Abstract
Na+-K+-ATPase activity in renal proximal tubule is regulated by several hormones including parathyroid hormone (PTH) and dopamine. The current experiments explore the role of Na+/H+ exchanger regulatory factor 1 (NHERF-1) in dopamine-mediated regulation of Na+-K+-ATPase. We measured dopamine regulation of ouabain-sensitive 86Rb uptake and Na+-K+-ATPase α1 subunit phosphorylation in wild-type opossum kidney (OK) (OK-WT) cells, OKH cells (NHERF-1-deficient), and OKH cells stably transfected with full-length human NHERF-1 (NF) or NHERF-1 constructs with mutated PDZ-1 (Z1) or PDZ-2 (Z2) domains. Treatment with 1 μM dopamine decreased ouabain-sensitive 86Rb uptake, increased phosphorylation of Na+-K+-ATPase α1-subunit, and enhanced association of NHERF-1 with D1 receptor in OK-WT cells but not in OKH cells. Transfection with wild-type, full-length, or PDZ-1 domain-mutated NHERF-1 into OKH cells restored dopamine-mediated regulation of Na+-K+-ATPase and D1-like receptor association with NHERF-1. Dopamine did not regulate Na+-K+-ATPase or increase D1-like receptor association with NHERF-1 in OKH cells transfected with mutated PDZ-2 domain. Dopamine stimulated association of PKC-ζ with NHERF-1 in OK-WT and OKH cells transfected with full-length or PDZ-1 domain-mutated NHERF-1 but not in PDZ-2 domain-mutated NHERF-1-transfected OKH cells. These results suggest that NHERF-1 mediates Na+-K+-ATPase regulation by dopamine through its PDZ-2 domain.
Keywords: D1 receptor, PKC-ζ, oximal tubule, sodium homeostasis
dopamine regulates sodium homeostasis through inhibition of Na+-K+-ATPase and sodium hydrogen exchanger-3 (NHE-3) in renal proximal tubules thereby reducing transepithelial sodium reabsorption (1, 11). Dopamine promotes internalization of Na+-K+-ATPase through clathrin-mediated endocytosis triggered by PKC-ζ-mediated phosphorylation of Na+-K+-ATPase α-subunit (4, 5, 10, 25). Similarly, we have demonstrated that PTH inhibits the activity of Na+-K+-ATPase by clathrin-coated endocytosis triggered by PKCα-mediated phosphorylation of the Ser-11 on the Na+-K+-ATPase α1-subunit (16). We have also demonstrated that PTH-mediated regulation of Na+-K+-ATPase requires expression of an intact PDZ (postsynaptic protein PSD-95/SAP90, Drosophila septate junction protein Discs-large, tight junction protein ZO-1) protein NHERF-1 (18). NHERF-1 contains two (PDZ-1 and PDZ-2) domains and a COOH-terminal ezrin-binding domain (EBD). Mutations in the PDZ-1 domain of NHERF-1 or deletion of EBD result in loss of PTH-mediated regulation of Na+-K+-ATPase (18). Recently, Chen et al. (3) demonstrated that Pals-associated tight junction protein (PATJ) functionally interacts with dopamine, angiotensin II, and insulin receptors in opossum kidney (OK) cells to regulate Na+-K+-ATPase activity. A recent study by Weinman et al. (28, 33) demonstrated that dopamine 1 receptor (D1-like receptor) binds to NHERF-1 to decrease sodium phosphate cotransporter in mice. These studies highlight the importance of protein-protein interactions that can organize large signaling complexes required for the regulation of sodium transport in the proximal tubules. PDZ containing proteins can provide the platform for formation of such large signaling complexes.
In the present study we focused on NHERF-1 as a potential scaffold for the dopamine-stimulated responses to regulate Na+-K+-ATPase in a proximal tubule cell culture model, the OK cells. NHERF-1 is highly expressed in renal proximal tubules and has been demonstrated to be important for expression and regulation of several renal proximal tubular ion transporters such as the sodium-phosphate cotransporter (NpT2a, 8, 15, 20, 29), NHE-3 (9, 27, 30, 35), the bicarbonate exchanger (NBCe-1, 2), and Na+-K+-ATPase (18) and several G protein-coupled receptors (21, 22, 31). Our data demonstrate that NHERF-1 association with the D1 receptor increases while association between NHERF-1 and Na+-K+-ATPase decreases with dopamine treatment. Additionally, we found that expression of an intact PDZ-2 domain is important for the association between NHERF-1 and D1 receptor and the regulation of Na+-K+-ATPase by dopamine.
EXPERIMENTAL METHODS
Materials.
Dopamine (3-hydroxytyramine-HCl, DA) was purchased from Calbiochem, La Jolla, CA. Polyclonal antibodies against Na+-K+-ATPase α1-subunit (RT-NASE, for immunoprecipitation) were kindly provided by Dr. Thomas Pressley (Texas Tech University, Lubbock, TX). Monoclonal D1 receptor antibodies (DRD1A, cat. no. MA1-46024) were purchased from Thermo Fisher (Pittsburgh, PA). PKC activity kit was purchased from Millipore (Waltham, MA). Monoclonal antibodies against Na+-K+-ATPase α1-subunit (α6F, for Western blot analyses) developed by Dr. D. M. Fambrough were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NIHCD and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). Phosphoserine antibodies were purchased from Zymed (San Francisco, CA). Anti-NHERF-1 antibodies were previously characterized by Weinman and his colleagues (32).
NHERF-1 mutations.
NHERF-1 mutations were previously described by Dr. Weinman and his colleagues (34). Briefly, the human NHERF-1 cDNA was inserted into pET-30-(a)+ (Novagen) to generate a hexahistidine-fused NHERF-1 (His-NHERF-1), which was then transferred to pcDNA3.1/Hygro+ for expression in mammalian cells. Similarly, cDNAs encoding NHERF-1 with alanine substitutions (GAGA) in the core peptide-binding sequence GYGF (AA 77–82 PDZ-1, and 217–222 PDZ-2), which inactivates the individual PDZ domains, were inserted into pcDNA3.1/Hygro+. All cDNAs were confirmed by double-stranded DNA sequencing.
Cell culture.
The OK cells are a continuous cell line derived from Virginia opossum and a widely used model for mammalian renal proximal tubule (34). OKH cells are a clonal subline of the parental OK cell line that lacks the expression of NHERF-1 (7, 20, 23). Vector (pcDNA 3.1Hygro+)-transfected, OK-WT and OKH cells, and OKH cells stably transfected with human full-length, or PDZ domains mutated NHERF-1 [hNHERF-1 (1–355)], were maintained at 37°C in a humidified atmosphere with 5% CO2 in DMEM/F12 medium (1:1) supplemented with 10% vol/vol FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), in the presence of 600 U/ml hygromycin. The cells were fed twice a week and split once a week at 1:4 ratio. All experiments were carried out using cells at 90–95% confluence. Cells grown on six-well culture plates were washed with serum-free medium 24 h before use.
NHERF-1 cDNA transfection.
Vector (pcDNA 3.1Hygro+) or human NHERF-1 and PDZ-mutated human NHERF-1 cDNA constructs in pcDNA 3.1Hygro+ was transfected into OKH cells using Geneporter transfection reagent according to the manufacturer's protocol. Briefly, the cDNA and the Geneporter reagent were diluted separately in serum-free medium. The diluted cDNA was mixed with diluted Geneporter reagent and incubated at room temperature for 30 min. The culture medium from the cells was replaced with the mixture containing Geneporter and NHERF-1 cDNA and incubated for 24 h at 37°C in 95% air-5% CO2. The transfected cells were selected by growing them in 1,000 U/ml hygromycin in a 96-well cell culture plate for 2 wk. Cells from the hygromycin-resistant wells were grown in DMEM F-12 containing 600 U/ml hygromycin and 10% FBS. Expression of NHERF-1 was confirmed by Western blot analysis (18).
Treatment with dopamine.
Unless otherwise stated, cells were treated for 15 min with 1 μM 3-hydroxytyramine-HCl (dopamine) at 37°C in 95% air-5% CO2.
Membrane preparation.
The cells were treated with 1 μM dopamine or vehicle, washed twice with PBS, and homogenized in 50 mM mannitol-5 mM Tris, pH 7.4, and the crude membranes were prepared as described previously (19).
Immunoprecipitation.
The crude membranes were solubilized in immunoprecipitation buffer containing 20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 1 mM sodium pyrophosphate, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1% Triton X-100, 0.5% Nonidet 40, and 0.5% SDS, and centrifuged at 70,000 g for 1 h in Beckman ultracentrifuge. Na+-K+-ATPase α1-subunit was immunoprecipitated from 100-μg supernatant protein overnight at 4°C as described previously (17). Western blotting was performed as previously reported (19).
Western blot, Na+-K+-ATPase activity, and 86Rb uptake.
Western blot analysis for NHERF-1, Na+-K+-ATPase α1-subunit, and PKC-ζ was performed exactly as previously described (19). Na+-K+-ATPase activity as K+-dependent pNPPase activity was measured according to the method of Hird et al. (12) with slight modifications as described previously (19). Ouabain-sensitive 86Rb uptake was measured as described previously (17).
Confocal imaging.
Multichambered coverglass wells (Nunc) were seeded with OK cells. Cells were washed with serum-free medium 24 h and treated with 1 μM DA for 15 min before fixation. Cells were rinsed three times with PBS containing calcium and magnesium, incubated in 4% paraformaldehyde in PBS for 10 min, rinsed five times with PBS, solubilized with 0.025% saponin in PBS for 15 min, incubated with an appropriate dilution of primary antibody (1:250 in PBS-Saponin for both NHERF-1 and Na+-K+-ATPase α1 subunit antibodies) at 20°C, rinsed five times with PBS-saponin, and incubated with appropriate Alexafluor secondary antibody (1:1,000) conjugated to different fluorescent tags at 20°C. Anti-rabbit antibodies attached to Alexafluor 488 were used for identification of NHERF-1 and anti-mouse antibodies attached to Alexafluor 555 for identification of the α-subunit. The cells were rinsed five times with PBS-saponin, incubated with 300 nM DAPI for 5 min, rinsed three times with PBS, and mounted with 300 μl/well PBS. Images were acquired using a Zeiss confocal microscope and analyzed using LSM510 software. Z-scan analysis on single cells was performed by scanning at 1-μm intervals and three-dimensional reconstruction of the fluorescent images. The images for NHERF-1 and α-subunit were merged in a single image to compare the cellular distribution of the two proteins.
Determination of PKC and PKA activities.
Cells were treated with 1 μM DA for 15 min, washed two times with PBS, pH 7.4, and lysed in lysis buffer containing 20 mM Tris, pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EGTA, 1 mM EDTA, 10 μl/ml protease inhibitor cocktail 1, 10 μl/ml phosphatase inhibitor cocktail 1, 0.5% Nonidet-40, and 1% Triton X-100. The lysates were sonicated on ice for 15 s three times with an interval of 15 s in between each sonication pulse. PKC activity was determined according to the manufacturer's protocol (Upstate Biotechnology, Waltham, MA) and as described previously (17). Briefly, 10 μl each of assay dilution buffer (ADB; 20 mM MOPS, pH 7.2, 25 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, and 1 mM CaCl2), substrate cocktail (500 μM PKC substrate), PKA/CaMK inhibitor cocktail (2 μM protein kinase A inhibitor peptide PKI, and 20 μM R24571 in ADB), PKC lipid activator (0.5 mg/ml phosphatidyl serine and 0.05 mg/ml diacylglycerol in ADB), and magnesium/ATP cocktail (75 mM MgCl2, 500 μM ATP, and 100 μCi [γ-32P]ATP) were added to the 10-μl sample (25–30 μg protein) and incubated at 30°C for 10 min. PKA activity was determined as incorporation of radioactive phosphate to PKA substrate peptide kemptide in the presence or absence of 2 μM protein kinase A inhibitor peptide PKI. Briefly, to 30 μl of assay buffer (25 mM HEPES, pH 7.4, 20 mM MgCl2, 20 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 2 mM dithiothreitol, and 10 μM PKC inhibitory peptide, 20 μM CaMK inhibitor R24571, 500 μM ATP, and 100 μCi [γ-32P]ATP), 10 μl PKA substrate peptide (600 μM kemptide, final concentration), 2 μM PKI or vehicle, a 10 μl sample (25–30 μg protein) was added and incubated at 30°C for 10 min. After 10 min a 25-μl sample was slowly transferred to the center of a P81 phosphocellulose paper and incubated at room temperature for 30 min. The P81 papers were washed three times with 0.75% phosphoric acid and once with acetone, dried, and transferred to scintillation vials. Bound radioactivity was quantitated by the addition of 3 ml of scintillation fluid and reading in a scintillation counter (Pharmacia). A substrate control was measured to correct for nonspecific binding along with the samples. Control counts were subtracted from the sample counts to calculate the PKC activity according to the manufacturer's protocol. To calculate PKA activity, control counts were subtracted from the sample counts, and the difference between total and PKI inhibitable counts were considered as PKA activity. The activities are expressed as pmoles phosphate incorporated into the PKC/PKA substrate peptide per minute per milligram protein.
Protein concentration was measured by BCA method (Sigma) using BSA as standard.
Statistics.
Data are shown as means of three different experiments ± SE. P value was calculated using GraphPad software utilizing ANOVA followed by Bonferroni analysis. A P value <0.05 was a priori considered statistically significant.
RESULTS
Identification of proteins associated with Na+-K+-ATPase α1 subunit: effect of dopamine.
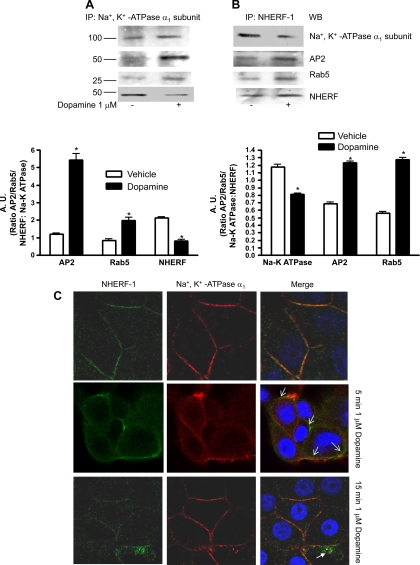
We previously demonstrated that Na+-K+-ATPase α1-subunit associates with NHERF-1 (18). Other investigators demonstrated that treatment with dopamine increases association of Na+-K+-ATPase α1-subunit with AP2 and Rab5 (4, 5, 10). To determine whether dopamine regulates Na+-K+-ATPase α1-subunit association with NHERF-1, OK-WT cells were treated for 15 min with 1 μM dopamine at 37°C, and Na+-K+-ATPase α1-subunit or NHERF-1 was immunoprecipitated using a polyclonal antibody against Na+-K+-ATPase α1-subunit (RT-NASE) or a polyclonal antibody against NHERF-1. The immunoprecipitated proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by Western blot analysis. As shown in Fig. 1A, treatment with dopamine increased association of Na+-K+-ATPase α-subunit with AP-2 and Rab5. Similarly, treatment with dopamine increased association of NHERF-1 with AP2 and Rab5 (Fig. 1B). However, treatment with dopamine decreased the association of Na+-K+-ATPase α1-subunit with NHERF-1 (Fig. 1A). Reciprocal immunoprecipitation with NHERF-1 confirmed decreased association of NHERF-1 with Na+-K+-ATPase α1-subunit (Fig. 1B). We confirmed the immunoprecipitation results by dual fluorescence confocal microscopy of cells treated for 5 and 15 min with 1 μM dopamine. As shown in Fig. 1C, treatment with vehicle showed association of Na+-K+-ATPase α1-subunit with NHERF. Treatment with dopamine for 5 or 15 min treatment resulted in dissociation of NHERF-1 from Na+-K+-ATPase α1-subunit.
Fig. 1.
Effect of dopamine on association of Na+-K+-ATPase α1-subunit with NHERF-1. A: wild-type (WT) opossum kidney (OK) cells were treated for 15 min with 1 μM dopamine at 37°C. Na+-K+-ATPase α1-subunit (A) or NHERF-1 (B) was immunoprecipitated from crude membranes and analyzed by Western blot analysis using antibodies against AP2, Rab5, NHERF-1, or Na+-K+-ATPase α1-subunit. Bar diagram shows cumulative data from three individual experiments as arbitrary units (A.U.) means ± SE ratio of protein to Na+-K+-ATPase α1-subunit band density. *P < 0.05 by ANOVA followed by Bonferroni's analysis from vehicle-treated cells. C: WT OK cells were treated for 5 or 15 min with 1 μM dopamine at 37°C. Cells were fixed and expression of NHERF-1 (green fluorescence) and Na+-K+-ATPase α1-subunit (red fluorescence) was determined by confocal microscopy. Representative images from three independent experiments are shown. Arrows indicate separation of NHERF-1 from Na+-K+-ATPase α1-subunit after dopamine treatment.
Effect of dopamine on Na+-K+-ATPase α1-subunit phosphorylation: role of PKC-ζ.
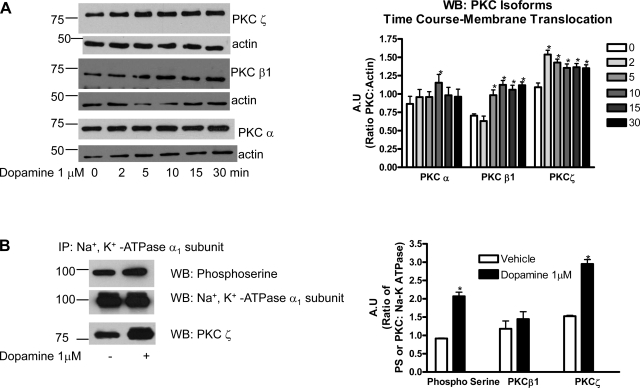
To determine the specific PKC isoform activated by dopamine in OK cells, time-dependent translocation of PKC-α, -β1, or -ζ from cytosol to membrane fraction was analyzed by Western blot analysis in dopamine-treated OK cells. As shown in Fig. 2A, treatment with dopamine increased membrane expression of PKC-ζ as early as 2 min after treatment, and the expression remained increased for 30 min after treatment. The membrane expression of PKC-β1 increased after 5 min treatment with dopamine, reached a maximum after 10 min of treatment, and remained increased for 30 min treatment. In contrast, dopamine increased membrane expression of PKCα only after 10 min treatment and returned to control levels after 15 and 30 min treatment. To correlate dopamine stimulated Na+-K+-ATPase α1-subunit phosphorylation with PKC-ζ translocation, Na+-K+-ATPase α1-subunit was immunoprecipitated after 15 min treatment with dopamine and analyzed by Western blot analysis using phospho-serine antibody. As shown in Fig. 2B, treatment with dopamine increased Na+-K+-ATPase α1-subunit phosphorylation. Dopamine treatment resulted in increased association of PKC-ζ, but not PKC-α or -β1, with Na+-K+-ATPase α1-subunit (Fig. 2B).
Fig. 2.
Effect of dopamine on PKC translocation and association with Na+-K+-ATPase α1-subunit. A: WT OK cells were treated for the indicated time with 1 μM dopamine at 37°C. Membranes were separated from the cytosol as described previously (17) and translocation of PKC-α, -β, and -ζ were analyzed by Western blot analysis. Representative Western blots of membrane fractions from three independent experiments are shown. Bar diagram represent data from three independent experiments as A.U. means ± SE ratio of PKC band density to actin band density. B: Na+-K+-ATPase α1-subunit was immunoprecipitated and analyzed by Western blot analysis using antibodies against phosphoserine, PKC-β1, or PKC-ζ. Representative Western blots from three independent experiments are shown. Bar diagram represent data from three independent experiments as A.U. means ± SE ratio of phosphoserine or PKC band density to Na+-K+-ATPase α1-subunit band density. *P < 0.05 by ANOVA followed by Bonferroni's analysis from vehicle-treated cells.
NHERF-1 association with Na+-K+-ATPase α1-subunit and dopamine 1 receptor (D1 receptor).
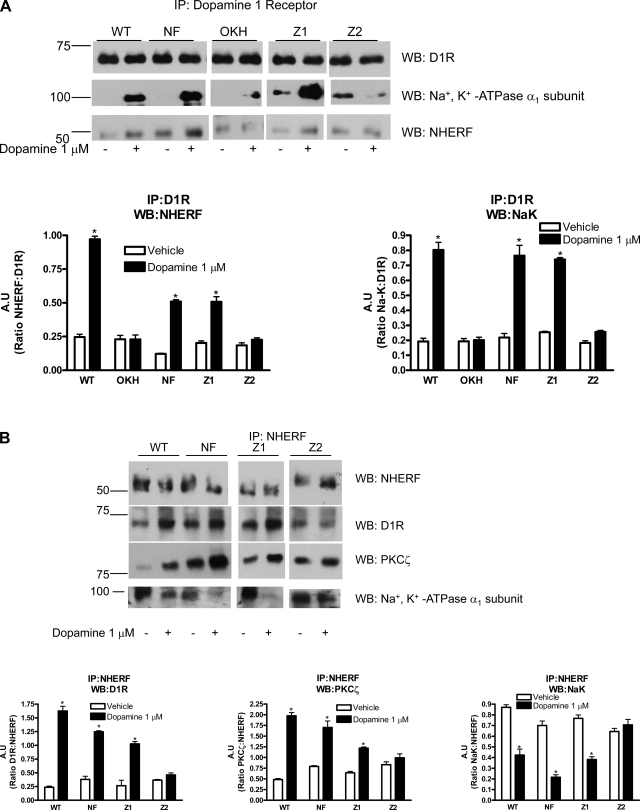
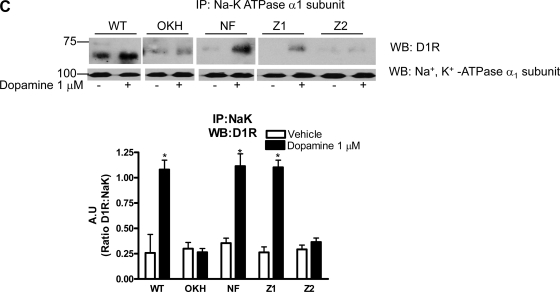
A recent report by Weinman et al. (28, 33) demonstrated that NHERF-1 co-immunoprecipitates with D1 receptor in mouse kidney proximal tubules. To determine the site on NHERF-1 responsible for interaction with D1 receptor, we used previously described OKH cells that express very low level of NHERF-1 and OKH cells stably transfected with full-length or PDZ domains-mutated human NHERF-1 (18). Cells were treated with vehicle or 1 μM dopamine, and D1 receptor was immunoprecipitated from crude membrane preparations and analyzed by Western blot anaylsis using NHERF-1 or Na+-K+-ATPase α1-subunit antibodies. As shown in Fig. 3A, 15 min treatment with 1 μM dopamine increased association of D1 receptor with NHERF-1 and Na+-K+-ATPase α1-subunit in OK-WT cells. In OKH cells that lack NHERF-1, there was no association between D1 receptor and Na+-K+-ATPase α1-subunit. The association was restored in OKH cells transfected with full-length (NF) or PDZ domain 1-mutated NHERF-1 (Z1) but not in OKH cells transfected with PDZ domain 2-mutated (Z2) NHERF-1. To confirm association of D1 receptor with NHERF-1, reciprocal immunoprecipitation with NHERF-1 was performed. As shown in Fig. 3B, treatment with dopamine increased association between D1 receptor and NHERF-1 in OK-WT cells but not in OKH cells. The association between D1 receptor and NHERF-1 was restored in OKH cells transfected with full-length or PDZ domain 1-mutated NHERF-1 but not in OKH cells transfected with mutated PDZ domain 2 (Fig. 3B). To determine whether NHERF-1 binds to PKC-ζ and Na+-K+-ATPase α1-subunit, NHERF-1 was immunoprecipitated from vehicle or dopamine-treated cells and analyzed by Western blot analysis. As shown in Fig. 3B, treatment with dopamine increased NHERF-1 association with PKC-ζ and D1 receptor in OK-WT cells but not in OKH cells. In contrast the NHERF-1-Na+-K+-ATPase α1-subunit association decreased, consistent with the data in Fig. 1. The same pattern of association was observed in OKH cells transfected with full-length or PDZ domain 1-mutated NHERF-1 but not in OKH cells transfected with mutated PDZ domain 2 (Fig. 3B). Immunoprecipitation with antibodies against Na+-K+-ATPase α1-subunit confirmed increased association of Na+-K+-ATPase α1-subunit and D1 receptor with dopamine treatment (Fig. 3C).
Fig. 3.
Effect of dopamine on Na+-K+-ATPase α1-subunit association with dopamine 1 receptor, NHERF-1, and PKC-ζ. Vector transfected WT and NHERF-1-deficient OK (OKH) cells, or OKH cells transfected with full-length NHERF-1 (NF), PDZ-1 (Z1)-mutated, or PDZ-2 (Z2)-mutated NHERF-1 were treated for 15 min with 1 μM dopamine at 37°C. Cells were lysed and dopamine 1 receptor (D1 receptor, A), NHERF-1 (B), or Na+-K+-ATPase α1-subunit (C) was immunoprecipitated and analyzed by Western blot analysis using antibodies against D1 receptor, NHERF-1, Na+-K+-ATPase α1-subunit, or PKC-ζ. A representative blot from three independent experiments is shown. Bar diagram represent data as A.U. means ± SE from three independent experiments. *P < 0.05 by ANOVA followed by Bonferroni's analysis from the respective vehicle-treated cells.
Role of NHERF-1 in dopamine-mediated regulation of Na+-K+-ATPase.
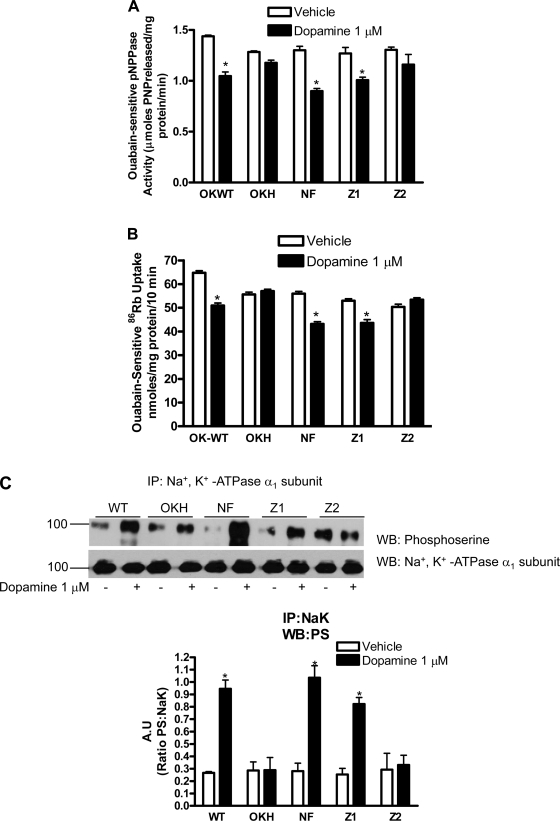
The above data suggest that both Na+-K+-ATPase α1-subunit and NHERF-1 associate with D1 receptor in OK cells after dopamine treatment. However, the NHERF-1-Na+-K+-ATPase α1-subunit association is decreased under the same conditions. To determine whether the changes in associations between NHERF-1-Na+-K+-ATPase α1-subunit and NHERF-1 and D1 receptor are important for the dopamine-mediated inhibition of Na+-K+-ATPase activity, Na+-K+-ATPase activity was measured in crude membrane preparations isolated from OK-WT and OKH cells expressing NHERF-1. The ouabain-sensitive, K+-dependent pNPPase activity (Fig. 4A) and ouabain-sensitive 86Rb uptake (Fig. 4B) was decreased in dopamine-treated OK-WT cells but not in NHERF-1-deficient OKH cells, indicating the requirement for NHERF-1 in dopamine-mediated inhibition of Na+-K+-ATPase activity. OKH cells transfected with full-length or PDZ-1 mutated NHERF-1 regained the dopamine inhibition of Na+-K+-ATPase activity. In contrast, dopamine had no effect on Na+-K+-ATPase-mediated pNPPase activity and 86Rb uptake in OKH cells tranfected with mutated PDZ-2.
Fig. 4.
Effect of NHERF-1 expression on dopamine-mediated Na+-K+-ATPase α1-subunit regulation. Vector-transfected WT and NHERF-1-deficient OK (OKH) cells, or OKH cells transfected with full-length NHERF-1 (NF), PDZ-1 (Z1)-mutated, or PDZ-2 (Z2)-mutated NHERF-1 were treated for 15 min with 1 μM dopamine at 37°C. A: ouabain-sensitive K+-pNPPase activity was measured as described previously (18). Each bar represents activity as mean ± SE (μmole pNPP released·mg protein−1·min−1) from four independent experiments. *P < 0.05 by ANOVA followed by Bonferroni's analysis from the respective vehicle-treated cells. B: ouabain-sensitive 86Rb uptake was measured as described previously (18). Each bar represents 86Rb uptake as means ± SE (nmole 86Rb uptake·mg protein−1·10 min−1). *P < 0.05 by ANOVA followed by Bonferroni's analysis from the respective vehicle-treated cells. C: cells were lysed after treatment with dopamine as above. Na+-K+-ATPase α1-subunit was immunoprecipitated from crude membranes and analyzed by Western blot analysis using phosphoserine antibodies. Bar diagram represents data as A.U. (ratio of phosphoserine band density to Na+-K+-ATPase α1-subunit) means ± SE from four independent experiments. *P < 0.05 by ANOVA followed by Bonferroni's analysis from the respective vehicle-treated cells.
To determine whether NHERF-1 regulates dopamine-mediated phosphorylation of Na+-K+-ATPase α1-subunit, the above cells were treated with 1 μM dopamine, and Na+-K+-ATPase α1-subunit was immunoprecipitated from crude membrane preparations and analyzed by Western blot using phospho-serine antibodies. As shown in Fig. 4C, treatment with dopamine increased phosphorylation of Na+-K+-ATPase α1-subunit in vector-transfected OK-WT cells but not in vector-transfected OKH cells. Transfection with full-length or PDZ-1-mutated NHERF-1 in OKH cells restored dopamine-mediated phosphorylation of Na+-K+-ATPase α1-subunit. In contrast, dopamine had no effect on Na+-K+-ATPase α1-subunit phosphorylation in cells transfected with PDZ-2.
Role of NHERF-1 in dopamine-stimulated PKA and PKC activity.
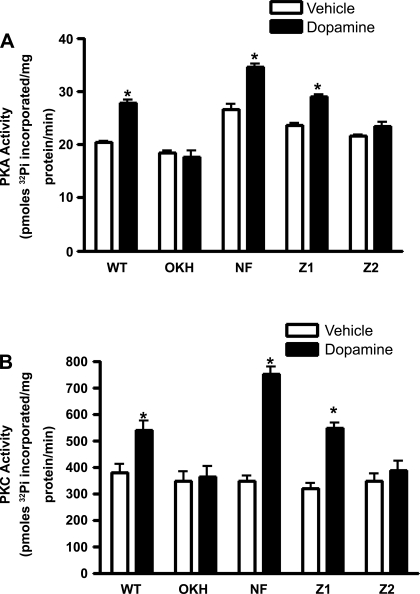
We have previously demonstrated that NHERF-1 is required for PTH-mediated stimulation of PKC activity in OK cells (18). Recently, Weinman and colleagues (28) demonstrated that dopamine failed to activate PKC or PKA in kidney slices and proximal tubule cells from NHERF-1 knock-out mice. Therefore, we determined the specific PDZ domains of NHERF-1 critical for dopamine-mediated PKC and PKA activation. As shown in Fig. 5, treatment of vector-transfected OK-WT cells with 1 μM dopamine increased PKA (Fig. 5A) and PKC (Fig. 5B) activities. In contrast, dopamine had no effect on PKA and PKC activities in vector-transfected OKH cells. Transfection with NHERF-1 or NHERF-1 with a mutation in PDZ-1 domain in OKH cells restored dopamine-stimulated PKA and PKC activities but not in OKH cells transfected with NHERF-1 with mutations in PDZ-2.
Fig. 5.
Effect of NHERF-1 expression on dopamine-mediated PKA and PKC activities. Vector-transfected WT and NHERF-1-deficient OK (OKH) cells, or OKH cells transfected with full-length NHERF-1 (NF), PDZ-1 (Z1)-mutated, or PDZ-2 (Z2)-mutated NHERF-1 were treated for 15 min with 1 μM dopamine at 37°C. PKA (A) and PKC (B) activities were measured in whole cell lysates as described in experimental methods. Each bar represents data as means ± SE [pmoles of 32Pi inserted in PKA (A) or PKC (B) specific peptides] from three independent experiments. *P < 0.05 by ANOVA followed by Bonferroni's analysis from the respective vehicle-treated cells.
DISCUSSION
Studies from several laboratories have established that dopamine regulates sodium reabsorption in the kidney proximal tubules primarily through activation of D1-like receptors (1). We and others demonstrated that dopamine decreases Na+-K+-ATPase activity in OK cells through activation of PKA and PKC (10, 18). The laboratories of Bertorello and Pedemonte demonstrated that dopamine through activation of PKC-ζ phosphorylates Na+-K+-ATPase at serine-11 and -18 resulting in association with AP2, Rab5, and 14-3-3-ζ (4, 5, 10, 26). A recent report from Dr. Bertorello's laboratory (3) suggests that dopamine-mediated regulation of Na+-K+-ATPase α1-subunit requires association with a six PDZ domain containing protein Pals with D1 receptors. Whereas the study suggested an important role for PDZ protein-mediated interactions in dopamine regulation of the sodium pump, the study was based on overexpression of a protein not normally expressed in OK cells. In contrast our study demonstrates the requirement for NHERF-1, an endogenously expressed PDZ domain containing protein in dopamine-mediated regulation of Na+-K+-ATPase. NHERF-1 is expressed in proximal tubules from human, mouse, rat, and opossum kidney and has been demonstrated to regulate the expression and trafficking of NpT2a (15, 28, 33) and regulation of NHE3 (30, 32, 34, 35), NBCe1 (2), and Na+-K+-ATPase (18). Prior studies (21, 31) demonstrated that NHERF-1 can associate with several G protein-coupled receptors including PTH receptor, β2-adrenergic receptor, and the κ-opioid receptor. A recent study by Weinman et al. (28) demonstrated that NHERF-1 associates with D1 receptor and that this association is critical for regulation of type IIa sodium-phosphate cotransporter in mouse proximal tubules. However, the role of NHERF-1 in dopamine-mediated regulation of Na+-K+-ATPase α1-subunit has not been explored. The present studies were conducted to identify the PDZ domain on NHERF-1 responsible for the association with D1 receptor and to determine whether the association between NHERF-1 and D1 receptor is required for dopamine-mediated regulation of Na+-K+-ATPase α1-subunit in OK cells. Our results show that treatment with dopamine increased association between NHERF-1 and D1 receptors in OK-WT cells but not in NHERF-deficient OKH cells. Mutations in the PDZ-2 domain (between amino acids 157–237) prevented dopamine-mediated association between NHERF-1 and D1 receptor. Chen et al. (3) speculated that D1 receptors express a type III PDZ binding site (E433, K434, and I435), whereas Na+-K+-ATPase α1-subunit expresses a potential type IV PDZ binding site (A498, S499, and E500). Thus D1 receptor and Na+-K+-ATPase α1-subunit can bind canonical PDZ containing proteins including NHERF-1 and PATJ. Our data also show that the NHERF-1 and D1 receptor association is important for the regulation of Na+-K+-ATPase by dopamine. Treatment with dopamine increased Na+-K+-ATPase α1-subunit phosphorylation and decreased Na+-K+-ATPase-mediated 86Rb uptake in OK-WT cells and in OKH cells transfected with full-length or PDZ-1-mutated NHERF-1 but not in OKH cells or OKH cells transfected with PDZ-2-mutated NHERF-1. We and others (18, 28) have previously demonstrated that NHERF-1 is required for receptor-mediated activation of downstream signaling cascades including PKA and PKC. The present study confirms that NHERF-1 is required for dopamine-mediated regulation of Na+-K+-ATPase through activation of PKA and PKC, also through the PDZ-2 domain.
Unlike D1 receptor association with NHERF-1 and Na+-K+-ATPase α1-subunit, association between Na+-K+-ATPase α1-subunit and NHERF-1 decreased in response to dopamine stimulation of OK cells. Studies from the laboratory of Dr. Weinman demonstrated that dopamine-induced PKC activation leads to phosphorylation of NHERF-1 at Ser77 (28, 33). Whether NHERF-1 phosphorylation is responsible for decreased association between Na+-K+-ATPase α1-subunit and NHERF-1 cannot be determined from our data. However, our data show an increase in association between PKC-ζ and NHERF-1 suggesting that PKC-ζ may phosphorylate NHERF-1. Further studies are required to confirm this observation.
Our studies further demonstrated that treatment with dopamine increased Na+-K+-ATPase α1-subunit association with AP2 and Rab5. Using a PKC-ζ-specific peptide, Effendiev et al. (10) demonstrated that dopamine increases phosphorylation of Ser11 and Ser18 on Na+-K+-ATPase α1-subunit through a PKC-ζ-mediated mechanism (4). This phosphorylation has been demonstrated to increase association of Na+-K+-ATPase α1-subunit with 14-3-3-ζ, AP2, and Rab5 leading to endocytosis (reviewed in Ref. 26). In contrast, Nowicki et al. (24) demonstrated translocation of PKC-α and -ε to the plasma membrane in LLC-PK1 cells. The data presented here demonstrate that treatment of OK cells with dopamine increases PKC-ζ and PKC-β1 translocation to the membrane in a time-dependent manner and increased association with Na+-K+-ATPase α1-subunit, consistent with the model proposed by Efendiev et al. (10). However, we cannot exclude the possibility that other PKC isoforms may play a role in regulation of Na+-K+-ATPase α1-subunit by dopamine. Further studies directed specifically to answer this question are required.
Based on our data we speculate that NHERF-1 may stabilize Na+-K+-ATPase α1-subunit in the membrane. Upon stimulation with dopamine, NHERF-1 may undergo phosphorylation resulting in its dissociation with Na+-K+-ATPase α1-subunit thereby allowing phosphorylation of Na+-K+-ATPase α1-subunit and association with AP2 and Rab5. Whether there exists a 1:1 relationship between the increased association between Na+-K+-ATPase α1-subunit and NHERF-1 with D1R remains to be elucidated. However, our data suggest that there may not be 1:1 relationship between the interactions.
In summary, the data presented in this study demonstrate novel interactions among D1 receptor, Na+-K+-ATPase α1-subunit, PKC-ζ, and NHERF-1 that are modulated by dopamine. The data further suggest that an intact PDZ-2 domain is required for this association and regulation of Na+-K+-ATPase by dopamine-mediated PKA and PKC activation in OK cells. These studies together with our previous studies (18) suggest that different NHERF-1 PDZ domains are required for stimulation of different PKC isoforms and that NHERF-1 may confer the specificity of different hormones to regulate Na+-K+-ATPase α1-subunit in renal proximal tubule cells.
GRANTS
The work was supported by Veteran Affairs Merit Review grant (to E. D. Lederer and E. J. Weinman), National Institutes of Health R01 to E. J. Weinman, and Scientist Development Grant (National, 0435153N) and Grant-in-Aid (DK-55881), Great River Affiliate from American Heart Association to S. J. Khundmiri (09GRNT 2130026).
DISCLOSURES
The opinions expressed in this manuscript do not reflect the opinions of the Department of Veteran Affairs. No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Thomas Pressley (Texas Tech University) for the RT-NASE antibodies against Na+-K+-ATPase α1 subunit and Dr. Evelyn Gozal (University of Louisville) for help with PKA activity assays.
REFERENCES
- 1. Asghar M, Chillar A, Lokhandwala MF. Renal proximal tubules from old Fischer 344 rats grow into epithelial cells in cultures and exhibit increased oxidative stress and reduced D1 receptor function. Am J Physiol Cell Physiol 295: C1326–C1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernardo AA, Kear FT, Santos AV, Ma J, Steplock D, Robey RB, Weinman EJ. Basolateral Na(+)/HCO(3)(−) cotransport activity is regulated by the dissociable Na(+)/H(+) exchanger regulatory factor. J Clin Invest 104: 195–201, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Z, Leibiger I, Katz AI, Bertorello AM. Pals-associated tight junction protein functionally links dopamine and angiotensin II to the regulation of sodium transport in renal epithelial cells. Br J Pharmacol 158: 486–493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Chibalin AV, Pedemonte CH, Katz AI, Féraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalytic alpha-subunit constitutes a triggering signal for Na+, K+-ATPase endocytosis. J Biol Chem 273: 8814–8819, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol 295: F1117–F1125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole JA, Forte LR, Krause WJ, Thorne PK. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol Renal Fluid Electrolyte Physiol 256: F672–F679, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Cunningham R, Biswas R, Brazie M, Steplock D, Shenolikar S, Weinman EJ. Signaling pathways utilized by PTH and dopamine to inhibit phosphate transport in mouse renal proximal tubule cells. Am J Physiol Renal Physiol 296: F355–F361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Efendiev R, Bertorello AM, Pedemonte CH. PKC-beta and PKC-zeta mediate opposing effects on proximal tubule Na+, K+-ATPase activity. FEBS Lett 456: 45–48, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of Na-K ATPase in human renal proximal tubule cells. Hypertension 54: 1070–1076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hird RB, Wakefield TW, Mukherjee R, Jones BU, Crawford FA, Andrews PC, Stanley JC, Spinale FG. Direct effects of protamine sulfate on myocyte contractile processes. Cellular and molecular mechanisms. Circulation 92: 433–446, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Jaitovich A, Bertorello AM. Salt, Na+, K+-ATPase and hypertension. Life Sci 86: 73–78, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 295: F1110–F1116, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Khundmiri SJ, Ahmad A, Bennett RE, Weinman EJ, Steplock D, Cole J, Baumann PD, Lewis J, Singh S, Clark BJ, Lederer ED. Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol 294: F840–F849, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED. Clathrin-mediated endocytosis of Na+, K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha1-subunit. J Biol Chem 279: 17418–17427, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Khundmiri SJ, Dean WL, McLeish KR, Lederer ED. PTH-mediated regulation of Na+-K+-ATPase requires ERK-dependent translocation of PKCα. J Biol Chem 280: 8705–8713, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, Barati M, Rane MJ, Lederer E. Parathyroid hormone regulation of Na+, K+ -ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol 16: 2598–2607, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lederer ED, Khundmiri SJ, Weinman EJ. Role of NHERF-1 in regulation of the activity of Na-K ATPase and sodium-phosphate co-transport in epithelial cells. J Am Soc Nephrol 14: 1711–1719, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Mahon MJ, Cole JA, Lederer ED, Segre GV. Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17: 2355–2364, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Mahon MJ, Donowitz M, Yun CC, Segre GV. Na (+)/H (+) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417: 858–861, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Mahon MJ, Segre GV. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem 279: 23550–23558, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Miyauchi A, Dobre V, Rickmeyer M, Cole J, Forte L, Hruska KA. Stimulation of transient elevations in cytosolic Ca2+ is related to inhibition of Pi transport in OK cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F485–F493, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol 279: C1812–C1818, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Ogimoto G, Yudowski GA, Barker CJ, Köhler M, Katz AI, Féraille E, Pedemonte CH, Berggren PO, Bertorello AM. G protein-coupled receptors regulate Na+, K+ -ATPase activity and endocytosis by modulating the recruitment of adaptor protein 2 and clathrin. Proc Natl Acad Sci USA 97: 3242–3247, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na, K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol 25: 322–327, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann NY Acad Sci 1165: 249–260, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Weinman EJ, Biswas R, Steplock D, Douglass TS, Cunningham R, Shenolikar S. Sodium-hydrogen exchanger regulatory factor 1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney. J Biol Chem 285: 13454–13460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, EX , Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412–3420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Weinman EJ, Cunningham R, Shenolikar S. NHERF and regulation of the renal sodium-hydrogen exchanger NHE3. Pflügers Arch 450: 137–144, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol 68: 491–505, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Weinman EJ, Steplock D, Donowitz M, Shenolikar S. NHERF associations with sodium-hydrogen exchanger isoform 3 (NHE3) and ezrin are essential for cAMP-mediated phosphorylation and inhibition of NHE3. Biochemistry 39: 6123–6129, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Weinman EJ, Steplock D, Zhang Y, Biswas R, Bloch RJ, Shenolikar S. Cooperativity between the phosphorylation of Thr95 and Ser77 of NHERF-1 in the hormonal regulation of renal phosphate transport. J Biol Chem 285: 25134–25138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinman EJ, Wang Y, Wang F, Greer C, Steplock D, Shenolikar S. A C-terminal PDZ motif in NHE3 binds NHERF-1 and enhances cAMP inhibition of sodium-hydrogen exchange. Biochemistry 42: 12662–12668, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na(+)/H(+) exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002 [DOI] [PubMed] [Google Scholar]