Abstract
The pancreatic ATP-sensitive potassium (KATP) channel consisting of four inwardly rectifying potassium channel 6.2 (Kir6.2) and four sulfonylurea receptor SUR1 subunits plays a key role in insulin secretion by linking glucose metabolism to membrane excitability. Syntaxin 1A (Syn-1A) is a plasma membrane protein important for membrane fusion during exocytosis of insulin granules. Here, we show that Syn-1A and KATP channels endogenously expressed in the insulin-secreting cell INS-1 interact. Upregulation of Syn-1A by overexpression in INS-1 leads to a decrease, whereas downregulation of Syn-1A by small interfering RNA (siRNA) leads to an increase, in surface expression of KATP channels. Using COSm6 cells as a heterologous expression system for mechanistic investigation, we found that Syn-1A interacts with SUR1 but not Kir6.2. Furthermore, Syn-1A decreases surface expression of KATP channels via two mechanisms. One mechanism involves accelerated endocytosis of surface channels. The other involves decreased biogenesis and processing of channels in the early secretory pathway. This regulation is KATP channel specific as Syn-1A has no effect on another inward rectifier potassium channel Kir3.1/3.4. Our results demonstrate that in addition to a previously documented role in modulating KATP channel gating, Syn-1A also regulates KATP channel expression in β-cells. We propose that physiological or pathological changes in Syn-1A expression may modulate insulin secretion by altering glucose-secretion coupling via changes in KATP channel expression.
Keywords: ATP-sensitive potassium channel, trafficking, biogenesis, sulfonylurea receptor, inwardly rectifying potassium channel 6.2
atp-sensitive potassium (KATP) channels residing in the plasma membrane of pancreatic β-cells have a critical role in glucose homeostasis by controlling insulin secretion according to blood glucose levels. The ability of KATP channels to couple glucose concentrations to insulin secretion lies in their sensitivities to intracellular nucleotides ATP and ADP, whose concentrations are determined by the rate of glucose metabolism (1, 38). ATP inhibits channel activity while ADP, in the presence of intracellular Mg2+, stimulates channels. As glucose concentration goes up, intracellular ATP concentration increases and ADP concentration decreases, resulting in KATP channel closure, membrane depolarization, Ca2+ influx, and exocytosis of insulin granules. Conversely, as glucose concentration goes down, the ratio of ATP to ADP is decreased, leading to KATP channel opening, membrane hyperpolarization, and termination of insulin secretion. The extent to which KATP channels control β-cell membrane potential is not only a function of gating but also expression of the channel at the cell surface. Reduced expression leads to inability of the β-cell to shut down insulin secretion when plasma glucose falls, whereas increased expression is expected to stabilize the cell near its resting membrane potential and raise the concentration of glucose needed to elicit an insulin response (4, 24, 50).
The KATP channel is a complex of four pore-forming inwardly rectifying potassium channel 6.2 (Kir6.2) subunits and four regulatory sulfonylurea receptor 1 (SUR1) subunits (8, 25, 47). The status of subunit assembly in the early secretory pathway is monitored via a RKR (arginine lysine-arginine) peptide endoplasmic reticulum (ER) retention/retrieval motif present in both SUR1 and Kir6.2 (57). Exposure of the signal in individually expressed or partially assembled channels prevents them from leaving the ER. Assembly of channel subunits into an octamer, however, conceals the signal to allow channels to exit the ER and traffic to the plasma membrane, thus providing a quality control mechanism ensuring only functional channels are expressed at the cell surface. The mechanism by which the RKR motif controls KATP channel trafficking from ER to the Golgi is not completely understood. Between the ER and Golgi, an ER-Golgi intermediate compartment (ERGIC) highly enriched with a 53-kDa membrane protein ERGIC-53 serves as a sorting station to separate forward trafficking proteins that are transported to the Golgi from retrograde trafficking proteins that are to be returned to the ER (19, 45). Recent evidence suggests the RKR motif likely serves as a retrieval signal by binding to coatomer protein complex I (COPI) thus directing incorrectly assembled channel subunits from Golgi/ERGIC back to the ER; whereas binding of 14-3-3 proteins to fully assembled channels allows forward trafficking from ERGIC to the Golgi (20, 49). As KATP channels traverse the trans-Golgi network (TGN), SUR1 is further modified at its N-linked glycosylation sites to give rise to the complex glycosylated form that migrates slower on SDS gel than the core-glycosylated form, referred to as the upper and lower band, respectively (52). In the plasma membrane, KATP channels have been shown to undergo clathrin-mediated endocytic trafficking (22, 33). Endocytosed channels are targeted either for lysosomal degradation or are recycled back to the plasma membrane (34).
Syntaxin 1A (Syn-1A) is a target-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (t-SNARE) protein in the plasma membrane that together with another t-SNARE protein SNAP-25 forms the trans-SNARE complex with v-SNARE protein synaptobrevin in the vesicle membrane in a Ca2+-dependent way to mediate membrane fusion and vesicle exocytosis (7). As such, Syn-1A plays an important role in hormone and neurotransmitter release in endocrine and neuronal cells. In pancreatic β-cells, cleavage of Syn-1A by botulinum toxin inhibits potassium chloride or glucose-induced insulin secretion (51), and Syn-1A knockout mice islets show impaired insulin secretion (39). In addition to its well-defined role in membrane fusion, Syn-1A has been shown to directly bind and regulate the activity and/or expression of a variety of ion channels and transporters including L-type Ca2+ channels, KV2.1 channels, cystic fibrosis transmembrane conductance regulator (CFTR), GABA transporters, and glutamate transporters (11, 14, 27, 30, 35, 37, 46, 55). These expanded roles of Syn-1A allow it to further modulate regulated secretion and synaptic transmission.
The connection between Syn-1A and KATP channels was first reported in 2004 (43). In vitro pull-down assays using purified recombinant bacterial fusion proteins and whole cell patch-clamp recordings showed that Syn-1A acutely inhibits KATP channel activity by interacting with the nucleotide-binding domains (NBD) of SUR1. Subsequent studies extended the finding to cardiac KATP channels composed of SUR2A and Kir6.2 and identified H3 domain of Syn-1A being responsible for mediating the interaction with SUR (12, 28). Based on these results, it was proposed that Syn-1A binds NBD1 and NBD2 of SUR1 to fine-tune KATP channel function during dynamic changes in cytosolic ATP and ADP concentrations (12, 43). Whether Syn-1A affects other aspects of KATP channel regulation has not been explored. In diabetic rodent and human β-cells, levels of Syn-1A and cognate SNARE proteins are known to be reduced (6, 17, 36, 40, 41). Interestingly, increased KATP channel current density in the absence of glucose has been observed in β-cells of Goto-Kakizaki (GK) rats, a rodent model of type 2 diabetes, in which Syn-1A levels are reduced (23). This prompts the question of whether changes in Syn-1A affect expression of KATP channels.
In this study, we show that interaction of Syn-1A with SUR1 leads to a decrease in KATP channel surface expression in both endogenous and ectopic expression models. We demonstrate that this regulation occurs at two levels: increased endocytosis of surface channels and impaired channel maturation and processing in the early secretory compartments. We propose that Syn-1A not only modulates KATP channel gating but also channel abundance at the cell membrane to influence the efficiency of coupling between glucose stimulation and insulin secretion.
MATERIALS AND METHODS
Molecular biology.
The plasmids Syn-1A and Syn-1A-EGFP were in the pcDNA3 vector. Wild-type (WT) hamster SUR1 or FLAG epitope (DYKDDDDK)-tagged SUR1 (referred to as fSUR1) cDNAs were cloned into pECE. WT rat Kir6.2 or hemagglutinin (HA) epitope (YPYDVPDYA)-tagged Kir6.2 cDNAs were cloned into pcDNA1, as described previously (4). Construction of recombinant adenoviruses carrying fSUR1, Kir6.2, or HA-tagged Kir6.2 cDNA was as described previously (32). The FLAG-tag for SUR1 was placed at the extracellular NH2-terminus of the protein. The HA-tag for Kir6.2 was inserted between amino acid 100 and 101 in the extracellular domain; an extra nine amino acids (DLYAYMEKG) were added between amino acid 98 and 99 to aid accessibility of the epitope. For imaging experiments, we used a SUR1 in which a minimal bungarotoxin-binding peptide (WRYYESSLEPYPD) was added to the NH2-terminus of the protein (BTX tag-SUR1). We have shown that these epitope tags do not affect the trafficking or function of the channels (4, 31). Scrambled small interfering RNA (siRNA) and three Syn-1A siRNA (no. 1:GAGCUCAUGUCGGACAUUAAGAAGA, no. 2:UCUUUCUUAAUGUCCGACAUGAGCUC, and no. 3:GCGAACAA AGUUCGCU CCAAGCUAA) were from Invitrogen.
Cell culture and transfection.
COSm6 cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively) (Invitrogen). The cells were transiently transfected with 0.6 μg fSUR1 and 0.4 μg Kir6.2 per 35-mm dish in the presence or absence of Syn-1A, Syn-1A-GFP, or empty vector pcDNA3 (amount indicated in figures) using FuGene 6 (Roche Applied Science, Indianapolis, IN). INS-1 cells clone 832/13 (kindly provided by Dr. Christopher Newgard, Duke University, Durham, NC) were plated in 10-cm plate and cultured in RPMI 1640 medium with 11.1 mM d-glucose (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol (20a). For overexpression of Syn-1A, INS-1 cells were transfected with 1 μg of Syn-1A using LipofectAMINE 2000 (Invitrogen). For knockdown of endogenous Syn-1A, INS-1 cells were transfected with Syn-1A siRNA at a final concentration of 50 nM using HiPerfect transfection reagent from Qiagen (Valencia, CA).
Western blot analysis and immunoprecipitation assay.
Transfected COSm6 or INS-1 cells were washed twice with ice-cold phosphate-buffered saline (in mM: 137 PBS, 2.68 NaCl, 10 KCl, 10 Na2HPO4, and 1.76 KH2PO4; pH 7.4) and lysed in 1% Triton X-100 buffer (20 mM HEPES, 125 mM NaCl, 4 mM EDTA, 1 mM EGTA, and 1% Triton X-100; pH 7.4) at 4°C with rotation for 30 min. Cell lysate was cleared by centrifugation at 14,000 rpm for 10 min at 4°C. Small aliquots of the lysates were used for protein determination by the Lowry method (Pierce, Rockford, IL) with bovine serum albumin as the standard. To immunoprecipitate fSUR1, HA-Kir6.2, endogenous SUR1, or Syn-1A, 500 μg-1 mg of protein was incubated overnight at 4°C with 50 μl of FLAG- or HA-antibody-conjugated agarose beads (Sigma-Aldrich, St. Louis, MO), or anti-Syn-1A (Sigma-Aldrich), or anti-SUR1-conjugated protein A agarose beads (Sigma-Aldrich), respectively. After being washed three times with lysis buffer, bound proteins were eluted by incubation with FLAG peptide (250 μg/ml for fSUR1 sample), HA peptide (10 μg/ml for HA-Kir6.2 sample), or SDS sample buffer containing 2.5% β-mercaptoethanol for 30 min at room temperature. Immunoprecipitated (ip) and non-ip (input) proteins were separated by SDS-polyacrylamide gel (7.5–12.5%) electrophoresis, and transferred onto PVDF membranes (Millipore, Bedford, MA). Membranes were incubated overnight at 4°C with a primary antibody diluted in the Tris-buffered saline plus 0.1% Tween-20 (TBST). The antibody against FLAG (1:500 dilution) was from Sigma-Aldrich. The antibodies against SUR1 and Kir6.2 (1:500 dilution) were made as described previously (54). The antibody against Syn-1A (1:2,000 dilution) was from Sigma. The antibody against α-tubulin (1:2,000) was purchased from Sigma-Aldrich. After three 10-min washes in TBST buffer, blots were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies in TBST buffer: 1:40,000 goat anti-rabbit IgG (GE Healthcare, Buckinghamshire, UK) for SUR1, Kir6.2, and Syn-1A; 1:80,000 horse anti-mouse IgG (GE Healthcare) for FLAG, HA, and α-tubulin. Finally, the blots were washed 3× for 10 min in TBST and developed using the enhanced chemiluminescence detection kit (Super Signal West Femto, Pierce). The signals were imaged by UVP Biospectrum system (Upland, CA). Blots were stripped and reprobed with anti-α-tubulin as a loading control. The blots were quantified with Image J (NIH) and normalized to the corresponding controls.
Surface biotinylation and detection of biotinylated fSUR1 and Kir6.2.
Transfected COSm6 or INS-1 cells were washed twice with cold PBS. Biotinylation of surface protein was carried out by incubating cells with 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL) in PBS for 30 min on ice. The reaction was terminated by incubating cells for 5 min with PBS containing 20 mM glycine, followed by three washes with cold PBS. Cells were lysed immediately in 1% Triton lysis buffer (30 min at 4°C with rotation). Cell lysate was cleared by centrifugation at 21,000 g for 45 min at 4°C, and biotinylated proteins were pulled down by incubation with Neutravidin-agarose beads (Pierce) overnight at 4°C. The beads were washed twice with lysis buffer, and proteins were eluted with SDS sample buffer containing 2.5% β-mercaptoethanol. Eluted proteins were then separated by SDS-PAGE, and fSUR1 was detected by Western blot using anti-Syn-1A, SUR1, or Kir6.2 antibodies.
Chemiluminescence assay.
COSm6 cells in 35-mm dishes were fixed with 2% paraformaldehyde for 20 min at room temperature 48 h posttransfection. Fixed cells were preblocked in PBS + 0.1% BSA for 1 h, incubated in M2 anti-FLAG antibody (10 μg/ml) for 1 h, washed 4× for 30 min in phosphate-buffered saline (PBS) + 0.1% bovine serum albumin (BSA), incubated in horseradish peroxidase-conjugated anti-mouse secondary antibodies (GE Healthcare, 1:1,000 dilution) for 30 min, washed again 4× for 30 min in PBS +0.1% BSA, and 2× for 5 min in PBS. For surface channel pulse-chase experiments, cells were incubated with anti-FLAG antibody in DMEM at 4°C for 1 h. This labeling medium was replaced with warm DMEM, and cells were chased for 0, 15, or 30 min at 37°C. At the end of each time point, cells were fixed and processed for chemiluminescence assays as described above. Chemiluminescence signal was read in a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA) after 10-s incubation in Power Signal ELISA luminol solution (Pierce). The results of each experiment are the average of two dishes. Signals observed in untransfected cells were subtracted as background. Data points shown in the figures are the average of 3–10 independent experiments as specified.
Metabolic labeling and immunoprecipitation.
COSm6 cells were transfected with KATP channels along with Syn-1A or control vector. Forty-eight hours later, cells were incubated in methionine/cysteine-free Dulbecco's modified Eagle's medium supplemented with 5% dialyzed fetal bovine serum for 30 min before being labeled with l-[35S]methionine (ICN Tran35S-Label, 150–250 μCi/ml) for 60 min at 37°C. Labeled cultures were chased in regular medium supplemented with 10 mM methionine at 37°C. At the end of each chase, cells were lysed in 500 μl lysis buffer as described above. For immunoprecipitation, 500 μl of lysate was incubated with 100 μl of FLAG-antibody conjugated agarose beads overnight at 4°C. The precipitate was washed 3× in the lysis buffer, and the proteins were eluted with FLAG-peptide. The eluted proteins were separated by 8% SDS-PAGE, and the dried gels were scanned and quantified by a PhosphorImager (Bio-Rad, Hercules, CA) and its software Quantity One.
86Rb+ efflux assay.
COSm6 or INS-1 cells were plated onto six-well plates and cultured for 2 days to confluency. Cells were incubated for 12 h in culture medium containing 86RbCl (1 μCi/ml). Before measurement of 86Rb+ efflux, cells were incubated for 30 min at room temperature in Krebs-Ringer solution (in mM: 118 NaCl, 2.5 CaCl2·H2O, 1.2 KH2PO4, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 10 HEPES; pH 7. 4) with metabolic inhibitors (2.5 μg/ml oligomycin and 1 mM 2-deoxy-d-glucose). At select time points the solution in the well was collected and fresh solution added. At the end of a 40-min period, cells were lysed. The 86Rb+ in the collected solution and the cell lysate was counted. The percentage efflux at each time point was calculated as the cumulative counts in the collected solution divided by the total counts from the solutions and the cell lysate.
Immunostaining and imaging.
COSm6 cells were transfected as described above. All imaging experiments were performed on a Zeiss LSM710 3-channel spectral confocal microscope using the ×63 1.4 numerical aperature and ×100 1.45 numerical aperature objectives (Carl Zeiss, Jena, Germany). All imaging data are from at least 14 cells/experiment. For pulse labeling and chase of surface channels, COSm6 cells were transfected with BTX tag-SUR1 and Kir6.2 along with Syn-1A or control empty vector. After 24 h cells were replated at the appropriate density onto 22-mm, no. 1 glass coverslips (Fisher Scientific). Imaging was performed 48 h posttransfection. For pulse-chase experiments, cells were incubated with a fluorescence dye-conjugated bungarotoxin, TRITC-BTX (Molecular Probes, Eugene, OR), at 1:200 diluted in DMEM at 4°C. After 1 h the dye was replaced with fresh DMEM, and cells were further incubated at 37°C for 7 min. Cells were then fixed and viewed under the microscope. Untransfected cells treated the same way served as control to exclude nonspecific binding of the dye. For studies with Syn-1A, cells were treated as described above, fixed, and stained with rabbit anti-Syn-1A (Sigma-Aldrich) and Alexa488 goat anti-rabbit secondary antibody (Invitrogen). Note, in Fig. 2 Syn-1A-transfected cells were incubated in warm labeling media for an extended period of time (30 min). This protocol allows for examination of protein distribution throughout the cell at steady-state rather than the 7-min chase characterizing internalization behavior. In the colocalization studies with ERGIC-53, COSm6 cells were transfected and treated as above and fixed. Cells were stained with mouse anti-FLAG (for SUR1) and rabbit anti-ERGIC-53 primary antibodies (Sigma-Aldrich) and anti-mouse Alexa568- and anti-rabbit Alexa488 secondary antibodies. After mounting was completed, cells were viewed under the microscope. Cells treated with secondary only and untransfected cells stained the same way served as negative controls. For image analysis to determine distribution of stained proteins the fluorescence intensity of individual cells was analyzed using linescan (MetaMorph, Molecular Devices, Downington, PA). Briefly, a line was drawn across the cell. The maximum intensity of fluorescence was determined for each point along the line and graphed as intensity versus pixel distance across the cell. The solid white lines in Fig. 6 indicate the length of the scan. For colocalization analysis a Metamorph colocalization routine was used. Pictures from the two different channels (488 and 561 nm) were thresholded for light objects. Colocalized objects had to be positive for staining in both channels. Appropriate secondary only and red or green only stained controls were employed.
Fig. 2.
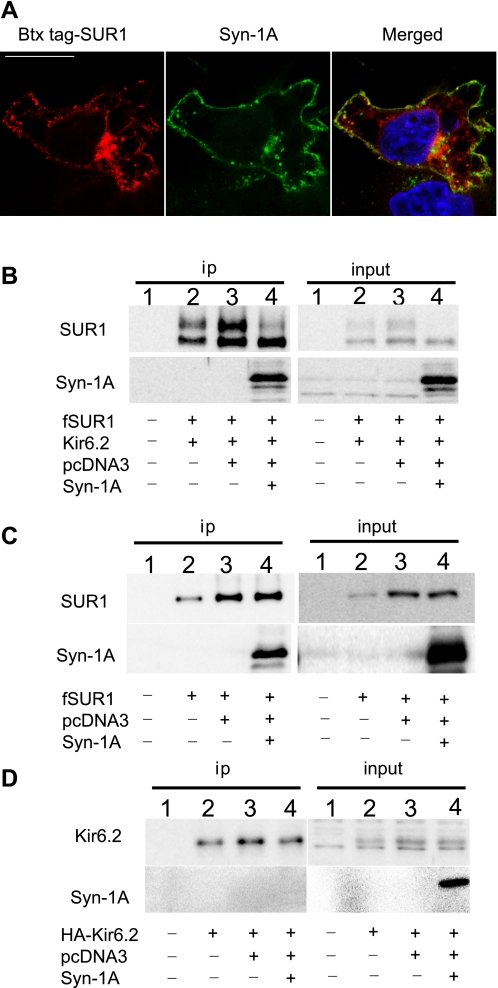
Colocalization and interaction of Syn-1A with KATP channels in COSm6 cells. A: COSm6 cells in each 35-mm dish were transfected with wild-type (WT) BTX tag-SUR1/Kir6.2 (0.6 μg SUR1 and 0.4 μg Kir6.2) and Syn-1A-GFP (0.2 μg). BTX tag-SUR1 was labeled by incubation with TRITC-BTX for 30 min at 37°C. Cells were fixed and counterstained with DAPI (blue). BTX tag-SUR1 (left, red) displayed extensive colocalization with Syn-1A-GFP (middle, green) at the cell surface, and in intracellular compartments (merge, yellow, right). Scale bar: 10 μm. B: immunoblotting of fSUR1 and Syn-1A in FLAG-antibody immunoprecipitated protein samples (left) from untransfected control cells (lane 1), cells transfected with WT KATP channel subunits fSUR1 and Kir6.2 (lane 2), and cells cotransfected with KATP channels (fSUR1 and Kir6.2) and empty vector pcDNA3 (lane 3) or Syn-1A (lane 4). Syn-1A was coimmunoprecipitated with fSUR1. Note in both ip and input samples (right), Syn-1A greatly reduced SUR1 upper band. C is same as B except that cells were transfected with SUR1 without Kir6.2 in lanes 2, 3, and 4. Syn-1A still coprecipitated with fSUR1 in the absence of Kir6.2. Note in the absence of Kir6.2, fSUR1 was present as the immature core-glycosylated band only. For both B and C, cells transfected with Syn-1A only were also analyzed as a negative control to exclude the possibility that the FLAG-antibody or HA-antibody cross reacts with Syn-1A (online supplemental Fig. S2). D is same as C except that cells were transfected with HA-Kir6.2 without fSUR1 in lanes 2, 3, and 4. HA-Kir6.2 was immunoprecipitated with anti-HA antibody-conjugated agarose beads. Syn-1A did not coprecipitate with HA-Kir6.2, although HA-Kir6.2 was clearly seen in the input samples.
Fig. 6.
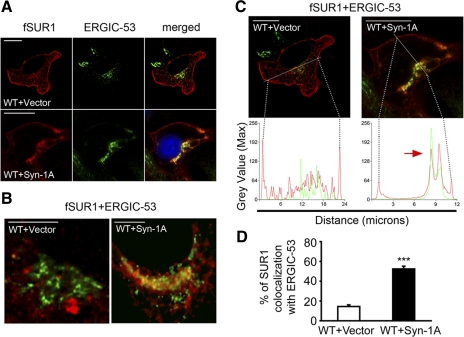
Syn-1A increases KATP channels localized to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). A: comparison of a cell expressing KATP channels (fSUR1/Kir6.2) stained with anti-FLAG antibody and anti-ERGIC-53 (top, scale bar 10 μm) and a cell coexpressing KATP channels and Syn-1A stained in the same manner (bottom). When expressed alone, WT channels displayed distinct surface staining as well as puncta throughout the cell (red, top). ERGIC-53 staining was perinuclear (green, top). In cells coexpressing KATP channels and Syn-1A, surface channel staining was much weaker (red, bottom), whereas intracellular staining was more concentrated and overlapped significantly with the perinuclear ERGIC-53 staining (merged, bottom). B: close-up views of SUR1 and ERGIC-53 colocalization in WT and WT+Syn-1A expressing cells (scale bar 5 μm). C: redistribution of fSUR1/Kir6.2 in cells coexpressing Syn-1A was analyzed by line scan. KATP channels stained for fSUR1 (left picture, scale bar 10 μm and graph) showed peak intensity values at the cell surface (marked by the black dashed lines, SUR1 fluorescence is shown in red) and several small peaks distributed along the line representing staining inside the cell that occasionally overlaps with ERGIC-53 staining (green line in graph). Coexpression of Syn-1A led to significant channel redistribution such that the red peaks corresponding to surface channel staining were much weaker in intensity while intracellular staining was highly concentrated as seen by the two main peaks that overlap significantly with ERGIC-53 staining (right picture and graph). D: quantification of SUR1 colocalization with ERGIC-53 in WT and WT+Syn-1A expressing cells (n = 5–6 cells /group, ***P < 0.001 unpaired Student's t-test).
Statistical analysis.
All data were analyzed with the program GraphPad Prism. Results are expressed as means ± SE. Differences were tested using either one-way or two-way analysis of variance (ANOVA) followed by the post hoc Dunnett's test for multiple comparisons. When only two groups were compared, unpaired Student's t-test was used. The level of statistical significance was set at P < 0.05.
RESULTS
Endogenous interactions between Syn-1A and KATP channels in INS-1 cells and effects on channel expression.
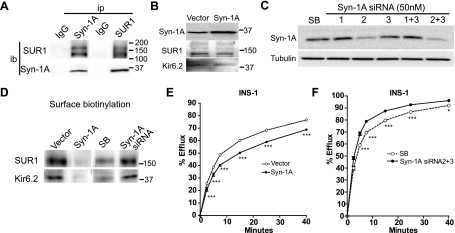
Previous studies examining the relationship between Syn-1A and KATP channels focused on in vitro protein-protein interactions and acute effects of perfused Syn-1A recombinant protein fragments on channel gating in whole cell patch-clamp recording experiments (12, 43). Whether physical interactions between Syn-1A and SUR1 occur physiologically has not been tested. We used a rat insulinoma cell line INS-1 that expresses endogenous Syn-1A and KATP channels and asked if the two proteins interact in coimmunoprecipitation experiments. Results show that endogenous SUR1 is clearly present in protein samples precipitated by anti-Syn-1A antibody, in contrast to control IgG. Conversely, Syn-1A is also present in protein samples precipitated using anti-SUR1 antibody complexed with protein-A beads (Fig. 1A). That endogenous Syn-1A and SUR1 copurify in insulin-secreting cells indicates that interactions between the two proteins do occur physiologically.
Fig. 1.
Interactions between syntaxin 1A (Syn-1A) and sulfonylurea receptor 1 (SUR1) in INS-1 cells affect ATP-sensitive K (KATP)channel surface expression and activity. A: INS-1 cells were immunoprecipitated (ip) by Syn-1A or SUR1 antibody conjugated with protein A agarose beads and immunoblotted (ib) with SUR1 and Syn-1A antibodies. Rabbit IgG served as a control for ip. Results indicate that there is an interaction between endogenous Syn-1A and SUR1. Molecular mass markers in this and subsequent panels of this figure are in kDa. B: INS-1 cells were transiently transfected with pcDNA3 empty vector or Syn-1A plasmids. Top: Western blot shows that Syn-1A levels are higher in Syn-1A-transfected cells than in cells transfected with control pcDNA3. Total endogenous SUR1 and inwardly rectifying K channel 6.2 (Kir 6.2) proteins from these samples are also shown (middle and bottom blots). C: INS-1 cells were treated with 50 nM of Syn-1A small interfering RNA (siRNA) (nos. 1, 2, 3, 1+3, or 2+3) or scrambled siRNA (SB) for 48 h. Afterwards, Syn-1A was immunoblotted to assess the knockdown effects, tubulin served as a loading control. Results show siRNA no. 2 and the combination of no.2 and 3 are most effective in knocking down endogenous Syn-1A. D: biotinylated surface SUR1 in INS-1 cells with elevated or reduced Syn-1A (as described in B and C). Biotinylated surface proteins were precipitated by streptavidin beads and immunoblotted by SUR1 and Kir6.2 antibodies. The signals of surface SUR1 and Kir6.2 are both significantly reduced in cells transfected with Syn-1A plasmids compared with control vector-transfected cells; whereas the signals were relatively higher in cells with reduced Syn-1A (siRNA) compared with control (SB). Note the overall SUR1 and Kir6.2 signals in the knockdown experiments were less due to the number of cells used in the experiments (1 × 105 cells per well in a 12-well plate for siRNA and 3 × 105 cells in a 35-mm dish for overexpression experiments) as the reduced cell density is necessary for optimal knockdown effects. E and F: representative 86Rb+ efflux profiles in INS-1 cells with elevated (E) or reduced (F) Syn-1A. Increased Syn-1A expression led to decreased endogenous KATP channel activity (E). By contrast, decreased Syn-1A protein levels accompanied an increase in endogenous KATP channel activity (F). (n = 5–6/group; *P < 0.05; ***P < 0.001 by two-way ANOVA and Dunnett′s post hoc test).
To determine whether the interaction between Syn-1A and SUR1 has effects on channel expression, we manipulated Syn-1A protein levels via overexpression of Syn-1A plasmid or siRNA-mediated knockdown of endogenous Syn-1A. To upregulate Syn-1A, INS-1 cells were transfected with a range of Syn-1A cDNA (0.2 to 1.5 μg per 35-mm dish). Transfection with 1.0–1.5 μg Syn-1A cDNA increased Syn-1A protein levels by approximately twofold compared with cells transfected with equal amount of pcDNA3 empty vector (Fig. 1B). Under this condition, steady-state levels of total endogenous SUR1 and Kir6.2 proteins appeared not to be grossly altered (Fig. 1B). We therefore chose to transfect 1.2 μg Syn-1A plasmid in all subsequent experiments. For knockdown of Syn-1A, we tested three Syn-1A siRNA sequences. Western blots show that siRNA no. 2 and the combination of nos. 2 and 3 resulted in the greatest knockdown (by 65% and 74%, respectively, compared with the scramble control; Fig. 1C). Subsequent knockdown experiments used the combination of siRNA nos. 2 and 3. Surface biotinylation followed by immunoprecipitation and immunoblotting was used to monitor the abundance of surface SUR1 protein in INS-1 cells overexpressing Syn-1A or with Syn-1A proteins knocked down. Figure 1D shows that increasing Syn-1A markedly decreased surface biotinylated SUR1 compared with vector control; conversely, decreasing Syn-1A expression by siRNA increased surface SUR1 protein compared with scramble (SB) siRNA control. Note the biotinylated SUR1 signal was stronger in overexpression than in knockdown experiments. This is due to the necessity to use a lower cell density for effective siRNA knockdown. We also probed the surface biotinylated protein samples with anti-Kir6.2 antibody and found reduced or increased Kir6.2 proteins in cells with increased or decreased Syn-1A, respectively (Fig. 1D). Because Kir6.2 lacks extracellular lysine residues that can be biotinylated, detection of Kir6.2 in the streptavidine pulled-down surface biotinylated protein samples indicates Kir6.2 was associated with biotinylated SUR1. Thus surface expression of SUR1 and Kir6.2 in the channel complex was affected in parallel, consistent with altered surface channel expression as a consequence of altered Syn-1A expression. To test how Syn-1A levels affect KATP channel activity, functional 86Rb+ efflux assays were conducted. In this assay, cells were preloaded with 86Rb+, and channels were activated by treating cells with metabolic inhibitors that lower the ATP-to-ADP ratio. The amount of 86Rb+ efflux observed over time indicates the level of channel activity. We observed decreased endogenous channel activity in cells overexpressing Syn-1A (Fig. 1E) and increased KATP channel activity in INS-1 cells treated with Syn-1A siRNA (Fig. 1F), consistent with Syn-1A having a role in regulating KATP channel function by modulating surface expression and/or gating. Taken together, the results provide strong evidence for a physiological interaction between Syn-1A and KATP channels in INS-1 cells and a role of this interaction in modulating surface expression and activity of KATP channels.
Syn-1A interacts with KATP channels in COSm6 cells and reduces channel expression at the cell surface.
To understand the mechanisms underlying Syn-1A regulation of KATP channels, we used COSm6 cells as the experimental platform for mechanistic studies as they are more amenable to molecular and biochemical manipulations without interference from endogenous channels. We first characterized the interactions between Syn-1A and KATP channels in COSm6 cells cotransfected with Syn-1A and the channel subunits SUR1 and Kir6.2. Immunofluorescent staining experiments were performed to survey the cellular distribution of Syn-1A and the channel. For these experiments, a SUR1 construct with a minimum bungarotoxin binding peptide inserted at the extracellular NH2-terminus (BTX tag-SUR1) was used. Channels were labeled by continuous incubation for 30 min at 37°C in medium containing a red fluorescent dye-conjugated bungarotoxin (TRITC-BTX) as described in materials and methods. We observed significant overlap between BTX tag-SUR1 and cotransfected Syn-1A tagged with the green fluorescent protein (GFP) both in the plasma membrane and intracellular compartments, suggesting Syn-1A colocalizes with KATP channels (Fig. 2A). To confirm physical interactions biochemically, coimmunoprecipitation experiments were conducted in COSm6 cells cotransfected with KATP channels (fSUR1/Kir6.2) and Syn-1A or control empty vector (pcDNA3). As shown in Fig. 2B, Syn-1A coimmunoprecipitated with fSUR1 (fSUR1 also coimmunoprecipitated with Syn-1A using anti-Syn-1A antibody; not shown), consistent with physical interactions between the channel and Syn-1A. To further investigate whether this interaction is mediated by SUR1 or Kir6.2, COSm6 cells cotransfected with Syn-1A and either SUR1 or Kir6.2 were subjected to coimmunoprecipitation. Whereas Syn-1A coimmunoprecipitated with SUR1, it was not detectable in the Kir6.2 immunoprecipitate (Fig. 2, C and D). These results show that interactions between Syn-1A and KATP channels occur in COSm6 cells as in INS-1 cells. Moreover, the interaction is likely mediated by the SUR1 subunit, consistent with previous reports (12, 43).
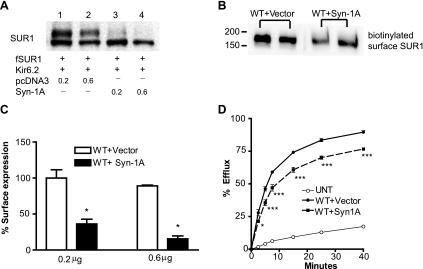
Next, we investigated whether Syn-1A affects surface expression of KATP channels similarly as that observed in INS-1 cells. In Western blot analysis of SUR1, there was a marked reduction of the complex-glycosylated mature form in cells transfected with KATP channels and Syn-1A compared with cells transfected with KATP channel subunits and the control empty vector. The extent of reduction is correlated with the amount of cotransfected Syn-1A (Fig. 3A). The abundance of the complex-glycosylated SUR1 is indicative of the amount of protein that has passed the ER quality control checkpoint and traversed the medial-Golgi and is usually reflective of the amount of channels expressed at the cell surface. Thus the reduced upper band is indicative of reduced surface expression of the channel. To directly test this, we used surface biotinylation followed by immunoprecipitation to monitor the amount of surface SUR1 in cells transfected with KATP channels in the absence or presence of Syn-1A. As shown in Fig. 3B, Syn-1A indeed reduced the amount of SUR1 at the cell surface. Further quantification of surface channel expression using a chemiluminescence assay described in materials and methods corroborated the results obtained by surface biotinylation (Fig. 3C). Moreover, 86Rb+ efflux assays were performed to assess surface channel activity. When compared with cells expressing KATP channels and empty vector, cells coexpressing Syn-1A exhibited significantly reduced efflux, consistent with what we observed in INS-1 cells. These results demonstrate that in the ectopic COS cell expression system, Syn-1A also decreases surface expression and function of KATP channels, an effect likely mediated by interaction with SUR1.
Fig. 3.
Syn-1A reduces surface expression of KATP channels in COSm6 cells. A: COSm6 cells were cotransfected with cDNAs for fSUR1, Kir6.2, and Syn-1A (0.2 or 0.6 μg; lanes 3 and 4) or empty vector (pcDNA3; lanes 1 and 2). Western blot analysis of SUR1 shows significantly reduced mature upper band in cells cotransfected with Syn-1A. B: biotinylated surface SUR1 in WT+Vector and WT+Syn-1A groups show that Syn-1A decreases surface SUR1 expression. Duplicates are shown for each group. Note there is only one form of fSUR1, the complex-glycosylated form as expected. C: COSm6 cells were transiently transfected with WT channels and 0.2 or 0.6 μg vector or Syn-1A. Surface SUR1 expression was assessed by chemiluminescence assay and expressed as percentage of that observed in cells transfected with WT+0.2 μg vector (n = 4/group, *P < 0.05 by unpaired t-test). D: representative 86Rb+ efflux profile of COSm6 cells cotransfected with WT channels and vector or Syn-1A. Cells coexpressing Syn-1A has significantly reduced channel activity compare with cells without Syn-1A (n = 7–8/group, *P < 0.05; ***P < 0.001 by two-way ANOVA and Dunnett′s post hoc test). Background efflux in untransfected cells is shown as a control.
Syn-1A enhances endocytic trafficking of KATP channels.
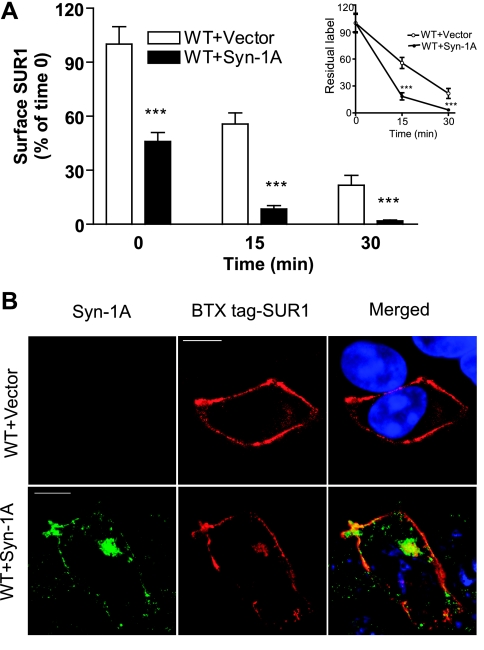
Surface protein expression is an equilibrium of protein insertion into and removal from the plasma membrane. We first examined the possibility that Syn-1A affects endocytic trafficking of surface KATP channels, since downregulation of surface KATP channels by Syn-1A was observed in both COSm6 cells and INS-1 cells. To monitor the rate of surface channel endocytosis, we performed a pulse-chase surface chemiluminescence assay in which surface channels were pulse-labeled at 4°C using anti-FLAG antibody and chased at 37°C for various times. Residual-labeled channels at each chase time point were then quantified using chemiluminescence assays. We found that coexpression of Syn-1A with KATP channels led to significantly faster channel disappearance from the cell surface, consistent with accelerated endocytosis (Fig. 4A). Channel endocytosis was further assessed by pulse-chase immunofluorescent staining experiments. Here, cells transfected with BTX tag-SUR1 and Kir6.2 with or without Syn-1A were pulse labeled in medium containing TRITC-BTX at 4°C and chased at 37°C for 7 min. Cells coexpressing Syn-1A had clearly more intracellular labeling after 7 min of chase compared with cells without Syn-1A (Fig. 4B). Thus accelerated endocytosis of surface channels is an underlying mechanism by which Syn-1A reduces surface expression of KATP channels.
Fig. 4.
Syn-1A accelerates endocytosis of surface KATP channels. A: COSm6 cells were cotransfected with fSUR1, Kir6.2, and vector or Syn-1A. Surface channels were pulse-labeled at 4°C using anti-FLAG antibody and endocytosis followed by chasing the labeled channels at 37°C for various times. Residual labeled surface channels at each chase time was quantified by the chemiluminescence assay (n = 4/ per group; ***P < 0.001 by two-way ANOVA and Dunnett′s post hoc test). Inset compares the rate of endocytosis in which residual surface label is expressed as percentage of time 0 of each group. B: endocytosis of surface channels was compared in cells coexpressing Syn-1A or not by immunofluorescent staining. Cells were transfected with BTX tag-SUR1 and Kir6.2 plus control vector or Syn-1A. Surface BTX tag-SUR1 was labeled with TRITC-BTX dye in the medium for 1 h at 4°C and chased for 7 min at 37°C in the absence of the dye. Cells were then stained for Syn-1A, counterstained with DAPI (shown in the merge pictures), and imaged. When compared with cells cotransfected with control vector, cells cotransfected with Syn-1A showed relatively reduced surface SUR1 staining and increased intracellular staining that colocalized with Syn-1A. Scale bar: 10 μm.
Syn-1A reduces biogenesis and forward trafficking of KATP channels.
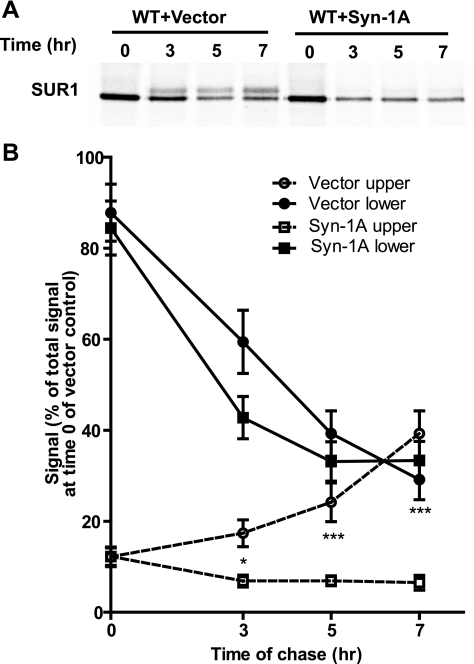
Next, we examined the possibility that Syn-1A reduces expression of channels at the surface by affecting their biogenesis and/or trafficking along the secretory pathway. Metabolic pulse-chase experiments were used to follow the rate of channel maturation in cells transfected with KATP channel subunits along with Syn-1A or control empty vector. As shown in Fig. 5A, in control cells at the beginning of the chase (time 0) after 1 h metabolic labeling, SUR1 was seen as a single core-glycosylated immature lower band. The intensity of this band decreased over the next 7 h of chase period with a concomitant increase of the complex-glycosylated mature upper SUR1 band. In contrast, cells cotransfected with Syn-1A exhibited no increase in the mature upper band over the entire chase period. In addition, quantification of the results revealed that Syn-1A not only impaired maturation of SUR1 but also increased the degradation of labeled protein, although it had little effect on the amount of SUR1 protein labeled during the pulse (Fig. 5B). This is clearly seen when the signal of total SUR1 (upper and lower) at the beginning (0 h) and end of the chase (7 h) is compared between control and Syn-1A-expressing cells (see supplemental Fig. S1 online at AJP-Cell Physiol website). The results provide clear evidence that Syn-1A diminishes maturation and increases degradation of SUR1 and the channel in the early secretory compartments.
Fig. 5.
Syn-1A impairs maturation and processing of KATP channels. Metabolic pulse-chase experiments were performed in COSm6 cells transfected with KATP channel subunits with or without Syn-1A. Cells were pulse-labeled with [35S]methionine/cysteine for 1 h and chased for the indicated times. fSUR1 was immunoprecipitated using anti-FLAG antibodies. A: autoradiographs show that in WT+Vector cells, a fraction of the immature lower fSUR1 band, was converted to the mature upper band over time. This conversion, however, was markedly reduced in WT+Syn-1A cells, indicating impaired channel maturation. B: signal of upper and lower band was quantified using a phosphorImager and was expressed as percentage of total signal observed in control (WT+Vector) at time 0 (n = 7–8/ group; *P < 0.05; ***P < 0.001 by two-way ANOVA and Dunnett′s post hoc test).
To gain further insight into the channel maturation/trafficking event that is affected by Syn-1A, we asked whether the channel proteins might be arrested in a membrane compartment between ER and the medial Golgi where complex-glycosylation occurs. It is now well established that proteins moving from the ER to the Golgi complex in mammalian cells pass through the tubulovesicular membrane compartment ERGIC, a major sorting station that separates proteins bound for Golgi and those to be transported back to the ER (2). By coimmunofluorescent staining of KATP channel subunit SUR1 and the ERGIC marker ERGIC-53 (2, 18), we found that Syn-1A significantly increased colocalization of KATP channels with ERGIC-53 (Fig. 6, A and B). A “line scan” method that analyzes the intensity profile of SUR1 and ERGIC-53 fluorescence signals revealed that in cells cotransfected with Syn-1A the profiles of intracellular green signal (ERGIC-53) and red signal (SUR1) were highly coincident, in contrast to cells cotransfected with control vector where there was much less overlap between the green and red signals (Fig. 6C). Quantitative analysis showed that whereas only 12.6 ± 1.7% SUR1 colocalized with ERGIC-53 in WT+Vector group, 47.8 ± 2.7% SUR1 colocalized with ERGIC-53 in the presence of Syn-1A (Fig. 6D). From these data, we conclude that Syn-1A causes KATP channels to be retained in ERGIC, thus preventing channel trafficking to the Golgi and further processing of SUR1 in the Golgi. Therefore, it appears that Syn-1A reduces surface expression of KATP channels by both increasing channel endocytosis and decreasing channel forward trafficking and maturation.
Syn-1A does not alter processing and expression of another inwardly rectifying potassium channel GIRK.
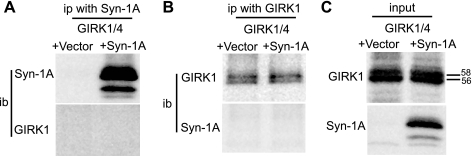
Because Syn-1A is a t-SNARE protein involved in membrane fusion, there is a possibility that overexpression of Syn-1A affects forward and endocytic trafficking by altering membrane trafficking in general (13). To exclude such a potential nonspecific effect, we examined the effect of Syn-1A on another ion channel, the G protein-coupled inwardly rectifying potassium channel GIRK (Kir3). Prior studies have shown that coexpression of GIRK4 (Kir3.4) with GIRK1 (Kir3.1) generates mature channels in which the N-linked glycosylation site in GIRK1 becomes complex-glycosylated and is recognized as an upper band that is distinct from the core-glycosylated form (Fig. 7, B and C). Unlike SUR1, GIRK1/4 does not coimmunoprecipitate with Syn-1A in cells cotransfected with GIRK1/4 and Syn-1A, using either GIRK1/4 or Syn-1A as the bait, similar to that reported previously by others (Fig. 7, A and B) (16, 42). Moreover, there is no significant difference in the glycosylation pattern of GIRK1/4 between cells overexpressing Syn-1A and those not expressing Syn-1A. These results provide strong evidence that Syn-1A exerts a specific effect on the processing and trafficking of KATP channels.
Fig. 7.
Syn-1A does not interact with GIRK1/4 channels and does not affect their processing. COSm6 cells were transfected with GIRK1 and GIRK4, and either empty vector or Syn-1A. Cells were immunoprecipitated (ip) using anti-Syn-1A- or GIRK1-conjugated protein A agarose beads. A: proteins pulled down by anti-Syn-1A antibody were immunoblotted (ib) with anti-Syn-1A or GIRK1 antibodies. No GIRK1 was detected in the Syn-1A immunoprecipitates. B: proteins pulled down with anti-GIRK1 antibody were immunoblotted with anti-GIRK1 or Syn-1A antibodies. No Syn-1A was detected in the GIRK1 immunoprecipitates. C: input samples for GIRK1/4+Vector and GIRK1/4+Syn-1A were immunoblotted with anti-GIRK1 or Syn-1A antibodies. Note, the doublet in GIRK1 blots corresponds to the core-glycosylated immature band (mol wt = 56 kDa) and complex-glycosylated mature band (mol wt = 58 kDa). No significant difference was observed in the relative intensity of the two bands between control and Syn-1A expressing cells.
DISCUSSION
As the key signal transduction protein complex linking glucose metabolism to insulin secretion, pancreatic β-cell KATP channels have been extensively studied in the past two decades. However, much remains to be learned about the mechanisms by which channel abundance in the plasma membrane–a critical parameter determining the efficiency of glucose stimulation-insulin secretion coupling–is regulated. In this study, we show that the t-SNARE protein Syn-1A is a negative regulator of KATP channel surface expression. We present evidence that Syn-1A interacts specifically with the SUR1 subunit in both INS-1 cells expressing endogenous Syn-1A and KATP channels and ectopic expression system. In both INS-1 and COSm6 cells, overexpression of Syn-1A led to decreased surface expression of KATP channels, whereas lack of or knocked-down expression of Syn-1A resulted in more KATP channels in the plasma membrane.
Mechanisms of KATP channel surface expression regulation by Syn-1A.
Studies over the past decade have revealed that Syn-1A, in addition to its well-defined role in membrane fusion, directly binds and regulates a variety of ion channels including L-type Ca2+ channels, KV2.1 channels, CFTR, and KATP channels (3, 5, 26, 27, 46). The regulation is not only limited to acute effects on activities of these channels/transporters but in many cases is also seen as changes in their surface expression. For example, Syn-1A has been shown to positively regulate surface expression of a number of proteins including Kv1.1, GABA transporters, dopamine transporters, and norepinephrine transporters to modulate neuronal transmission (15, 21, 29, 48). On the other hand, Syn-1A has also been reported to negatively regulate the surface expression of Kv2.1, CFTR, the epithelial sodium channel ENaC, and the glutamate transporter EAAC1 (9, 30, 44, 55). The mechanisms underlying the expression regulation of different membrane proteins by Syn-1A are not all clear. However, altered endocytic trafficking (55) or altered insertion of vesicles carrying the affected proteins into the plasma membrane have been proposed (9, 48).
Our studies in COSm6 cells suggest that Syn-1A exerts its effect on KATP channel expression in at least two ways: increased endocytosis of surface channels and reduced channel maturation and processing in the early secretory pathway. An earlier study of the glutamate transporter EAAC1 has proposed that Syn-1A promotes endocytosis of the transporter by facilitating the assembly of clathrin-coated pits, as Syn-1A has been shown in some cells to associate with dynamin and synaptotagmin, proteins involved in clathrin-coated pit formation. Since KATP channels are known to be endocytosed by the clathrin-mediated, dynamin-dependent pathway (55), a similar mechanism may explain the effect of Syn-1A. When Syn-1A was coexpressed with SUR1 only without the other channel subunit Kir6.2, interactions between Syn-1A and SUR1 were still observed (Fig. 2C). This suggests that the two proteins already interact in the ER, since in the absence of Kir6.2 SUR1 is retained in the ER (as evidenced by the lack of the complex-glycosylated SUR1 band; Fig. 2C) (10). The interaction in the early secretory compartment culminates in a profound effect on maturation of the channel complex when both SUR1 and Kir6.2 are coexpressed. How does Syn-1A reduce maturation and processing efficiency of the KATP channel complex? Based on metabolic pulse-chase experiment results, Syn-1A is unlikely to affect translation of channel proteins as SUR1 signal intensity after 1 h pulse labeling appeared comparable in cells cotransfected with control vector or Syn-1A. Rather, the reduced processing efficiency seems to result from arrest of the channel in the ER to Golgi intermediate compartment ERGIC. Inability to traffic forward to the Golgi may lead to degradation of channel proteins by the ubiquitin-proteasome-mediated, ER-associated degradation pathway(53), explaining the increased degradation of pulse-labeled SUR1 we observed (Fig. 5, supplemental Fig. S1) and in part the somewhat reduced overall steady-state channel protein levels (Fig. 2B and Fig. 3A). Interestingly, an early study has shown that syntaxin 5 is involved in membrane trafficking between ER and the Golgi and that overexpression of syntaxin 5 disrupts this forward trafficking (13). Overexpression of Syn-1A has also been shown to interfere with ER to Golgi trafficking; however, this effect is much less pronounced compare with syntaxin 5 and appears to require high levels of overexpression. While one might argue that in our study Syn-1A overexpression could cause gross perturbation of membrane flow between secretory compartments by upsetting the balance of general transport factors such as other SNARE proteins, thereby stalling forward trafficking of KATP channels in the early secretory pathway, this is unlikely the case since maturation and processing of another ion channel complex GIRK1/4 was not affected (Fig. 7).
Retention of KATP channels in the ERGIC compartment in the presence of Syn-1A suggests an intriguing possibility that Syn-1A interaction with SUR1 may interfere with recognition of trafficking signals that direct assembled channels from ERGIC to the Golgi. One trafficking regulation event that could be affected by Syn-1A is binding of 14-3-3 proteins to assembled KATP channel complex. Previous studies have shown that both COPI proteins and 14-3-3 recognize the RKR motifs (56). Whereas binding of COPI to unassembled or partially assembled channel subunits retrieve channel proteins back to ER thus causing ER retention, binding of 14-3-3 proteins to fully assembled channels relieve COPI-mediated retrograde trafficking and promote forward trafficking of channels to the Golgi (20). It will be interesting to determine in future studies whether Syn-1A interaction with SUR1 prevents 14-3-3 from binding to assembled channel complex to prevent channels from reaching the Golgi for further maturation and processing.
Physiological implications and relevance to disease.
Regulation of KATP channels by Syn-1A was first reported by Pasyk et. al (43). Using in vitro binding assays and whole cell patch-clamp recordings, they showed that Syn-1A binds to both NBD1 and NBD2 of SUR1 and that perfusion of recombinant Syn-1A causes acute inhibition of KATP currents (43). Our study shows that in addition to the acute gating effects, Syn-1A markedly reduces surface expression of KATP channels. Importantly, we demonstrate that the interactions between SUR1 and Syn-1A occur physiologically in INS-1 cells expressing endogenous Syn-1A and KATP channels. Moreover, up- or downregulation of Syn-1A result in opposite regulation of KATP channel expression in the INS-1 cell membrane. These cell biological changes are consistent with the altered channel activity assessed by the 86Rb+ assays. Whereas we did not attempt to tease apart the contributions from gating regulation and expression regulation to the altered 86Rb+ efflux activities seen in our experiments because the mechanism by which Syn-1A modulates KATP channel gating is not completely understood, the results nevertheless demonstrate that altering the extent of Syn-1A-KATP channel interactions will have significant functional impact on channel response to metabolic signals. Although speculative, coregulation of Syn-1A and KATP channel expression and function in opposite directions may augment the effects of both on insulin secretion such that reduced Syn-1A levels will not only reduce secretion by impairing membrane fusion but also by increasing KATP channel conductance to raise the threshold of glucose stimulation and vice versa. It is important to bear in mind that Syn-1A not only regulates expression and gating of KATP channels but also other channels such as voltage-gated Ca2+ channels and Kv2.1 involved in membrane excitability important for the coupling between glucose stimulation and insulin secretion. It would be important to understand the spatial and temporal relationships between these regulatory events to gain full understanding of the insulin secretion machinery.
In diabetic rodent and human β-cells, Syn-1A expression levels are reduced (6, 17, 36, 40, 41). It is possible that increased surface expression of KATP channels as a result of reduced Syn-1A could contribute to or exacerbate the diabetes phenotype. In this regard, our finding may present a new therapeutic opportunity. In other proteins such as CFTR, which is also negatively regulated by Syn-1A, it has been shown that the effect of Syn-1A could be abrogated by Munc-18, a high-affinity Syn-1A binding protein, soluble Syn-1A peptide, or botulinum neurotoxin C1, which cleaves Syn-1A. Thus it may be possible to uncouple KATP from Syn-1A regulation by similar manipulations under certain pathological conditions where it is advantageous to do so. Additionally, minimizing interactions between Syn-1A and KATP channels in β-cells may be exploited to boost surface expression of mutant channels exhibiting trafficking defects that cause congenital hyperinsulinism.
In conclusion, our study has uncovered Syn-1A as a regulator of KATP channel expression at the β-cell membrane. Together with earlier studies demonstrating an acute effect of Syn-1A on channel gating (12, 43), they show Syn-1A to be a versatile regulator of KATP channels acting at the level of biogenesis, endocytic trafficking, and gating.
GRANTS
This work was supported by National Institutes of Health grant DK57699 (to S.-L. Shyng) and the March of Dimes Research Grant Foundation Grant 1-2001-707 (to S.-L. Shyng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Joel W. Gay for plasmids preparation and the staff of Advanced Light Microscopy Core at Jungers Center (OHSU, Portland, OR) for their expert help with image acquisition and image analysis.
REFERENCES
- 1. Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20: 101–135, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci 119: 2173–2183, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem 77: 972–985, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Cartier EA, Conti LR, Vandenberg CA, Shyng SL. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci USA 98: 2882–2887, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann NY Acad Sci 868: 144–159, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chan CB, MacPhail RM, Sheu L, Wheeler MB, Gaisano HY. Beta-cell hypertrophy in fa/fa rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes 48: 997–1005, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Clement JP, 4th, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K(ATP) channel subunits. Neuron 18: 827–838, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Condliffe SB, Zhang H, Frizzell RA. Syntaxin 1A regulates ENaC channel activity. J Biol Chem 279: 10085–10092, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Conti LR, Radeke CM, Shyng SL, Vandenberg CA. Transmembrane topology of the sulfonylurea receptor SUR1. J Biol Chem 276: 41270–41278, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL. CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex. Proc Natl Acad Sci USA 99: 12477–12482, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui N, Kang Y, He Y, Leung YM, Xie H, Pasyk EA, Gao X, Sheu L, Hansen JB, Wahl P, Tsushima RG, Gaisano HY. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J Biol Chem 279: 53259–53265, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Dascher C, Matteson J, Balch WE. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J Biol Chem 269: 29363–29366, 1994 [PubMed] [Google Scholar]
- 14. Deken SL, Beckman ML, Boos L, Quick MW. Transport rates of GABA transporters: regulation by the N-terminal domain and syntaxin 1A. Nat Neurosci 3: 998–1003, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Feinshreiber L, Chikvashvili D, Michaelevski I, Lotan I. Syntaxin modulates Kv1.1 through dual action on channel surface expression and conductance. Biochemistry 48: 4109–4114, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Fili O, Michaelevski I, Bledi Y, Chikvashvili D, Singer-Lahat D, Boshwitz H, Linial M, Lotan I. Direct interaction of a brain voltage-gated K+ channel with syntaxin 1A: functional impact on channel gating. J Neurosci 21: 1964–1974, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S. Abnormal expression of pancreatic islet exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology 143: 4218–4226, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gilbert A, Jadot M, Leontieva E, Wattiaux-De Coninck S, Wattiaux R. Delta F508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells. Exp Cell Res 242: 144–152, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci 113: 587–596, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J Cell Sci 119: 4353–4363, 2006 [DOI] [PubMed] [Google Scholar]
- 20a. Hohmeier HE, BeltrandelRio H, Clark SA, Henkel-Rieger R, Normington K, Newgard CB. Regulation of insulin secretion from novel engineered insulinoma cell lines. Diabetes 46: 968–977, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Horton N, Quick MW. Syntaxin 1A up-regulates GABA transporter expression by subcellular redistribution. Mol Membr Biol 18: 39–44, 2001 [PubMed] [Google Scholar]
- 22. Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hughes SJ, Faehling M, Thorneley CW, Proks P, Ashcroft FM, Smith PA. Electrophysiological and metabolic characterization of single beta-cells and islets from diabetic GK rats. Diabetes 47: 73–81, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Huopio H, Shyng SL, Otonkoski T, Nichols CG. KATP channels and insulin secretion disorders. Am J Physiol Endocrinol Metab 283: E207–E216, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett 409: 232–236, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Ji J, Yang SN, Huang X, Li X, Sheu L, Diamant N, Berggren PO, Gaisano HY. Modulation of L-type Ca(2+) channels by distinct domains within SNAP-25. Diabetes 51: 1425–1436, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kang Y, Huang X, Pasyk EA, Ji J, Holz GG, Wheeler MB, Tsushima RG, Gaisano HY. Syntaxin-3 and syntaxin-1A inhibit L-type calcium channel activity, insulin biosynthesis and exocytosis in beta-cell lines. Diabetologia 45: 231–241, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, Tsushima RG, Light PE, Gaisano HY. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem 279: 47125–47131, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res 29: 1405–1409, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Leung YM, Kang Y, Gao X, Xia F, Xie H, Sheu L, Tsuk S, Lotan I, Tsushima RG, Gaisano HY. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J Biol Chem 278: 17532–17538, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Lin CW, Yan F, Shimamura S, Barg S, Shyng SL. Membrane phosphoinositides control insulin secretion through their effects on ATP-sensitive K+ channel activity. Diabetes 54: 2852–2858, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin YW, Bushman JD, Yan FF, Haidar S, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism. J Biol Chem 283: 9146–9156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mankouri J, Taneja TK, Smith AJ, Ponnambalam S, Sivaprasadarao Kir6 A. 2 mutations causing neonatal diabetes prevent endocytosis of ATP-sensitive potassium channels. EMBO J 25: 4142–4151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control KATP channel surface density. J Biol Chem 285: 5963–5973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michaelevski I, Chikvashvili D, Tsuk S, Singer-Lahat D, Kang Y, Linial M, Gaisano HY, Fili O, Lotan I. Direct interaction of target SNAREs with the Kv2.1 channel Modal regulation of channel activation and inactivation gating. J Biol Chem 278: 34320–34330, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes 48: 2367–2373, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Naren AP, Quick MW, Collawn JF, Nelson DJ, Kirk KL. Syntaxin 1A inhibits CFTR chloride channels by means of domain-specific protein-protein interactions. Proc Natl Acad Sci USA 95: 10972–10977, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, Matsushima S, Kawakami H, Watanabe T, Akagawa K, Nagamatsu S. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol 177: 695–705, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ostenson CG, Chen J, Sheu L, Gaisano HY. Effects of palmitate on insulin secretion and exocytotic proteins in islets of diabetic Goto-Kakizaki rats. Pancreas 34: 359–363, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes 55: 435–440, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Pabon A, Chan KW, Sui JL, Wu X, Logothetis DE, Thornhill WB. Glycosylation of GIRK1 at Asn119 and ROMK1 at Asn117 has different consequences in potassium channel function. J Biol Chem 275: 30677–30682, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem 279: 4234–4240, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Peters KW, Qi J, Watkins SC, Frizzell RA. Syntaxin 1A inhibits regulated CFTR trafficking in xenopus oocytes. Am J Physiol Cell Physiol 277: C174–C180, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Schweizer A, Fransen JA, Bachi T, Ginsel L, Hauri HP. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol 107: 1643–1653, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seagar M, Takahashi M. Interactions between presynaptic calcium channels and proteins implicated in synaptic vesicle trafficking and exocytosis. J Bioenerg Biomembr 30: 347–356, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol 110: 655–664, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci 23: 1697–1709, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taneja TK, Mankouri J, Karnik R, Kannan S, Smith AJ, Munsey T, Christesen HB, Beech DJ, Sivaprasadarao A. Sar1-GTPase-dependent ER exit of KATP channels revealed by a mutation causing congenital hyperinsulinism. Hum Mol Genet 18: 2400–2413, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Taschenberger G, Mougey A, Shen S, Lester LB, LaFranchi S, Shyng SL. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J Biol Chem 277: 17139–17146, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122: 893–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yan F, Lin CW, Weisiger E, Cartier EA, Taschenberger G, Shyng SL. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem 279: 11096–11105, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Yan FF, Lin CW, Cartier EA, Shyng SL. Role of ubiquitin-proteasome degradation pathway in biogenesis efficiency of β-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol 289: C1351–C1359, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 56: 2339–2348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu YX, Shen L, Xia P, Tang YW, Bao L, Pei G. Syntaxin 1A promotes the endocytic sorting of EAAC1 leading to inhibition of glutamate transport. J Cell Sci 119: 3776–3787, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol 13: 638–646, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.