Bromocriptine is a sympatholytic D2-dopamine agonist that has been approved for the treatment of type 2 diabetes. Based on animal and human studies, timed bromocriptine administration within 2 h of awakening is believed to augment low hypothalamic dopamine levels and inhibit excessive sympathetic tone within the central nervous system (CNS), resulting in a reduction in postmeal plasma glucose levels due to enhanced suppression of hepatic glucose production. Bromocriptine has not been shown to augment insulin secretion or enhance insulin sensitivity in peripheral tissues (muscle). Addition of bromocriptine to poorly controlled type 2 diabetic patients treated with diet alone, metformin, sulfonylureas, or thiazolidinediones produces a 0.5–0.7 decrement in HbA1c. Bromocriptine also reduces fasting and postmeal plasma free fatty acid (FFA) and triglyceride levels. In a 52 double-blind, placebo-controlled study in type 2 diabetic patients, bromocriptine reduced the composite cardiovascular end point by 40%. The mechanism of the drug’s beneficial effect on cardiovascular disease remains to be determined.
Introduction
Type 2 diabetes is a chronic metabolic disorder characterized by insulin resistance, impaired β-cell function, and multiple other metabolic/endocrine abnormalities (1). Because of its multifactorial pathogenesis, restoration of normoglycemia is difficult to achieve and requires multiple antidiabetic medications that have different mechanisms of action and can be used in combination to produce an additive effect (1,2). Therefore, the development of antidiabetic agents that have novel mechanisms of action and can be used in combination with currently approved medications for the treatment of type 2 diabetes is highly desirable.
Type 2 diabetic patients are at high risk for atherosclerotic cardiovascular complications (3). Although hyperglycemia is a risk factor for coronary artery disease and stroke, it is a relatively weak risk factor compared with other more established risk factors such as dyslipidemia, hypertension, obesity, and the insulin resistance (metabolic) syndrome (4,5). However, even after correction of dyslipidemia, hypertension, and dysglycemia, type 2 diabetic patients still remain at high risk for atherosclerotic cardiovascular complications (6). Therefore, antidiabetic agents that not only improve glycemia but also reduce cardiovascular risk are desirable.
Recently, timed-release bromocriptine (Cycloset), a sympatholytic dopamine D2 receptor agonist, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of type 2 diabetes. This centrally acting antidiabetic agent has a novel mechanism of action; reduces plasma glucose, triglyceride, and FFA levels; and in a prospective 1-year study reduced cardiovascular events. In this review, we will examine the mechanism of action, pharmacokinetic properties, glucose-lowering efficacy, potential antiatherogenic benefits, and safety of Cycloset.
Mechanism of action
Bromocriptine is unique in that it does not have a specific receptor that mediates its action on glucose and lipid metabolism. Rather, its effects are mediated via resetting of dopaminergic and sympathetic tone within the CNS (7). Because the human brain is not accessible to sampling, much of what we have learned about the mechanism of action of bromocriptine has been derived from animal studies.
Mammalian species living in the wild have an incredible ability to alter their metabolism from the insulin-sensitive/glucose-tolerant state to the insulin-resistant/glucose-intolerant state at exactly the right time of the year to survive long periods when food is sparse (rev. in 7). During transition to the insulin-resistant state, basal lipolytic activity increases to spare glucose utilization by peripheral (muscle) tissues, fat oxidation becomes predominant, and hepatic glucose production and gluconeogenesis rise to supply glucose to the CNS during prolonged periods (seasons) of food deprivation. At the end of the season, animals revert back to their insulin-sensitive/glucose-tolerant state. Such seasonal metabolic changes are characteristic of all migrating birds and hibernating animals and are governed by changes in monoaminergic concentrations/activity in the suprachiasmatic nuclei (SCN) of the hypothalamus—the mammalian circadian pacemaker—and in the ventromedial hypothalamus (VMH) (7). These neurogenic and metabolic changes are consistent with the thrifty gene hypothesis (8), which proposes that conversion to the obese, insulin-resistant state during periods of inadequate food supply provides a survival advantage. It is noteworthy that development of the insulin-resistant state during these periods of seasonal change precisely mimics the type 2 diabetic state: insulin resistance in muscle and liver, accelerated hepatic glucose production/gluconeogenesis, hyperglycemia, adipocyte insulin resistance and increased lipolysis, enhanced fat oxidation, increased plasma FFA and triglyceride levels, and obesity. These changes also mimic those observed in people with the insulin resistance syndrome (5,9).
A large body of evidence implicates endogenous dopaminergic and serotonergic rhythms in SCN and VMH in the transition from the insulin-sensitive to insulin-resistant state. The VMH has multiple connections with other hypothalamic nuclei and plays a pivotal role in modulating autonomic nervous system function, hormonal secretion, peripheral glucose/lipid metabolism, and feeding behavior (10–13).
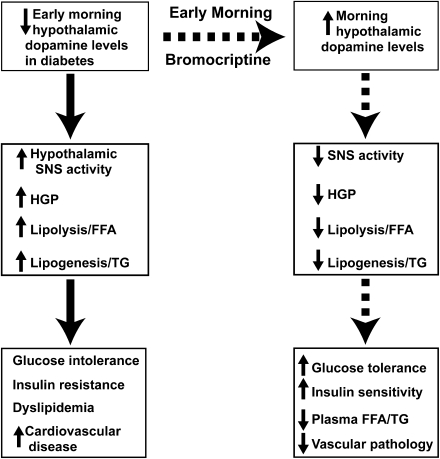
Within the VMH, multiple studies have documented that both serotonin and noradrenergic levels and activity are increased during the insulin-resistant state and decrease to normal with return to the insulin-sensitive state in animals that undergo seasonal changes in metabolism (14–19). Conversely, dopamine levels are low during the insulin-resistant state and increase to normal following return of the insulin-sensitive state (20,21). Further, selective destruction of dopaminergic neurons in the SCN causes severe insulin resistance (22), and animal models of nonseasonal obesity (i.e., ob/ob mouse [16], Zucker fatty rat [23], high energy–fed male Sprague-Dawley rats [24]) have reduced dopamine levels in VMH and lateral hypothalamic nuclei. Chronic infusion of norepinephrine and/or serotonin into the VMH of insulin-sensitive animals causes severe insulin resistance, glucose intolerance, and accelerated lipolysis in hamsters and rats (19,25). Conversely, systemic (20,26,27) and intracerebral (28) bromocriptine administration in insulin-resistant animals leads to a decrease in elevated VMH noradrenergic and serotonergic levels (measured in vivo by microdialysis) with a resultant decline in hepatic glucose production/gluconeogenesis, reduced adipose tissue lipolysis, and improved insulin sensitivity. Systemic bromocriptine also inhibits VMH responsiveness to norepinephrine (17), and, conversely, norepinephrine infusion into the VMH antagonizes the beneficial effect of bromocriptine on glucose tolerance and insulin sensitivity (29). Consistent with these observations in animals, systemic bromocriptine administration improves glycemic control and dyslipidemia without change in body weight in type 2 diabetic and obese nondiabetic humans (29–31). The proposed mechanism of action of bromocriptine to improve glucose tolerance is summarized in Fig. 1.
Figure 1.
Proposed mechanism of action of bromocriptine to improve glucose homeostasis and insulin sensitivity. HGP, hepatic glucose production; TG, triglyceride.
In summary, in vertebrates circadian rhythms of target tissue response to insulin, i.e., lipolysis, hepatic glucose production, and muscle insulin sensitivity, are mediated via circadian rhythms within the CNS, i.e., the SCN and VMH, and act temporarily to regulate seasonal changes in metabolism and body fat stores/muscle mass.
How do these circadian rhythms apply to humans and what are the implications for bromocriptine as a therapy for type 2 diabetes since humans do not manifest these pronounced circadian oscillations/seasonal changes in metabolism? As reviewed by Cincotta and colleagues (7,29,32), hypothalamic centers (SCN and VMH) that regulate these circadian rhythms not only receive photic inputs via the optic chiasm but also receive input from other centers throughout the CNS, neurogenic stimuli from peripheral tissues and gastrointestinal tract, hormonal signals, and signals from circulating metabolites. The net result after all of these inputs are integrated within the hypothalamus needs not to be circadian in nature. Nonetheless, interventions, such as bromocriptine, which alter monoamine neurotransmitter levels within these hypothalamic circadian centers, can exert significant effects on glucose and lipid metabolism.
Pharmacokinetics and dose
Following ingestion, Cycloset (bromocriptine mesylate) tablets are rapidly dissolved and absorbed within 30 min (29). When ingested on an empty stomach, the maximum plasma concentration is reached within 60 min. Absorption is delayed by food and peak plasma levels are achieved at ∼120 min in the fed state. There is extensive hepatic first-pass extraction and metabolism, and only 5–10% of the ingested dose reaches the systemic circulation (33–35). Ninety-eight percent of ingested bromocriptine is excreted via the biliary route with an elimination half-life of ∼6 h. Within the liver, bromocriptine is extensively metabolized by the cytochrome P450 system, specifically CYP3A4. There are some 20–30 metabolites, but their biologic activity is largely unknown.
Cycloset differs from traditional bromocriptine formulations, such as Parlodel, in its quick release that provides peak concentrations within 60 min. There is no AB-rated equivalent for Cycloset. Cycloset comes as 0.8 mg tablets. The starting dose is 0.8–1.6 mg/day and can be titrated to a maximum of 4.6 mg/day. Cycloset is administered as a once daily dose within 2 h of awaking. Type 2 diabetic individuals are believed to have an early morning dip in dopaminergic tone, which leads to increased sympathetic activity (25). In vertebrate species circadian plasma prolactin levels closely parallel changes in hypothalamic levels of dopamine and insulin sensitivity (rev. in 7 and 32). In lean, normal glucose-tolerant insulin-sensitive humans, plasma prolactin concentrations peak at night during sleep. In contrast, obese insulin-resistant individuals have elevated (twofold) day time plasma prolactin levels (29), consistent with reduced dopaminergic tone (36). Administration of Cycloset within 2 h of awakening reduces the elevated prolactin levels (29,37,38) and is thought to restore dopaminergic activity, thereby reducing postprandial plasma glucose, triglyceride, and FFA concentrations without increasing plasma insulin levels (see subsequent discussion).
Mechanism of action—human trials
A number of phase 2 trials have been performed to examine the mechanism of action of Cycloset. In a small study of 12 nondiabetic obese hyperinsulinemic (≥20 μU/mL) subjects (38), bromocriptine (1.6 mg/day for 2 weeks) reduced the fasting and postprandial (standardized meals) glucose levels without change in body weight and with a decrease in both fasting and postprandial plasma insulin concentrations by ∼50% (Supplementary Fig. 1).
In a similar study, 8 weeks of timed bromocriptine reduced day-long plasma glucose, triglyceride, and FFA levels without change in body weight and a small decrease in plasma insulin concentration in 13 nondiabetic obese women (Supplementary Fig. 2) (31). Insulin-stimulated glucose disposal, measured with the insulin suppression test, was not altered. Since the insulin suppression test primarily reflects insulin-mediated glucose disposal in muscle, the improvement in postprandial plasma glucose levels (31) most likely reflects enhanced suppression of hepatic glucose production by insulin, similar to what has been described in animals (26). However, to date, no study has examined hepatic glucose production following glucose ingestion or in response to a physiologic increase in plasma insulin concentration in man. The decline in postprandial plasma FFA and triglyceride concentrations (32) is similar to observations in animals (26,38).
In a 16-week double-blind, placebo-controlled study in 22 obese type 2 diabetic subjects treated with Cycloset for 16 weeks, HbA1c declined by 1.2%, fasting plasma glucose by 54 mg/dL, and mean plasma glucose during oral glucose tolerance test by 46 mg/dL without change in plasma insulin concentration, body weight, or percent body fat (30) (Supplementary Fig. 3). During a two-step euglycemic-hyperinsulinemic clamp, there was no improvement in insulin sensitivity during the first, physiologic insulin clamp step (80 μU/mL), although maximally insulin-stimulated (377 μU/mL) glucose disposal was increased. These results are consistent with those observed with the insulin suppression test (31) and demonstrate that, within the physiologic range of hyperinsulinemia, Cycloset does not improve insulin action in muscle. During the first insulin clamp step, suppression of hepatic glucose production was not enhanced by Cycloset. However, the steady-state plasma insulin concentration, although physiologic for muscle, was far above that required for half-maximal suppression of hepatic glucose production, making it difficult to ascertain whether hepatic insulin sensitivity was enhanced by insulin.
In a provocative study (39), insulin-treated type 2 diabetic subjects were randomized to placebo (n = 11) or Cycloset (n = 21) (4.8 mg/day) for 12 weeks. Compared with placebo, Cycloset reduced HbA1c by 0.7% and mean plasma glucose concentration (7 a.m. to 7 p.m.) by 8% without change in body weight. These results are consistent with an improvement in insulin sensitivity, although the site, i.e., liver versus muscle, remains to be defined.
In a small (n = 17) double-blind, placebo-controlled study (38) in nondiabetic obese individuals with elevated diurnal prolactin levels (changes in plasma prolactin parallel seasonal changes in insulin sensitivity and glucose tolerance in vertebrates [7]), Cycloset (1.6–2.4 mg/day) plus a 25% calorie-restricted diet significantly reduced body weight and body fat content compared with placebo plus diet. However, in a larger (n = 387) placebo-controlled 24-week study, Cycloset plus diet failed to produce a greater weight loss than placebo plus diet in obese nondiabetic subjects (29). Of interest, a post hoc analysis revealed that obese subjects with elevated diurnal prolactin levels (who represented ∼1/4 of the group) lost more weight (5.7 vs. 3.0 kg) than subjects with a normal prolactin rhythm.
Phase 3 efficacy trials
Four phase 3 trials have evaluated the efficacy of Cycloset versus placebo in the treatment of type 2 diabetic patients (29,40,41). In all studies, Cycloset was administered in the morning within 2 h of awaking and individuals with atypical day-night work shifts were excluded from study. These four studies included 1) 24-week monotherapy trial (n = 159) (29), 2) two 24-week add-on to sulfonylurea trials (n = 494) (30), and 3) 52-week add-on to various oral antidiabetic agents trial (41) (Table 1). Results of these four studies consistently demonstrated a placebo-subtracted decline in HbA1c of 0.5–0.7%. Prior to randomization and after 6 months, type 2 diabetic subjects in the monotherapy and add-on to sulfonylurea studies (29) were admitted to the clinical research center for 12 h (7 a.m. to 7 p.m.) and received standardized meals for breakfast (0700 h), lunch (1200 h), and dinner (1700 h). Serum glucose, insulin, FFA, and triglyceride concentrations were measured prior to and at 1 and 2 h postmeal ingestion. Relative to placebo, Cycloset significantly reduced the fasting, postbreakfast, postlunch, and postdinner glucose concentrations (Supplementary Fig. 4) without change in serum insulin level or body weight. Cycloset also significantly reduced fasting and postmeal serum FFA and triglyceride concentrations (Supplementary Fig. 5).
Table 1.
Phase 3 Cycloset efficacy trials
| N | Duration (months) | Baseline HbA1c (%) | Placebo-subtracted change in HbA1c (%) | P | |
|---|---|---|---|---|---|
| Monotherapy |
159 |
6 |
8.7 |
−0.56 |
<0.02 |
| Add-on to SU |
494 |
6 |
9.4 |
−0.55 |
<0.0001 |
| Add-on to various OHAs | 3,095 | 12 | 8.3 | −0.6 to −0.9 | <0.01–0.001 |
SU, sulfonylurea.
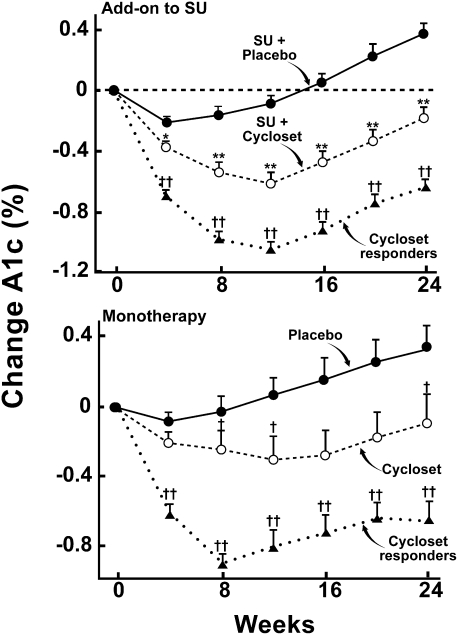
In the monotherapy and add-on to sulfonylurea studies, a prespecified analysis was performed on Cycloset responders (minimum HbA1c decrease from baseline = 0.3% at week 8) versus the entire Cycloset-treated group (Fig. 2). In monotherapy and add-on to sulfonylurea trials, the decrements in HbA1c from baseline were −0.65 and −0.63, respectively, and responders represented 63 and 65% of the total Cycloset-treated group (29). The placebo-subtracted difference in HbA1c in responders was 1% in both monotherapy and add-on to sulfonylurea trials (Fig. 2).
Figure 2.
Change in HbA1c in Cycloset (total group) and placebo-treated diabetic subjects. Cycloset responders (defined as a ≥0.3% decrease in HbA1c at week 8) had a significantly greater decline in HbA1c (placebo-subtracted ΔHbA1c = 1.0%) than the total group (ref. 29). SU, sulfonylurea. *P < 0.01; **P < 0.001; †P < 0.04 for Cycloset responders vs. total Cycloset group; ††P < 0.0001 for Cycloset responders vs. placebo.
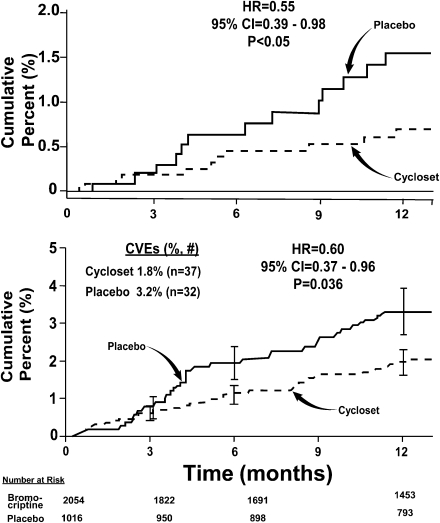
Figure 3.
Top: Kaplan-Meier plot of time to first cardiovascular event (myocardial infarction, stroke, hospitalization for angina or CHF, coronary revascularization, and death) in 3,070 type 2 diabetic subjects treated with Cycloset or placebo for 52 weeks (ref. 41). Bottom: Kaplan-Meier plot of time to first cardiovascular (MACE) event (myocardial infarction, stroke, and death) in type 2 diabetic subjects treated with Cycloset or placebo for 52 weeks (ref. 41). CVEs, cardiovascular events.
Efficacy data also are available from a large 52-week randomized, double-blind, placebo-controlled trial in which Cycloset was added to therapy in poorly controlled (HbA1c >7.5%) type 2 diabetic patients who were taking one to two oral hypoglycemic agents (OHAs) (41–43). Mean baseline HbA1c was 8.3%, mean age 58 years, mean BMI 33 kg/m2; 63% were male. In type 2 diabetic subjects who completed 24 weeks of treatment and who took ≥80% of their medication, the placebo-subtracted decrease in HbA1c ranged from 0.6 to 0.9% and was consistent in subjects failing any OHA, failing metformin ± OHA, failing metformin + sulfonylurea, and failing thiazolidinedione ± OHA (Supplementary Fig. 6) (41–43).
Safety and tolerability
In the Cycloset monotherapy and add-on to sulfonylurea trials (29), side effects that occurred more commonly in Cycloset versus placebo were nausea (26 vs. 5%), asthenia (15 vs. 8%), constipation (11 vs. 4%), dizziness (11 vs. 6%), and rhinitis (8 vs. 5%). In general, these side effects were mild and transient. Thirteen percent of Cycloset-treated subjects withdrew because of adverse events compared with 3–5% of placebo-treated subjects (P < 0.01). There was no increase in serious adverse events in the Cycloset compared with placebo groups (2.4 vs. 4.3%, respectively). There was no difference in the incidence of hypoglycemia between the Cycloset and placebo-treated groups in any trial.
All-cause safety trial
A large (n = 3,070) 52-week, randomized, placebo-controlled (2:1), double-blind trial evaluated overall and cardiovascular safety of Cycloset in type 2 diabetic patients treated with diet alone, one to two OHAs, or insulin alone or with one OHA (41). Mean age was 60 years, mean BMI 32.4 kg/m2, and mean HbA1c 8.3%. Fifty-seven percent of subjects were male, and ethnic background was 68% Caucasian and 17% African American. Cycloset therapy was initiated at 0.8 mg/day and titrated to 4.8 mg/day, as tolerated. After 3 months, alteration in other antidiabetic medications was permitted.
Overall, there were 8.6% serious adverse events in the Cycloset group and 9.6% serious adverse events in the placebo group (hazard ratio [HR] 0.89, P = NS). Thirty-two diabetic patients (3.2%) in the Cycloset group compared with 37 patients (1.8%) in the placebo group experienced a prespecified cardiovascular event (myocardial infarction, stroke, hospitalization for angina, hospitalization for congestive heart failure [CHF], coronary revascularization, and death) (HR 0.60, two-sided 95% CI 0.37–0.96, P = 0.036) (Fig. 3). Using the major adverse cardiac events (MACE) end point (myocardial infarction, stroke, and death), the HR was reduced in the Cycloset compared with placebo-treated subjects (HR 0.55, two-sided 95% CI 0.39–0.98, P < 0.05) (Fig. 3). Based on the composite cardiovascular outcome, 79 diabetic patients need to be treated for 1 year to avoid one cardiovascular event.
Adverse events that occurred at a frequency >5% and numerically were greater in the Cycloset group included nausea (32.2 vs. 7.6%), dizziness (14.8 vs. 9.2%), fatigue (13.9 vs. 6.7%), headache (11.4 vs. 8.3%), vomiting (8.1 vs. 3.1%), diarrhea (8.1 vs. 8.0%), and constipation (5.8 vs. 5.1%).
The mechanism(s) via which timed bromocriptine reduces cardiovascular events in type 2 diabetic patients remains to be defined. In the cardiovascular safety trial (41), bromocriptine reduced HbA1c by 0.6% (P < 0.0001), blood pressure by 2/1 mmHg (P < 0.02), heart rate by 1 bpm (P = 0.02), and plasma triglyceride by 0.08 mmol/L (P = 0.02). However, these changes were modest and seem unlikely to explain the 40% decrease in composite cardiovascular outcome. Although not measured in the safety trial, reductions in postprandial FFA levels have been observed in other trials with bromocriptine (30,31). In animal studies, bromocriptine has been shown to attenuate the effect of CNS sympathetic overactivity on the vasculature (44,45), and a direct inhibitory effect of bromocriptine on mitogen-stimulated proliferation of rat vascular muscle cells and human aortic smooth muscle cells has been demonstrated in vitro (46). In the high fat–fed, spontaneously hypertensive rat (SHR)—an animal model of the insulin resistance syndrome and eNOS uncoupling—bromocriptine reduced pathologically elevated endothelial nitric oxide synthase (eNOS) and inducible NOS (iNOS) levels (47). Uncoupled eNOS increases NAD[P]-H oxidase levels, resulting in generation of reactive oxygen species and decreased nitric oxide production, a potent vasodilator and antiatherogenic agent. All of these biochemical abnormalities were reversed in the aorta of bromocriptine-treated SHR, and aortic stiffness was markedly reduced. Thus, a number of biochemical/molecular abnormalities involved in the development of atherosclerosis, as well as multiple circulating cardiovascular risk factors (hyperglycemia, hypertriglyceridemia, and elevated FFA) and hypertension, improve with bromocriptine therapy. At present, it is unclear whether the salutatory effect of bromocriptine to reduce cardiovascular events (41) is related to the drug’s beneficial effect on any of these pathologic processes/atherosclerotic risk factors. Further mechanistic studies and a large, long-term prospective study will be required to establish the mechanism of action and cardiovascular protective benefit of bromocriptine therapy.
Summary
Both as monotherapy and in combination with other OHAs, timed bromocriptine (Cycloset) causes a 0.6–0.7% reduction in HbA1c and reduces plasma triglyceride and FFA concentrations in type 2 diabetic patients. In a 52-week safety study, Cycloset decreased the cardiovascular composite end point by 40%. Other advantages of Cycloset include absence of hypoglycemia since insulin secretion is not stimulated, weight neutrality, no need for dose adjustment in patients with moderate renal insufficiency, lack of edema and CHF, and good side effect profile.
Supplementary Material
Acknowledgments
R.A.D. has received grants from Takeda and Amylin Pharmaceuticals and has been a board member of and consulted for Takeda, Amylin Pharmaceuticals, Isis Pharmaceuticals, and Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
Lorrie Albarado (University of Texas Health Science Center, San Antonio, TX) provided expert secretarial assistance in preparation of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0064/-/DC1.
References
- 1.Defronzo RA. Banting Lecture: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 3.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven GM. Banting Lecture 1988: Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 6.Rosenzweig JL, Ferrannini E, Grundy SM, et al. Endocrine Society Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3671–3689 [DOI] [PubMed] [Google Scholar]
- 7.Cincotta AH. Hypothalamic role in the insulin resistance syndrome. In Insulin Resistance Syndrome. Hansen B, Shaffrir E, Eds. London, Taylor and Francis, 2002, p. 271–312 [Google Scholar]
- 8.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–362 [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–194 [DOI] [PubMed] [Google Scholar]
- 10.Morgane PJPJ. Hypothalamic Control ofMmetabolism. New York, Marcel Dekker, 1980, p. 519–555 [Google Scholar]
- 11.Luiten PG, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol 1987;28:1–54 [DOI] [PubMed] [Google Scholar]
- 12.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997;99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev 1987;3:185–206 [DOI] [PubMed] [Google Scholar]
- 14.Luo SHS, Cincotta AH. Increased daily turnover of noradrenaline and serotonin in the ventral medial hypothalamus (VMH) of obese versus lean Zucker rats assessed by in vivo microdialysis. Society for Neuroscience Abstracts 1996;22:605 [Google Scholar]
- 15.Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res 1995;88:127–131 [DOI] [PubMed] [Google Scholar]
- 16.Oltmans GA. Norepinephrine and dopamine levels in hypothalamic nuclei of the genetically obese mouse (ob/ob). Brain Res 1983;273:369–373 [DOI] [PubMed] [Google Scholar]
- 17.Kraszewski KZ, Cincotta AH. Increased responsiveness of ventromedial hypothalamic neurons to norepinephrine in obese versus lean mice: relation to the metabolic syndrome. Int J Mol Med 2000;5:349–355 [DOI] [PubMed] [Google Scholar]
- 18.Boundy VA, Cincotta AH. Hypothalamic adrenergic receptor changes in the metabolic syndrome of genetically obese (ob/ob) mice. Am J Physiol Regul Integr Comp Physiol 2000;279:R505–R514 [DOI] [PubMed] [Google Scholar]
- 19.Cincotta AH, Luo S, Zhang Y, et al. Chronic infusion of norepinephrine into the VMH of normal rats induces the obese glucose-intolerant state. Am J Physiol Regul Integr Comp Physiol 2000;278:R435–R444 [DOI] [PubMed] [Google Scholar]
- 20.Luo S, Meier AH, Cincotta AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology 1998;68:1–10 [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Luo J, Cincotta AH. Suprachiasmatic nuclei monoamine metabolism of glucose tolerant versus intolerant hamsters. Neuroreport 1999;10:2073–2077 [DOI] [PubMed] [Google Scholar]
- 22.Luo S, Luo J, Meier AH, Cincotta AH. Dopaminergic neurotoxin administration to the area of the suprachiasmatic nuclei induces insulin resistance. Neuroreport 1997;8:3495–3499 [DOI] [PubMed] [Google Scholar]
- 23.Meguid MM, Fetissov SO, Blaha V, Yang ZJ. Dopamine and serotonin VMN release is related to feeding status in obese and lean Zucker rats. Neuroreport 2000;11:2069–2072 [DOI] [PubMed] [Google Scholar]
- 24.Levin BE, Dunn-Meynell AA. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. Am J Physiol 1997;272:R1365–R1370 [DOI] [PubMed] [Google Scholar]
- 25.Luo S, Luo J, Cincotta AH. Chronic ventromedial hypothalamic infusion of norepinephrine and serotonin promotes insulin resistance and glucose intolerance. Neuroendocrinology 1999;70:460–465 [DOI] [PubMed] [Google Scholar]
- 26.Cincotta AH, Meier AH. Bromocriptine inhibits in vivo free fatty acid oxidation and hepatic glucose output in seasonally obese hamsters (Mesocricetus auratus). Metabolism 1995;44:1349–1355 [DOI] [PubMed] [Google Scholar]
- 27.Scislowski PW, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. Int J Obes Relat Metab Disord 1999;23:425–431 [DOI] [PubMed] [Google Scholar]
- 28.Luo S, Liang Y, Cincotta AH. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology 1999;69:160–166 [DOI] [PubMed] [Google Scholar]
- 29.Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999;8:1683–1707 [DOI] [PubMed] [Google Scholar]
- 30.Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care 2000;23:1154–1161 [DOI] [PubMed] [Google Scholar]
- 31.Kamath V, Jones CN, Yip JC, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care 1997;20:1697–1701 [DOI] [PubMed] [Google Scholar]
- 32.Meier AHCA. Circadian rhythms regulate the expression of the thrifty genotype/phenotype. Diabetes Reviews 1996;4:464–487 [Google Scholar]
- 33.Maurer G, Schreier E, Delaborde S, Loosli HR, Nufer R, Shukla AP. Fate and disposition of bromocriptine in animals and man. I: structure elucidation of the metabolites. Eur J Drug Metab Pharmacokinet 1982;7:281–292 [DOI] [PubMed] [Google Scholar]
- 34.Maurer G, Schreier E, Delaborde S, Nufer R, Shukla AP. Fate and disposition of bromocriptine in animals and man. II: Absorption, elimination and metabolism. Eur J Drug Metab Pharmacokinet 1983;8:51–62 [DOI] [PubMed] [Google Scholar]
- 35.Parkes D. Drug therapy: bromocriptine. N Engl J Med 1979;301:873–878 [DOI] [PubMed] [Google Scholar]
- 36.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet 2001;357:354–357 [DOI] [PubMed] [Google Scholar]
- 37.Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol 2003;480:125–131 [DOI] [PubMed] [Google Scholar]
- 38.Cincotta AH, Meier AH. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care 1996;19:667–670 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz S. Bromocriptine (Ergoset) improves glycemic control in type 2 diabetes on insulin. Diabetes 1999;48(Suppl. 1):A99 [Google Scholar]
- 40.Scranton R, Cincotta A. Bromocriptine—unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmacother 2010;11:269–279 [DOI] [PubMed] [Google Scholar]
- 41.Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010;33:1503–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scranton REFW, Ezrokhi M, Gaziano JM, Cincotta AH. Quick release bromocriptine (Cycloset TM) improves glycaemic control in patients with diabetes failing metformin/sulfonylurea combination therapy. Diabetologia 2008;51:S372–S373 [Google Scholar]
- 43.Cincotta AHGJ, Ezrokhi M, Scranton R. Cycloset (Quick-Release Bromocriptine Mesylate), a novel centrally acting treatment for type 2 diabetes. Diabetologia 2008;51:S22 [Google Scholar]
- 44.Franchi F, Lazzeri C, Barletta G, Ianni L, Mannelli M. Centrally mediated effects of bromocriptine on cardiac sympathovagal balance. Hypertension 2001;38:123–129 [DOI] [PubMed] [Google Scholar]
- 45.Liang Y, Cincotta AH. Increased responsiveness to the hyperglycemic, hyperglucagonemic and hyperinsulinemic effects of circulating norepinephrine in ob/ob mice. Int J Obes Relat Metab Disord 2001;25:698–704 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Cincotta AH. Inhibitory effects of bromocriptine on vascular smooth muscle cell proliferation. Atherosclerosis 1997;133:37–44 [DOI] [PubMed] [Google Scholar]
- 47.Ezrokhi MTY, Luo S, Cincotta AH. Timed dopamine agonist treatment ameliorates both vascular nitrosative/oxidative stress pathology and aortic stiffness in arteriosclerotic, hypertensive SHR rats. Diabetes 2010;59(Suppl. 1):252-OR [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.