Abstract
OBJECTIVE
The study objective was to assess the relationship between β-cell function and HbA1c.
RESEARCH DESIGN AND METHODS
A total of 522 Mexican American subjects participated in this study. Each subject received a 75-g oral glucose tolerance test (OGTT) after a 10- to 12-h overnight fast. Insulin sensitivity was assessed with the Matsuda index. Insulin secretory rate was quantitated from deconvolution of the plasma C-peptide concentration. β-Cell function was assessed with the insulin secretion/insulin resistance (IS/IR) (disposition) index and was related to the level of HbA1c.
RESULTS
At HbA1c levels <5.5%, both the Matsuda index of insulin sensitivity and IS/IR index were constant. However, as the HbA1c increased >5.5%, there was a precipitous decrease in both the Matsuda index and the IS/IR index. Subjects with HbA1c = 6.0–6.4% had a 44 and 74% decrease in the Matsuda index and the IS/IR index, respectively, compared with subjects with HbA1c <5.5% (P < 0.01 for both indices). Subjects with normal glucose tolerance and HbA1c <5.7% had β-cell function comparable to that of subjects with normal glucose tolerance with HbA1c = 5.7–6.4%. However, subjects with impaired fasting glucose or impaired glucose tolerance had a marked decrease in β-cell function independent of their HbA1c level.
CONCLUSIONS
The results of the current study demonstrate that in Mexican Americans, as HbA1c increases >6.0%, both insulin sensitivity and β-cell function decrease markedly. Performing an OGTT is pivotal for accurate identification of subjects with impaired β-cell function.
In 1997, the American Diabetes Association (ADA) revised its criteria for the diagnosis of type 2 diabetes and determined that subjects with fasting plasma glucose (FPG) >126 mg/dL and 2-h plasma glucose ≥200 mg/dL are considered to have type 2 diabetes (1). These cut points were chosen on the basis of the increased incidence of diabetic retinopathy rather than on the presence of metabolic abnormalities (i.e., insulin resistance and β-cell dysfunction) that are responsible for type 2 diabetes (1).
Impaired β-cell function is the principal factor responsible for the development and progression of type 2 diabetes (2). In addition to β-cell dysfunction, subjects with type 2 diabetes manifest severe insulin resistance in skeletal muscle, liver, and adipocytes (3–6). Insulin resistance is the earliest metabolic abnormality detected in subjects destined to develop type 2 diabetes. In response to insulin resistance, the β-cell appropriately increases insulin secretion and normal glucose tolerance (NGT) is maintained. However, when β-cell failure ensues, glucose intolerance develops. Initially, this is manifest as impaired glucose tolerance (IGT) and subsequently as overt diabetes (1). Thus, impaired β-cell function is an essential condition in the development of type 2 diabetes (1).
Although normal β-cell function is pivotal to the maintenance of NGT, β-cell failure develops long before hyperglycemia becomes evident. Recent studies have demonstrated that the decrease in β-cell function begins in the range considered to be well within NGT according to the 1997 ADA criteria (7–10). Studies that have related β-cell function to FPG (7,8) and 2-h plasma glucose (9,10) concentrations reported that β-cell function progressively declined with the increase in both FPG and 2-h plasma glucose from the low normal range to the high normal range, to the impaired glucose tolerant and diabetic ranges. These results indicate that the decrease in β-cell function, which is the primary factor responsible for the deterioration of glucose tolerance, is a continuum with no threshold above which β-cell dysfunction develops.
ADA recently changed the diagnostic criteria for type 2 diabetes to include individuals with HbA1c ≥6.5%; high-risk individuals are defined as having an HbA1c = 5.7–6.4% (11,12). No data are available relating the HbA1c to β-cell function. Therefore, the aim of the current study was to examine the relationship between β-cell function and HbA1c.
RESEARCH DESIGN AND METHODS
Subjects
The participants in this study included 522 subjects of Mexican American descent who were part of the San Antonio Veterans Administration Genetic Epidemiology Study (5). In the Veterans Administration Genetic Epidemiology Study, Mexican American families with one diabetic and one nondiabetic parent and two siblings with type 2 diabetes were recruited through advertising within the medical center and in local newspapers. Subjects responding to the advertisement were screened with a 75-g oral glucose tolerance test (OGTT). All family members who responded to the advertisement and fulfilled the inclusion criteria agreed to participate in the study. This study reports on 522 subjects who were free of diabetes and received a 75-g OGTT and had NGT, IGT, impaired fasting glucose (IFG), or type 2 diabetes based on the 2003 glucose criteria established by ADA (13). None of the subjects with type 2 diabetes knew that he/she had diabetes, and type 2 diabetes was diagnosed for the first time with the OGTT. Thus, no type 2 diabetic subject in the study had used antidiabetic medications.
All subjects had normal liver, cardiopulmonary, and kidney function as determined by medical history, physical examination, screening blood tests, electrocardiogram, and urinalysis. No subject with NGT, IFG, IGT, or type 2 diabetes was taking any medication known to affect glucose tolerance. Body weight was stable (±2 kg) for at least 3 months before the study in all subjects. No subject participated in an excessively heavy exercise program. The study protocol was approved by the institutional review board of the University of Texas Health Science Center, San Antonio, and informed written consent was obtained from all subjects before their participation. All studies were performed at the General Clinical Research Center of the University of Texas Health Science Center at 0800 h after a 10- to 12-h overnight fast.
OGTT
Before the start of the OGTT, a small polyethylene catheter was placed into an antecubital vein, and blood samples were collected at −30, −15, 0, 15, 30, 45, 60, 75, 90, 105, and 120 min for the measurement of plasma glucose, C-peptide, and insulin concentrations. On the day of the OGTT, height, weight, and waist circumference were determined at the narrowest part of the torso, and a blood sample was obtained for HbA1c measurement.
Analytic techniques
Plasma glucose concentration was measured by the glucose oxidase reaction (Glucose Oxidase Analyzer, Beckman, Fullerton, CA). Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Linco Research, St. Louis, MO). HbA1c was measured with high-performance liquid chromatography.
Calculations
Insulin secretory rate (ISR) during the OGTT was calculated from deconvolution of the plasma C-peptide concentration as previously described (8), and the incremental area under the ISR curve was related to the incremental area under the plasma glucose curve (ΔISR[AUC]0–120/ΔG[AUC]0–120). The insulin secretion/insulin resistance (IS/IR) (disposition) index was determined by dividing ΔISR/ΔG by the severity of insulin resistance [ΔISR(AUC)0–120/ΔG(AUC)0–120 ÷ IR], as measured by the inverse of the Matsuda index (14). The Matsuda index incorporates both hepatic and muscle components of insulin resistance, correlates well with the measurement of insulin sensitivity from the euglycemic insulin clamp, and was calculated as follows:
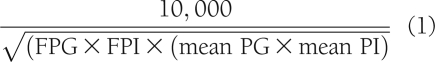 |
The incremental area under the ISR curve [ΔISR(AUC)0–120] and the incremental area under the plasma glucose concentration curve [ΔG(AUC)0–120] were calculated according to the trapezoid rule.
Statistical analysis
Subjects were divided into deciles based on the HbA1c, and the mean HbA1c in each decile was related to the mean IS/IR index in the same decile. Data are presented as the mean ± SD. For comparison between two groups, Student t test was used. To compare the mean of more than two groups, ANOVA was used. Significant differences were confirmed by the Bonferroni test. Statistical significance was considered at P < 0.05.
RESULTS
Table 1 presents the characteristics of the study participants. The 21.5% of subjects had NGT, 35.9% had IFG and/or IGT, and 42.6% had type 2 diabetes according to the 2003 ADA criteria (13). However, if subjects were classified on the basis of the HbA1c level according to the ADA clinical practice recommendation (12), only 29.5% had type 2 diabetes (HbA1c ≥6.5%) and 21.4% were characterized as high-risk individuals (HbA1c = 5.7–6.4%), whereas 49.1% had NGT (HbA1c <5.7%) (Table 2).
Table 1.
Characteristics of study participants: diagnosis of glucose tolerance status is based on the 2003 ADA criteria
| Age (years) | 47 ± 1 |
| Sex (M/F) | 178/344 |
| BMI (kg/m2) | 33.0 ± 0.3 |
| Waist circumference (cm) | 100.9 ± 0.8 |
| FPG (mg/dL) | 120 ± 2 |
| HbA1c (%) | 6.2 + 0.07 |
| NGT (%) | 21.5 |
| IFG and/or IGT (%) | 35.9 |
| Type 2 diabetes (%) | 42.6 |
Table 2.
Relationship between HbA1c and diagnostic category (NGT, IFG and/or IGT, and type 2 diabetes) based on glucose criteria during the OGTT according to the 2003 ADA criteria
| NGT | IFG and/or IGT | Type 2 diabetes | Total | |
|---|---|---|---|---|
| HbA1c <5.7% | 97 | 128 | 31 | 256 |
| HbA1c = 5.7–6.4% | 15 | 56 | 40 | 111 |
| HbA1c ≥6.5% | 0 | 3 | 151 | 154 |
| HbA1c <5.5% | 90 | 103 | 28 | 221 |
| HbA1c = 5.5–6.0% | 18 | 62 | 21 | 101 |
| HbA1c >6.0% | 5 | 22 | 172 | 199 |
We divided subjects with HbA1c <5.7% (columns A and B in Table 3) and high-risk individuals with HbA1c = 5.7–6.4% (columns D and E in Table 3) into two groups based on plasma glucose values during the OGTT: i) NGT (FPG <100 mg/dL and 2-h plasma glucose <140 mg/dL, columns A and D) and ii) IFG and/or IGT (FPG = 100–125 mg/dL or 2 h plasma glucose = 140–199 mg/dL, columns B and E), and compared the metabolic characteristics of the various groups. Table 3 demonstrates that, in subjects with NGT with HbA1c = 5.7–6.4% (column D), the Matsuda index of insulin sensitivity was decreased by 35% compared with NGT subjects with HbA1c <5.7% (column A) (3.1 ± 0.5 and 4.5 ± 0.4, respectively, P = 0.03). However, the IS/IR (disposition) index was comparable between the two groups (Table 3). Likewise, in subjects with IFG and/or IGT and an HbA1c = 5.7–6.4% (column E), the Matsuda index of insulin sensitivity was significantly reduced compared with subjects with HbA1c <5.7% (column B) (3.1 ± 0.5 and 2.2 ± 0.2, respectively, P < 0.001). However, the IS/IR index in subjects with IFG and/or IGT was similarly reduced in both groups (Table 3). Changing the HbA1c cut points to <5.5%, 5.5–6%, 6.0–6.49%, and 6.5–7.0% (Table 3) demonstrated that NGT groups with HbA1c <5.5% (column G) and HbA1c = 5.5–6.0% (column H) were more insulin-sensitive and had a greater insulin secretion/insulin resistance (disposition) index compared with subjects with IFG and/or IGT with HbA1c <5.5% (column H) and HbA1c = 5.5–6% (column K). However, subjects with HbA1c = 6–6.5% (column M) and 6.5–7% (column N) had a marked decrease in the insulin secretion/insulin resistance index (0.1 ± 0.01 and 0.07 ± 0.02, respectively) compared with subjects with HbA1c <6.0%.
Table 3.
Relationship between HbA1c and measures of glucose tolerance, insulin sensitivity, and β-cell function
| HbA1c <5.7% |
HbA1c = 5.7–6.5% |
HbA1c <5.5% |
HbA1c = 5–5.9% |
HbA1c = 6.0–6.49% | HbA1c = 6.5–7.0% | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NGT | IFG and/or IGT | P value | NGT | IFG and/or IGT | P value | NGT | IGT and/or IFG | P value | NGT | IGT and/or IFG | P value | ||||
| Column no. | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O |
| n | 122 | 105 | 25 | 46 | 90 | 103 | 18 | 61 | 46 | 29 | |||||
| HbA1c (%) | 4.9 ± 0.1 | 5.1 ± 0.05 | <0.05 | 5.8 ± 0.04 | 6.0 ± 0.05 | NS | 4.8 ± 0.07 | 4.9 ± 0.05 | NS | 5.6 ± 0.03 | 5.7 ± 0.02 | NS | 6.2 ± 0.02 | 6.8 ± 0.03 | <0.001 |
| FPG (mg/dL) | 90 ± 1 | 99 ± 1 | <0.0001 | 90 ± 2 | 109 ± 2 | <0.0001 | 89 ± 1 | 99 ± 1 | <0.0001 | 90 ± 1 | 100 ± 1 | <0.0001 | 110 ± 2 | 135 ± 4 | <0.001 |
| 2-h PG (mg/dL) | 116 ± 2 | 163 ± 3 | <0.0001 | 121 ± 4 | 192 ± 5 | <0.0001 | 116 ± 2 | 151 ± 2 | <0.0001 | 118 ± 4 | 157 ± 3 | <0.0001 | 197 ± 8 | 261 ± 9 | <0.001 |
| ∆G0–120 (mg/dL) | 75 ± 3 | 124 ± 3 | <0.0001 | 88 ± 7 | 150 ± 5 | <0.0001 | 75 ± 3 | 112 ± 3 | <0.0001 | 85 ± 8 | 112 ± 5 | <0.0001 | 162 ± 7 | 174 ± 8 | NS |
| ∆ISR0–120 | 11.5 ± 0.7 | 10.6 ± 0.7 | NS | 13.9 ± 1.9 | 8.0 ± 0.7 | NS | 11.2 ± 0.7 | 10.8 ± 0.9 | NS | 14.3 ± 1.7 | 11.4 ± 1.0 | NS | 7.5 ± 0.9 | 4.7 ± 1 | <0.05 |
| ∆ISR/∆G0–120 | 0.17 ± 0.01 | 0.1 ± 0.01 | <0.0001 | 0.16 ± 0.02 | 0.07 ± 0.01 | <0.0001 | 0.17 ± 0.01 | 0.11 ± 0.01 | <0.0001 | 0.2 ± 0.04 | 0.11 ± 0.01 | <0.0001 | 0.05 ± 0.01 | 0.03 ± 0.01 | <0.05 |
| Matsuda index | 4.5 ± 0.4 | 3.1 ± 0.2 | <0.001 | 3.1 ± 0.5 | 2.2 ± 0.2 | <0.001 | 4.6 ± 0.4 | 3.0 ± 0.2 | <0.0001 | 3.7 ± 0.4 | 2.7 ± 0.2 | <0.01 | 2.2 ± 0.2 | 2.3 ± 0.2 | NS |
| ∆ISR/∆G0–120 × Matsuda | 0.56 ± 0.09 | 0.25 ± 0.02 | <0.0001 | 0.46 ± 0.07 | 0.15 ± 0.03 | <0.0001 | 0.56 ± 0.03 | 0.27 ± 0.03 | <0.0001 | 0.64 ± 0.08 | 0.3 ± 0.04 | <0.0001 | 0.1 ± 0.01 | 0.07 ± 0.02 | NS |
PG, plasma glucose; NS, not significant.
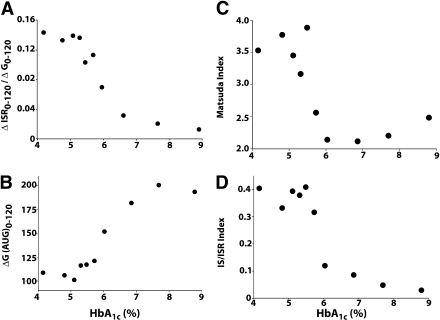
When all subjects were pooled into one group and the Matsuda index of insulin sensitivity and IS/IR index were related to the HbA1c as a continuous variable, the relationship between the two was highly nonlinear. Whole-body insulin sensitivity, measured with the Matsuda index, remained unchanged up to an HbA1c = 5.5%. However, as the HbA1c increased >5.5%, there was a steep decrease in the Matsuda index (Fig. 1). Subjects with HbA1c = 6.0–6.4% had a 44% decrease in insulin sensitivity compared with subjects with HbA1c <5.5% (P < 0.01).
Figure 1.
Relationship between HbA1c and ΔISR0–120/ΔG0–120 (A), ΔG(AUC)0–120 (B), Matsuda index of insulin sensitivity (C), and IS/IR index (D) in all 521 subjects.
The postload plasma glucose concentration, measured as the incremental area under the plasma glucose curve (∆G0–120), remained unchanged with the increase in HbA1c up to a value = 5.5%, and, as observed when the HbA1c exceeded 5.5%, ∆G0–120 progressively increased with the increase in HbA1c. Likewise, insulin secretion, measured as the incremental area under the ISR (∆ISR0–120/∆G0–120) curve, remained unchanged up to an HbA1c = 5.5%, and, as observed when the HbA1c increased >5.5%, ∆ISR0–120/∆G0–120 progressively decreased with further increases in the HbA1c. β-Cell function, measured with the IS/IR (disposition) index, did not change significantly up to an HbA1c = 5.7%. However, as the HbA1c increased >5.7%, there was a marked decrease in β-cell function. Subjects with HbA1c = 6.0% had a 62% decrease in the IS/IR index compared with subjects with HbA1c <5.7%.
CONCLUSIONS
Although the HbA1c represents the mean plasma glucose level (fasting and postprandial) throughout the day, the results of the current study demonstrate that, in Mexican Americans, the relationship between β-cell function and HbA1c differs significantly from the relationship between β-cell function and the fasting and 2-h plasma glucose concentrations (7–10). Similarly, the relationship between insulin sensitivity and HbA1c differs from that obtained using the fasting and 2-h plasma glucose concentrations (3). Although both insulin sensitivity (measured with the euglycemic insulin clamp) (3) and β-cell function (measured with the IS/IR index) progressively decreased with the increase in both fasting (8) and 2-h (9) plasma glucose concentrations, they remained unchanged with the increase in HbA1c up to a value = 5.5%; thereafter, both insulin sensitivity and β-cell function precipitously decreased with increasing HbA1c levels >5.5%.
ADA and the International Diabetes Federation recently revised their criteria for the diagnosis of type 2 diabetes and high-risk individuals (11,12). With both criteria, subjects with an HbA1c >6.5% are diagnosed with type 2 diabetes. The ADA criteria state that subjects with HbA1c = 5.7–6.5% are at high risk to develop diabetes, whereas the international expert committee suggested that subjects with HbA1c = 6.0–6.5% represent high-risk individuals. The results of the current study demonstrate that, in Mexican Americans, subjects with HbA1c = 6.0% already manifest severe forms of both core defects that are characteristic of type 2 diabetes, i.e., insulin resistance (44% decrease in insulin sensitivity) and β-cell dysfunction (62% decrease in IS/IR index). In subjects with HbA1c = 6.0–6.4%, insulin sensitivity, measured with the Matsuda index, did not differ from that in subjects with HbA1c ≥6.5%, and the IS/IR index was decreased by 74% compared with subjects with HbA1c <5.5%. Furthermore, the majority of studies that have evaluated the relationship between the incidence of diabetic retinopathy and HbA1c have reported a significant increase in the incidence of diabetic retinopathy as the HbA1c increased >6.0% (15–20). For example, the threshold for the increase in retinopathy was 5.5% in the 2005–2006 National Health and Nutrition Examination Survey (15) and the Hisayama study (16), 6.0% in the National Health and Nutrition Examination Survey III (17), 6.2% in the Pima Indian study (18), and 6.3% in the Egyptian study (19). Moreover, when the risk for heart attack and stroke was related to HbA1c in nondiabetic subjects in the Atherosclerosis Risk in Communities Study (21), subjects with HbA1c = 6–6.4% had a 78% increase in the risk for heart attack and stroke compared with subjects with HbA1c <5.5%. Taken together, these results indicate that subjects with HbA1c = 6.0–6.4% manifest maximal insulin resistance with ∼75% decrease in β-cell function and increased risk of diabetic retinopathy and cardiovascular disease. On the basis of these pathophysiologic and anatomic abnormalities, these subjects should be considered to have type 2 diabetes, and an HbA1c cut point of 6.0% seems more appropriate for the diagnosis of type 2 diabetes than the HbA1c cut point of ≥6.5% established by both ADA and the international expert committee. Moreover, consistent with other studies (22–25), an HbA1c cut point of 6.5% underdiagnoses many subjects with type 2 diabetes. The prevalence of type 2 diabetes in this cohort was only 29.5% (HbA1c ≥6.5%) compared with 42.6% with the 2003 ADA criteria based on fasting and 2-h plasma glucose levels (Table 2). Conversely, if an HbA1c cut point of 6.0% is used to diagnose type 2 diabetes, the prevalence of type 2 diabetes in this cohort would be 38%, which is comparable to that with the ADA glucose criteria. Thus, an HbA1c cut point of 6.5% for the diagnosis of type 2 diabetes would leave ∼30% of subjects with type 2 diabetes undiagnosed. Moreover, in subjects with HbA1c = 6.0–6.5%, only ∼5% were nondiabetic and ∼95% had type 2 diabetes based on the results of the OGTT (Table 2). Thus, decreasing the HbA1c cut point for type 2 diabetes from 6.5 to 6.0% would result in only a small number of false positives.
Although both insulin sensitivity and β-cell function remained unchanged in individuals with HbA1c <5.7%, approximately half of the subjects in this group had IFG and/or IGT and therefore are at increased risk of future type 2 diabetes and could benefit from an intervention program aimed to reduce their future type 2 diabetes risk, e.g., weight loss and exercise. However, according to the new ADA criteria (HbA1c <5.7%), this large group of individuals would be considered to have NGT and would remain unidentified as having glucose intolerance. Conversely, when subjects were stratified on the basis of the results of the OGTT, those with IFG and or IGT (despite having HbA1c <5.7%) had a marked decrease in β-cell function compared with subjects with NGT (55% decrease in IS/IR index, Table 3). Likewise, subjects with NGT with HbA1c = 5.7–6.4% had comparable β-cell function compared with subjects with NGT with HbA1c <5.7%. Subjects with IFG and/or IGT with HbA1c = 5.7–6.4% had a further decrease in β-cell function. These results demonstrate that the OGTT provides a better tool to identify subjects with β-cell failure compared with the HbA1c. It is likely that challenging the β-cell with a glucose load provides a “stress test” to the β-cell and exposes more subtle decreases in β-cell function compared with measurements taken during the fasting state, e.g., HbA1c. Because β-cell function is the principal factor responsible for the development of type 2 diabetes, these results underscore the importance of performing an OGTT for the assessment of β-cell health and identification of subjects at increased future risk for type 2 diabetes.
A limitation to this study is that both insulin sensitivity and β-cell function were measured with OGTT-derived indices. Although these indices were validated with the insulin clamp, additional studies with the gold standard measurements (euglycemic insulin clamp and hyperglycemic clamp) are desirable to provide definitive evidence. Because of increased rate of obesity in Mexican Americans (mean BMI = 33), β-cell dysfunction could become evident at an earlier stage of glucose intolerance compared with other ethnic groups. Therefore, validation of the present findings in other ethnic groups is warranted.
In summary, the results of the current study demonstrate that Mexican American subjects with HbA1c >6% manifest both core defects of type 2 diabetes in severe form (44 and 74% decrease in insulin sensitivity and β-cell function, respectively). In addition, a cut point of HbA1c = 6.0% is comparable to the OGTT in identifying subjects with type 2 diabetes. These observations, together with the increase in diabetic microvascular complications in subjects with HbA1c >6.0%, favor using a cut point of HbA1c = 6.0% for the diagnosis of type 2 diabetes. Furthermore, the results of this study demonstrate that the OGTT represents a better tool for the identification of subjects with β-cell dysfunction who are at increased future risk for type 2 diabetes.
Acknowledgments
This work was supported by American Heart Association Grant 10SDG4470014 (to M.A.A.-G.). M.K. is supported by the Turkish Diabetes, Obesity, and Nutrition Association, the Turkish Diabetes Foundation, and the University of Abant Izzet Baysal.
No potential conflicts of interest relevant to this article were reported.
C.J., D.W., L.N., and N.A. contributed to data generation. M.K. and M.A.A.-G. performed the data analysis. M.A.A.-G. wrote the article. R.A.D. reviewed the manuscript.
The authors thank the nurses, James King, John Kincaid, Rose Kaminski-Graham, and Norma Diaz (BRU, Audie Murphy VA Hospital) for assistance in performing the OGTT studies and providing excellent care of the patients throughout the study. Lorrie Albarado (Diabetes Division, UT Health Science Center at San Antonio) provided expert secretarial assistance in manuscript preparation.
References
- 1.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep 2009;9:193–199 [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 6.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godsland IF, Jeffs JA, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, Matsuda M, Jani R, et al. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 2008;295:E401–E406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2003;47:31–39 [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 11.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;(Suppl. 1)S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 15.Cheng YJ, Gregg EW, Geiss LS, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care 2009;32:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki M, Kubo M, Kiyohara Y, et al. Hisayama study Comparison of diagnostic methods for diabetes mellitus based on prevalence of retinopathy in a Japanese population: the Hisayama study. Diabetologia 2004;47:1411–1415 [DOI] [PubMed] [Google Scholar]
- 17.Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA 1999;281:1203–1210 [DOI] [PubMed] [Google Scholar]
- 18.McCance DR, Hanson RL, Charles MA, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994;308:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelgau MM, Thompson TJ, Herman WH, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care 1997;20:785–791 [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, Liew G, Tapp RJ, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 2008;371:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Pang Z, Gao W, et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 2010;33:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van ’t Riet E, Alssema M, Rijkelijkhuizen JM, Kostense PJ, Nijpels G, Dekker JM. Relationship between A1C and glucose levels in the general Dutch population: the new Hoorn study. Diabetes Care 2010;33:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care 2010;33:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]