Abstract
This study evaluated the potential utility of albuminuria as a “biomarker” of acute kidney injury (AKI) and tested whether AKI induces renal expression of the normally silent albumin gene. Urine albumin concentrations were measured in mice with five different AKI models (maleate, ischemia-reperfusion, rhabdomyolysis, endotoxemia, ureteral obstruction). Albumin gene induction in renal cortex, and in antimycin A-injured cultured proximal tubular cells, was assessed (mRNA levels; RNA polymerase II binding to the albumin gene). Albumin's clinical performance as an AKI biomarker was also tested (29 APACHE II-matched intensive care unit patients with and without AKI). Results were contrasted to those obtained for neutrophil gelatinase-associated lipocalin (NGAL), an established “AKI biomarker” gene. The experimental and clinical assessments indicated albumin's equivalence to NGAL as an AKI biomarker (greater specificity in experimental AKI; slightly better receiver-operating curve in humans). Furthermore, experimental AKI markedly induced the albumin gene (mRNA/RNA polymerase II binding increases; comparable to those seen for NGAL). Albumin gene activation in patients with AKI was suggested by fivefold increases in RNA polymerase II binding to urinary fragments of the albumin gene (vs. AKI controls). Experimental AKI also increased renal cortical mRNA levels for α-fetoprotein (albumin's embryonic equivalent). A correlate in patients was increased urinary α-fetoprotein excretion. We conclude that AKI can unmask, in the kidney, the normally silent renal albumin and α-fetoprotein genes. In addition, the urinary protein data independently indicate that albuminuria, and perhaps α-fetoprotein, have substantial utility as biomarkers of acute tubular injury.
Keywords: biomarkers, neutrophil gelatinase-associated lipocalin, monocyte chemoattractant protein-1, RNA polymerase II, HK-2 cells
acute kidney injury (AKI) is typically a silent clinical event, usually being identified only after its occurrence by the onset of progressive azotemia. Because of this, there has been a concerted effort to identify AKI “biomarkers,” with the hope of facilitating timely prophylactic and/or early therapeutic interventions. Toward this end, three general diagnostic approaches have been used. First, increased excretion of “resident” proximal tubule proteins has been sought. Candidate molecules include both proximal tubular cell transport proteins (34) and enzymes (e.g., N-acetylglucosaminidase; alkaline phosphatase, γ-glutamyltransferase) (2, 10, 14, 25, 42). A second approach has been to document proximal tubular dysfunction, as denoted by decreased tubular reabsorption of freely filtered low-molecular-weight proteins (e.g., β2-microglobin, lysozyme, or cystatin C) (17, 26, 39). Third, and the approach which has received the greatest recent attention, has been to quantify the urinary excretion of tubular proteins that are acutely overexpressed in response to AKI. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) are two such examples (3–5, 8, 9, 18, 28).
It is noteworthy that each of these three general approaches is based on quantifying the concentrations of a specific urinary protein. To better interpret these results, it could prove useful to compare each of these protein biomarkers to either total urinary protein (uTP) or urinary albumin (uAlb) content. Although total protein and albumin excretion remain “gold standards” in the evaluation of chronic glomerulopathies, their potential utility as AKI biomarkers has received surprisingly little attention and remains poorly defined. Hence this study has sought to clarify whether urinary albumin might provide useful information in this regard. Furthermore, we tested the novel hypothesis that the albumin gene, which is normally repressed in kidney, could potentially be upregulated in response to AKI and thus express characteristics of an acute renal tubular stress reactant (e.g., analogous to NGAL or KIM-1). To evaluate these possibilities, we have measured uTP and uAlb concentrations during the early phases (2–4 h; 24 h) of five different mouse models of AKI (ischemia-reperfusion, maleate nephrotoxicity, glycerol-induced rhabdomyolysis, urinary tract obstruction, and endotoxemia). Potential activation of the albumin gene was assessed by quantifying renal cortical albumin mRNA. Proximal tubule cell-specific results were sought using cell culture experiments. Finally, potential translational value of these experimental data was sought by studying both urinary albumin concentrations, and levels of RNA polymerase II (Pol II) binding to urinary fragments of the albumin gene in critically ill patients with (AK+) and without AKI (AKI−). These experiments, described below, provide evidence for AKI-mediated albumin gene induction in the renal cortex and support the concept that albuminuria may, indeed, have AKI biomarker utility.
METHODS
Animal Experiments
Male CD-1 mice (25–35 g; Charles River Laboratories, Wilmington, MA) were used for all animal experiments. Surgeries were performed under deep pentobarbital sodium (40–50 mg/kg ip) using protocols that were approved by the Institutional Animal Care and Use Committee. Five different models of renal injury were studied as follows.
Maleate nephrotoxicity.
Maleate-mediated proximal tubule injury was induced by Na maleate injection (600 mg/kg ip) (36). Either 4 or 24 h later (n = 5 at each time), the mice were anesthetized, a midline abdominal incision was made, and then plasma, urine, and kidney samples were obtained. Matched samples from five normal mice served as controls. Plasma samples were used to quantify blood urea nitrogen (BUN) concentrations as an index of kidney injury. The kidneys were resected, iced, and cortical tissue samples were extracted for total RNA with an RNeasy Kit (Qiagen) for subsequent RT-PCR analyses (36). Urine samples were assayed for total protein, albumin, and creatinine concentrations (see below).
Rhabdomyolysis-induced AKI.
Five mice were injected with 9 ml/kg of 50% glycerol, administered in equally divided doses into the hindlimbs, as previously described (37). Either 4 or 24 h later, plasma, urine, and kidney samples were obtained as noted above. Four normal mice served as controls.
Ischemia-reperfusion-induced AKI.
Twelve mice were subjected to 25 min of bilateral renal pedicle occlusion, conducted through a midline abdominal incision. Body temperature was maintained at 37°C. Following vascular clamp removal, the abdominal cavity was closed with sutures in two layers (38). The mice were then divided into two groups: early reperfusion (4 h; n = 6) and late reperfusion (∼24 h; n = 6). The mice used for early reperfusion studies received 1 mg of furosemide via a tail vein injection to induce a diuresis. Four hours later, urine samples were obtained from the urinary bladder and then the kidneys were resected for subsequent renal cortical mRNA analysis. Six sham-operated mice, also injected with furosemide, were used to obtain control urine and renal cortical tissue samples. The late reperfusion mice (n = 6) were allowed to recover from anesthesia. At the approximate 24-h time point, they were reanesthetized, a urine sample was obtained from the bladder (without furosemide administration), a terminal plasma sample was collected for BUN analysis, and then the kidneys were resected for mRNA analysis. Six sham-operated mice served as controls.
Endotoxemia-induced renal failure.
Endotoxemia induces a “prerenal” form of acute renal failure in mice, mediated by a primary increase in renal vasoconstriction in the absence of tubular necrosis (e.g., Refs. 32 and 34). Thus we used this model to assess whether albuminuria/proteinuria result. Mice were subjected to intraperitoneal injection of Escherichia coli endotoxin (10 mg/kg; 0111:B4; L-2630) (35). Either 2 or ∼24 h later (n = 5 at each time point), the mice were anesthetized, and plasma, urine, and kidney samples were obtained, as noted above. Four normal mice served as controls.
Unilateral ureteral obstruction/obstruction release.
Six mice were anesthetized and subjected to left ureteral obstruction, created at the ureteral midpoint with a silk ligature (40, 42). Approximately 24 h later, the mice were reanesthetized. Three of the mice had the dilated left renal pelvis aspirated with a syringe fitted with a 26-gauge needle, and the urine samples were saved. The obstructed left kidneys and the unobstructed right kidneys were then resected. The remaining three mice had their urinary bladders emptied, and the left ureteral ligature was removed to permit urinary drainage. Then, the right ureter was ligated. By so doing, all subsequent urine within the urinary bladder originated from the postobstructed left kidney. The abdominal cavities were then closed by resuturing. Approximately 24 h later, they were reanesthetized, urine was collected from the bladder, a plasma sample was obtained for BUN analysis, and the kidneys were resected. An equal number of sham-operated mice served as controls.
Analysis of mouse tissue and urine samples.
Mouse uTP and uAlb concentrations were determined by the pyrogallol red-molybdate (24) and the bromocresol green (Bioassays, Hayward, CA) methods, respectively. The uTP and uAlb results were expressed as absolute urine concentrations (mg/dl) and as uTP/uCr and uAlb/uCr ratios. The percentage to which uAlb contributed to the total urine protein uTP (uAlb/uTP %) was also determined (uAlb/uTP × 100%). RNA was extracted from renal cortices with the RNeasy kit method (Qiagen), and the samples were assayed for albumin mRNA by RT-PCR using the primers presented in Table 2. For comparison, NGAL mRNA levels were also assessed (20). α-Fetoprotein mRNA was also quantified by RT-PCR using primers from R&D Systems (RDP337). The albumin, NGAL and α-fetoprotein mRNA results were factored by simultaneously obtained GAPDH product, used as a “housekeeping” gene.
Table 2.
Primary pairs used for PCR analyses, as described in the text
| Sequences | Product Size | |
|---|---|---|
| Primers for RT-PCR mRNA | ||
| Human albumin | 5′-ACA AGA GTG AGG TTG CTC ATC GGT-3′ | 872 bp |
| 5′-CAC TTC GGC AAT GCA GTG GGA TTT-3′ | ||
| Mouse albumin | 5′-TGT CTT CCT GGG CAC ATT CTT GTA-3′ | 336 bp |
| 5′-TTG GTG CCC ACT CTT CCT AGG TTT-3′ | ||
| Primers for ChIP gene | ||
| Human albumin | 5′-CGA AAC ACA CCC CTG GAA TA-3′ | 131 bp |
| 5′-TGC TAA TTT CCC TCC GTT TG-3′ | ||
| Mouse albumin | 5′-GCT GGG GTT GTC ATC TTT GT-3′ | 104 bp |
| 5′-TTC CAA ACC TCC GTG AAA AC-3′ |
ChIP, chromatin immunoprecipitation assay.
Cultured Human Proximal Tubule (HK-2) Cell Experiments
mRNA assessments.
The following experiments were conducted to ascertain whether acutely damaged proximal tubule cells respond with an increase in albumin mRNA. To this end, HK-2 cells, an immortalized proximal tubule cell line derived from normal human kidney, were used (27). They were maintained in T75 flasks with keratinocyte serum-free medium (KSF-M) supplemented with pituitary extract and l-glutamine, as previously described (27). For experimentation, they were split by trypsinization and seeded into T25 Costar flasks. Approximately 6 h later, the flasks were divided equally into three groups (n = 7 flasks/group): 1) control incubation with KSF-M; 2) incubation with antimycin A (7.5 μM); or 3) incubation with antimycin A+Ca ionophore A23187 (10 μM) (11). After completing an overnight incubation, the cells were harvested for albumin mRNA analysis using the primers presented in Table 2. As a comparison, NGAL mRNA levels were also assessed (13). The mRNA results were factored by the simultaneously determined values for GAPDH.
RNA Poll II binding assessments.
Pol II binding increases at the transcription start site of genes during active transcription. Therefore, to gain further support for the concept that injury induces albumin and NGAL gene transcription, the combined antimycin A/Ca ionophore challenge was repeated in seven flasks of HK-2 cells and in eight controls flasks. After overnight incubations, chromatin was harvested, fixed in formalin, and sheared (20). Pol II binding to exon 1 of the albumin and NGAL genes was assessed by chromatin immunoprecipitation assay (ChIP), as previously described (20). The albumin primers used are presented in Table 2. The results were factored by the amount of Pol II detected at exon 1 of the β-actin housekeeping gene.
Clinical Study
Patient cohorts.
Urine samples were obtained from a subset of patients enrolled in a large prospective observational study of critically ill adult patients treated in multiple intensive care units (ICU) at Vanderbilt University (20, 29). The research project was reviewed and approved by the Vanderbilt University Institutional Review Board (IRB). Informed consent was obtained from the patient or surrogate, whenever possible; however, given the minimally invasive nature of the study, a waiver of consent was deemed permissible by the Vanderbilt IRB. Samples were collected between February 2006 and March 2009. They were collected on the morning of ICU day 2 from 14 critically ill patients who developed AKI (AKI+), defined as ≥50% or ≥26.5 μmol/l increase in serum creatinine from the value measured closest to enrollment. ICU control samples were collected from 15 critically ill patients who did not develop AKI (AKI−), but who had comparable overall illness severity as judged by Acute Physiology and Chronic Health Evaluation II scores (APACHE II; see Table 1). The AKI+ and AKI− populations were also matched for age, race, gender, and sepsis status. As normal controls, six urine samples were obtained from healthy volunteers (data not included in Table 1). Thus a total of 35 urine samples were used for the clinical portion of this study. All of the AKI+ patients in the study population had a presumptive nephrological diagnosis of “acute tubular necrosis,” as judged by consulting nephrologists.
Table 1.
Clinical characteristics of 14 critically ill patients with acute kidney injury and 15 critically ill control patients
| Clinical Characteristic | Critically Ill Controls | Patients with AKI |
|---|---|---|
| Age, yr | 63 ± 15 | 52 ± 17 |
| Male | 6 (40%) | 10 (71%) |
| Caucasian | 12 (80%) | 12 (86%) |
| Current smoker | 6 (40%) | 1 (7%) |
| Alcohol abuse | 5 (33%) | 2 (14%) |
| ICU location | ||
| Medical ICU | 8 (53%) | 10 (71%) |
| Surgical ICU | 5 (33%) | 2 (14%) |
| Cardiac ICU | 2 (13%) | 2 (14%) |
| APACHE II score | 27 ± 4 | 33 ± 7 |
| Comorbidities | ||
| Diabetes | 3 (20%) | 2 (14%) |
| Chronic liver disease | 3 (20%) | 2 (14%) |
| Hypertension | 6 (40%) | 6 (43%) |
| Congestive heart failure | 2 (13%) | 3 (21%) |
| Coronary artery disease | 2 (13%) | 2 (14%) |
| Peripheral vascular disease | 1 (6.7%) | 2 (14%) |
| Chronic kidney disease | 0 (0%) | 0 (0%) |
| Sepsis at enrollment | 11 (73%) | 11 (79%) |
| Creatinine, mg/dl | ||
| Baseline | 0.81 ± 0.18 | 0.83 ± 0.34 |
| Admission | 0.86 ± 0.25 | 2.38 ± 1.69 |
| Enrollment | 0.84 ± 0.29 | 2.65 ± 1.21 |
| Peak | 0.98 ± 0.30 | 3.68 ± 1.5 |
| Probable primary cause of AKI | ||
| Sepsis | NA | 11 (79%) |
| Shock (nonseptic) | NA | 3 (21%) |
| Outcomes | ||
| ICU days | 7 ± 6 | 15 ± 14 |
| Hospital mortality | 0 (0%) | 4 (29%) |
| Renal replacement therapy | 0 (0%) | 8 (57%) |
Values are means ± SD or n (%). AKI, acute kidney injury; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; NA, not applicable.
Total protein, albumin, NGAL, and MCP-1 analyses.
Two-milliliter urine samples were centrifuged (10,000 rpm), and the supernatants were assayed for total protein (pyrogallol red-molybdate) and albumin concentrations (immunoturbidity; Beckman Coulter; model DxC 600). As comparators, the results were compared with those for either NGAL or to MCP-1, the latter being comparable to NGAL as an AKI biomarker (20). NGAL and MCP-1 levels were measured by ELISAs, performed by the Cytokine Shared Resource Laboratory, Fred Hutchinson Cancer Research Center. The results were factored by corresponding urine creatinine concentrations.
ChIP assay.
The urine pellets obtained from the above centrifugations were formalin fixed, sheared, and used to determine Pol II binding to exon 1 of the albumin gene. For comparison, Pol II binding was also assessed at exon 1 of the NGAL and MCP-1 genes (20). The methods used were as previously described (20). The albumin primers used for ChIP assay are presented in Table 2. All Pol II binding results were factored by the amount of Pol II at exon 1 of the β-actin housekeeping gene (20).
Calculations and Statistics
All values are presented as means ± SE, unless otherwise indicated. Statistical comparisons were performed by an unpaired Student's t-test. If multiple comparisons were made, the Bonferroni correction was applied. Receiver-operating curve characteristics (ROC) for clinical data were computed parametrically using a bi-negative exponential model [PASW Statistics 18.0 for Macintosh (IBM)].
RESULTS
Maleate Model of Nephrotoxic AKI
Absolute urine protein concentrations.
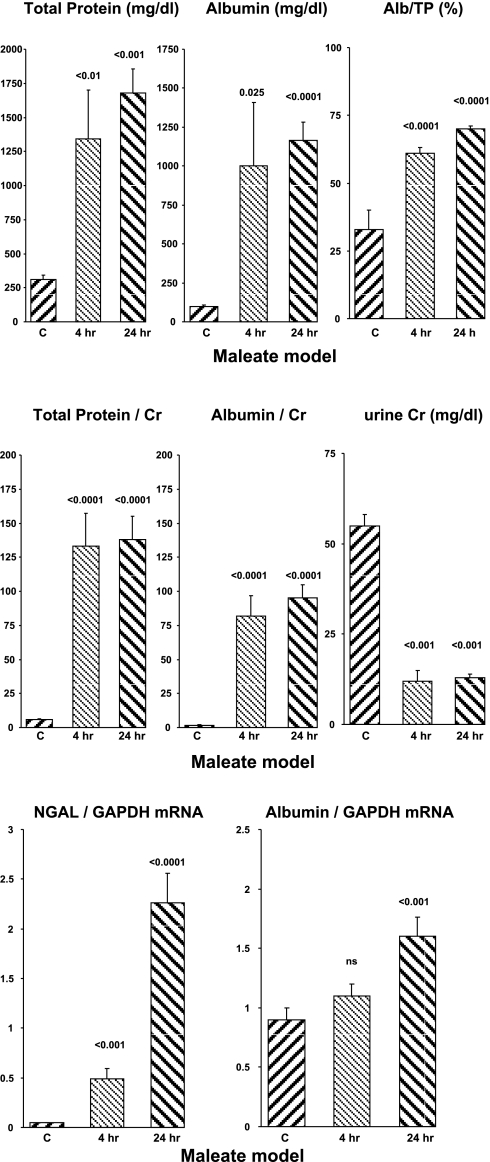
Within 4 h of maleate injection, there were marked increases in both uAlb and uTP concentrations (Fig. 1, top). The albumin increase was larger, and this resulted in a doubling of albumin's contribution to the total urinary protein content (uAlb/uTP × 100%; rising from 30 to 60%; top right). By 24 h post-maleate injection, further increases in both albumin and total protein concentrations were observed. The uAlb/uTP % remained elevated at ∼65%.
Fig. 1.
Maleate nephrotoxicity in mice. Urinary total protein (uTP) and urinary albumin (uAlb) are expressed as either absolute concentrations (top) or as ratios to urinary creatinine (middle) at the 4- and 24-h time points. The percentage to which albumin contributed to the urine total protein concentration is presented at top right, and absolute urine creatinine concentrations are presented at middle right. Neutrophil gelatinase-associated lipocalin (NGAL) mRNA and albumin mRNA concentrations in renal cortex at 4 and 24 h post-maleate injection are shown at the bottom. C, controls.
Urine protein/creatinine ratios.
Factoring the uTP and uAlb concentrations by the urinary creatinine concentration magnified the differences between the control and maleate-treated animals. For example, at 4 h post-maleate injection, the uTP/uCr and uAlb/uCr values were ∼20- to 40-fold higher than seen in the control animals (Fig. 1, middle). This was due to a concomitant increase in both uTP and uAlb concentrations, as well as corresponding reductions in urine creatinine values (Fig. 1, middle, far right).
Renal cortical mRNA concentrations.
As expected, maleate induced dramatic stepwise increases in NGAL mRNA levels. Surprisingly, stepwise increases in renal cortical albumin mRNA values (Fig. 1, bottom right) were also observed.
Severity of AKI.
Maleate caused severe AKI, as denoted by marked increases in the 24-h BUN concentrations (98 ± 13 mg/dl; controls, 26 ± 1; P < 0.01).
Glycerol Model of Rhabdomyolysis-Induced AKI
Absolute urine protein concentrations.
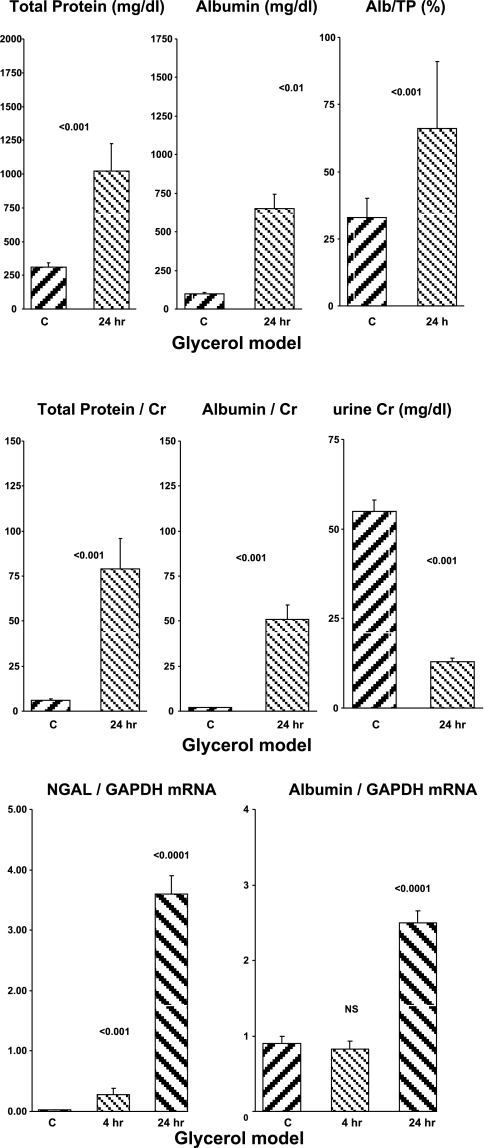
Urine samples that were collected at 4 h post-glycerol injection were deep red in color, reflecting myohemoglobin excretion, as is typical of this acute renal failure model. This precluded accurate urinary protein assessments at this time, given that the assays are colorimetric. However, by 24 h post-glycerol injection, the urine had returned to normal color and hence, these were assayed. Up to sixfold increases in both urinary total protein and urinary albumin concentrations were observed (Fig. 2). As with maleate, the % contribution of albumin to total urine protein increased, rising from 33 to 66% (Fig. 2, top right).
Fig. 2.
Glycerol-mediated rhabdomyolysis in mice. Urine total protein and urine albumin concentrations at 24 h post-glycerol injection are expressed as absolute concentrations (top) or as albumin/creatinine ratios (middle). Bottom: 4- and 24-h postglycerol renal cortical NGAL and albumin mRNA levels.
Urine protein/creatinine ratios.
As with maleate, factoring the uTP and uAlb concentrations by urine creatinine magnified the differences between the control and AKI urine samples (∼10- to 20-fold greater in the post-glycerol vs. the control samples) (Fig. 2, middle). Again, as with maleate, this was due to the fact that the urine creatinine concentrations were reduced in the postglycerol urine samples (Fig. 2, middle right).
Renal cortical mRNA levels.
NGAL mRNA demonstrated the expected stepwise increase post-glycerol injection (Fig. 2, bottom). An accompanying increase in albumin mRNA was also observed (3-fold control values at the 24-h time point).
Severity of AKI.
All of the glycerol-treated mice developed severe renal failure (BUN, 146 ± 9 mg/dl; controls, 26 ± 1; P < 0.01).
Ischemia-Reperfusion-Induced AKI
Absolute urine protein concentrations.
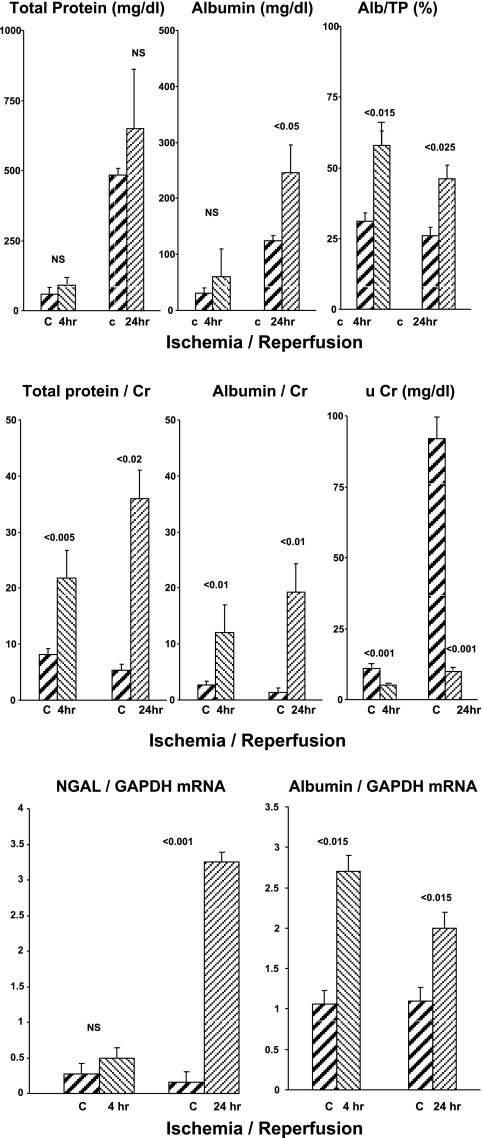
At both 4 and 24 h postischemia, uTP and uAlb concentrations were elevated, compared with control mice, but only the 24-h uAlb values achieved statistical significance (Fig. 3, top). However, the uAlb/uTP % was significantly higher in the postischemia groups at both the 4- and 24-h time points (∼60% vs. ∼30% for the controls; consistent with both the maleate and glycerol models).
Fig. 3.
Ischemia-reperfusion injury model in mice. uTP, uAlb, renal cortical albumin mRNA, and NGAL mRNA levels at 4- and 24-h time points are presented (top, middle, and bottom, respectively). Two sets of controls were used, one with (4 h) and one without (24 h) furosemide use to stimulate a diuresis.
Urine protein/creatinine ratios.
Factoring the absolute uTP and uAlb levels by urine creatinine concentrations revealed ∼5- to 20-fold higher values for both at 4- and 24-h postischemia mice compared with control mice (Fig. 3, middle). As noted in the glycerol and maleate models, these dramatic increases reflected both increased protein and albumin concentrations and corresponding decreases in urine creatinine concentrations (see Fig. 3, middle, far right).
Renal cortical mRNA values.
NGAL mRNA was significantly elevated at 24 h, but not at 4 h, postischemia (Fig. 3, bottom). Conversely, the albumin mRNA values were elevated at both time points.
Severity of AKI.
The ischemia-reperfusion protocol induced severe renal failure, as denoted by the 24-h BUN concentrations (156 ± 3; vs. controls, 27 ± 1 mg/dl; P < 0.001).
Mouse Model of Endotoxemia
Absolute urine protein concentrations.
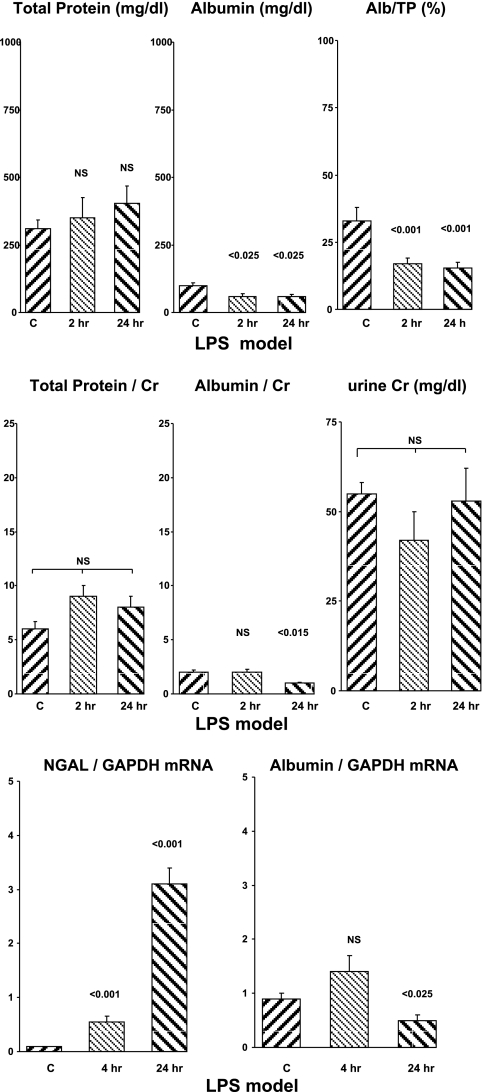
No increases in uTP or uAlb concentrations were observed at either 2 or 24 h post-LPS injection (Fig. 4, top). Rather, LPS significantly reduced uAlb levels compared with the control values. Thus, unlike the above structural models of AKI which induce tubular necrosis, this prerenal form of AKI did not evoke increased albumin excretion.
Fig. 4.
LPS model of renal injury in mice. uTP, uAlb, renal cortical albumin mRNA, and NGAL mRNA levels at 2-and 24-h time points are presented (top, middle, and bottom, respectively). See Fig. 1 legend for details.
Urine protein/creatinine ratios.
LPS did not alter urine creatinine concentrations (Fig. 4, middle). Thus, factoring urine protein concentrations by uCr did not change the relative amounts of albumin or total protein between the endotoxemic and control animals.
Renal cortical mRNA concentrations.
LPS caused a marked stepwise increase in renal cortical NGAL mRNA (Fig. 4, bottom). Conversely, albumin mRNA was unchanged at 2 h post-LPS. and it was significantly suppressed by ∼50% at the 24-h post-LPS time point (P < 0.025).
LPS-induced azotemia.
LPS injections evoked mild azotemia, as reflected by 24-h BUN concentrations (55 ± 14 vs. 24 ± 2 mg/dl; P < 0.025).
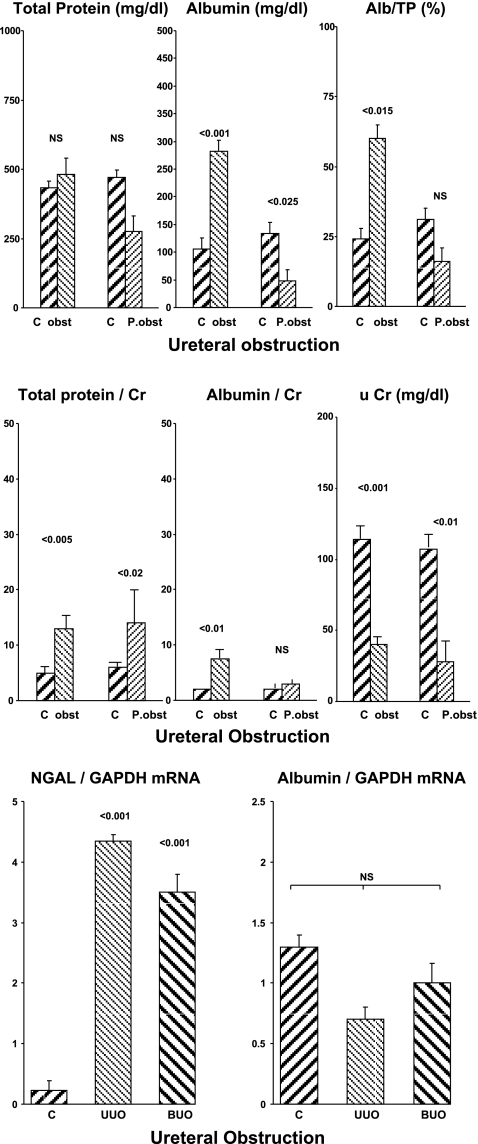
Mouse Model of Ureteral Obstruction
Absolute urine protein concentrations.
Urine aspirated from the renal pelvis of kidneys subjected to 24 h of ureteral obstruction had normal uTP, but elevated uAlb, concentrations (Fig. 5, top). This resulted in the uAlb/uTP% rising from 24 to ∼60% (Fig. 5, right). However, by 24 h post-release of ureteral obstruction, the uAlb, uTP, and uAlb/uTP % values all returned to normal levels.
Fig. 5.
Ureteral obstruction (obst) and postobstruction (P. obst) in mice. Top: absolute urinary total protein and albumin concentrations. Middle: urinary protein/creatinine ratios. The assessments were made after 24 h of ureteral obstruction ± 24 h of obstruction release. NGAL and albumin mRNAs were assessed 24 h after either unilateral ureteral obstruction (UUO) or 24 h bilateral after ureteral obstruction (BUO). Bottom: mRNA results.
Urine protein/creatinine ratios.
The urine creatinine concentrations were markedly reduced both during and after the period of ureteral obstruction (Fig. 5, middle). This raised the uTP/uCr and uAlb/uCr ratios in the presence of ureteral obstruction. However, by 24 h post-release of obstruction, no increase in uAlb/uCr concentrations was observed.
Renal cortical mRNA concentrations.
Unilateral ureteral obstruction (UUO) caused an approximate 15-fold increase in renal cortical NGAL mRNA (Fig. 5, bottom). Conversely, no increase in albumin mRNA was observed. Of note, UUO and bilateral ureteral obstruction (BUO) can exert differing effects on renal parenchyma (e.g., Ref. 19). Thus NGAL and albumin mRNAs were also assessed in five additional mice that had been subjected to 24 h of BUO. Again, a marked increase in NGAL, but not in albumin mRNA levels, was observed.
Azotemia.
As expected, the presence of UUO and BUO caused mild and severe azotemia, respectively (control 24 ± 41; UUO, 32 ± 2; and 126 ± 6 mg/dl).
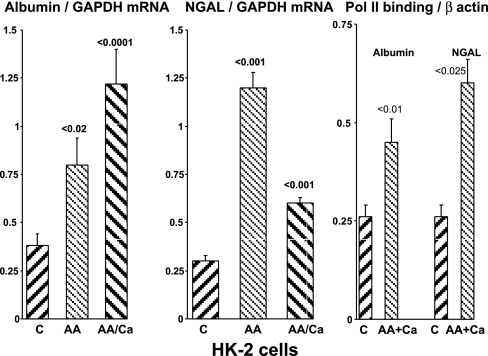
HK-2 cell assessments.
When cultured proximal tubule cells were challenged with a mitochondrial inhibitor, antimycin A, or with antimycin A+a Ca2+ ionophore (Ca), marked and comparable increases in albumin mRNA and NGAL mRNA resulted (Fig. 6). As shown in Fig. 6, right, the NGAL and albumin mRNA increases were associated with significant increases in Pol II binding to exon 1 of these two genes, implying that the mRNA increases likely arose from increased transcription.
Fig. 6.
HK-2 cell analyses. Albumin and NGAL mRNA levels, and RNA polymerase II (Poll II) binding to the albumin and NGAL genes, were assessed 24 h following control incubations or exposure to antimcyin A (AA) or AA+Ca2+ ionophore A23187 (Ca) addition. AA alone or AA+Ca ionophore each induced marked increases in albumin mRNA and NGAL mRNA. In addition, antimycin A+Ca ionophore induced an approximate 2- to 3-fold increase in Pol II binding to exon 1 of the albumin and NGAL genes (right), implying that the increase in mRNA values reflected increased gene transcription.
Clinical Assessments
Fifteen critically ill, ICU hospitalized, patients with AKI (8 of whom eventually required hemodialysis; see Table 2), and 14 APACHE II matched critically ill ICU patients who did not develop azotemia (AKI−) formed the patient cohort for this part of the study (Table 2, Figs. 7–9). The patients were enrolled in an IRB approved observational cohort study at Vanderbilt University (29), the goal of which is to identify biomarkers of critical illness. The serum creatinine concentrations for the AKI+ and AKI− groups at the time of urine sampling were 2.65 ± 0.31 and 0.84 ± 0.1 mg/dl, respectively (P < 0.01). Selected clinical demographics and associated conditions for these two patient groups are presented in Table 1.
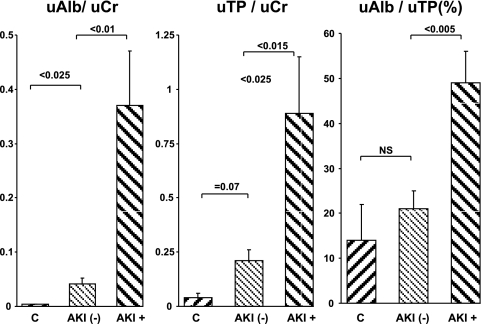
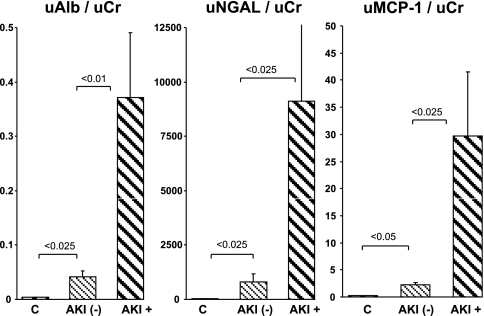
Fig. 7.
Clinical assessments of uAlb/uCr ratios, uTP/Cr ratios, and the percentage to which albumin contributed to the total protein concentrations in control subjects (C) and in ICU patients with and without acute kidney injury (AKI; AKI+ and AKI−, respectively). There was a trend toward higher urine protein concentrations in the AKI-− vs. the control group (implying subclinical renal injury). The AKI+ patients manifested striking increases in both albumin and total urine protein, with albumin showing a preferential increase (reflected by a significant increase in albumin's contribution to the total urine protein concentration, ∼50%). This uAlb/uTP ratio closely agreed with the values observed in the mouse AKI studies.
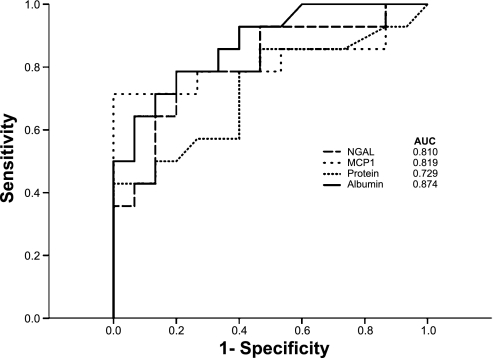
Fig. 9.
Clinical assessments of receiver-operator characteristics curves (ROC) for acute kidney injury, derived by using uAlb/Cr, uTP/Cr, MCP-1/Cr, and NGAL/Cr for the 29 AKI+ and AKI− patients who are described in the text. The greatest area under the curve was for albumin (0.874), followed by MCP-1 (0.819), NGAL (0.810), and uTP.
The urine protein concentrations are presented as uTP/uCr and uAlb/uCr ratios. The AKI negative (AKI−) patients had modestly increased uTP/Cr and uAlb/Cr ratios compared with the normal (control) volunteers (Fig. 7). However, the % contribution of albumin to the total urine protein levels remained unchanged for the AKI− group (∼15%). Conversely, the AKI+ group had markedly elevated uAlb/uCr and uTP/uCr values (e.g., ∼10-fold higher than AKI− patients), and the contribution of albumin to the total urine protein concentration rose from control values of ∼15 to ∼50%. Of note, the uAlb/uTP% values for the AKI+ patients (∼50%) were highly comparable to the values observed in the three experimental intrarenal models of AKI (50–65%), thereby representing a strong experimental-clinical correlate.
A comparison of uAlb/uCr ratios with the ratios for the two reference biomarker proteins, NGAL and MCP-1, is presented in Fig. 8. The same profiles were observed for these three proteins: modest, but significant (P < 0.025) NGAL and MCP-1 protein elevations in the AKI- patient group (vs. normal volunteer samples), and marked elevations of each in the AKI+ groups. When the performances of uAlb/uCr, uNGAL/uCr, uMCP-1/uCr, and uTP/uCr ratios were compared by ROC, the degrees of utility were comparable. However, uAlb/uCr had the highest AUC (0.874; Fig. 9).
Fig. 8.
Clinical assessments ot the comparison of urinary alb/Cr, NGAL/Cr, and MCP-1/Cr ratios in normal control subjects (C) and ICU patients without (AKI−) or with AKI (AKI+). See text for description.
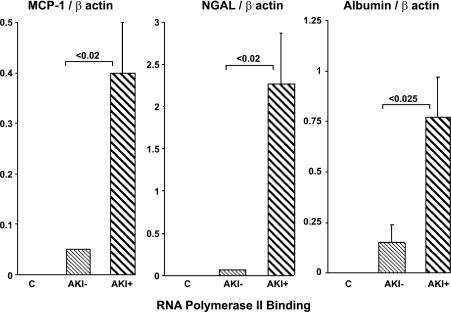
As an index of in vivo gene transcription, levels of Pol II binding to urinary fragments of the albumin, MCP-1, and NGAL genes (exon 1) were assessed, and the results were factored by values at the housekeeping β-actin gene. As shown in Fig. 10, the AKI− and control patients had either minimal or no evidence of increased Pol II binding to any of these three genes. Conversely, the AKI+ patients had striking (5- to 20-fold) increases that quantitatively paralleled the observed increases in NGAL, MCP-1, and urine albumin levels.
Fig. 10.
Clinical assessments of Pol II binding to exon 1 or human NGAL, MCP-1, and albumin gene fragments in urine from 29 ICU patients, as described in the text.
Assessments of α-Fetoprotein Gene Expression Following Experimental and Clinical AKI
Experimental AKI.
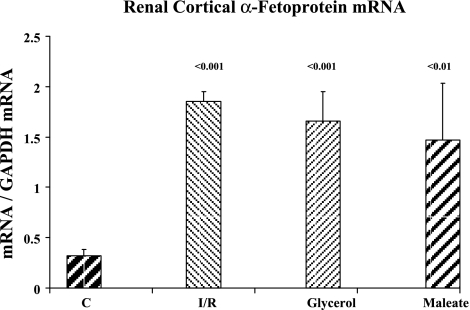
Because the above experimental and clinical data indicated that AKI activated the albumin gene, we assessed whether this same type of change could be observed with a second gene which is normally associated with the liver, rather than the kidney. To this end, α-fetoprotein mRNA levels were measured in control renal cortex and in the renal cortex obtained 24 h post-ischemic renal injury and 24 h post-maleate and -glycerol injections. As shown in Fig. 11, each of these three intrarenal AKI models induced approximately fourfold increases in α-fetoprotein mRNA levels compared with values observed in control kidneys.
Fig. 11.
Renal cortical α-fetoprotein mRNA levels in control mice and in mice with post-ischemia-reperfusion (I/R)-, glycerol-, and maleate-induced acute renal failure. These assessments were made ∼24 h after the initial renal insult. P values compare results to that observed in normal renal cortical tissues.
Clinical AKI.
To seek a clinical correlate, urinary α-fetoprotein levels were measured in the clinical urine samples. The AKI+ group had a fourfold increase in α-fetoprotein/Cr ratios (149 ± 28 pg/mg Cr) compared with normal controls (38 ± 14 pg/mg creatinine; P < 0.025). However, even the AKI− group had elevated α-fetoprotein levels (114 ± 26 pg/mg Cr), suggesting the potential of subclinical renal injury in this patient population [analogous to elevated urine NGAL levels in AKI− patients (793 ± 387 ng/mg Cr) compared with normal urine samples (28 ± 5 ng/mg; P < 0.025, as depicted in Fig. 8)].
DISCUSSION
Albuminuria is a well-accepted biomarker for various glomerulopathies, and its degree conveys both diagnostic and prognostic information. However, its potential utility as an AKI biomarker has not been rigorously tested. There are at least three potential reasons to suggest potential utility in this regard. First, both ischemic and toxic forms of AKI can alter glomerular structure and function (1, 15, 30, 31, 33), and hence, potentially increase albumin filtration. Second, proximal tubular injury almost certainly decreases tubular albumin reabsorption, in a fashion analogous to that observed for freely filtered low-molecular-weight serum proteins, such as β2-microglobulin and cystatin C (17, 26, 39). Third, and as will be discussed at length below, it appears that the albumin gene, which is normally silent within the kidney, is induced by AKI, in a fashion analogous to NGAL or KIM-1. Given the relatively large amount of albumin that is normally filtered, it seems quite unlikely that renal tubular albumin gene induction, with possible albumin synthesis, would make more than a trivial contribution to total urinary albumin excretion. Nevertheless, that AKI can induce the albumin gene helps to place the interpretation of urinary albumin excretion in a new theoretical context.
To gain support for the concept that albuminuria may, indeed, have value as an AKI biomarker, we first sought “proof of concept” using five different mouse models of AKI. Three of these, ischemia-reperfusion injury, maleate nephrotoxicity, and the glycerol model of rhabdomyolysis, are classic examples of so called “intrarenal acute renal failure,” as defined by the presence of severe proximal tubular necrosis, apoptosis, intraluminal cast formation, and resultant filtration failure (e.g., Refs. 6, 7, 21, 22, 36). Each of these three models evoked marked increases in urinary albumin excretion, as assessed by either absolute urine albumin concentrations and/or after they were factored by corresponding urine creatinine levels. Of further note, albumin's contribution to the total urinary protein concentration approximately tripled in each of these intrarenal AKI models, rising from control values of ∼17% to as high as 65%. This suggests that a preferential increase in urinary albumin excretion had occurred. Finally, the increase in urinary albumin levels were observed as early as 4 h after initiation of renal injury and either remained stable, or increased thereafter. Thus an increase in urinary albumin concentrations would appear to have potential utility as an early AKI biomarker (i.e., before the emergence of significant azotemia).
A highly desirable feature of an AKI biomarker is an ability to differentiate between intrarenal vs. so-called “prerenal” (renal vasoconstriction-induced; e.g., as induced by LPS) or “postrenal” (obstructive) acute renal failure. This can be particularly problematic for several biomarkers, given that sepsis syndrome, even in the absence of tubular injury, can raise urinary biomarker levels (e.g., NGAL and MCP-1) (20). The present results suggest that albuminuria does not share this deficiency, given that urine albumin concentrations were significantly reduced at both 2 and 24 h following LPS injection. Further suggesting a degree of specificity for intrarenal forms of AKI was the finding that urine samples obtained from postobstructed kidneys also retained normal albumin and albumin/total protein ratios. Given these experimental findings, future clinical testing of albumin's utility in differentiating prerenal, postrenal, and intrarenal AKI seems warranted.
Because the above results indicated that urinary albumin acutely rises with the induction of experimental renal tubular injury, we next explored what we initially considered to be a highly implausible hypothesis: that the albumin gene, normally silent in the kidney, might be injury-inducible within the renal cortex, and more specifically, within proximal tubular cells. To test this hypothesis, renal cortical albumin mRNA levels were measured in both normal renal cortex and in renal cortex following induction of each of the five AKI models described above. As a point of reference, the results were contrasted with those obtained for NGAL mRNA, a well-accepted AKI biomarker gene. Surprisingly, albumin mRNA could be detected in normal renal cortex, albeit at very low levels. Furthermore, with each of the three models of proximal tubular injury (ischemia-reperfusion, maleate, and glycerol), significant albumin mRNA increases were observed. Indeed, both the patterns and relative degrees of its expression fully paralleled those seen for NGAL mRNA. In contrast, whereas both endotoxemia and ureteral obstruction induced marked renal cortical NGAL mRNA increases, no increases in albumin mRNA were observed. In sum, these mRNA results suggest the following: 1) albumin mRNA can be detected, albeit at very low levels, in normal renal cortex; 2) it, like NGAL, appears to be an injury-inducible gene; and 3) albumin mRNA appears to have greater specificity for intrarenal forms of AKI than does NGAL, based on the results of the ureteral obstruction and endotoxemia experiments. Indeed, these mRNA findings completely paralleled the urinary albumin (i.e., protein) assessments, which also demonstrated urinary albumin increases with intrarenal-induced, but not with endotoxemic- or ureteral obstruction-induced renal failure.
Given the heterogeneity of cell types within the renal cortex, we next questioned whether the injury-induced increases in albumin mRNA reflected proximal tubular cell events. Of note in this regard is that the proximal tubule is the principal site of injury in most forms of intrarenal acute renal failure (including that induced by maleate, ischemia, and rhabdomyolysis). To address this issue, proximal tubular cell- specific results were sought using human-derived proximal tubular HK-2 cells (12, 27). When these cells were subjected to an antimycin A ± Ca ionophore challenge, marked albumin mRNA increases were observed, paralleling those seen with NGAL. Furthermore, these mRNA increases were paralleled by increased Pol II recruitment to the albumin and NGAL genes. Given that Pol II is the enzyme that drives transcription, the degree of Pol II gene binding serves as an indirect index of transcription rates (16, 23). Thus these HK-2 results support the concept that AKI can increase albumin gene transcription within proximal tubular cells.
To seek clinical correlates of the animal and cell culture data, urinary albumin and total protein concentrations were measured in ICU patients with early AKI (AKI+), and the results were contrasted with those obtained from AKI− ICU patient controls. Urine NGAL and MCP-1 protein concentrations were also assayed, serving as two reference biomarkers (20). As shown in Fig. 7, ∼10-fold higher albumin/Cr ratios were observed in the AKI+ vs. the AKI− patients, and these results were essentially paralleled by urinary NGAL and MCP-1 increases (Fig. 8). Indeed, a direct comparison of the urinary albumin/Cr vs. NGAL/Cr vs. MCP-1/Cr ratios by ROC curves demonstrated that albumin was at least equivalent (AUC 0.874) to these two comparator biomarker proteins (NGAL, 0.810; MCP-1, 0.819; Fig. 9). In an attempt to gain clinical support for the experimental finding that AKI can activate the albumin gene, Pol II binding to urinary albumin gene fragments was assessed and the results were factored by Pol II levels at the β actin housekeeping gene. As shown in Fig. 10, the AKI+ patients manifested approximately fivefold increases in Pol II-albumin gene binding, compared with the AKI− ICU controls. Clearly, ChIP analyses of urinary gene fragments can only serve as an indirect gauge of in vivo events. However, in the absence of direct tissue assessments (not possible without a renal biopsy), urine ChIP analysis would appear to be the most useful available technique for evaluating this issue.
Finally, we questioned whether AKI-mediated albumin gene induction might extend to other genes that are predominantly expressed in the liver. In this regard, α-fetoprotein is the embryonic equivalent of albumin, and indeed, both the albumin and α-fetoprotein gene reside on the same chromosome (chromosome 4). Thus we tested whether α-fetoprotein might, like albumin, be AKI inducible. Using commercially available primers, we probed RNA extracts from postglycerol, postmaleate, and postischemic kidney samples, and approximately fourfold α-fetoprotein mRNA elevations were observed in each AKI model. Furthermore, when the clinical AKI+ urine samples were analyzed for α-fetoprotein levels by ELISA, a fourfold increase was observed compared with normal urine samples. Thus, when these results are considered alongside of the albumin mRNA data, we speculate that AKI might induce a renal “hepatization” response: i.e., whereby the damaged kidney expresses hepatic cell markers that are normally suppressed. In other words, AKI induces selected markers of an hepatic phenotype.
Four limitations of this study require mention. First, although AKI induced dramatic increases in albumin mRNA (renal cortex, HK-2 cells) and increased Pol II-albumin gene binding (HK-2 cells; AKI+ patient urine samples), it remains unknown whether increased in vivo renal albumin synthesis occurred. Indeed, it remains possible that in vivo renal tubular injury could block albumin translation. If so, then the injury- induced increases in urinary albumin excretion would solely reflect increased albumin filtration/decreased tubule albumin reabsorption. Second, the AKI+ patient cohort consisted of patients who already had established, albeit relatively early, acute renal failure. Thus, despite the fact that albuminuria was seen as early as 4 h post-AKI induction in mice, albumin's utility as an early AKI biomarker in patients (i.e., appearing before the onset of azotemia) remains unknown. Third, the present study was a case-controlled, rather than a cohort, study. This could potentially limit the ability to generalize the current results to a more broad-based patient population. Finally, it is notable that with each of the four tested biomarkers (albumin, NGAL, MCP-1, α-fetoprotein), increased urinary concentrations were observed in the AKI− patients compared with the normal volunteer group. This implies that the distinction between AKI+ vs. AKI− is somewhat arbitrary. Rather it appears more likely that a spectrum of renal injury exists in critically ill (ICU) patients which can be observed even in the absence of filtration failure.
In sum, this study documents that quantifying urinary albumin levels has utility as a biomarker of intrarenal forms of acute tubular injury. Indeed, given the universal availability of a urine albumin assay, its relatively low cost, and albumin's comparable performance to at least two other urinary protein AKI biomarkers (NGAL, MCP-1), it would appear to be a useful comparator test when making clinical AKI biomarker assessments. The current study also puts forth a new concept: that the albumin gene, which is normally silent within the kidney, is rapidly induced by AKI, as indicated by increases in renal cortical albumin mRNA expression and by increased Pol II binding to the albumin gene (e.g., as in urine samples from AKI+ vs. AKI− controls). In addition, this study demonstrates for the first time that experimental AKI can also increase the expression of the renal α-fetoprotein gene. This implies that AKI-mediated albumin gene induction is part of a more broad-based response: whereby the injured kidney assumes selected components of an hepatic phenotype. Given the relatively large amount of albumin that is normally filtered by the glomerulus, it is seems quite unlikely that any potential increase in tubular albumin synthesis would have more than a trivial effect on urinary albumin concentrations. Nevertheless, that the albumin gene is upregulated following AKI places studies of albumin handling by the kidney in a new context: that albumin is an acute stress reactant in the kidney, analogous to that of other stress-activated proteins. The potential implications of this albumin “stress response” vis à vis the evolution of renal injury remains unknown. A compelling question in this regard is whether induction of the albumin or α-fetoprotein gene might exert protective effects on injured tubules, such as occurs with other stress-activated proteins (e.g., heme oxygenase 1) (21, 22). Perhaps the results of the present study can stimulate future investigations of this issue.
GRANTS
This work was supported by grants from the National Institutes of Health (DK384342; R21-DK083315; HL081332; HL-103836; DK082192) and from nonrestricted research funds from the Kidney Research Institute, Seattle, WA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Ms. Kirsten Becker for expert technical support, John Ruzinski and Dr. Jonathan Himmelfarb for providing assays of human albumin concentrations, and Stuart Tenney and Bonnie Kraskouskas for administrative support.
REFERENCES
- 1. Avasthi PS, Evan AP, Huser JW, Luft FC. Effect of gentamicin on glomerular ultrastructure. J Lab Clin Med 98: 444–454, 1981 [PubMed] [Google Scholar]
- 2. Blaikley J, Sutton P, Walter M, Lapsley M, Norden A, Pugsley W, Unwin R. Tubular proteinuria, and enzymuria following open heart surgery. Intensive Care Med 29:1364–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bonventre JV. Kidney injury molecule-1 (K.I.M.-1): a specific, and sensitive biomarker of kidney injury. Scand J Clin Lab 241: 78S–83S, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bonventre JV. Kidney injury molecule-1 (K.I.M.-1): a urinary biomarker, and much more. Nephrol Dial Transplant 24: 3265–3268, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis 52: 395–399, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Glaumann B, Glaumann H, Berezesky IK, Trump BF. Studies on cellular recovery from injury. II. Ultrastructural studies on the recovery of the pars convoluta of the proximal tubule of the rate kidney from temporary ischemia. Virchows Arch B Cell Pathol 24: 1–18, 1977 [PubMed] [Google Scholar]
- 7. Glaumann B, Trump BF. Studies on the pathogenesis of ischemic cell injury. III. Morphological changes of the proximal pars recta tubules (P3) of the rat kidney made ischemic in vivo. Virchows Arch B Cell Pathol 19: 303–323, 1975 [PubMed] [Google Scholar]
- 8. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Meta-analysis Investigator Group: accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis, and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, Philipp T, Kribben A. Prognostic value of tubular proteinuria, and enzymuria in nonoliguric acute tubular necrosis. Clin Chem 50: 552–528, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Iwata M, Herrington J, Zager RA. Protein synthesis inhibition induces cytoresistance in cultured human proximal tubular (HK-2) cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1154–F1163, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Iwata M, Herrington J, Zager RA. Sphingosine: a mediator of acute renal tubular injury, and subsequent cytoresistance. Proc Natl Acad Sci USA 92: 8970–8974, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson AC, Becker K, Zager RA. Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Physiol Renal Physiol 299: F426–F435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kharasch ED, Frink EJ, Jr, Zager R, Bowdle TA, Artru A, Nogami WM. Assessment of low-flow sevoflurane, and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology 86: 1238–1253, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi S, Nagase M, Honda N, Hishida A. Glomerular alterations in uranyl acetate-induced acute renal failure in rabbits. Kidney Int 26: 808, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Kornberg RD. The molecular basis of eukaryotic transcription. Proc Natl Acad Sci USA 104: 12955–12961, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma Y, Li Q, Wang J, Xu Z, Song C, Zhuang R, Yang K, Yang A, Jin B. Cystatin C, a novel urinary biomarker for sensitive detection of acute kidney injury during haemorrhagic fever with renal syndrome. Biomarkers 15: 410–417, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Modi KS, Harris KP, Klahr S. Effects of unilateral or bilateral release of bilateral ureteral obstruction on renal function in rat. Nephron 64: 235–241, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, Himmelfarb J, Zager RA. Monocyte chemoattractant protein-1 gene activation as a marker of acute kidney injury. J Am Soc Nephrol. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nath KA, Balla J, Croatt AJ, Vercellotti GM. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro, and in vivo. Kidney Int 47: 592–602, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikolov DB, Burley SK. RNA polymerase II transcription initiation: a structural view. Proc Natl Acad Sci USA 94: 15–22, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orsonneau JL, Coiuet P, Masssoubre P, Lustenerger P, Bernard S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin Chem 35: 2233–2236, 1989 [PubMed] [Google Scholar]
- 25. Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A. A panel of urinary biomarkers to monitor reversibility of renal injury, and a serum marker with improved potential to assess renal function. Nat Biotechnol 28: 486–494, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Parikh CR, Lu JC, Coca SG, Devarajan P. Tubular proteinuria in acute kidney injury: a critical evaluation of current status, and future promise. Ann Clin Biochem 47: 301–312, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2 an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 20: 1823–1832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solez K, Racusen LC, Whelton A. Glomerular epithelial cell changes in early postischemic acute renal failure in rabbits, and man. Am J Pathol 103: 163–173, 1981 [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki T, Mostofi FK. Electron microscopic studies of acute tubular necrosis. Early changes in the rat kidney after subcutaneous injection of glycerin. Lab Invest 23: 8–14, 1970 [PubMed] [Google Scholar]
- 32. Wang W, Falk SA, Jittikanont S, Gengaro PE, Edelstein CL, Schrier RW. Protective effect of renal denervation on normotensive endotoxemia-induced acute renal failure in mice. Am J Physiol Renal Physiol 283: F583–F587, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Williams RH, Thomas CE, Navar LG, Evans AP. Hemodynamic, and single nephron function during the maintenance phase of ischemic acute renal failure in the dog. Kidney Int 19: 503–515, 1981 [DOI] [PubMed] [Google Scholar]
- 34. Zager RA. Lysozyme, and albumin radioimmunoassays. New techniques for the study of proteinuria. Invest Urol 17: 526–528, 1980 [PubMed] [Google Scholar]
- 35. Zager RA. Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int 47: 628–637, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Zager RA. Uremia induces proximal tubular cytoresistance, and heme oxygenase-1 expression in the absence of acute kidney injury. Am J Physiol Renal Physiol 296: F362–F368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zager RA. Urinary protein markers of tubulointerstitial nephritis. Invest Urol 8: 197–202, 1980 [PubMed] [Google Scholar]
- 38. Zager RA, Carpenter CB. Radioimmunoassay for urinary renal tubular antigen: a potential marker of tubular injury. Kidney Int 13: 505–512, 1978 [DOI] [PubMed] [Google Scholar]
- 39. Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol 290: F1453–F1462, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury, and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187–F197, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Zager RA, Johnson AC. Progressive histone alterations, and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury. Am J Physiol Renal Physiol 298: F827–F837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zager RA, Johnson AC. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems, and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol 296: F1032–F1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]