Abstract
Urine outflow obstruction activates a variety of profibrotic factors, including the intrarenal renin-angiotensin system. However, the obstruction also nullifies the transmural hydraulic pressure difference across the glomerular capillary wall, an established inducer of glomerulosclerosis. In the present study, we investigated whether, and by what mechanism, urine outflow obstruction affects the process of progressive glomerulosclerosis. For this purpose, we tested the effect of unilateral ureteral obstruction (UUO) of 7 days duration in two distinct mouse models of glomerulosclerosis. In the human immunodeficiency virus (HIV) nephropathy model, where HIV-1 genes are selectively expressed in podocytes and develop progressive podocyte damage and glomerulosclerosis, UUO protected against sclerosis with preservation of podocytes morphologically and immunohistochemically. In contrast, the nonobstructed contralateral kidneys of these mice, as well as sham-operated HIV-1 mouse kidneys, developed severe podocyte injury and glomerulosclerosis. The protection against glomerulosclerosis imparted by ureteral obstruction was also documented in the NEP25 model of podocyte injury, in which a single injection of immunotoxin, LMB2, triggers selective podocyte injury followed by glomerulosclerosis, both of which were protected by UUO. Notably, intervention with an angiotensin II type 1 receptor antagonist provided only a partial protective effect in each of the models. These results demonstrate that urine outflow obstruction protects the glomerulus from progressive sclerosis. The results further reveal that this protection occurs at a very early stage of the pathologic process, namely, damage of podocytes.
Keywords: glomerular transmural pressure difference, angiotensin II
obstruction of urine outflow causes fibrotic changes in the tubulointerstitium (13). Numerous studies have repeatedly shown that outflow obstruction leads to activation of the renin-angiotensin system (RAS) and that pharmacological inhibition of the RAS attenuates tubulointerstitial fibrosis (11). These observations have led to the currently prevailing notion that the RAS has an important intermediary role in the tubulointerstitial fibrosis seen in urinary tract obstruction. It has been puzzling, however, that although the intrarenal RAS is a potent profibrotic stimulus, the glomerular architecture remains remarkably well preserved after ureteral obstruction (10, 11). It is notable that obstruction nullifies the glomerular transmural hydraulic pressure difference, a factor incriminated in the progressive destruction of glomerular architecture in many human and animal forms of progressive glomerulosclerosis (3, 6, 8, 9, 17).
The present study ascertains whether, and by what mechanism, urine outflow obstruction affects glomerular injury. For this purpose, we performed unilateral ureteral obstruction (UUO) in two transgenic mouse models of glomerulosclerosis. First, the human immunodeficiency virus (HIV)-1 model, a transgenic mouse line in which regulatory and accessory genes of HIV-1 including vpr and nef are regulated by a nephrin promoter and expressed selectively in podocytes (23). HIV-1 mice on a FVB/N genetic background start to develop podocyte injury at ∼30 days of age, which rapidly escalates to collapsing glomerulosclerosis by 37 days of age. The NEP25 model expresses human (h) CD25 (i.e., IL2 receptor) selectively on podocytes. By injecting a hCD25-targeted recombinant immunotoxin, anti-Tac(Fv)-PE38 (LMB2) (14), podocyte-selective injury can be induced (16). We investigated potential protective effects imparted by obstruction, compared with nonobstructed and sham kidneys in these two models, as well as to effects of intervention with an angiotensin II type 1 receptor antagonist (ARB).
MATERIALS AND METHODS
Animal experiments.
The Animal Experimentation Committee of Tokai University School of Medicine and the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all protocols, in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals.
HIV-1 transgenic mice (line HIV13) (23), which carry a nephrin promoter and a part of the HIV-1 genome, were maintained by backcrossing with the C57BL/6 strain. This strain with FVB/N genetic background develops severe podocyte injury and rapidly progressive glomerulosclerosis with no gender difference in podocyte injury (23). To minimize the influence of genetic background, all HIV-1 transgenic mice used in this study were obtained from mating a single male mouse in the N4 generation with wild-type FVB/N female mice. Nine (3 males, 6 females) HIV-1 transgenic mice were euthanized at 30 days of age to obtain basal histological data. UUO (n = 8, 4 males and 4 females) or sham (n = 10, 4 males and 6 females) operation was performed in HIV-1 mice at 30 days of age. UUO was done by ligating the left ureter at the level of the lower pole of the kidney under pentobarbital sodium anesthesia [60 μg/g body wt (BW)]. These mice were euthanizied 7 days later at 37 days of age.
Adult NEP25 transgenic mice (16), backcrossed with the C57BL/6 strain more than eight times, were used. Since there is no gender difference in response to LMB2, both male and female NEP25 mice were used. UUO or sham operation was performed in NEP25 mice 1 day after the injection of LMB2 (2.5 ng /g BW iv) and euthanized 4 or 8 days after LMB2 injection (n = 5 for each time point). Ten additional NEP25 mice were injected with LMB2 (2.5 ng/g BW), subjected to either sham operation (n = 5) or UUO (n = 5) 4 days after LMB2 injection, and euthanized 8 days after the injection. In some mice, half of the kidney was frozen for RNA or Western blot analyses.
Ten additional NEP25 mice injected with 2.5 ng/g BW of LMB2 were treated with losartan (0.5 g/l in drinking water, ∼25 μg/g BW; n = 5) or left untreated (n = 5). These mice were euthanized 8 days after LMB2 injection.
Morphological analysis.
Glomerular injury was evaluated in periodic acid-Schiff (PAS)-stained paraffin sections (2 μm thick). Indices for epithelial (both parietal and visceral) and mesangial injury were determined on a scale of 0 to 4, from none to most severe injury in PAS-stained sections, as previously reported (16).
For evaluating podocyte injury, paraffin sections were stained with guinea pig polyclonal anti-nephrin antibody (GP-N2, 1:200; Progen, Heidelberg, Germany) and monoclonal anti-synaptopodin antibody (Progen). For semiquantification of the staining, each quadrant of each glomerulus was scored as 0 (no staining), 1 (diminished), or 2 (normal), with total glomerular score range calculated from 0 (complete loss) to 8 (normal). The staining indices were determined by averaging scores for all glomeruli (>60), on a section for each mouse. In our previous study (15), the nephrin index was correlated well with urinary albumin excretion.
In addition, the following primary antibodies were used at the indicated dilution: goat anti-mouse IgA (1:100, ICN), goat anti-mouse C3 (1:1,000, ICN), rabbit anti-WT1 (1:100, Santa Cruz Biotechnology), rabbit anti-α-actinin-4 (1:1,000, ImmunoGlobe Antikörpertechnik), and rabbit anti-mouse albumin (1:5,000, Nordic Immunology, Tilburg, The Netherlands). In each kidney, >60 glomeruli were inspected.
Western blot analysis.
Western blot analysis was performed as previously reported (18). Briefly, kidney proteins (20 μg) were extracted from NEP25 mice without LMB2, NEP25 mice with LMB2 (2.5 ng/g BW) and sham operation, and NEP25 mice with LMB2 and UUO (each n = 5), separated on SDS-PAGE gels under denatured conditions, and transferred onto polyvinylidene difluoride membranes. Nephrin was detected with an anti-nephrin antibody (1:2,000) and β-actin with an anti-β-actin antibody (1:10,000, Sigma-Aldrich Japan, Tokyo, Japan).
mRNA analysis.
Total RNA was extracted from the kidney of NEP25 mice. Real-time RT-PCR for nephrin and 18S rRNA was performed using TaqMan primer probe sets and Applied Biosystems 7300 Real Time PCR Systems. The relative amount of nephrin mRNA was determined by the ΔΔCT method. Data are shown as the percentage of the mean of nephrin mRNA amounts simultaneously measured in five age-matched NEP25 kidneys without LMB2.
Statistical analysis.
To compare epithelial and mesangial injury indices, synaptopodin and nephrin staining indices, and relative amounts of nephrin mRNA and albumin droplet accumulation, nonparametric multiple comparisons were performed with the Steel-Dwass procedure using KyPlot software. The number of WT1-positive cells were compared by ANOVA with the Turley-Kramer method and are represented as means ± SD. Values were regarded as significant at P < 0.05.
RESULTS
Effects of urine outflow obstruction on the glomerulus in HIV-1 transgenic mice.
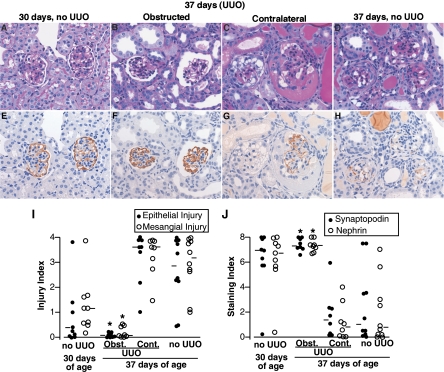
We performed UUO or sham operation in HIV-1 mice at 30 days of age, a time point when proteinuria becomes evident. At this stage, HIV-1 transgenic mice showed mild glomerular damage and focal proteinaceous casts with near-normal synaptopodin and nephrin staining (Fig. 1), except for one mouse, which showed severe glomerular damage with remarkable downregulation of nephrin and synaptopodin staining. The HIV-1 transgenic mice examined 7 days after sham operation, i.e., at 37 days of age, had the characteristic severe glomerular damage, evidenced by severe vacuolar degeneration of podocytes and parietal epithelial cells, along with adhesions, hyalinosis, mesangial matrix expansion, and occasional collapse. In contrast, 7 days after UUO, the ipsilateral obstructed kidneys of HIV-1 mice had remarkably well-preserved glomerular architecture. Notably, glomerular preservation was seen together with obstruction-related tubulointerstitial injury (Supplemental Fig. S1; all supplementary material for this article are available on the journal web site). Moreover, similar to sham-operated HIV-1 mice, glomeruli of the contralateral nonobstructed kidneys of HIV mice had severe damage. The median glomerular epithelial injury and mesangial indices were 0.05 and 0.07 in the ipsilateral obstructed kidneys (scale 0–4, with 0 representing no injury), which are markedly less than in the contralateral kidneys (3.60 and 3.61) or sham-operated kidneys (2.84 and 3.16), respectively (Fig. 1).
Fig. 1.
Protection by unilateral ureteral obstruction (UUO) of glomerular injury in human immunodeficiency virus (HIV)-1 transgenic mice. A–D: periodic acid-Schiff (PAS). E–H: synaptopodin immunostaining in the adjacent section. Magnification ×400. A and E: at 30 days of age, most glomeruli of HIV-1 transgenic mice were not damaged with intact synaptopodin staining. B and F: glomerular morphology as well as synaptopodin expression was almost completely preserved in obstructed kidneys of transgenic mice with UUO at 37 days of age. C and G: in contrast, the contralateral kidney in the same mouse showed severe vacuolar degeneration in epithelial cells, loss of podocytes, hyalinosis, mesangial expansion, and downregulation of synaptopodin. Note that cuboidal epithelial cells on the surface of the right glomerulus are negative for synaptopodin, potentially representing undifferentiated cells migrating from Bowman's capsule. D and H: similar glomerular damage and synaptopodin downregulation were observed in the HIV transgenic mice without UUO at 37 days of age. I: glomerular epithelial cell injury and mesangial cell injury were semiquantified (0–4 scale) in all glomeruli in a PAS-stained section for each kidney. ●, Epithelial injury indices; ○, mesangial injury indices for individual kidneys. J: nephrin and synaptopodin staining [0 (complete loss) to 8 (normal) scale] were semiquantified in all glomeruli in a section for each kidney. ●, Synaptopodin indices; ○, nephrin staining indices for individual kidneys. In obstructed kidneys from mice with UUO (Obst.), all 4 indices were improved compared with contralateral kidneys from mice with UUO (Cont.) and mice with sham operation. Bars represent median values. *P < 0.05 compared with Cont. and no UUO at 37 days of age.
Synaptopodin and nephrin immunostaining was markedly downregulated in the contralateral nonobstructed as well as sham-operated kidneys. The median staining indices for synaptopodin and nephrin were 1.37 and 0.79 in the contralateral kidney and 1.00 and 0.78 in the sham-operated kidneys (0–8 scale), respectively. The depressed values contrasted with the essentially normal synaptopodin and nephrin staining indices observed in obstructed kidneys, 7.32 and 7.32, respectively (Fig. 1). The number of WT-1-positive cells in the obstructed kidney of HIV-1 mice was, on average, 6.7 ± 0.7/glomerulus and not significantly different from that in wild-type controls, 7.7 ± 0.33/glomerulus. In contrast, WT-1-positive cells were markedly reduced in the nonobstructed kidney of HIV-1 mice (1.9 ± 1.8) and in HIV-1 mice without UUO (1.5 ± 0.9) (P < 0.05 vs. obstructed).
Effect of urine outflow obstruction on the glomerulus in NEP25 mice.
Because disease progression in the HIV-1 model can be somewhat variable, we also performed UUO in an inducible nephropathy model (NEP25). The toxin LMB2 used to target podocytes in NEP25 is rapidly cleared from the circulation, with a half-life of 35 min (14). NEP25 mice without UUO showed no proteinuria, no abnormal light microscopic phenotype, and normal nephrin and synaptopodin staining 24 h after injection of 2.5 ng/g BW of LMB2. Thereafter, NEP25 mice developed progressive podocyte and glomerular injury by 8 days and died within 14 days. We therefore performed UUO or sham operation 24 h after the injection of LMB2.
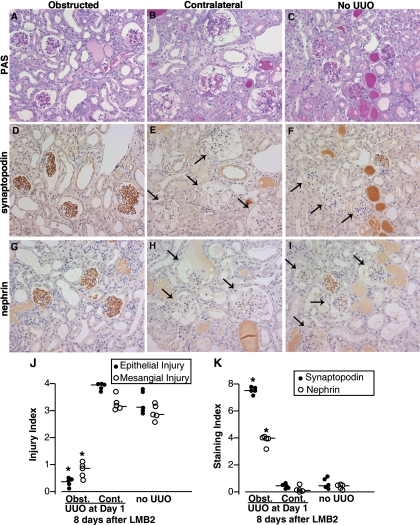
Eight days after LMB2 injection, glomeruli in the nonobstructed kidneys had severe damage. The glomeruli had prominent vacuolar degeneration in podocytes and parietal epithelial cells, adhesions, proliferation of parietal epithelial cells, hyalinosis, and expansion of mesangial matrix (Fig. 2). The median indices for epithelial and mesangial injury were 3.95 and 3.18 in the contralateral kidneys and 3.14 and 2.83 in the sham-operated kidneys (Fig. 2). By contrast, in the obstructed kidneys, glomeruli were almost completely preserved, with median epithelial and mesangial injury of 0.37 and 0.86, respectively.
Fig. 2.
Protection of glomerular injury in NEP25 mice by UUO. A–C: PAS. D–F: synaptopodin immunostaining. G–I: nephrin immunostaining. Magnification ×200. NEP25 mice had UUO or sham operation 24 h after LMB2 toxin injection [2.5 ng/g body wt (BW)], and injury was analyzed 8 days after LMB2. A, D, and G: in the obstructed kidneys of NEP25 mice with UUO, glomerular structure as well as synaptopodin and nephrin staining were well preserved. B, E, and H: in sharp contrast, in the contralateral kidneys in the same mice, glomeruli were severely damaged and synaptopodin and nephrin staining completely disappeared (arrows). C, F, and H: NEP25 mice with sham operation showed similar or slightly less severe glomerular injury and downregulation of synaptopodin and nephrin. J and K: semiquantification of glomerular injury and podocyte proteins in NEP25 mice. Epithelial injury index (● in J), mesangial injury index (○ in J), synaptopodin index (● in K) and nephrin index (○ in K) were determined for individual kidneys. Bars represent median values in each group. In obstructed kidneys from mice with UUO (Obst.), all 4 indices were improved compared with contralateral kidneys from mice with UUO (Cont.) and mice with sham operation. *P < 0.05 compared with Cont. and no UUO 8 days after 2.5 ng/g BW of LMB2.
Synaptopodin and nephrin staining was markedly downregulated in the nonobstructed kidneys after LMB2-induced injury. The median staining indices for synaptopodin and nephrin were 0.45 and 0.45 in the sham-operated kidneys and 0.45 and 0.16 in the contralateral kidneys. In remarkable contrast, most glomeruli in the obstructed kidneys showed essentially normal synaptopodin, with a median synaptopodin staining index of 7.53. The median nephrin index was 4.02, significantly higher than in the contralateral and sham-operated kidneys. A protective effect, albeit less remarkable, was also observed when UUO was performed 4 days after LMB2 injection (Supplemental Fig. S2). A similar protective effect of UUO was observed in NEP25 mice injected with 1.25 ng/g BW of LMB2 (data not shown). Western blot analysis confirmed that the obstructed kidney contained more nephrin protein than sham-operated or contralateral kidneys (Fig. 3), corresponding to the immunostaining. UUO in NEP25 mice without LMB2 did not affect nephrin as well as hCD25, which is driven by the nephrin promoter.
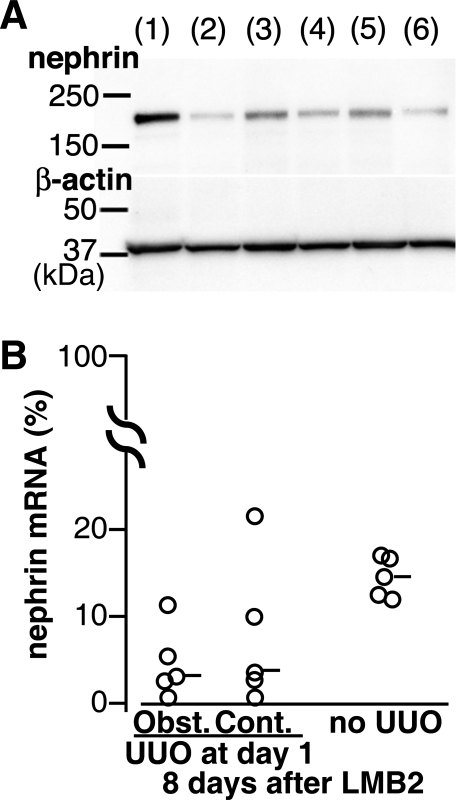
Fig. 3.
UUO preserved nephrin protein, but not mRNA, in NEP25 mice. A: Western blot analysis for nephrin and β-actin. Lane 1, NEP25 mouse without LMB2; lane 2, NEP25 mouse with LMB2 and sham operation; lanes 3 and 5, obstructed kidneys of NEP25 mice with LMB2 and UUO; lanes 4 and 6, contralateral kidneys of NEP25 mice with LMB2 and UUO. Results in lanes 3 and 4 and lanes 5 and 6 are from the same mice, respectively. B: UUO or sham operation was performed in NEP25 mice 1 day after LMB2 (2.5 ng/g BW). The relative amount of kidney nephrin mRNA was determined 8 days after LMB2. Data from RT-PCR with 18S rRNA as internal control are presented as percentage of the mean of nephrin mRNA amounts simultaneously measured in 5 NEP25 kidneys without LMB2.
We also quantified nephrin mRNA by real-time RT-PCR analysis. Twenty-four hours after LMB2, i.e., at the time of UUO, nephrin mRNA was already decreased, on average, to 51.3% of levels observed in NEP25 mice without LMB2. Eight days after LMB2, nephrin mRNA decreased further similarly in both obstructed and nonobstructed kidneys, suggesting that UUO did not affect nephrin mRNA (Fig. 3).
Similarly to synaptopodin, α-actinin-4, another actin binding protein preferentially expressed in podocytes, was also dramatically decreased in the sham-operated kidneys and contralateral kidneys of both HIV-1 mice and of NEP25 mice. In contrast, α-actinin-4 staining was preserved in the obstructed kidneys of both transgenic mouse models (Fig. 4).
Fig. 4.
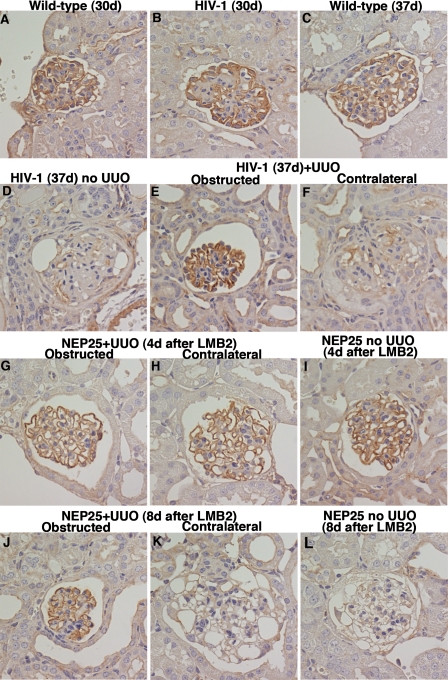
Protection of α-actinin-4 in HIV-1 and NEP25 mice by UUO. At 30 days of age, α-actinin-4 staining was not downregulated in HIV-1 transgenic mice (B) compared with wild-type littermates (A). By 37 days of age, α-actinin-4 staining has nearly completely disappeared in HIV-1 mice (D) in contrast to wild-type littermates (C). HIV-1 mice were subjected to UUO at 30 days and analyzed at 37 days. α-Actinin-4 staining was almost completely preserved in obstructed kidneys (E), but disappeared in contralateral kidneys (F). NEP25 mice had UUO or sham operation 24 h after LMB2 injection (2.5 ng/g BW) and then analyzed 4 (G–I) or 8 (J–L) days after LMB2. Four days after LMB2 injection, α-actinin-4 staining was well preserved, and UUO did not change the staining pattern (G–I). By 8 days after LMB2 injection, α-actinin-4 staining disappeared in contralateral (K) and sham-operated (L) kidneys in NEP25 mice. In sharp contrast, α-actinin-4 staining was well preserved in obstructed kidneys (J). Magnification ×400.
Electron microscopy revealed that podocytes in the nonobstructed kidneys were severely injured, showing large blebs, extensive effacement of foot processes, and necrosis. In contrast, podocytes in the glomeruli of obstructed kidneys were largely preserved. They were devoid of blebs and necrosis, although the foot processes were found to be effaced (Fig. 5).
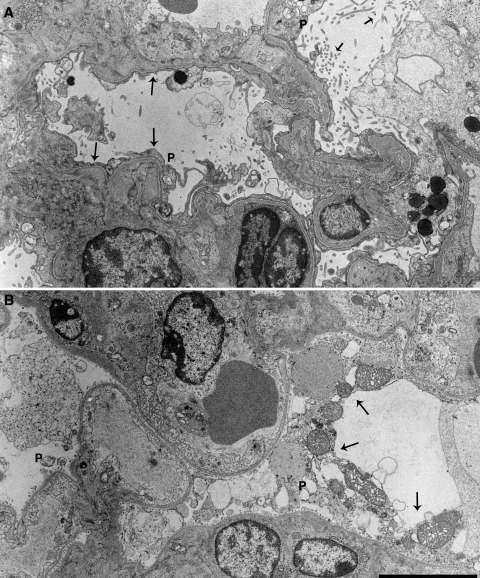
Fig. 5.
UUO protected glomerular ultrastructure. Electron micrograph of obstructed (A) and contralateral (B) kidneys of NEP25 mice injected with 2.5 ng/g BW of LMB2. In the contralateral kidneys, podocytes (P) were severely damaged (arrows). In the obstructed kidneys, podocyte cell bodies were remarkably preserved, although foot process effacement (long arrows) and microvillous transformation (short arrows) were extensive. Bar = 5 μm.
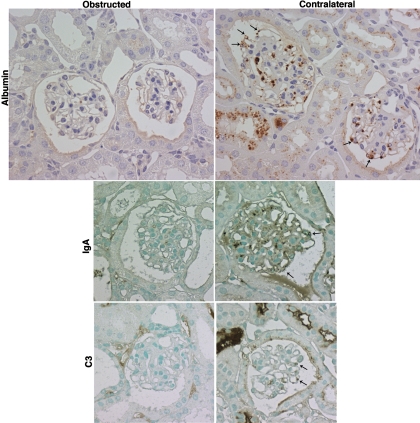
In both the NEP25 and HIV-1 models, plasma proteins, including albumin, IgA, and C3, were present in podocytes in nonobstructed contralateral kidneys, but their accumulation was minimal in glomeruli of obstructed kidneys (data not shown). Additional experiments in NEP25 mice revealed that the dramatic prevention of plasma protein accumulation by UUO was evident 4 days after LMB2 injection (Fig. 6), when nephrin staining and light microscopic morphology of podocytes still remained intact in both obstructed and nonobstructed kidneys (Supplemental Fig. S1). Thus, at this time point, on average 90.7 ± 6.8% of glomeruli in the contralateral kidneys of UUO mice (n = 5) and 74.3 ± 4.0% of glomeruli in sham-operated kidneys (n = 5) showed podocytes incorporating albumin droplets, whereas this value in obstructed kidneys was only 5.4 ± 4.0%. (P < 0.05 vs. contralateral and sham-operated kidneys.)
Fig. 6.
Attenuation by UUO of glomerular plasma protein accumulation. Immunostaining for albumin, IgA, and C3 in contralateral and obstructed kidneys of NEP25 mice 4 days after LMB2 injection (2.5 ng/g BW). In the contralateral kidneys, plasma proteins were accumulated in podocytes (arrows). In contrast, the accumulation was markedly attenuated in glomeruli of obstructed kidneys. Magnification ×400.
Comparison between effects of UUO vs. ARB.
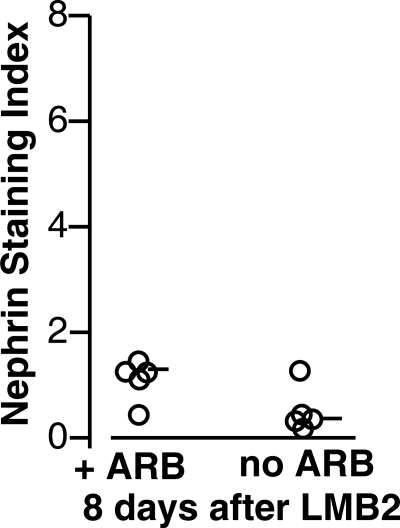
We previously showed that losartan, an ARB, partially protected against renal injury in NEP25 mice injected with a low dose (0.625 ng/g BW) of LMB2 (15). We now compared the protective effects of UUO with that provided by ARB. NEP25 mice injected with 2.5 ng/g BW of LMB2 were treated with a high dose of losartan (0.5 g/l of drinking water, ∼25 μg/g BW) starting 24 h later, i.e., at the same time as UUO. Eight days after LMB2, ARB-treated NEP25 mice showed the expected attenuation of injury compared with non-ARB-treated NEP25 mice. However, the nephrin staining in ARB-treated mice was severely downregulated, with a median nephrin index of 1.21 (Fig. 7). Thus, compared with 4.02 achieved with UUO (Fig. 2), ARB was much less effective in protecting against progressive podocyte injury.
Fig. 7.
Angiotensin II type 1 receptor antagonist (ARB) was far less effective in protecting podocyte injury than UUO. NEP25 mice were injected with LMB2 (2.5 ng/g BW), and a subset was treated with a high dose of the ARB losartan starting the next day. Eight days after LMB2, nephrin staining was severely downregulated compared with UUO in both groups, although NEP25 mice treated with ARB showed slightly less nephrin loss than untreated NEP25 mice.
DISCUSSION
The present studies examined the puzzling phenomenon that urine outflow obstruction does not cause sclerosis in the glomerulus although fibrosis is induced in the tubulointerstitium. This study makes the novel observation that ureteral obstruction actively prevents sclerosis in the glomerulus. Since the protection was even greater than with ARB administration, we verified the observation using two different transgenic models. The two models impart distinct insults, but share the same target, i.e., the podocyte. Our studies in these models revealed that the protective effect of urine outflow obstruction on the glomerular architecture acts at the level of the podocyte, the site of the initial pathological process leading to glomerulosclerosis.
While considerable efforts have been extended to define the effects of transmural pressure difference in the glomerular sclerosing process, the transgenic models of podocyte injury used in the current study offer several advantages over the previous models of glomerular injury and methods of manipulating transmural pressure difference. For example, the subtotal nephrectomy model cannot be used to study the effects of superimposed ureteral obstruction, as this precipitates prompt death from uremia. In diabetic nephropathy models, the progression of glomerular damage is too slow to document any beneficial effect of UUO of 7 days duration, as a longer duration of UUO causes secondary changes in glomerular contraction along with marked thinning of the cortex. In addition, UUO employed in the current study effectively nullifies glomerular capillary pressure and transmural pressure difference, with the magnitude of this effect being substantially greater than that documented with pharmacological interventions in the RAS (2, 22, 24). In the current study, UUO completely abrogated progression of podocyte injury in the HIV-1 model. Morphological preservation was accompanied by prevention of downregulation of synaptopodin and nephrin proteins even in the face of continued podocyte expression of toxic HIV-1 gene products. These results suggest that intense or continuous molecular insults that directly attack podocytes are insufficient alone to bring about the full spectrum of podocyte injury and glomerular damage in the obstructed kidneys. At the same time, the data do not imply that all the pathogenic mechanisms are channeled through physical forces, i.e., that physical forces alone can bring about podocyte injury and glomerulosclerosis.
Although the protective effect of UUO found in the present study is not clinically germane, it provides a unique opportunity to identify the pathogenic mechanisms underlying podocyte injury and glomerulosclerosis. The study reveals that, whereas 7 days of UUO protected the immunohistochemical pattern of podocyte nephrin expression, it had little effect on its mRNA level, indicating that UUO affected posttranscriptional regulation of nephrin. Notably, in human minimal change disease, Kim et al. (12) documented the discrepancy between nephrin mRNA and protein, suggesting a disturbance in posttranscriptional regulation of nephrin biosynthesis, specifically in nephrin plasma membrane localization. Of note, Tryggvason et al. (20) further showed a critical effect of endoplasmic reticulum stress in posttranslational dysregulation of nephrin.
The mechanism(s) for the protective effects of ureteral obstruction appears multifactorial. An injurious role of increased glomerular pressure, hence transmural pressure difference, i.e., glomerular pressure minus Bowman's space pressure, on glomerular architecture has been demonstrated in a variety of animal models using numerous therapeutic maneuvers, including dietary manipulations and angiotensin receptor antagonists, which have been shown to protect the glomerulus while normalizing high glomerular capillary hydraulic pressure (3, 6, 8, 9, 17). In the present study, the net ultrafiltration pressure was nullified by raising Bowman's space hydraulic pressure, i.e., by increasing the force opposing the glomerular capillary hydraulic pressure. In the obstructed kidney, the net ultrafiltration pressure becomes close to zero (5), i.e., far lower than that achieved by ARB. In view of the superior protective effect of UUO than ARB, the present study suggests that the “normal” baseline level of glomerular pressure may still not be ideal for preservation of the glomerular architecture, particularly in the face of existing injury.
Another potential mechanism for the UUO-induced protection of podocytes involves diminution of forces contributing to leakage of serum macromolecules into Bowman's space, thereby protecting podocytes from exposure to these potentially toxic macromolecules. In support of this idea are results in nonobstructed kidneys of HIV-1 and NEP25 mice which showed marked accumulation of plasma proteins in podocytes (Fig. 6). By contrast, ureteral obstruction prevented this accumulation. It is therefore conceivable that plasma macromolecules may be toxic to podocytes and parietal epithelial cells as they activate lysosomal systems within these cells (7) or by dysregulating cytokines and growth factors (1). Such mechanisms could also affect parietal-podocyte transition as parietal epithelial cells in the niche adjacent to the capillary loop can serve as stem cells to replenish injured podocytes (4, 19). Of note, the potentially harmful effects of high transmural pressure difference vs. leaked plasma protein are not mutually exclusive, since, quantitatively, the protein leakage is determined by the pressure where the barrier function of the capillary wall is already compromised (21).
As expected, ureteral obstruction caused tubulointerstitial injury in both HIV-1 and NEP25 models, although protecting against glomerulosclerosis (Supplementary Fig. S1). UUO-induced tubulointerstitial injury involves inflammatory cytokines and fibrogenic growth factors as well as activation of the local RAS (13). Our results show that the protective effects of UUO on glomerular injury surpass these potentially harmful influences (11). The beneficial effects of UUO were particularly striking in the HIV-1 model since podocytes in this model are under continuous local, direct attack by the HIV-1 genes they express.
In summary, urine outflow obstruction has a protective effect on the glomerular architecture from sclerosing processes and this protective mechanism operates at the level of podocytes.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants DK37868 and DK44757, the Research for the Future Program and Grant-in Aid for Scientific Research of Japan Society for the Promotion of Science, MEXT, HAITEKU, and in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
DISCLOSURES
T. Matsusaka received a research grant from Chugai Pharmaceutical Co., Ltd.
Supplementary Material
ACKNOWLEDGMENTS
We thank Akira Akatsuka, Shiho Imai, Naoko Sasaoka, Suguri Niwa, and Chie Sakurai for technical assistance and Dr. Ayumi Shintani for advice on statistical analysis.
Parts of this study were presented in abstract form at the annual meetings of the American Society of Nephrology in 2003 and 2004.
REFERENCES
- 1. Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G. Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77: 1993–2000, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson S, Vora JP. Current concepts of renal hemodynamics in diabetes. J Diabetes Complications 9: 304–307, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arendshorst WJ, Finn WF, Gottschalk CW. Nephron stop-flow pressure response to obstruction for 24 hours in the rat kidney. J Clin Invest 53: 1497–1500, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 7. Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34: 43–59, 1976 [PubMed] [Google Scholar]
- 8. Dworkin LD, Hostetter TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest 73: 1448–1461, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol 23: 194–199, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ishidoya S, Morrissey J, McCracken R, Klahr S. Delayed treatment with enalapril halts tubulointerstitial fibrosis in rats with obstructive nephropathy. Kidney Int 49: 1110–1119, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int 47: 1285–1294, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Kim BK, Hong HK, Kim JH, Lee HS. Differential expression of nephrin in acquired human proteinuric diseases. Am J Kidney Dis 40: 964–973, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res 58: 968–975, 1998 [PubMed] [Google Scholar]
- 15. Matsusaka T, Asano T, Niimura F, Kinomura M, Shimizu A, Shintani A, Pastan I, Fogo AB, Ichikawa I. Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor-independent. Hypertension 55: 967–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Meyer TW, Anderson S, Rennke HG, Brenner BM. Reversing glomerular hypertension stabilizes established glomerular injury in renal ablation. J Hypertens Suppl 4: S239–S241, 1986 [PubMed] [Google Scholar]
- 18. Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, Ichikawa I. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74: 1262–1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 13: 1385–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Yoshioka T, Rennke HG, Salant DJ, Deen WM, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res 61: 531–538, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 77: 1925–1930, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong J, Zuo Y, Ma J, Fogo AB, Jolicoeur P, Ichikawa I, Matsusaka T. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int 68: 1048–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Ziai F, Nagano H, Kusaka M, Coito AJ, Troy JL, Nadeau KC, Rennke HG, Tilney NL, Brenner BM, MacKenzie HS. Renal allograft protection with losartan in Fisher→Lewis rats: hemodynamics, macrophages, and cytokines. Kidney Int 57: 2618–2625, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.