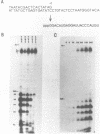
Abstract
A method is described to synthesize small RNAs of defined length and sequence using T7 RNA polymerase and templates of synthetic DNA which contain the T7 promoter. Partially single stranded templates which are base paired only in the -17 to +1 promoter region are just as active in transcription as linear plasmid DNA. Runoff transcripts initiate at a unique, predictable position, but may have one nucleotide more or less on the 3' terminus. In addition to the full length products, the reactions also yield a large amount of smaller oligoribonucleotides in the range from 2 to 6 nucleotides which appear to be the result of abortive initiation events. Variants in the +1 to +6 region of the promoter are transcribed with reduced efficiency but increase the variety of RNAs which can be made. Transcription reaction conditions have been optimized to allow the synthesis of milligram amounts of virtually any RNA from 12 to 35 nucleotides in length.
Full text
PDF





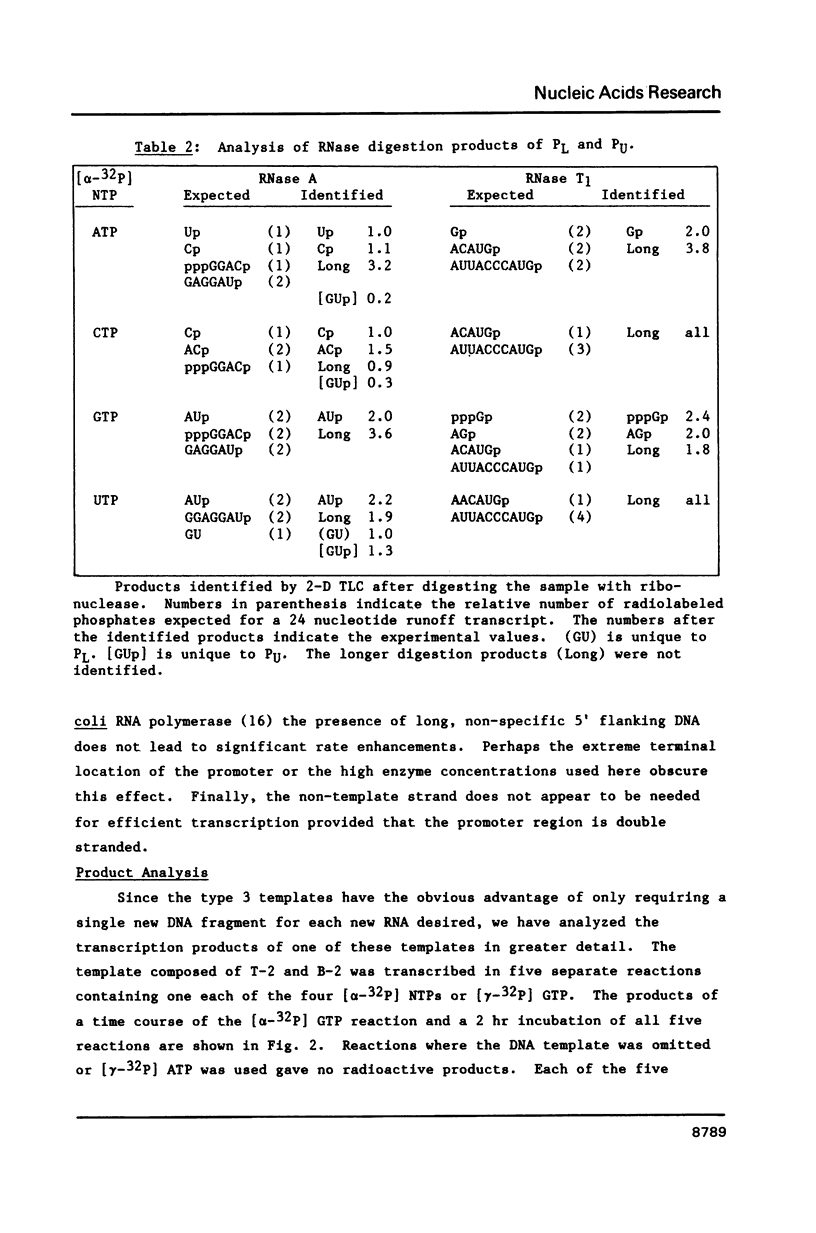




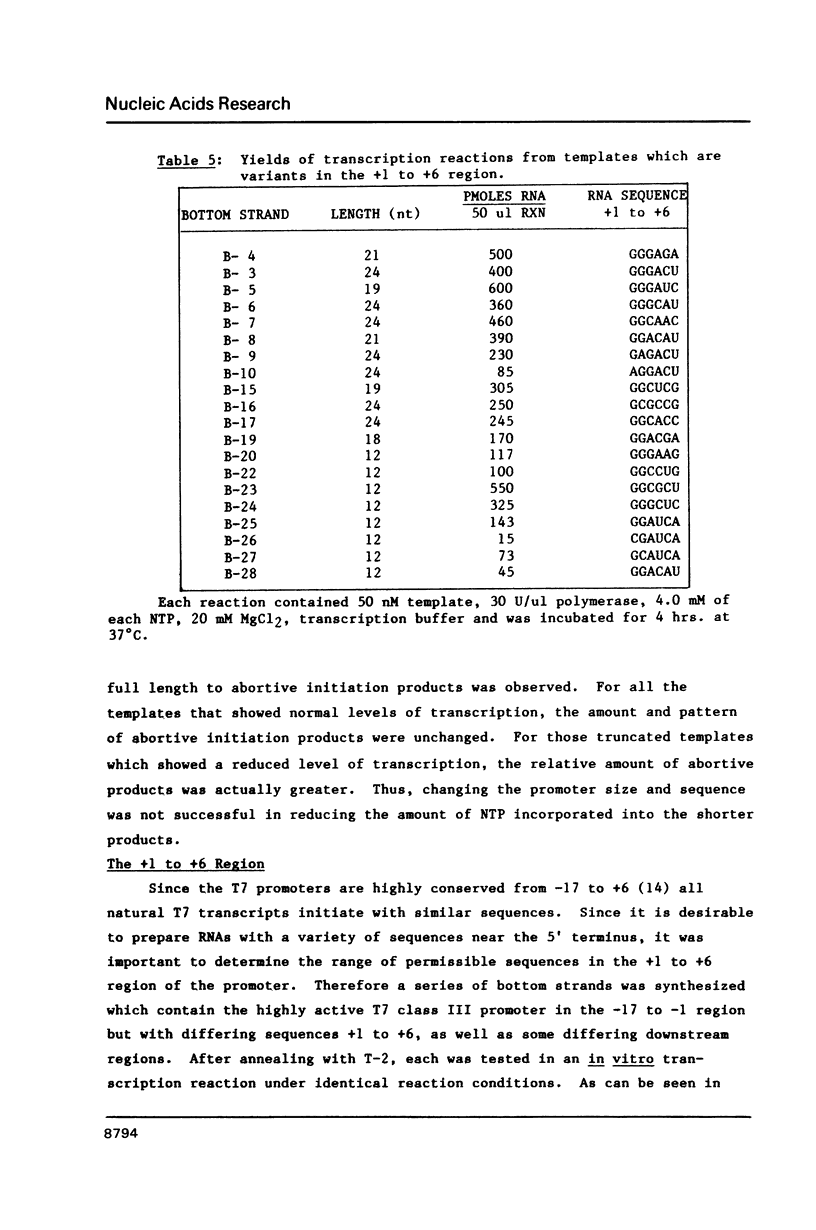




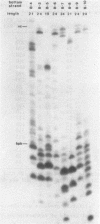
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., Ring J. Characterization of T7-specific ribonucleic acid polymerase. 1. General properties of the enzymatic reaction and the template specificity of the enzyme. J Biol Chem. 1973 Mar 25;248(6):2235–2244. [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Ikeda R. A., Richardson C. C. Interactions of the RNA polymerase of bacteriophage T7 with its promoter during binding and initiation of transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3614–3618. doi: 10.1073/pnas.83.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Wu C. W. Studies on SP6 promoter using a new plasmid vector that allows gene insertion at the transcription initiation site. Nucleic Acids Res. 1987 Mar 11;15(5):2279–2294. doi: 10.1093/nar/15.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Ishizaki Y., Hiraoka N., Obayashi A. Nucleotide sequence and expression of the cloned gene of bacteriophage SP6 RNA polymerase. Nucleic Acids Res. 1987 Mar 25;15(6):2653–2664. doi: 10.1093/nar/15.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. R., Krummel B., Chamberlin M. J. Isolation and properties of transcribing ternary complexes of Escherichia coli RNA polymerase positioned at a single template base. J Mol Biol. 1987 Jul 5;196(1):85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Coleman J. E. Kinetic analysis of T7 RNA polymerase-promoter interactions with small synthetic promoters. Biochemistry. 1987 May 19;26(10):2690–2696. doi: 10.1021/bi00384a006. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Gupta R. C., Randerath E. 3H and 32P derivative methods for base composition and sequence analysis of RNA. Methods Enzymol. 1980;65(1):638–680. doi: 10.1016/s0076-6879(80)65065-4. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Carey J., Romaniuk P. J., Lowary P. T., Beckett D. Interaction of R17 coat protein with its RNA binding site for translational repression. J Biomol Struct Dyn. 1983 Oct;1(2):539–552. doi: 10.1080/07391102.1983.10507460. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]