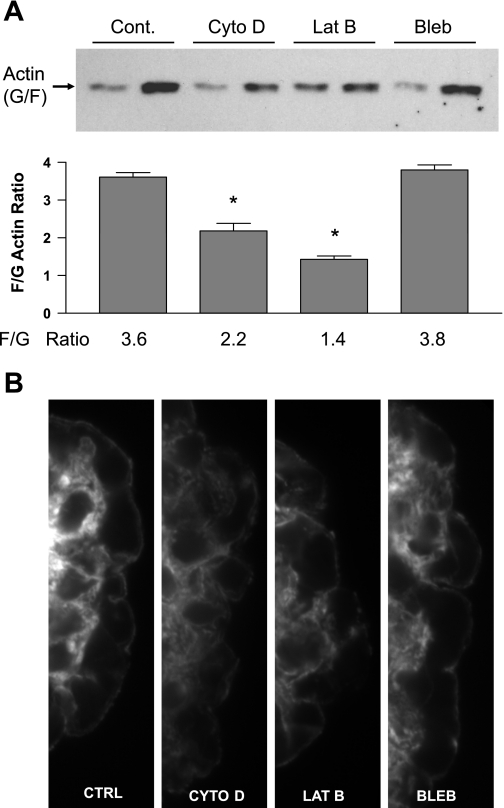
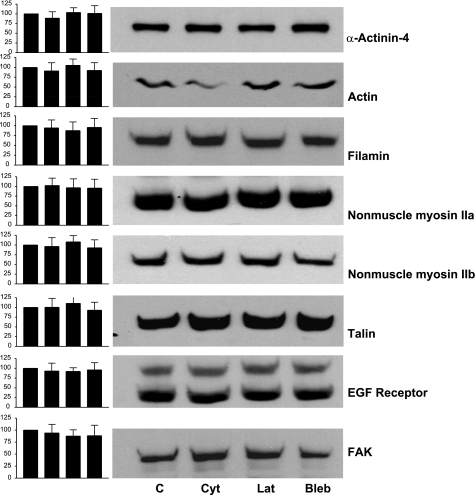
Isolated rat glomeruli were treated with cytochalasin D (5 μm), latrunculin B (500 nm), or blebbistatin (100 μm). After 18 h of exposure, F/G actin ratios were determined for the glomeruli to confirm that the agents had their predicted biochemical effects (Fig. 1A). Control glomeruli had an F/G actin ratio of 3.6, cytochalasin D reduced it to 2.2, and latrunculin B reduced it to 1.4. F-actin may not have been completely depolymerized because of the ability of cytochalasin and latrunculin to penetrate all regions of the glomerulus, and latrunculin may have had a greater effect because it is reported to penetrate tissues better than cytochalasin D. Blebbistatin did not result in a reduction in F actin (3.8 F/G ratio). Immunofluorescence studies of these glomeruli using rhodamine-phalloidin staining demonstrate a reduction in staining in the capillary walls of the glomeruli treated with cytochalasin D and latrunculin B, whereas the capillary walls of control glomeruli and glomeruli treated with blebbistatin had similar levels of staining (Fig. 1B). These studies also demonstrate that the capillary loop architecture of the glomeruli was intact. Figure 2 demonstrates that the level of expression of a number of proteins that affect the mechanical properties of cells and glomeruli (actin, α-actinin-4, filamin, nonmuscle myosins II and IIb, talin, and p125FAK) were not altered by the treatments. The EGF receptor was used as a control, because it is not a structural protein or directly involved in control of actin polymerization. These results demonstrate that the actin depolymerizing agents have the desired biochemical effect, but that they do not disrupt glomerular structure.
Fig. 1.
Effects of cytoskeleton disrupting agents on glomeruli. A: F/G actin ratios for glomeruli treated with cytochalasin D (Cyt D, 5 μM), latrunculin B (Lat B, 500 nM), and blebbistatin (Bleb, 100 μm). Top: representative blot for the G and F actin from glomeruli in each experimental condition, with the agent shown above the blot. Bottom: summary data, with the mean ratio below the corresponding bar. Each bar represents a mean ± SD of four independent experiments (*P < 0.05 vs. control). B: immunofluorescence images of capillary loops from single glomeruli stained with rhodamine phalloidin (×100 under oil) with treatment conditions as shown.
Fig. 2.
Expression of cytoskeletal and focal adhesion proteins in whole glomeruli. Glomeruli were maintained in tissue culture medium (lane 1) or tissue culture medium with 5 μM Cyto D (lane 2), 500 nM Lat B (lane 3), or 100 μM Bleb (lane 4) for 18–25 h. Approximately 50 glomeruli were dissolved in sample buffer, the samples were normalized for protein, and 10 μg of protein was loaded in each lane and size-fractionated on 10% gels. The individual proteins were identified with specific antisera. The bands representing the different proteins were quantified with densitometry (NIH Image software). The values for the samples from the treated glomeruli were normalized to the untreated glomeruli, which were assigned a value of 100%. Left, bar graphs of the representative blots represent means ± SD (n = 4). No statistically significant differences were found.
Mechanical properties of glomeruli determined by capillary micromechanics.
To directly quantify the mechanical properties of whole glomeruli, we employed the newly developed capillary micromechanics method that allows measurement of the mechanical response of glomeruli to deformations at the scale of the entire structure. This method involves deforming a single glomerulus in a tapered capillary and measuring the change in shape and volume in response to pressure behind the glomerulus as shown schematically in Fig. 3 (44). This technique enables us to characterize the full elastic response of glomeruli, expressed in terms of both the compressive modulus (volume change) and the shear modulus (shape change), and then to calculate the Young's modulus for comparison to the AFM data (44). To perform this analysis, we equated the pressure-induced externally applied stress with the internal elastic stress that results in changes in either the volume or the shape of the glomerulus. While the external stress is controlled by the pressure difference applied to the capillary, the changes in volume and shape are quantified directly using optical microscopy in combination with image analysis.
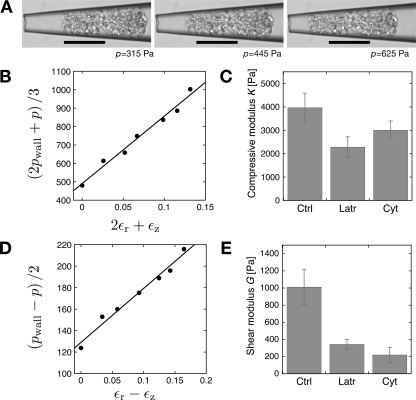
A typical experiment is shown as a series of images in Fig. 4A. With increasing pressure (from left to right) the glomerulus moved progressively toward the tip of the capillary and changed shape as a result of the externally applied stress. The glomerulus also underwent a significant change in volume, which indicated that glomeruli exhibit a finite compressive modulus, presumably due to the ability of water to flow from a tissue, in contrast to liquid droplets in an emulsion, which for all practical purposes can be considered to be incompressible.
Fig. 4.
Measurement of glomerular compressive and shear moduli using capillary micromechanics. Rat glomeruli were treated with Cyto D (5 μM), Lat B (500 nM), or left untreated. A: microscope images of an untreated glomerulus deforming due to the applied pressure difference (p), which is increasing from left to right (315, 445, and 625 Pa). The scale bars correspond to 100 μm. B: typical line for the analysis of the compressive modulus of the glomerulus shown in A. Plot of the stress relevant for compression versus the volumetric strain deformation. The slope of the line is the compressive modulus. C: average values of the compressive modulus for untreated glomeruli (n = 4), for glomeruli treated with Cyto D (n = 3), and Lat B (n = 5); the error bars are the corresponding means SE. The values for cytochalasin- and latrunculin-treated glomeruli were different from control (P < 0.05, ANOVA) but not from each other. D: typical line for the analysis of the shear modulus; plot of the stress relevant for shear deformation versus the relevant strain εr − εz. Slope of the line is the shear modulus for the sample shown in A. E: average values of the shear modulus for untreated glomeruli (n = 4) and glomeruli treated with Cyto D (n = 3), and Lat B (n = 5); the error bars represent the corresponding means SE. The values for cytochalasin and latrunculin-treated glomeruli were different from control (P < 0.05, ANOVA) but not from each other.
The two elastic moduli (compressive and shear) were derived from the data by plotting the relevant stress as a function of the relevant strain for each mode of deformation over a range of stress values. The slopes of these lines determine the compressive and shear moduli. To derive the compressive modulus (K), we plotted (2pwall + p)/3 as a function of the volumetric strain deformation ΔV/V, as shown in Fig. 4B. The slope of this line is the compressive modulus, and a typical measurement for the control glomeruli is shown in Fig. 4B.
To assess the contribution of the F-actin cytoskeleton to the elastic moduli of glomeruli, we treated glomeruli with two actin depolymerizing agents cytochalasin D and latrunculin B. We expected that a significant portion of the elastic moduli of glomeruli would be due to F-actin and the cytoskeletal structure of glomerular cells. We also wanted to demonstrate that the glomeruli were intact biochemically by demonstrating that they responded to the actin deoplymerizing agents. Figure 4C shows summary data where the compressive modulus in control glomeruli (3,970 ± 620 Pa) and glomeruli treated with cytochalasin D (2,280 ± 440 Pa) or latrunculin B (3,010 ± 390 Pa) were compared.
To derive the shear modulus, G, we plot (pwall − p)/2 as a function of the relevant strain deformation εr − εz, as shown in Fig. 4D, for a control glomerulus. The slope of this line is the shear modulus. Summary data are shown in Fig. 4E for the shear modulus of control glomeruli (1,020 ± 200 Pa) and glomeruli treated with cytochalasin D (340 ± 60 Pa) and latrunculin B (220 ± 90 Pa).
A comparison of untreated glomeruli and glomeruli treated with different actin depolymerizing agents (Fig. 4, C and E) showed distinct effects of the drugs on the compressive and shear moduli. The compressive modulus was reduced by 35% by latrunculin and 25% by cytochalasin. However, the effect on the shear modulus appeared even more dramatic, with a reduction of 60% for cytochalasin and a reduction of 75% for latrunculin. The difference in the effects of latrunculin and cytochalasin were not significant from each other for either the compressive or shear modulus.
Mechanical properties of glomeruli determined by AFM.
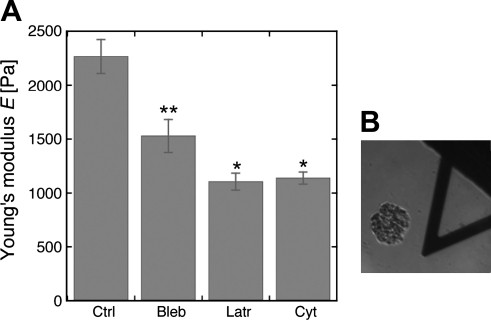
AFM probes submicron areas of membrane over a subsecond time scale, and as such, reflects the intrinsic characteristics of a structure since cells and tissues cannot actively remodel their cytoskeletons or membranes over this time scale (41). The external surface of a glomerulus is composed primarily of exposed capillaries, the majority of which are covered by podocyte foot processes as well as some podocyte cell bodies. When the AFM probe touches the surface of a glomerulus, it is most likely to encounter an external capillary wall. At least 10 measurements were made on each glomerulus so that the majority of measurements will reflect the characteristics of capillary walls. Figure 5 shows that the Young's modulus measured by AFM is 2,300 ± 160 Pa and demonstrated that agents that alter the cytoskeleton (cytochalasin D and latrunculin B that depolymerize F-actin) and blebbistatin that inhibits the activity of nonmuscle myosins IIa and IIb reduce the elastic modulus of the glomerular capillary wall. Figure 5B shows a glomerulus in relation to the AFM cantilever. The Young's modulus values were reduced ∼37% by blebbistatin (1,500 ± 140 Pa), 50% by cytochalasin D (1,100 ± 60 Pa), and 50% by latrunculin B (1,100 ± 80 Pa). These results provide a measurement of the Young's modulus of normal glomerular capillaries and demonstrate that a significant portion of the Young's modulus of glomerular capillaries depends on F-actin and nonmuscle myosins. The effect of blebbistatin was tested because nonmuscle myosins are believed to be involved in force sensing and consequently determination of the elastic propertis of cells (11). Failure of the elastic modulus to change in response to blebistatin would also indicate that the metabolism of the glomeruli was disrupted.
Fig. 5.
Measurement of the Young's modulus of the glomerular capillary wall by atomic force microscopy. A: atomic force microscopy (AFM) data from normal rat glomeruli treated with agents that alter with the cytoskeleton (5 μM Cyto D, 500 nM Lat B, and 100 μm Bleb). Each bar represents the mean ± SD of 10 independent measurements of 10 different glomeruli. The values for Cyto D and Lat B-treated glomeruli are statistically different from control and Bleb-treated glomeruli (P < 0.05), but not from each other, and those for the Bleb-treated glomeruli are different from control. *Statistically different from control and Bleb (P < 0.05), but not from each other; **Values for Bleb are different from control and the Latr- or Cyt-treated glomeruli. B: image of glomerulus in relation to the AFM cantilever.
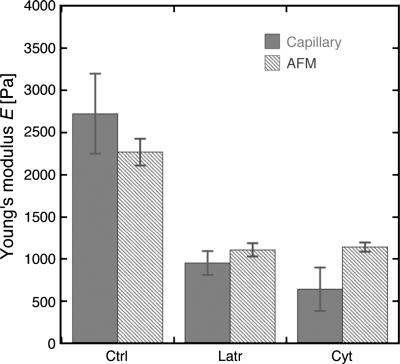
When comparing the results of the AFM measurements where the Young's modulus is measured directly to the capillary micromechanics assay where the Young's modulus is calculated from the compressive and shear moduli, we found that both the magnitude and trend in the data were surprisingly consistent between the two independent methods (Fig. 6). Our results indicate that the drug treatments had a similar effect on the mechanics of the whole glomerulus as well as on a more local scale on the mechanics of the glomerular capillary walls.
Fig. 6.
Comparison of Young's modulus values obtained using atomic force microscopy and capillary micromechanics for untreated glomeruli and glomeruli treated with 5 μM Cyto D and 500 nM Lat B. Data are from Figs. 3 and 4. Dark bars represent capillary micromechanics data, and light bars represent AFM data.
Mechanical properties of glomeruli from two mouse disease models.
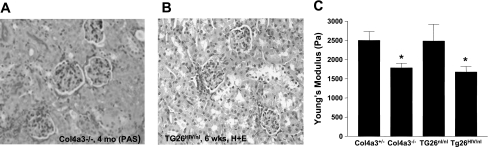
To determine whether glomerular disease is associated with biomechanical abnormalities, we used AFM to compare the Young's moduli of glomeruli from two mechanistically distinct mouse models of disease: Col4a3−/− mice a model of Alport syndrome, and Tg26HIV/nl mice, a model of HIV-associated nephropathy (HIVAN) (1, 25, 30). Col4a3−/− mice were compared with their Col4a3+/− littermate controls, which are phenotypically normal (B6 background), and Tg26HIV/nl mice were compared with their normal Tg26nl/nl littermates (FVB/N background). Col4a3−/− mice lack the collagen α3α4α5(IV) network and are a faithful model of Alport syndrome with abnormal GBMs and progression to renal failure by 8 mo. Tg26HIV/nl mice express a subgenomic HIV-1 proviral DNA lacking the gag and pol genes and are a good model of human HIVAN. HIVAN appears to be a disease of podocytes as these cells have marked structural and functional abnormalities (27, 29, 41). Studies were performed early in the disease for each model when glomerulosclerosis was minimal, 4 mo for the Col4a3−/− mice, and approximately 40 days for the Tg26HIV/nl mice (1, 25). Figure 7 shows that the glomeruli of both the Col4a3−/− (Fig. 7A) and Tg26HIV/nl (7B) mice have minimal evidence of sclerosis at the light microscopic level. At ∼40 days, the urine protein dipsticks for the Tg26HIV/nl mice had readings of 2.7 ± 0.45 (n = 20), which corresponds to ∼100–300 mg/dl protein. The dipstick reading for the normal control littermates was 1.4 ± 0.45 (n = 8), which corresponds to 30–100 mg/dl protien. Tg26HIV/nl mice with fully developed renal disease have 4+ proteinuia, which corresponds to >2,000 mg/dl protein, indicative of severe podocyte injury. At 4 mo, the Col4a3−/− mice had urine protein-to-creatinine ratios of 19.6 ± 4.3 g/g (n = 6), whereas their Col4a3+/− controls had urine protein-to-creatine ratios of 12.1 ± 3.9 g/g (n = 6). Albumin-to-creatinine ratios, a more sensitive index of glomerular injury, were 31.6 ± 7.6 g/g (n = 6) and 0.08 ± .088 g/g (n = 6). In later stages of disease, the Col4a3−/− mice had urine protein-to-creatinine ratios on the order of 75–100 g/g (1). At 4 mo, the Col4a3−/− mice had serum creatinine values 0.5–0.6 mg/dl, indistinguishable from controls, whereas at 40 days, the Tg26HIV/nl mice had serum BUN levels of ∼55 mg/dl, twice that of controls (1, 25). Figure 7C shows the results of AFM measurements of glomeruli from each of these mouse models. The Young's modulus values for the control glomeruli from both sets of mice were similar to those for rat glomeruli, approximately 2,500 Pa (Col4a3+/− 2,600 ± 230 Pa and Col4a3+/− 2,500 ± 440 Pa). However, the Col4a3−/− glomeruli and Tg26HIV/nl glomeruli were significantly softer than their respective controls, 1,800 ± 130 Pa for the Col4a3−/−, and 1,700 ± 150 Pa for the Tg26HIV/nl glomeruli. These results demonstrate that at an early stage of disease, a point before light microscopic evidence of glomerulosclerosis was present, the glomeruli from both disease models exhibited significant mechanical abnormalities and were significantly more deformable than normal glomeruli.
Fig. 7.
Microscopic appearance of kidney cortex from Col4a3−/− (A) and Tg26HIV/nl (B) mice, and AFM measurement of Young's modulus of glomeruli form the same animals. Kidney sections and glomeruli were prepared from Col4a3−/− mice at 4 mo and Tg26HIV/nl mice at 40 days. The sections were stained with periodic acid Schiff and hematoxylin and eosin, respectively, and photographed at ×40. AFM studies were performed on isolated glomeruli (Col4a3−/− and Col4a3+/−) or Tg26HIV/nl and Tg26nl/nl (C). Each bar represents the mean value from at least 10 measurements of at least 10 glomeruli ± SD, *P < 0.05 vs. respective control (ANOVA).
DISCUSSION
Different tissues have specific mechanical properties that are optimized for the function of a particular tissue. For example, bone is stiff as would be expected for a supporting structure, and blood vessels are compliant to accommodate pulsatile blood flow. These tissue-specific mechanical properties also provide the appropriate mechanical environment for the differentiation and maintenance of differentiated cells (10, 11, 16, 20). Abnormal tissue or cell mechanical properties can result in disease by two mechanisms, loss of the structural integrity of the tissue in response to physiological or pathophysiological forces, or loss of the differentiated characteristics of cells as a result of an inappropriate mechanical environment.
The mechanical properties of renal glomeruli are not well defined but are likely to be important and tightly controlled in normal physiology and abnormal in disease. Clearly, in late stages of disease, glomeruli acquire abnormal structure with fibrosis and should be substantially stiffer than normal. In an effort to begin to define the mechanical properties of glomeruli, some of the factors that control these properties and the potential role of biomechanical factors in disease, we used two methods to measure the elastic moduli of glomeruli, studied the effects of agents that affect the state of the actin cytoskeleton on their elastic moduli, and studied glomeruli from two mechanistically distinct disease models Col4a3−/− and Tg26HIV/nl mice. Remarkably, we found that in both disease models rather than being stiffer than normal, glomeruli are significantly more deformable than normal, an unexpected finding that suggests that at early stages of disease, mechanical abnormalities of the tissue are present that may represent an important component of glomerular injury.
Our studies are a first step toward characterization of the mechanical properties of normal and diseased glomeruli. The Young's modulus of normal rat and mouse glomerular capillary walls and whole rat glomeruli is on the order of 2,500 Pa. The value for the elastic modulus obtained for both rat and mouse glomeruli of 2,500 Pa is similar to that obtained using two other techniques, microaspiration of glomerular capillary walls (2,600 ± 320 Pa, n = 4, FJ Byfield, I Levental, RT Miller, and PA Janmey, unpublished observations), and microindentation of whole glomeruli (2,800 ± 320 Pa, n = 3) (26). These results suggest that a Young's modulus of 2,500 Pa may be a general property of mammalian glomeruli. This value is in the range where mechanical characteristics of capillary walls could be compatible with physiological deformation in response to hemodynamic force due to a net transcapillary pressure of 30–40 mmHg (∼4,000 Pa).
Treatment of glomeruli with cytochalasin D or latrunculin B, which depolymerize F-actin, reduced the Young's modulus of rat glomeruli from ∼2,500 to ∼1,000 Pa using values from both AFM and microcapillary mechanics. Blebbistatin inhibits nonmuscle myosins IIa and IIb, the activities of which are required for cells to respond to mechanical stimuli and reduced the elastic modulus of glomeruli to ∼1,400 Pa. These results indicate that the mechanical characteristics of glomeruli and capillaries depend on the state of the actin cytoskeleton and the activity of nonmuscle myosins. The contributions of intermediate GBM were not addressed in these studies.
AFM measurements reflect local mechanical characteristics at the cellular or subcellular level (41). In these studies, AFM measurements probably reflect the aggregate mechanical properties of the capillary wall. The AFM cantilever has a 1-μm bead tip to avoid puncturing the cell membrane. The excursion of the tip is ∼300 nm, less than the thickness of the capillary and negligible compared with the size of a glomerulus (100–120 μm) or a glomerular capillary (∼10 μm) but on the scale of the thickness of the GBM (250–350 nm).
The capillary micromechanics assay involves measuring the deformation of an entire glomerulus in response to a known force and so the measurement represents the aggregate mechanical characteristics of the cells and matrix that form a glomerulus. The deformation takes place over seconds to minutes and consequently may allow for active remodeling of the cytoskeleton and or membrane. Nevertheless, capillary micromechanics determines the full elastic behavior of the glomeruli by measuring both their compressive and their shear moduli and provides a quantitative measure of an aggregate tissue property, which represents the mechanical environment for the cells of the glomerulus.
The capillary micromechanics assay, in particular the measurment of the compressive modulus, volume change, could appear to resemble that described by the Savin and Harper groups in which the ultrafiltration coefficient of isolated glomeruli is measured as a change in glomerular volume in response to a sudden change in external oncotic force (36, 37). However, the oncotic assay involves responses over a subsecond time scale, whereas the capillary micromechanics assay assess changes in volume over seconds to minutes and so is unlikely to depend on the filtration characteristics of the glomerular filtration barrier. Parenthetically, the studies of Qiu et al. (35) on the effects of VEGF165b on glomerular permeability can also be interpreted as showing that VEGF165b reduces the deformability of glomerular capillaries and glomeruli. The structural alterations observed in glomerular endothelial cells are compatible with either altered permeability or altered mechancial characteristics.
A number of recent studies show that changes in cell or matrix elastic moduli have direct effects on cell structure and function. When brain cortical cells are plated on soft substrates (150–300 Pa), neurons grow selectively, but when plated on rigid substrates (2,000 Pa), glia grow selectively (14, 15, 28). Mesenchymal stem cells differentiate along three lineages depending on the rigidity of their substrate (measurement of differentiation markers by Western blot, gene array, and morphology). Substrates in the range of 0.1–1 kPa lead to neuronal cells, substrates in the range of 8–17 kPa lead to myocyte-like cells, and substrates in the range of 25–40 kPa result in osteoblast-like cells (11). Skeletal muscle stem cells require a mechanical environment similar to that of muscle, ∼12 kPa, to maintain their capacity for renewal and differentiation (16). The cell cycle is also affected by substrate stiffnesses (23). Mesenchymal stem cells remain quiescent for prolonged times when cultured on substrates too soft to support their differentiation into tissue-specific cell types (43). These and other results demonstrate that cellular responses to the mechanical properties of their environment are sufficient to specify cell lineage or a disease phenotype. Mechanical force and environment, then, are important factors in the differentiation and maintenance of cell phenotype in addition to better-known soluble and cell-attached factors.
Factors that control organ and tissue stiffness through effects on the cytoskeleton and extracellular matrix may be essential components of injury or disease processes (11, 13, 15, 20, 33). Several Mendelian forms of glomerular disease are caused by mutations in cytoskeletal or matrix proteins including α-actinin-4, inverted formin 2 (INF-2), nonmuscle myosin IIa (MYH9), laminin-2, and type IV collagen (Alport syndrome) (2, 4, 17, 22, 34, 39). The effects of agents that disrupt the cytoskeleton (cytochalasin D and latrunculin B) and that inhibit the activity of nonmuscle myosins may approximate the effects of loss-of-funtion mutations in cytoskeletal proteins. Podocytes from Tg26HIV/nl mice have disordered cytoskeletons with reduced levels of filamin and are markedly more deformable than controls (27, 41). The causative role of alterations in structural proteins in glomerular disease supports the possibility that alterations in cell or matrix mechanics may be important in the pathogenesis of glomerular disease.
We expected glomeruli at early stages of disease to be normal mechanically or to show slightly increased stiffness with the initiation of fibrosis as is seen in the CCl4 model of hepatic fibrosis in the rat (13). However, in two mechanistically distinct models of glomerular disease (Col4α3−/− and Tg26HIV/nl mice) the Young's modulus of the external glomerular capillaries is significantly reduced by ∼30% when compared with controls. We also found a similar reduction in stiffness in glomeruli of ColXVIII−/− mice, also at an early stage of renal disease (Kinnunen et al., unpublished observations). As described above, reduced stiffness or increased compliance could result in greater distension of glomerular capillaries by pulsatile blood flow and could in part be responsible for capillary dilation seen in disease models (9, 18, 31, 32). Distension could result in altered filtration barrier characteristics due to either physical disruption of slit diaprhagm geometry or stretching of the GBM leading to reduce permselectivity. Presumably, greater degrees of distension would lead directly to injury or increased release or activation of cytokines such as TGF-β (42). These processes could result in subsequent stiffening of the glomerulus through increased production of matrix and fibroblasts, in effect acting as compensation for the reduced initial stiffness of the glomerulus.
Reduced glomerular stiffness could alter cell behavior. Cells grown on soft matrix have smaller focal adhesions, adhere less well, and spread to a lesser degree than those on a stiff matrix (7, 20). Differences in matrix stiffness of a few hundred Pa can result in selection for growth of either neuronal or glial cells in brain cortical homogenates and affect the cytologic appearance and gene and protein expression of breast cells (15, 33). Changes in stiffness of this order of magnitude are found between normal and diseased glomeruli and could affect the adhesion of podocytes to each other or the GBM, possibly explaining podocyte loss in glomerular disease or apparent differentiation. Reduced stiffness of podocytes could also relate to the increased migratory phenotype of diseased podocytes described by others (3, 12). These possibilities remain to be tested experimentally.
Reduced stiffness of the Col4a3−/− glomeruli can be rationalized by the finding that the composition of their GBMs is different from normal glomeruli with absence of Col4α3, α4, and α5, increased levels of Col4α1 and α2, and altered laminin expression (30). The GBM of the Col4a3−/− glomeruli could be less stiff than normal because Col4α3 α4,and α5 are cross-linked to a higher degree than Col4α1 and α2. The cytoskeletal structure of Tg26HIV/nl podocytes is grossly abnormal with smaller, less spread cells, reduced stress fibers, reduced levels of filamin, reduced Rho activity, and less stiff cells as measured by AFM and microaspiration (27, 41). Such abnormal structure of the podocytes could explain reduced stiffness of whole glomeruli.
Our results demonstrate that glomeruli from rats and mice have a consistent Young's modulus of ∼2,500 Pa. Although it cannot be proven at this time, it is likely that the principal determinant of the capillary wall mechanical properties is the podocytes and that the principal determinant of overall glomerular mechanical properties is the capillary walls. In glomeruli from three disease models, Col4a3−/− and Tg26HIV/nl mice described in this paper, and ColXVIII−/− mice described elsewhere, glomeruli are significantly softer than normal controls at “early clinical” stages of disease. The exact meaning of this finding is not clear at this time, but the fact that significant biomechanical abnormalities occur early in disease and are associated with distinct effects on cell behavior suggests that they could represent an important disease mechanism.
GRANTS
This work was supported by a Veterans Affairs Mertit Review award to R. T. Miller, the Leonard Rosenberg Research and Education Foundation, the Rammelkamp Center for Research and Education, and awards from the NIH (RO1DK-083592 to L. A. Bruggeman, J. M. Henderson, P. A. Janmey, R. T. Miller, M. R. Pollak, and D. A. Weitz; DK-59588 to M. R. Pollak, K08-DK-073091 to J. M. Henderson, and R01DK-078314 to J. H. Miner), the NSF to D. A. Weitz (DMR-1006546) and the Harvard MRSEC to D. A. Weitz (DMR-0820484).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank John Sedor for his indulgence and sense of humor.
Current address for H. Wyss: Eindhoven University of Technology, Eindhoven, The Netherlands; current address for T. Franke: University of Augsburg, Augsburg, Germany; current address for E. Mele: Universitá del Salento, Centre for Biomolecular Nanotechnologies, CBN-II T, Italy.
REFERENCES
- 1. Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol 160: 721–730, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arrondel C, Vodovar N, Knebelmann B, Grunfeld JP, Gubler MC, Antignac C, Heidet L. Expression of nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol 13: 65–74, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signaling. Nature Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nature Genetics 42: 72–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 100: 84–92, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruggeman LA, Ross MD, Tanji N, Cara A, D'Agati VD, Burns GC, Dikman S, Gordon RE, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site for HIV-1 infection. J Am Soc Nephrol 11: 2079–2087, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Byfield FJ, Wen Q, Levental I, Nordstrom K, Durian DJ, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to substrate stiffness on gels coated with collagen but not fibronectin. Biophys J 96: 5095–5102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniels BS, Hauser EB, Deen WM, Hostetter TH. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol Renal Fluid Electrolyte Physiol 262: F919–F926, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Dworkin LD, Hostetter TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest 73: 1448–1461, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on matrix with heart-like elasticitry: scar-like rigidity inhibits beating. J Cell Sci 121: 3794–3802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nature Med 14: 931–938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver preceds matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol 98: 1547–1553, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Georges PC, Miller WJ, Meaney DF, Sawyer E, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 105: 3012–3018, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Trun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gubler MC. Inherited diseases of the glomerular basement membrane. Nat Clin Prat Nephrol 4: 24–37, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Fluid Electrolyte Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Milestones in nephrology: hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol 12: 1315–1325, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9: 1–34, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Janmey PA, Schliwa M. Quick guide-rheology. Current Biol 18: R1–R2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguiz-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nature Genetics 24: 251–256, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian R. Cell cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current Biol 19: 1511–1518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein ID, Predescu DN, Sharma T, Knezevic I, Malik AB, Predescu S. Intersectin-2L regulates caveola endocytosis secondary to Cdc42-mediated actin polymerization. J Biol Chem 284: 25953–25961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulsclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA 89: 1577–1581, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levental I, Levental KR, Klein EA, Assoian R, Miller RT, Wells RG, Janmey PA. A simple indentation device for measuring micrometer-scale tissue stiffness. J Phys Condens Matter 22: 194120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE. HIV-1 Nef disrupts the podocyte cytoskeleton by interacting with diaphenous interacting protein. J Biol Chem 283: 8173–8182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolberg H, Gluck J, Janmey P, Wei EQ, Kas J, Reichenbach A. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA 103: 17759–17754, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinka S, Bruggeman LA. Persistent NF-kB activation in renal epithelial cells in transgenic mouse model of HIV-associated nephropathy. Am J Physiol Renal Physiol 290: F657–F665, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miner JH, Sanes JR. Molecular and functional defects in kidneys from mice lacking collagen α3(IV): implications for Alport syndrome. J Cell Biol 135: 1403–1413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagata M, Kriz W. Glomerular damage after uninephrectomy in young rats. II Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 42: 148–160, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Nagata M, Scharer K, Kriz W. Glomerular damage after uninephrectomy in young rats. I Hypertrophy and distortion of capillary architecture. Kidney Int 42: 136–147, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Pecci A, Panza E, Pujol-Moix N, Klersy C, Di Bari F, Bozzi V, Gresele P, Lethagen S, Fabris F, Dufour C, Granata A, Doubek M, Pecoraro C, Koivisto PA, Heller PG, Iolascon A, Alvisi P, Schwabe D, De Candia E, Rocca B, Russo U, Ramenghi U, Norris P, Seri M, Balduini CL, Savoia A. Position of nonmuscle myosin heavy chain IIa (NMMHC-IIa) mutation predicts the natural history of MYH9-related disease. Hum Mutat 29: 409–417, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Qiu Y, Ferguson J, Oltean S, Neal CR, Kaura A, Bevan H, Wood E, Sage LM, Lanati S, Nowak DG, Salmon AHJ, Bates D, Harper SJ. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol 21: 1498–1509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salmon AHJ, Neal CR, Bates DO, Harper SJ. Vascular endothelial growth factor increases the ultrafiltration coefficient in isolated intact Wistar rat glomeruli. J Physiol 570: 141–156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Schlondorff D. Preparation and study of isolated glomeruli. Methods Enzymol 191: 130–140, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Seri M, Savino M, Bordo D, Cusano R, Rocca B, Meloni I, Di Bari F, Koivisto PA, Bolognesi M, Ghiggeri GM, Landolfi R, Balduini CL, Zelante L, Ravazzolo L, Renieri A, Savoia A. Epstein syndrome: another renal disorder with mutations in the nonmuscle myosin heavy chain 9 gene. Hum Genet 110: 182–186, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Takemoto M, Asker N, Gerhart G, Lundkvist A, Johansson BR, Saito Y, Betsholz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tandon R, Levental I, Huang C, Byfield FJ, Ziembicki JA, Schelling JR, Bruggeman LA, Sedor JR, Janmey PA, Miller RT. Altered golmerular podocyte cytoskeletal composition results in distinct cellular mechanical properties. Am J Physiol Renal Physiol 292: F701–F710, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-β the insoluble and soluble meet. Sci Signal 1: pe13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemcial and mechanical stimuli. Tissue Eng part A 15: 147–154, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Wyss HM, Franke T, Mele E, Weitz DA. Capillary micromechanics: measuring the elasticity of mesoscopic soft objects. Soft Matter 6: 4550–4555, 2010 [Google Scholar]