Abstract
Pulmonary fluid clearance is regulated by the active transport of Na+ and Cl− through respiratory epithelial ion channels. Ion channel dysfunction contributes to the pathogenesis of various pulmonary fluid disorders including high-altitude pulmonary edema (HAPE) and neonatal respiratory distress syndrome (RDS). Nasal potential difference (NPD) measurement allows an in vivo investigation of the functionality of these channels. This technique has been used for the diagnosis of cystic fibrosis, the archetypal respiratory ion channel disorder, for over a quarter of a century. NPD measurements in HAPE and RDS suggest constitutive and acquired dysfunction of respiratory epithelial Na+ channels. Acute lung injury (ALI) is characterized by pulmonary edema due to alveolar epithelial-interstitial-endothelial injury. NPD measurement may enable identification of critically ill ALI patients with a susceptible phenotype of dysfunctional respiratory Na+ channels and allow targeted therapy toward Na+ channel function.
Keywords: acute respiratory distress syndrome, ENaC, sodium channel
proper regulation and maintenance of pulmonary fluid balance is crucial to health. In utero, the lungs are net fluid secretors with the volume of fluid being vital for optimal lung growth (100). Pulmonary hypoplasia occurs when too little fluid is produced, such as in oligohydramnios (143), whereas excess fluid produces pulmonary hyperplasia (144, 234). Importantly, fetal fluid secretion is under the control of Cl− secretion by the respiratory epithelium (133–134). At birth the sudden dependency on pulmonary gas exchange requires a dramatic change in lung fluid dynamics. As gaseous exchange requires a relatively dry alveolus the respiratory epithelium transforms to a net fluid absorber in a remarkably short space of time. In the few days before birth the lungs begin to produce less fluid, and during labor the physical passage of the fetus through the birth canal forces fluid from the lungs (20). Simultaneous increases in fetal sympathetic output and catecholamine levels help activate alveolar Na+ channels necessary for fluid absorption (29, 169). Additionally, the manifold increase in fetal oxygenation ex utero further stimulates Na+ channels (9, 172).
After birth, a small volume of airway surface liquid (ASL) is required for normal airway function (i.e., microbial killing and clearance). The ASL consists of a periciliary layer, in which the cilia beat, of ∼7 μm height (215) and an overlying mucus blanket of variable volume (dependent on the amount of mucus produced). Excessive ASL volume interferes with airway function, which most notably is observed in cystic fibrosis (CF), resulting in airway narrowing and blockage (185).
Optimal alveolar gas exchange is dependent on an even thinner alveolar lining fluid (ALF) of 0.1–0.2 μm height (15). The ALF consists of an analogous dual layer, with surfactant covering an aqueous subphase. ALF evens the air-liquid interface, enables surfactant precursors to reach the surfactant layer, and allows movement of surfactant within this layer. Pulmonary edema results in fluid deposition in the alveolar region. The resolution of pulmonary edema is vitally dependent on the active absorption of Na+ and Cl− from the alveolar air space into the interstitium creating an osmotic gradient for the movement of water out of the alveolar air space (150). From the interstitium, water is cleared by the lymphatic system (107, 229).
Acute lung injury (ALI) is a form of increased permeability pulmonary edema seen in critically ill patients. It is a significant problem, with a yearly incidence of 86 per 100,000 person-years, translating to 190,600 cases per year in the USA (186). Mortality rates range from 20–60% (30, 130) and an estimated 74,500 Americans die from this condition annually (186). Survivors suffer from muscle wasting, weakness, fatigue, pulmonary dysfunction, cognitive disability, and affective disorders, and just 50% return to work (5, 86).
ALI results from an inflammatory injury to the alveolar epithelial-interstitial-endothelial complex (177) caused by either a pulmonary or extrapulmonary insult (1, 16). The neutrophil-mediated disruption of this physical barrier causes increased permeability pulmonary edema (230). Alveolar flooding is dependent on the balance of pulmonary edema formation and clearance (194).
Dysfunction of any of the components needed for alveolar fluid clearance (AFC) can predispose to the development of pulmonary edema. This review will evaluate the evidence for a spectrum of Na+ channel function, with decreased Na+ channel function predisposing to both the development of, and a worse outcome from, ALI. Additionally, we review the possible role nasal potential difference (NPD) measurement could play in the identification of this susceptible phenotype and suggest implications if this hypothesis is correct.
Alveolar Epithelial Cell Types
The alveolar epithelium constitutes ∼99% of the internal surface area of the lung (45) and is one of the tightest epithelia in the body forming transepithelial resistances of >2,000 Ω·cm2 (71, 117). It is ∼0.1–0.2 μm thick and is composed of two cell types: large, squamous type 1 alveolar cells (AT1) with a diameter of 50–100 μm (44) and smaller cuboidal type 2 alveolar cells (AT2) with a diameter of 10 μm (150). Alveoli are ∼250 μm in diameter (233) and at its thinnest the alveolar interstitium consists of only a fused basement membrane between the epithelial and endothelial layers (207). Although AT1 cells compose 66% of the cell population of the alveolus, because of their large size they constitute >95% of the alveolar surface (207). Alveolar epithelial cells form tight junctions, which represent a barrier between the functionally different apical and basolateral cell membranes with specifically localized ion channels and pumps (195). This functional division of apical and basolateral membranes is a prerequisite for vectorial transport of ions and water across the alveolar epithelial layer.
Major Alveolar Channels, Transporters, and Receptors
Na+ channels.
Apical membrane Na+ channels are expressed in the alveolar epithelium and contribute to Na+ absorption (13, 72) (see Fig. 1). Two main classes of Na+ channels have been found: the epithelial Na+ channel (ENaC) and the cyclic nucleotide-gated channel (CNG).
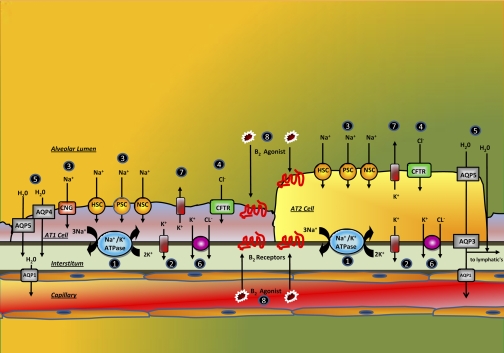
Fig. 1.
Schematic diagram of alveolar type 1 and 2 cells showing distribution of ion channels, pumps, aquaporins (AQP), and β-receptors (see text, under Mechanism of alveolar Na+transport and fluid absorption, for explanations of circled numbers in figure). HSC, highly selective cation channel; PSC, poorly selective cation channel; NSC, nonselective cation channel; CNG, cyclic nucleotide-gated channel; CFTR, cystic fibrosis transmembrane conductance regulator.
Three subtypes of ENaC have been described on the basis of the subunit composition: a highly selective cation channel (HSC) that is usually referred to as the ENaC channel; two types of poorly selective cation channel (PSC), types 1 and 2, differentiated by their unit conductance; and a nonselective cation channel (NSC) (56, 125). ENaC channels are present in the apical membranes of both AT1 and AT2 cells (91). HSC is a heterotrimeric channel composed of α, β, and γ-ENaC subunits (33). It has a unit conductance of 4–5 picoSiemens (pS; a measure of the ease of movement of ions through the ion channel) and a Na+/K+ selectivity of >40 (91, 125). PSC is composed of a combination of α- with either β- or γ-ENaC subunits and has a Na+/K+ selectivity of 5–8, with type 1 PSCs having unit conductance of 8–9 pS and type 2 PSCs a unit conductance of 56 pS (56, 125). NSC is composed solely of α-ENaC subunits (33), has a Na+/K+ selectivity of 1.5 and a unit conductance of 19–24 pS (91). Efficient Na+ transport requires all three subunits (33, 138). HSC, PSC, and NSC channels are inhibitable by amiloride (Table 1) (125).
Table 1.
Activators and inhibitors of ion channels and pumps
| Activator | Inhibitor | |
|---|---|---|
| ENaC | β-Agonists | Amiloride |
| Mechanical stimulation | ||
| CNG | Pimozide | |
| l-cis-Diltiazam | ||
| CFTR | Isoprenaline | Glibenclamide |
| Forskolin | GlyH-101 | |
| CFTR-Inh-172 | ||
| Na+-K+-ATPase | Ouabain |
ENaC, epithelial Na+ channel; CNG, cyclic nucleotide gated channel; CFTR, cystic fibrosis transmembrane conductance regulator.
One isoform of the CNG channel, CNG1, is present in the distal airway and AT1 cells (54) and is likely to be involved in distal airway and alveolar fluid balance (93, 97, 196). This channel is nonselective, amiloride insensitive, but pimozide sensitive and has a unit conductance of 2–8 pS (91). Other Na+- dependent cotransporters, such as glucose or amino acid cotransporters, may contribute to amiloride-insensitive Na+ absorption (14, 52, 212). In addition, atypical ENaC channels, composed of different subunit stoichiometries, rendering them potentially insensitive to amiloride, could also contribute to amiloride-insensitive Na+ transport (165, 178).
Cl− channels.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated apical membrane Cl− channel expressed in both AT1 and AT2 cells (28, 91, 153). Alveolar CFTR has a unit conductance of ∼4 pS (AT1, similar to airway CFTR) to 8 pS (AT2) and can be stimulated by forskolin (91). CFTR consists of two membrane-spanning domains, two nucleotide-binding domains, and a regulatory domain (197). Both airway (213) and alveolar (66) CFTR-mediated apical Cl− movement may be bidirectional and under the control of adenosine. Although the absence of CFTR does not hinder basal alveolar fluid balance, it is necessary for maximal AFC during β-agonist therapy (167). CFTR activation increases amiloride-sensitive Na+ absorption (68, 181), suggesting that both Na+ and Cl− transport determine alveolar fluid absorption (69, 147). The exact interaction between CFTR and ENaC remains to be determined.
K+ channels.
Respiratory epithelial K+ channels mediate a diverse range of physiological functions including oxygen sensing, inflammation, and epithelial repair (8). The primary role of these channels is to regulate the cell membrane potential to allow maintenance of the transepithelial driving force for ion movement and subsequent regulation of the airway and alveolar liquid layers (8). K+ channels have been localized to the apical and the basolateral membrane where they serve differing functions (167). Apical K+ channels support K+ secretion into the ALF resulting in the relatively high K+ concentration of the ALF (59). In contrast, basolateral K+ channels support recycling of K+ across the basolateral membrane in support of the Na+-K+-ATPase (see Na+-K+-ATPase) and to hyperpolarize the cell. Classes of K+ channels found in alveolar cells include inwardly rectifying K+ channels (K+ ir), Ca2+-activated K+ channels, and voltage-gated K+ channels (K+ v) (167). K+ ir channels have been localized to the basolateral membrane, and the α-subunit of the K+ v channel has been localized to the apical membrane. A barium-sensitive K+ channel with a unit conductance of 5–6 pS has been identified in the apical membrane of AT1 (91).
ATP-sensitive K+ channels (K+ATP) have also been described in the alveolus. Inhibition of K+ATP reduced amiloride-sensitive Na+ currents and forskolin-stimulated Cl− currents in AT2, whereas K+ATP activation increased both ion fluxes (110–111). Similarly, ENaC and CFTR expression responded to K+ATP modification, with increased expression occurring with K+ATP activation and decreased expression seen with K+ATP inhibition (111). The interplay between K+, ENaC, and CFTR provides further evidence for the complexity of the relationship between epithelial ion channels.
Na+-K+-ATPase.
The Na+-K+-ATPase is ubiquitously expressed and is the primary active transport process that generates the gradients necessary for epithelial Na+ absorption. It is a heterodimeric protein composed of an α- and a β-subunit and is located in the basolateral membrane of AT1 (91–92) and AT2 (91, 168) cells. The α-subunit allows exchange of three intracellular Na+ for two extracellular K+ during ATP hydrolysis (198). The β-subunit is required for protein assembly and insertion into the cell membrane (136). Four α- and three β-subunits exist and different associations of an α- and a β-subunit, plus different posttranscriptional processing, creates a range of Na+-K+-ATPase isoenzymes with different functional characteristics (19). AT1 cells contain α1-, α2-, and β1-subunits, whereas AT2 cells express α1- and β1-subunits (92, 182).
The regulation of Na+-K+-ATPase and ENaC occurs in parallel, with increased apical Na+ transport being matched by increased Na+-K+-ATPase activity (43). Upregulating stimuli, such as glucocorticoids (47) and catecholamines (141), increase expression and function of both proteins. Likewise, downregulating stimuli, such as hypoxia, reduce levels (237) and function (225) of these membrane transporters.
Water channels.
The aquaporins (AQP) are a family of small (∼30-kDa monomer), integral membrane proteins that function as mercury-sensitive water channels. AQP5 has been located in the apical membrane of both AT1 cells (154) and AT2 cells (127), AQP3 is located in the basolateral membrane of AT2 cells (105), and AQP1 is located in microvascular endothelia. AQP4 is also probably present in AT1 cells (105). Water permeability is required in both apical and basolateral membranes for efficient transcellular water transport, although paracellular water transport also occurs. AQPs increase the water permeability of epithelial membranes by 5- to 50-fold (23) with AT1 cells having the highest water permeability of any mammalian cell (55).
Murine alveolar osmotic water permeability is reduced ∼10-fold by AQP1 or AQP5 deletion (6), and >30-fold reduced by combined AQP1/AQP5 deletion (119). Active, near-isosmolar alveolar fluid clearance (AFC) was unaffected, however. Similarly, AQP deletion did not affect the rate of AFC in newborn mice or mice with experimentally induced pulmonary edema including ALI (203), suggesting that other pathways for water exist. AQPs do not seem to play a major role in the maintenance of the ASL (204). The lack of effect of AQP deletion has been suggested to be due to the slower rate of active fluid absorption in the lung compared with organs such as the renal proximal tubule or the salivary gland (131). It appears that AQPs are not required for physiologically relevant functions in pulmonary fluid balance (224).
β-Adrenergic receptors.
Four β-adrenergic receptor subtypes exist (149), with over 90% of all pulmonary β-receptors being located in the alveoli, predominantly in the form of the β2-receptor (34). Both β1- and β2-receptors are expressed on the cell membranes of both AT1 (114) and AT2 (64) cells. β-Stimulation may increase the activity of ENaC or Na+-K+-ATPase, improve pulmonary lymphatic flow (201), or enhance CFTR function (167). Basal rates of AFC may not be dependent on β-adrenergic receptor signaling (148). Upregulated AFC is largely dependent on β2-adrenergic receptor signaling (148), although β1-adrenergic stimulation may also increase alveolar Na+ transport (191). β-Stimulation increases rates of AFC in normal lungs (36, 128), in models of hydrostatic pulmonary edema (77), and in models of ALI (132). Overexpression of alveolar β2-receptors increases alveolar Na+ transport and AFC (58, 137, 148).
Respiratory Ion Transport and Fluid Balance
Development of the theory of osmosis-dependent alveolar fluid clearance.
Traditionally AFC was thought to be under the control of Starling forces, i.e., hydrostatic and oncotic forces (208). Work from the early 1980s onward, however, has shown that AFC is an active osmotic process dependent on the epithelial absorption of Na+ and Cl− (131). Both AT1 and AT2 cells allow passive diffusion of Na+ through apical membrane ENaC channels along its electrochemical gradient into the cells, and active extrusion of Na+ via the Na+-K+-ATPase in the basolateral membrane (91–92). Na+ also enters AT1 cells via apical CNG1 channels (91) and possibly AT2 cells (96), although culture conditions may have influenced the phenotype of these cells. The apical membrane Na+ channels are the rate-limiting step for Na+ absorption accounting for 90% of the resistance to Na+ movement (91). Approximately 30% of rat AT1 and AT2 cell Na+ transport is inhibited by amiloride (92), and, correspondingly, up to 60% of AFC is amiloride insensitive (163). The contribution of amiloride-sensitive Na+ transport varies across species (131) and is ∼40–50% for humans (188–189).
The importance of air space Na+ uptake via apical membrane Na+ channels was demonstrated in several species by a reduction in AFC with amiloride (89, 131, 162, 164). Studies in anesthetized sheep demonstrated that alveolar air space isosmolar water and ion clearance takes place against an increasing oncotic gradient (131). Further evidence for the role of ENaC in AFC has come from knockout mice models. Newborn mice lacking α-ENaC die within 40 h from pulmonary edema (88). Consistent with this the use of small interfering RNA to inactivate α-ENaC protein production reduced AFC, which was resistant to further reduction with amiloride (112). Mice lacking β- or γ-ENaC subunits have lower rates of respiratory fluid clearance than wild-type mice and live for ∼40 days before dying from electrolyte imbalances (135). Knockout of the β-ENaC subunit results in decreased baseline AFC and impairs the upregulation in AFC seen with β2-agonists (178). The significance of Cl− transport on AFC has been investigated by use of CFTR knockout mice. Basal AFC rates were unaffected compared with wild-type mice, but β-adrenergic-mediated upregulation of AFC was lessened, suggesting that β-agonist-mediated upregulation is at least partly dependent on CFTR function and Cl− transport (69).
K+ channel activity appears to play a role in AFC. The functional significance of K+ATP was suggested by pharmacological stimulation in human lungs (190). In that study, activation of KATP channels resulted in increased K+ secretion into the alveolar space, suggesting a role in ALF regulation.
The contribution of Na+-K+-ATPase to AFC was confirmed with its specific inhibitor ouabain in animal perfused lung preparations (81). In the ex vivo human lung ouabain reduced AFC by almost 50% (187–188). Similarly, overexpression of either the β1- or α2-subunit of Na+-K+-ATPase increases AFC (65, 182, 217). The exact degree of Na+-K+-ATPase inhibition required to impair AFC is unknown. Neither the α1- nor α2-subunits function near their maximal capacity under normal conditions (118, 232). Mice that are 50% protein deficient in both α1- and α2-subunits have a submaximal response to stimulated AFC, although a normal basal rate. This suggests a partial impairment of the Na+-K+-ATPase may not be a rate-limiting factor for Na+ transport and correspondingly AFC.
Transepithelial electrolyte transport from the alveolar space toward the interstitium is followed by osmotically obliged water via both cellular and paracellular pathways resulting in clearance of pulmonary edema. The contribution of AQPs is felt to be of minor significance as AQPs do not appear to contribute to either regulation of the ASL or AFC in knockout mice (223). Thus, although all components of AFC are important, it would seem that the function of ion channels, or β-adrenergic receptors, are likely to be of greater significance than Na+-K+-ATPase or AQPs in determining AFC abilities.
Mechanism of alveolar Na+ transport and fluid absorption.
See Fig. 1; numbered paragraphs below refer to circled numbers in the figure.
1) The Na+-K+-ATPase pump exchanges intracellular Na+ for extracellular K+. The activity of the Na+-K+-ATPase hyperpolarizes the basolateral membrane potential and establishes an outward K+ and an inward Na+ gradient.
2) Intracellular K+ follows its chemical gradient across basolateral K+ channels, which hyperpolarizes the basolateral membrane. In an epithelial setting, basolateral hyperpolarization results in apical hyperpolarization by electrical coupling of the two membranes across the tight junctions.
3) This creates a large electrochemical gradient for entry of luminal Na+ through the apical membrane Na+ channels, including HSC, PSC, NSC, and CNG channels.
4) The resulting Na+ absorption depolarizes the apical membrane potential to allow for Cl− entry across CFTR.
5) The osmotic gradient created by the movement of Na+ and Cl− causes water to move from the air space to the interstitium both transcellularly and paracellularly.
6) Cl− leaves the basolateral membrane through an as yet undetermined pathway thought to be a Cl−/K+ cotransporter to maintain electroneutrality (91).
7) K+ secretion through the apical membrane enables maintenance of the transepithelial electrochemical gradient needed for ion and water movement.
8) AFC may be upregulated in a catecholamine-dependent or independent fashion (239). Na+ movement occurs by using the same Na+-K+-ATPase driven mechanism, but in an upregulated manner. The exact means of upregulation remains unclear but several theories have been suggested (65). The upregulation may be primarily due to an increase in Na+ absorption (80) caused by an increase in any of the following: ENaC delivery to the apical membrane (32), ENaC open probability (124, 212), Na+-K+-ATPase delivery to the basolateral membrane (17), Na+-K+-ATPase α-phosphorylation (109) and Na+-K+-ATPase activity (212). In addition, the increase, in AFC may be mediated through CFTR and Cl− conductance. Increased Cl− movement may be required electrically to initiate or maintain increased Na+ movement (90, 99, 166). Studies utilizing CFTR knockout mice have demonstrated an inhibition of upregulated AFC (69–70). The exact role of CFTR and ENaC in AFC remains uncertain, but there are indications that both are required for efficient water clearance.
ALI as an Ion Channelopathy
It is intuitive that a disorder of any of the components necessary for the regulation of the respiratory tract surface liquid layer could be implicated in disorders of pulmonary fluid balance. Decreased expression of ENaC has been reported in experimental models of ALI including bleomycin-induced injury (73), viral pneumonia (218), and canine ischemia-reperfusion injury (210). The ability to maintain a maximal or submaximal rate of AFC (129, 231), and similarly a smaller magnitude of extravascular lung water (42), has been associated with improved survival in ALI. Although this varying ability to resorb edema fluid could represent differing degrees of alveolar injury, it is also possible that it could represent underlying Na+ channel function, with those with the most functional Na+ channels having the best outcome. Cardiogenic pulmonary edema serves as a useful comparative model as it is a condition with much less physical injury to the alveolus (41, 53). Patients with this condition who maintain an intact rate of AFC also have superior outcomes to those who do not (222). This suggests that rates of AFC in the critically ill may depend on more than just the degree of alveolar epithelial injury.
Consistent with this theory of a spectrum of Na+ channel activity being associated with abnormal pulmonary fluid handling, genetic variation in the β2-adrenergic receptor has been associated with a susceptibility to the development of pulmonary edema (201). A large body of evidence exists linking Na+ channel activity with pulmonary edema (see Nasal Potential Difference as a Surrogate Measure of Alveolar Ion Channel Function).
Potential Difference as a Measure of Ion Channel Function
The movement of ions across epithelia results in a transepithelial voltage, or potential difference (PD). The transepithelial PD is determined by the sum of apical and basolateral membrane PDs. As transepithelial conductances or ion gradients change, the PD changes accordingly. Placing electrodes on both sides of an epithelial membrane allows this PD to be measured via a high-impedance voltmeter. Topically perfusing the apical membrane with compounds to activate or inhibit ion channels allows the functionality of these channels to be investigated.
Respiratory epithelial PD has been measured throughout the respiratory tract, including nose (104), trachea (103), bronchi (50, 103), and alveolus (157–159). Although the alveolus is the site of interest, the measurement of alveolar PD is highly invasive, requiring alveolar micropuncture with microelectrode insertion, and to our knowledge has not been performed in humans. Lower airway PD measurement is also invasive requiring bronchoscopy.
NPD measurement is easily performed in humans, is well tolerated, and has been shown to correlate closely with the PD found in the distal respiratory epithelium (101, 103). NPD has been used as a diagnostic technique in cystic fibrosis for over 25 years. The basal NPD is largely determined by the potential generated by epithelial Na+ absorption (104). Initial studies established the Na+ dependence and amiloride sensitivity of NPD (104). Later, Knowles and colleagues (102) employed a simple modification of the perfusate to a Cl− free solution to establish a Cl− gradient across the epithelium. This allowed a measure of Cl−-selective NPD, which was a key approach to study the defective Cl− conductance in CF.
Over the years, and across the globe, the technique has been refined by local investigators to suit their needs and preferences, however, no common standard approach for NPD has been established (2, 202). Large variation in the multiple components of the NPD circuit (NPD catheter, solutions, electrodes, bridges, voltmeters, etc.), the technique of measuring NPD (location in the nose, method of passage and fixation of catheter, solution flow rate and temperature, etc.) and methods of scoring readings have made comparisons between studies less clear. At present NPD has been optimized for the measurement of Cl− transport in a relatively healthy outpatient population for the purpose of diagnosing cystic fibrosis. The optimal method of measuring baseline NPD, and thus Na+ channel function, has been a secondary consideration in the CF literature. However, a few studies have examined the effects of components of the measurement technique on baseline/basal NPD. Baseline/basal NPD as measured on the floor of the nose is similar to that measured under the inferior turbinate (4). Room temperature solutions are equivalent to body temperature solutions (27), and ECG cream is similar to agar for completing the electrical circuit (202).
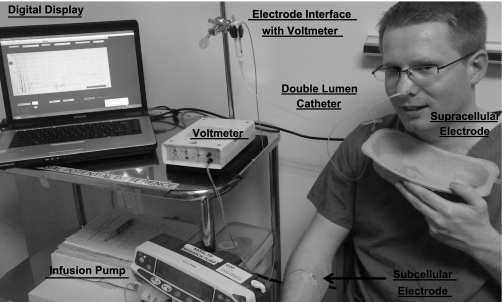
Here we describe a generic technique to measure Na+ transport (Fig. 2). Measurement of NPD involves the placement of a double-lumen catheter, which acts as the measuring (luminal) electrode, in the nose. A reference (electrically contraluminal) electrode is placed either subcutaneously (using an electrolyte/agar-filled needle) or over an area of abraded skin, typically on the forearm (3). Abrading the skin breaks the epithelial skin barrier and short-circuits the skin potential, allowing electrical contact to the subepithelial space. The measuring electrode catheter is placed either under the inferior turbinate (104) or along the floor of the nose (4), and the site of greatest PD is sought. The catheter is secured at this site. Different solutions are infused through the double lumen catheter to activate or inhibit specific ion channels and enable in vivo ion channel function to be investigated. For Na+ transport, the following measurements can be made (see Fig. 3).
Fig. 2.
Measurement of nasal potential difference (NPD).
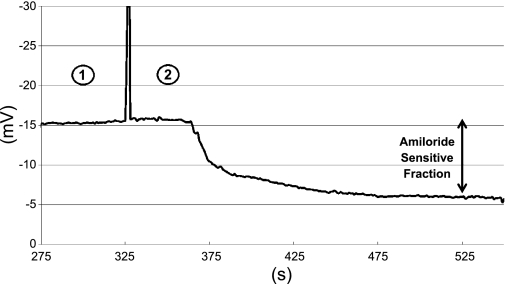
Fig. 3.
Typical NPD tracing from a healthy volunteer (see text for details). 1 = Perfusion with Ringers solution; 2 = perfusion with amiloride solution; vertical line indicates onset of amiloride perfusion.
Baseline NPD.
This is a composite value of all ion transport but is predominantly reflective of Na+ transport.
Amiloride infusion.
Amiloride (0.1 mM) blocks the ENaC class (HSC, PSC, NSC) of Na+ channels, leading to luminal Na+ retention and a fall in NPD (due to the accumulation of the positively charged Na+ cation making a negative PD less negative).
Nasal Potential Difference as a Surrogate Measure of Alveolar Ion Channel Function
There are several reasons why NPD potentially could serve as a noninvasive, surrogate measure of alveolar ion channel function.
Firstly, the epithelium of the respiratory tract has a continuous function: to regulate the depth of the overlying fluid layer (8). At baseline each nostril contains ∼800 μl fluid (94). Human lungs hold 3–7 ml/kg body wt of extravascular water (98) including 20–30 ml of ALF (31). The mechanism of nasal (200), airway (205), and alveolar (43, 116) fluid regulation is largely the same: the modification of transepithelial Na+ and Cl− movement. In the basal state Na+ absorption dominates, generating a negative potential difference and limiting the height of the liquid layer; under stimulated conditions CFTR mediated Cl− secretion increases the height of this liquid layer (21). Airway epithelia can modify the function of apical ENaC and CFTR channels to continuously change between secretory and absorptive modes to finely regulate the ASL volume; these effects are probably controlled by adenosine under basal conditions and also involve ATP under phasic motion conditions (213–214). C-type natriuretic peptide could also play such a role (95). Alveoli are also capable of both Na+ absorption and Cl− secretion (26, 93, 116, 156). Similar to ASL regulation an analogous adenosine controlled mechanism enables AT1 and AT2 cells to adjust the volume of ALF by alternating between Cl− secretion and Na+ absorption (66).
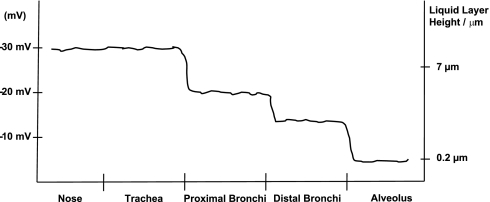
This changing requirement for respiratory liquid layer depth is reflected by the changing epithelial PD through the respiratory tract. Just as the surface fluid layer thins from proximal airway to alveolus, so too does the PD become smaller. Lower airway PD measurements show tracheal PDs of approximately −30 mV with a decrease of ∼10 mV in large proximal bronchi and a further ∼6 mV decrease in more distal segmental and subsegmental bronchi (101, 103). Alveolar PD has been determined in cell-based (37) and animal studies (157, 159) as being less than −10 mV (see Fig. 4). This relationship of decreasing respiratory PD has been consistently demonstrated in vivo and ex vivo, in both animal (24–25, 60) and human studies (50, 103–104).
Fig. 4.
Schematic representation of respiratory potential differences with associated height of respiratory liquid layers. Data adapted from Refs. 33, 81, 84, 127, 129.
Secondly, indirect evidence for the utility of NPD as a surrogate measure of more distal ion channel function comes from human studies of disorders of the airway liquid layer. CF is the archetypal respiratory ion channel disorder. It is a genetic disease of CFTR with autosomal recessive inheritance. To date over 1,600 mutations have been described (49), leading to varying amounts of the CFTR gene being expressed and CFTR protein being produced, assembled, trafficked to the cell membrane, inserted, and functioning correctly (185). The exact pathophysiology of CF remains debated; however, the low ASL volume theory proposes that CFTR dysfunction causes excessive airway Na+ resorption via ENaC (126) and an inability to secrete Cl− to increase ASL height when necessary (213). This results in decreased ASL causing a dry, inspissated mucus layer, which hinders airway clearance and promotes a cycle of repeated airway infection, bronchial damage, and the development of bronchiectasis. NPD readings from patients with CF typically demonstrate a large baseline NPD [approximately −45 mV (236) to −70 mV (101)], a large amiloride-sensitive fraction, and a lack of response to low Cl− and isoprenaline perfusion. This identifies Na+ hyperabsorption, an inability of CFTR to allow Cl− movement, and the interdependency of ENaC and CFTR function. CF patients with lower, more normal NPDs have a milder phenotype than those with higher NPDs (67).
Systemic pseudohypoaldosteronism is a rare autosomal recessive disorder of ENaC caused by loss-of-function mutations (35, 209). Excessive ASL occurs due to an inability to absorb Na+ and thus water from the air space (97). This is partly compensated for by increased removal via mucociliary clearance. The excess air space fluid causes luminal narrowing, wheezing and repeated pulmonary infection (40, 238). Baseline NPD is approximately one-third that of healthy controls and not affected by amiloride, reflecting abnormal ENaC-mediated Na+ absorption. In addition, nasal surface liquid Na+ concentration is increased and liquid volume more than doubled compared with healthy volunteers (97).
Thirdly, and most importantly, further indirect evidence comes from human studies associating abnormal NPD with alveolar dysfunction in the form of pulmonary edema. Neonatal RDS is a form of pulmonary edema suffered by premature babies. Both surfactant and ENaC subunit (216, 226) production are dependent on gestational age, and if birth occurs before sufficient production has occurred respiratory dysfunction is likely. RDS occurs with a deficiency of both surfactant and ENaC, whereas transient tachypnea of the newborn (TTN) occurs with ENaC deficiency alone (63). Premature babies with RDS have lower mRNA levels of all three ENaC subunits than premature babies without RDS (85). Similarly, premature babies with RDS have lower NPDs than otherwise healthy premature babies (10). As these babies grow older their NPD increases in keeping with enhanced ENaC production. Dexamethasone upregulates ENaC expression (216) and pretreatment for the premature fetus decreases the incidence of RDS (115), albeit via numerous mechanisms (7). Babies with TTN have a lower amiloride-sensitive fraction of NPD than healthy babies, with this value recovering to normal after 3 days (83). Interestingly, newborn babies' NPD varies with mode of delivery (83).
High-altitude pulmonary edema (HAPE) is a form of pulmonary edema typically suffered at altitudes above 2,500 m. The pathophysiology is complex, consisting of excessive hypoxic pulmonary vasoconstriction, endothelial stress failure, excessive inflammation, nitric oxide dysfunction, and, importantly, impaired amiloride-sensitive Na+ transport (12). When measured at low altitude, mountaineers prone to the development of HAPE have significantly reduced baseline NPD compared with those resistant to the condition (192). This reduction was due to the amiloride-sensitive fraction of Na+ transport, localizing the defective Na+ movement to the ENaC class of Na+ channels. Pretreating this group of HAPE prone mountaineers with salmeterol, a β2-agonist known to upregulate respiratory Na+ transport, more than halved the incidence of HAPE upon reexposure to high altitude. When HAPE prone and resistant mountaineers underwent NPD measurement at high altitude, both groups had reduced baseline NPDs compared with their low-altitude values. This decrease was due to impaired amiloride-insensitive Na+ transport and localized the defect to CNG channels (193).
These findings suggest that a constitutive defect in the ENaC class of Na+ channels, by interfering with the mechanism of AFC, may predispose certain individuals to the development of pulmonary edema and that an acquired transient defect in CNG channels, perhaps due to an environmental factor such as hypoxia, may compound this constitutive defect and further impair AFC.
Fourthly, there is direct evidence from animal studies that nasal epithelial function may be a surrogate for alveolar epithelium. Transgenic mice, with endogenous murine α-ENaC replaced by partly functional rat α-ENaC, have been used to investigate the possible role of a constitutively impaired ENaC in the generation of pulmonary edema (60). These mice grow normally and appear otherwise healthy until physiologically challenged. The baseline NPD of these mice is reduced by ∼50%, with the defect, as expected, being linked to the amiloride-sensitive fraction. Similarly, the rate of AFC is reduced by ∼50% compared with wild-type mice. When subjected to experimentally induced lung injury, the transgenic mice are more prone to the development of pulmonary edema, develop a more severe pulmonary edema, and exhibit slower rates of AFC. NPD was also significantly correlated with AFC. Further comparison of NPD and AFC comes from another mouse study investigating the effects of inducible nitric oxide synthase (iNOS), a possible regulator of amiloride-sensitive Na channels, on AFC (84). iNOS(+/+) mice had higher NPD and similar AFC values to iNOS(−/−) mice. In the presence of amiloride both NPD and AFC decreased in the controls but there was little change in the knockout mice, again suggesting that nasal Na+ transport reflects alveolar Na+ transport.
Several factors could limit the relationship between measured NPD and alveolar ion transport, especially in the intensive care unit (ICU). The nasal epithelium is subjected to a different environment than the alveolus. It has been suggested that altered NPDs seen at high altitude may be reflective of a lack of stimulated Cl− secretion at this cold dry climate (121). This is less likely to be of significance in an ICU. Abrasion of the nasal epithelium abolishes NPD (101) and therefore placement of nasogastric and nasotracheal tubes may affect NPD measurements. However, NPD does not vary between nostrils (104), allowing either nostril to be used.
Even relatively mild ischemic tissue injury may lead to loss of the polarized cellular structure affecting Na+ transport, as has been shown for renal tubular cells (221). During severe ALI denudement of the alveolar epithelium may lead to an electrical discordance between a functioning nasal epithelium and a physically damaged, poorly functioning alveolar epithelium. This could be a strength of the procedure, by permitting measurement of the underlying constitutive epithelial function and not losing this signal due to alveolar epithelial injury. Exogenous infusions of catecholamines could potentially upregulate either nasal or alveolar ion transport, or both. To date, no association has been shown between catecholamine therapy for cardiovascular support and rates of AFC in patients with ALI (231). Similarly, anesthesia and sedation could have effects on Na+, Cl−, and K+ channels affecting the applicability of readings in the ICU. Lower airway PD values are the same when measured during general anesthesia or sedation (103). Sedation is not required for the measurement of NPD and is not expected to alter its measurement. Volatile general anesthetics have inconsistent effects on AFC (155, 179). Volatile anesthetics are rarely used as a means of sedation in ICU. In addition, importantly mechanical ventilation does not appear to modify NPD in a mouse model (60).
Modifiers of NPD and AFC in ALI
Although NPD can identify a defect in ion transport, it cannot identify the cause of the defect. A detailed review of the many potential causes of defects in Na+ transport are outside the scope of this article. However, it is interesting to briefly touch on three factors present in ICU that could affect both NPD and AFC: hypoxia, glucocorticoids, and inflammatory mediators.
The effect of hypoxia on NPD has been variable, perhaps dependent on the setting in which the measurement is made (120). Hypoxia has inhibitory effects on Na+ transport via the impairment of ENaC and Na+-K+-ATPase at multiple levels. In hypoxic conditions, alveolar ENaC suffers from reduced mRNA expression (174–175), apical membrane abundance (174–175), and reduced activity (225). Hemoxygenase acts as an O2 sensor and regulates ENaC function, inhibiting the channel during hypoxia (227). Na+-K+-ATPase is similarly affected at transcription (175, 237), translation (237), and posttranslational modification (46, 211).
The specific effects of hypoxia on Cl− channel function are poorly understood. Inhibition of alveolar apical membrane Na+ transport, via amiloride and phloridzin, a blocker of the Na+-glucose transporter, causes Cl−-driven fluid secretion into the alveolus in the setting of hypoxia (151). This suggests that unopposed Cl− secretion generates a gradual accumulation of pulmonary fluid and could reflect the basic blueprint for respiratory ion channel function (Cl− secretion in hypoxia, i.e., in utero; Na+ absorption in normoxia, i.e., ex utero). Although ALI patients are relatively hypoxemic (usually PaO2 <11 kPa), they are rarely absolutely hypoxemic (PaO2 <8 kPa) with few (<10%) dying from refractory hypoxemia (145, 206).
Glucocorticoids are often proposed for their anti-inflammatory and antifibrotic effects in ALI/acute RDS (ARDS) (139). The presence of a glucocorticoid response element in the α-ENaC subunit is suggestive of a role in the regulation of alveolar Na+ transport (38). Inhibition of cortisol synthesis decreases ENaC mRNA and AFC, whereas administration of dexamethasone reverses this effect (161). Dexamethasone modulates expression and activity of both ENaC and Na-K-ATPase (11, 47, 108), with resulting increases in transepithelial current (47), and AFC (74, 160).
Inflammatory mediators are present in virtually all critically ill patients and have been suggested as a possible cause of ALI/ARDS (18, 61, 82). Several mediators have been shown to inhibit AFC, via negative effects on the expression and function of Na+ transport proteins. Conversely, proinflammatory cytokines may have positive effects on Na+ transport and edema resolution, making the overall picture difficult to interpret at present.
Reduction of pulmonary Na+ transport occurs in association with a range of cytokines, including interleukin-1β (184), TGF-β1 (76), and TNF-α (48). TNF-α can, however, also increase AFC (22, 78, 180). Reactive oxygen and nitrogen species (RONS) similarly diminish Na+ transport and AFC in the setting of mycoplasma infection (87) and ventilator-induced lung injury (75). Interleukin-4 (IL-4) (79) can lower β- and γ-ENaC subunit expression and amiloride-sensitive current in vitro, while increasing CFTR mRNA, CFTR function, and Cl− current, suggesting a net effect of increased fluid secretion (79). Against this, TGF-β1 decreases CFTR gene and protein expression with an associated reduction in nasal Cl− transport (176) and cAMP-mediated AFC (183). Non-CFTR Cl− channels are also affected (39, 240).
K+ channel function could also be regulated by inflammation, as has been briefly reviewed by Bardou et al. (8). There is little direct evidence, but bronchial cells change from a Na+-absorbing to a Cl−-secreting state, along with an increase in SK4 K+ transport, when subjected to IL-3 (57).
Na+-K+-ATPase gene expression and function is similarly affected by cytokines, increasing with IL-1β (152), TGF-β1 (235), and leukotriene D4 (199) with concomitant elevated rates of ion transport and fluid clearance. In contrast, mitochondrial reactive oxygen species downregulate alveolar Na+-K+-ATPase function during hypoxia (46). AQPs may equally be affected, with TNF-α, via activation of NF-κB, decreasing aquaporin 5 mRNA and protein expression in murine lung epithelial cells (219). Likewise, β-receptors could be susceptible to effects of both infection and inflammatory mediators. Respiratory syncytial virus directly (146), and indirectly via inflammatory mediators such as CXCR8 (51, 220), induces β2-receptor insensitivity. Nitric oxide (173) and neutrophil-mediated oxidant injury (142), generated by severe hemorrhagic shock, also decrease alveolar β-receptor function and β-agonist-mediated upregulation of AFC.
The differences in Na+ transport shown between diverse models may be due to multiple effectors, not just cytokines, including severity and duration of injury, degree of hypoxia, generation of RONS, and cell or animal model used (149). The dominant influence of inflammatory mediators on Na+ transport and AFC remains to be conclusively determined (228).
Future Directions
ALI patients capable of maintaining maximal or submaximal AFC, as measured by increasing protein concentration from sequentially aspirated tracheal fluid samples, have increased survival (231). β2-Agonists can increase alveolar Na+ transport (170). Salbutamol, a β2-agonist, has been shown to decrease extravascular lung water in ALI when administered intravenously (171) and in a retrospective study was associated with improved outcomes when inhaled (122). Inhaled salbutamol may increase the resolution of pulmonary edema after lung resection (113). The recently completed ALTA study investigating the effect of inhaled albuterol on outcome in ALI reported negative results (130). Similarly, the recently terminated BALTI-2 study, which investigated the effects of intravenous salbutamol in ALI, was stopped for futility (Gao F et al., unpublished at present). With the increasing understanding of the importance of matching treatment to an individual's phenotype, it is possible that this trial was negative because of an inability to identify those with constitutively dysfunctional Na+ channels who would benefit from pharmacological upregulation. To date, Na+ channel function, as assessed by NPD, has been studied in pediatric meningococcal-associated pulmonary edema and shown not to be affected (62).
If NPD proves to be a useful technique in the ICU, there are possible avenues for its development. Bedside parameters for the purposes of managing pulmonary edema are currently limited. Many ICUs perform daily chest X-rays, partly with the aim of quantifying the degree of pulmonary edema (123). This quantification is known to be poor (106). Cardiac filling pressures, via measurements of central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP), are commonly used to avoid hypervolemia and the risk of pulmonary edema. Both CVP and PAOP have been shown to be poor indicators of fluid status in ICU (140). Extravascular lung water can be more accurately measured by the single indicator transpulmonary thermodilution technique, via a PiCCO device (98); however, this remains a largely research tool. NPD could potentially improve on these monitoring techniques, by identifying an individual's Na+ transport phenotype and thus the potential to develop, and recover from, pulmonary edema. Sequential NPD measures could also, in theory, map an individual's Na+ transport ability during critical illness, enabling optimization of both respiratory and hemodynamic therapy. Clearly these potential applications require future prospective investigation before implementation.
NPD could potentially be used to predict the development of, and even outcome from, ALI. The knowledge of an individual's constitutive capacity for AFC could help guide fluid balance with the aim of optimizing fluid resuscitation without precipitating pulmonary edema. Similarly, future ALI studies could stratify for underlying Na+ transport ability. It would be attractive to distinguish the degree of pulmonary edema and respiratory failure due to constitutively limited Na+ transport rather than alveolar injury per se. Such knowledge could influence trial outcomes and design.
Conclusion
A spectrum of respiratory epithelial Na+ channel function may exist and contribute to both the development of, and outcome from, ALI. NPD is an in vivo measure of respiratory epithelial ion channel function. Although it is likely to have limitations, substantial evidence supports the possibility this technique could act as a surrogate measure of alveolar Na+ channel function.
GRANTS
R. Mac Sweeney is supported by the Research and Development Office, Northern Ireland. H. Fischer was supported by National Heart, Lung, and Blood Institute Grant HL86323.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Agarwal R, Srinivas R, Nath A, Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? Chest 133: 1463–1473, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Ahrens RC, Standaert TA, Launspach J, Han SH, Teresi ME, Aitken ML, Kelley TJ, Hilliard KA, Milgram LJH, Konstan MW, Weatherly MR, McCarty NA. Use of nasal potential difference and sweat chloride as outcome measures in multicenter clinical trials in subjects with cystic fibrosis. Pediatr Pulmonol 33: 142–150, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Alton EW, Currie D, Logan-Sinclair R, Warner JO, Hodson ME, Geddes DM. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J 3: 922–926, 1990 [PubMed] [Google Scholar]
- 4. Alton EW, Hay JG, Munro C, Geddes DM. Measurement of nasal potential difference in adult cystic fibrosis, Young's syndrome, and bronchiectasis. Thorax 42: 815–817, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1389–1394, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bai C, Fukuda N, Song Y, Ma T, Matthay M, Verkman A. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest 103: 555–561, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballard PL. Scientific rationale for the use of antenatal glucocorticoids to promote fetal development. Neoreviews 1: e83–e90, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Bardou O, Trinh NTN, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L145–L155, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Barker PM, Gatzy JT. Effect of gas composition on liquid secretion by explants of distal lung of fetal rat in submersion culture. Am J Physiol Lung Cell Mol Physiol 265: L512–L517, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Barker PM, Gowen CW, Lawson EE, Knowles MR. Decreased sodium ion absorption across nasal epithelium of very premature infants with respiratory distress syndrome. J Pediatr 130: 373–377, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Barquin N, Ciccolella DE, Ridge KM, Sznajder JI. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 273: L825–L830, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Basnyat B, Murdoch DR. High-altitude illness. Lancet 361: 1967–1974, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Basset G, Crone C, Saumon G. Fluid absorption by rat lung in situ: pathways for sodium entry in the luminal membrane of alveolar epithelium. J Physiol 384: 325–345, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basset G, Saumon G, Bouchonnet F, Crone C. Apical sodium sugar transport in pulmonary epithelium in situ. Biochim Biophys Acta 942: 11–18, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Bastacky J, Lee CY, Goerke J, Koushafar H, Yager D, Kenaga L, Speed TP, Chen Y, Clements JA. Alveolar lining layer is thin and continuous: low-temperature scanning electron microscopy of rat lung. J Appl Physiol 79: 1615–1628, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 20: 225–232, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Bertorello AM, Komarova Y, Smith K, Leibiger IB, Efendiev R, Pedemonte CH, Borisy G, Sznajder JI. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell 14: 1149–1157, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202: 145–156, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Bland RD, Hansen TN, Haberkern CM, Bressack MA, Hazinski TA, Raj JU, Goldberg RB. Lung fluid balance in lambs before and after birth. J Appl Physiol 53: 992–1004, 1982 [DOI] [PubMed] [Google Scholar]
- 21. Blouquit-Laye S, Chinet T. Ion and liquid transport across the bronchiolar epithelium. Respir Physiol Neurobiol 159: 278–282, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Borjesson A, Norlin A, Wang X, Anderson R, Folkesson H. TNF-α stimulates alveolar liquid clearance during intestinal ischemia-reperfusion in rats. Am J Physiol Lung Cell Mol Physiol 278: L3–L12, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Borok Z, Verkman AS. Lung edema clearance: 20 years of progress. Invited review: role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol 93: 2199–2206, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Boucher RC, Jr, Bromberg PA, Gatzy JT. Airway transepithelial electric potential in vivo: species and regional differences. J Appl Physiol 48: 169–176, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Boucher RC, Stutts MJ, Gatzy JT. Regional differences in bioelectric properties and ion flow in excised canine airways. J Appl Physiol 51: 706–714, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Bove P, Rogers T, Grubb B, Boucher R. Ion transport and alveolar liquid regulation in human alveolar type II cells (Abstract). Am J Respir Crit Care Med 179: A4994, 2009. [Google Scholar]
- 27. Boyle MP, Diener-West M, Milgram L, Knowles M, Foy C, Zeitlin P, Standaert T. A multicenter study of the effect of solution temperature on nasal potential difference measurements. Chest 124: 482–489, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Brochiero E, Dagenais A, Prive A, Berthiaume Y, Grygorczyk R. Evidence of a functional CFTR Cl− channel in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L382–L392, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol 344: 137–152, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F, ALIVE Study Group Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30: 51–61, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Burri PH. Structural aspects of prenatal and postnatal development and, growth of the lung. In: Lung Growth and Development, edited by McDonald JAE. New York: Dekker, 1997, p. 1–35(Lung Biology in Health and Disease 100) [Google Scholar]
- 32. Butterworth MB, Helman SI, Els WJ. cAMP-sensitive endocytic trafficking in A6 epithelia. Am J Physiol Cell Physiol 280: C752–C762, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Canessa CM. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Carstairs J, Nimmo A, Barnes P. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis 132: 541–547, 1985 [DOI] [PubMed] [Google Scholar]
- 35. Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Charron PD, Fawley JP, Maron MB. Effect of epinephrine on alveolar liquid clearance in the rat. J Appl Physiol 87: 611–618, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Cheek JM, Kim KJ, Crandall ED. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol Cell Physiol 256: C688–C693, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Chow YH, Wang Y, Plumb J, O'Brodovich H, Hu J. Hormonal regulation and genomic organization of the human amiloride-sensitive epithelial sodium channel α subunit gene. Pediatr Res 46: 208–214, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Chu S, Blaisdell CJ, Bamford P, Ferro TJ. Interferon-γ regulates ClC-2 chloride channel in lung epithelial cells. Biochem Biophys Res Commun 324: 31–39, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Clark JC, Weaver TE, Iwamoto HS, Ikegami M, Jobe AH, Hull WM, Whitsett JA. Decreased lung compliance and air trapping in heterozygous SP-B-deficient mice. Am J Respir Cell Mol Biol 16: 46–52, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Cottrell TS, Levine OR, Senior RM, Wiener J, Spiro D, Fishman AP. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res 21: 783–798, 1967 [DOI] [PubMed] [Google Scholar]
- 42. Craig TR, Duffy MJ, Shyamsundar M, McDowell C, McLaughlin B, Elborn JS, McAuley DF. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med 38: 114–120, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med 163: 1021–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982 [DOI] [PubMed] [Google Scholar]
- 45. Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. Morphometric characteristics of cells of the alveolar region of mammalian lungs. Am Rev Respir Dis 128: S42–S46, 1983 [DOI] [PubMed] [Google Scholar]
- 46. Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 111: 1057–1064, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dagenais A, Denis C, Vives M, Girouard S, Masse C, Nguyen T, Yamagat T, Grygorczyk C, Kothary R, Berthiaume Y. Modulation of α-ENaC and α1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L217–L230, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Dagenais A, Frechette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, Brochiero E, Masse C, Berthiaume Y. Downregulation of ENaC activity and expression by TNF-α in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L301–L311, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Davies JC, Alton EW, Brush A. Cystic fibrosis. Br Med J 335: 1255–1259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davies JC, Davies M, McShane D, Smith S, Chadwick S, Jaffe A, Farley R, Collins L, Bush A, Scallon M, Pepper J, Geddes DM, Alton EWFW. Potential difference measurements in the lower airway of children with and without cystic fibrosis. Am J Respir Crit Care Med 171: 1015–1019, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to β-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Prost N, Saumon G. Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol 159: 331–337, 2007 [DOI] [PubMed] [Google Scholar]
- 53. DeFouw D, Berendsen P. Morphological changes in isolated perfused dog lungs after acute hydrostatic edema. Circ Res 43: 72–82, 1978 [DOI] [PubMed] [Google Scholar]
- 54. Ding C, Potter ED, Qiu W, Coon SL, Levine MA, Guggino SE. Cloning and widespread distribution of the rat rod-type cyclic nucleotide-gated cation channel. Am J Physiol Cell Physiol 272: C1335–C1344, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the air space and vasculature in rat lung. Proc Natl Acad Sci USA 95: 2991–2996, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dobbs LG, Johnson MD. Alveolar epithelial transport in the adult lung. Respir Physiol Neurobiol 159: 283–300, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Dulong S, Bernard K, Ehrenfeld J. Enhancement of P2Y6-induced Cl− secretion by IL-13 and modulation of SK4 channels activity in human bronchial cells. Cell Physiol Biochem 20: 483–494, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Dumasius V, Sznajder J, Azzam Z, Boja J, Mutlu G, Maron M, Factor P. Beta2-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res 89: 907–914, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Effros RM, Feng NH, Mason G, Sietsema K, Silverman P, Hukkanen J. Solute concentrations of the pulmonary epithelial lining fluid of anesthetized rats. J Appl Physiol 68: 275–281, 1990 [DOI] [PubMed] [Google Scholar]
- 60. Egli M, Duplain H, Lepori M, Cook S, Nicod P, Hummler E, Sartori C, Scherrer U. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J Physiol Online 560: 857–865, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eisenhut M. Changes in ion transport in inflammatory disease. J Inflamm 3: 5, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eisenhut M, Wallace H, Barton P, Gaillard E, Newland P, Diver M, Southern KW. Pulmonary edema in meningococcal septicemia associated with reduced epithelial chloride transport. Pediatr Crit Care Med 7: 119–124, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Elias N, O'Brodovich H. Clearance of fluid from airspaces of newborns and infants. Neoreviews 7: e88–e94, 2006 [Google Scholar]
- 64. Fabisiak JP, Vesell ES, Rannels DE. Interactions of beta adrenergic antagonists with isolated rat alveolar type II pneumocytes. I. Analysis, characterization and regulation of specific beta adrenergic receptors. J Pharmacol Exp Ther 241: 722–727, 1987 [PubMed] [Google Scholar]
- 65. Factor P, Dumasius V, Saldias F, Brown LAS, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase β1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther 11: 2231–2242, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, Kass D, Martino JM, Bellmeyer A, Albazi JS, Emala C, Lee HT, Dobbs LG, Matalon S. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 104: 4083–4088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fajac I, Hubert D, Bienvenu T, Richaud-Thiriez B, Matran R, Kaplan JC, Dall'Ava-Santucci J, Dusser DJ. Relationships between nasal potential difference and respiratory function in adults with cystic fibrosis. Eur Respir J 12: 1295–1300, 1998 [DOI] [PubMed] [Google Scholar]
- 68. Fang X, Barbry P, Fukuda N, Matthay MA. Upregulation of isosmolar alveolar fluid clearance in mice depends on CFTR (Abstract). Pediatr Pulmonol 20: 69A, 2000 [Google Scholar]
- 69. Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fang X, Song Y, Hirsch J, Galietta LJV, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 292: L1432–L1443, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Fischer H, Van Driessche W, Clauss W. Evidence for apical sodium channels in frog lung epithelial cells. Am J Physiol Cell Physiol 256: C764–C771, 1989 [DOI] [PubMed] [Google Scholar]
- 73. Folkesson H, Nitenberg G, Oliver B, Jayr C, Albertine K, Matthay M. Upregulation of alveolar epithelial fluid transport after subacute lung injury in rats from bleomycin. Am J Physiol Lung Cell Mol Physiol 275: L478–L490, 1998 [DOI] [PubMed] [Google Scholar]
- 74. Folkesson HG, Norlin A, Wang Y, Abedinpour P, Matthay MA. Dexamethasone and thyroid hormone pretreatment upregulate alveolar epithelial fluid clearance in adult rats. J Appl Physiol 88: 416–424, 2000 [DOI] [PubMed] [Google Scholar]
- 75. Frank J, Pittet J, Lee H, Godzich M, Matthay M. High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 284: L791–L798, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF. Transforming growth factor-β1 decreases expression of the epithelial sodium channel αENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939–43950, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Frank JA, Wang Y, Osorio O, Matthay MA. β-Adrenergic agonist therapy accelerates the resolution of hydrostatic pulmonary edema in sheep and rats. J Appl Physiol 89: 1255–1265, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay M. Mechanisms of TNF-α stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 280: L1258–L1265, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Galietta LJV, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168: 839–845, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Goodman BE, Anderson JL, Clemens JW. Evidence for regulation of sodium transport from airspace to vascular space by cAMP. Am J Physiol Lung Cell Mol Physiol 257: L86–L93, 1989 [DOI] [PubMed] [Google Scholar]
- 81. Goodman BE, Kim KJ, Crandall ED. Evidence for active sodium transport across alveolar epithelium of isolated rat lung. J Appl Physiol 62: 2460–2466, 1987 [DOI] [PubMed] [Google Scholar]
- 82. Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 14: 523–535, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Gowen CWJ, Lawson EE, Gingras J, Boucher RC, Gatzy JT, Knowles MR. Electrical potential difference and ion transport across nasal epithelium of term neonates: correlation with mode of delivery, transient tachypnea of the newborn, and respiratory rate. J Pediatr 113: 121–127, 1988 [DOI] [PubMed] [Google Scholar]
- 84. Hardiman KM, Lindsey JR, Matalon S. Lack of amiloride-sensitive transport across alveolar and respiratory epithelium of iNOS−/− mice in vivo. Am J Physiol Lung Cell Mol Physiol 281: L722–L731, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Helve O, Pitkanen O, Andersson S, O'Brodovich H, Kirjavainen T, Otulakowski G. Low expression of human epithelial sodium channel in airway epithelium of preterm infants with respiratory distress. Pediatrics 113: 1267–1272, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 89. Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol 76: 2636–2642, 1994 [DOI] [PubMed] [Google Scholar]
- 90. Jiang X, Ingbar DH, O'Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am J Physiol Cell Physiol 275: C1610–C1620, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 99: 1966–1971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Junor RWJ, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. A novel role for cyclic nucleotide-gated cation channels in lung liquid homeostasis in sheep. J Physiol Online 520: 255–260, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kaulbach H, White M, Igarashi Y, Hahn B, Kaliner M. Estimation of nasal epithelial lining fluid using urea as a marker. J Allergy Clin Immunol 92: 457–465, 1993 [DOI] [PubMed] [Google Scholar]
- 95. Kelley TJ, Cotton CU, Drumm ML. Regulation of amiloride-sensitive sodium absorption in murine airway epithelium by C-type natriuretic peptide. Am J Physiol Lung Cell Mol Physiol 274: L990–L996, 1998 [DOI] [PubMed] [Google Scholar]
- 96. Kemp PJ, Kim KJ, Borok Z, Crandall ED. Re-evaluating the Na+ conductance of adult rat alveolar type II pneumocytes: evidence for the involvement of cGMP-activated cation channels. J Physiol Online 536: 693–701, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR, Homolya V, Keenan B. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med 341: 156–162, 1999 [DOI] [PubMed] [Google Scholar]
- 98. Khan S, Trof RJ, Groeneveld AJ. Transpulmonary dilution-derived extravascular lung water as a measure of lung edema. Curr Opin Crit Care 13: 303–307, 2007 [DOI] [PubMed] [Google Scholar]
- 99. Kim KJ, Cheek JM, Crandall ED. Contribution of active Na+ and Cl− fluxes to net ion transport by alveolar epithelium. Respir Physiol 85: 245–256, 1991 [DOI] [PubMed] [Google Scholar]
- 100. Kitterman JA. Fetal lung development. J Dev Physiol 6: 67–82, 1984 [PubMed] [Google Scholar]
- 101. Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med 305: 1489–1495, 1981 [DOI] [PubMed] [Google Scholar]
- 102. Knowles M, Paradiso A, Boucher R. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther 6: 445–455, 1995 [DOI] [PubMed] [Google Scholar]
- 103. Knowles MR, Buntin WH, Bromberg PA, Gatzy JT, Boucher RC. Measurements of transepithelial electrical potential differences in the trachea and bronchi of human subjects in vivo. Am Rev Respir Dis 126: 108–112, 1982 [DOI] [PubMed] [Google Scholar]
- 104. Knowles MR, Carson JL, Collier AM, Gatzy JT, Boucher RC. Measurement of nasal transepithelial electrical potential differences in normal human subjects in vivo. Am Rev Respir Dis 124: 484–490, 1981 [DOI] [PubMed] [Google Scholar]
- 105. Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol 24: 224–234, 2001 [DOI] [PubMed] [Google Scholar]
- 106. Lange NR, Schuster DP. The measurement of lung water. Crit Care 3: R19–R24, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lauweryns J, Baert J. Alveolar clearance and the role of the pulmonary lymphatics. Am Rev Respir Dis 115: 625–683, 1977 [DOI] [PubMed] [Google Scholar]
- 108. Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na+ channels by dexamethasone. Am J Physiol Cell Physiol 279: C762–C770, 2000 [DOI] [PubMed] [Google Scholar]
- 109. Lecuona E, Garcia A, Sznajder JI. A novel role for protein phosphatase 2A in the dopaminergic regulation of Na,K-ATPase. FEBS Lett 481: 217–220, 2000 [DOI] [PubMed] [Google Scholar]
- 110. Leroy C, Dagenais A, Berthiaume Y, Brochiero E. Molecular identity and function in transepithelial transport of KATP channels in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L1027–L1037, 2004 [DOI] [PubMed] [Google Scholar]
- 111. Leroy C, Prive A, Bourret JC, Berthiaume Y, Ferraro P, Brochiero E. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L1207–L1219, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Li T, Folkesson HG. RNA interference for α-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol 290: L649–L660, 2006 [DOI] [PubMed] [Google Scholar]
- 113. Licker M, Tschopp JM, Robert J, Frey JG, Diaper J, Ellenberger C. Aerosolized salbutamol accelerates the resolution of pulmonary edema after lung resection. Chest 133: 845–852, 2008 [DOI] [PubMed] [Google Scholar]
- 114. Liebler JM, Borok Z, Li X, Zhou B, Sandoval AJ, Kim KJ, Crandall ED. Alveolar epithelial type I cells express β2-adrenergic receptors and G-protein receptor kinase 2. J Histochem Cytochem 52: 759–767, 2004 [DOI] [PubMed] [Google Scholar]
- 115. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515–525, 1972 [PubMed] [Google Scholar]
- 116. Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol 36: 688–696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lubman RL, Crandall ED. Polarized distribution of Na+-H+ antiport activity in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 266: L138–L147, 1994 [DOI] [PubMed] [Google Scholar]
- 118. Lytton J, Lin JC, Guidotti G. Identification of two molecular forms of (Na+,K+)-ATPase in rat adipocytes relation to insulin stimulation of the enzyme. J Biol Chem 260: 1177–1184, 1985 [PubMed] [Google Scholar]
- 119. Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest 105: 93–100, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mairbäurl H. Role of alveolar epithelial sodium transport in high altitude pulmonary edema (HAPE). Respir Physiol Neurobiol 151: 178–191, 2006 [DOI] [PubMed] [Google Scholar]
- 121. Mairbaurl H, Weymann J, Mohrlein A, Swenson ER, Maggiorini M, Gibbs JSR, Bartsch P. Nasal epithelium potential difference at high altitude (4,559 m): evidence for secretion. Am J Respir Crit Care Med 167: 862–867, 2003 [DOI] [PubMed] [Google Scholar]
- 122. Manocha S, Gordon A, Salehifar E, Groshaus H, Walley K, Russell J. Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury. Crit Care 10: R12, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin GS, Ely EW, Carroll FE, Bernard GR. Findings on the portable chest radiograph correlate with fluid balance in critically ill patients. Chest 122: 2087–2095, 2002 [DOI] [PubMed] [Google Scholar]
- 124. Marunaka Y, Niisato N, O'Brodovich H, Eaton DC. Regulation of an amiloride-sensitive Na+-permeable channel by a beta2-adrenergic agonist, cytosolic Ca2+ and Cl− in fetal rat alveolar epithelium. J Physiol 515: 669–683, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Matalon S, Lazrak A, Jain L, Eaton DC. Lung edema clearance: 20 years of progress. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol 93: 1852–1859, 2002 [DOI] [PubMed] [Google Scholar]
- 126. Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 127. Matsuzaki T, Hata H, Ozawa H, Kuniaki T. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem Cytochem 42: 159–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Matthay M, Folkesson H, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 129. Matthay M, Wiener-Kronish J. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990 [DOI] [PubMed] [Google Scholar]
- 130. Matthay MA, Brower R, Thompson BT, Schoenfeld D, Eisner MD, Carson S, Moss M, Douglas I, Hite D, MacIntyre N, Liu KD, ARDS Network. Randomized, placebo controlled trial of an aerosolized beta-2 adrenergic agonist (albuterol) for the treatment of acute lung injury (Abstract). Am J Respir Crit Care Med 179: A2166, 2009. [Google Scholar]
- 131. Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 132. McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 32: 1470–1476, 2004 [DOI] [PubMed] [Google Scholar]
- 133. McCray PB, Jr, Reenstra WW, Louie E, Johnson J, Bettencourt JD, Bastacky J. Expression of CFTR and presence of cAMP-mediated fluid secretion in human fetal lung. Am J Physiol Lung Cell Mol Physiol 262: L472–L481, 1992 [DOI] [PubMed] [Google Scholar]
- 134. McCray PBJ, Bettencourt JD, Bastacky J, Denning GM, Welsh MJ. Expression of CFTR and a cAMP-stimulated chloride secretory current in cultured human fetal alveolar epithelial cells. Am J Respir Cell Mol Biol 9: 578–585, 1993 [DOI] [PubMed] [Google Scholar]
- 135. McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PBJ, Stokes JB, Welsh MJ, Williamson RA. Disruption of the β subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci USA 96: 1727–1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. McDonough AA, Geering K, Farley RA. The sodium pump needs its beta subunit. FASEB J 4: 1598–1605, 1990 [DOI] [PubMed] [Google Scholar]
- 137. McGraw DW, Fukuda N, James PF, Forbes SL, Woo AL, Lingrel JB, Witte DP, Matthay MA, Liggett SB. Targeted transgenic expression of β2-adrenergic receptors to type II cells increases alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 281: L895–L903, 2001 [DOI] [PubMed] [Google Scholar]
- 138. McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol 109: 681–692, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med 34: 61–69, 2008 [DOI] [PubMed] [Google Scholar]
- 140. Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients. Chest 121: 2000–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 141. Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of β-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 275: L414–L422, 1998 [DOI] [PubMed] [Google Scholar]
- 142. Modelska K, Matthay MA, Brown LAS, Deutch E, Lu LN, Pittet JF. Inhibition of β-adrenergic-dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock. Am J Physiol Lung Cell Mol Physiol 276: L844–L857, 1999 [DOI] [PubMed] [Google Scholar]
- 143. Moessinger AC, Collins MH, Blanc WA, Rey HR, James LS. Oligohydramnios-induced lung hypoplasia: the influence of timing and duration in gestation. Pediatr Res 20: 951–954, 1986 [DOI] [PubMed] [Google Scholar]
- 144. Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest 86: 1270–1277, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with adult respiratory distress syndrome. Am Rev Respir Dis 132: 485–489, 1985 [DOI] [PubMed] [Google Scholar]
- 146. Moore PE, Cunningham G, Calder MM, DeMatteo AD, Jr, Peeples ME, Summar ML, Peebles RS., Jr Respiratory syncytial virus infection reduces beta2-adrenergic responses in human airway smooth muscle. Am J Respir Cell Mol Biol 35: 559–564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, Balagani R, Traver G, Sznajder JI, Factor P. Interdependency of β-adrenergic receptors and cftr in regulation of alveolar active Na+ transport. Circ Res 96: 999–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 148. Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on β2-adrenergic receptor signaling. Circ Res 94: 1091–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 149. Mutlu GM, Koch WJ, Factor P. Alveolar epithelial β2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med 170: 1270–1275, 2004 [DOI] [PubMed] [Google Scholar]
- 150. Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol 289: L685–L695, 2005 [DOI] [PubMed] [Google Scholar]
- 151. Nagyova B, O'Neill M, Dorrington KL. Inhibition of active sodium absorption leads to a net liquid secretion into in vivo rabbit lung at two levels of alveolar hypoxia. Br J Anaesth 87: 897–904, 2001 [DOI] [PubMed] [Google Scholar]
- 152. Nair PK, Li T, Bhattacharjee R, Ye X, Folkesson HG. Oxytocin-induced labor augments IL-1β-stimulated lung fluid absorption in fetal guinea pig lungs. Am J Physiol Lung Cell Mol Physiol 289: L1029–L1038, 2005 [DOI] [PubMed] [Google Scholar]
- 153. Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, Clancy JP. A macromolecular complex of β2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA 100: 342–346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol Cell Physiol 273: C1549–C1561, 1997 [DOI] [PubMed] [Google Scholar]
- 155. Nielsen VG, Baird MS, Geary BT, Matalon S. Halothane does not decrease amiloride-sensitive alveolar fluid clearance in rabbits. Anesth Analg 90: 1445–1449, 2000 [DOI] [PubMed] [Google Scholar]
- 156. Nielsen VG, Duvall MD, Baird MS, Matalon S. cAMP activation of chloride and fluid secretion across the rabbit alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 275: L1127–L1133, 1998 [DOI] [PubMed] [Google Scholar]
- 157. Nielson DW. Electrolyte composition of pulmonary alveolar subphase in anesthetized rabbits. J Appl Physiol 60: 972–979, 1986 [DOI] [PubMed] [Google Scholar]
- 158. Nielson DW, Goerke J, Clements JA. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc Natl Acad Sci USA 78: 7119–7123, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nielson DW, Lewis MB. Effects of amiloride on alveolar epithelial PD and fluid composition in rabbits. Am J Physiol Lung Cell Mol Physiol 258: L215–L219, 1990 [DOI] [PubMed] [Google Scholar]
- 160. Noda M, Suzuki S, Tsubochi H, Sugita M, Maeda S, Kobayashi S, Kubo H, Kondo T. Single dexamethasone injection increases alveolar fluid clearance in adult rats. Crit Care Med 31: 1183–1189, 2003 [DOI] [PubMed] [Google Scholar]
- 161. Norlin A, Baines DL, Folkesson HG. Role of endogenous cortisol in basal liquid clearance from distal air spaces in adult guinea-pigs. J Physiol 519: 261–272, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Norlin A, Finley N, Abedinpour P, Folkesson H. Alveolar liquid clearance in the anesthetized ventilated guinea pig. Am J Physiol Lung Cell Mol Physiol 274: L235–L243, 1998 [DOI] [PubMed] [Google Scholar]
- 163. Norlin A, Lu LN, Guggino SE, Matthay MA, Folkesson HG. Contribution of amiloride-insensitive pathways to alveolar fluid clearance in adult rats. J Appl Physiol 90: 1489–1496, 2001 [DOI] [PubMed] [Google Scholar]
- 164. O'Brodovich H, Hannam V, Seear M, Mullen JB. Amiloride impairs lung water clearance in newborn guinea pigs. J Appl Physiol 68: 1758–1762, 1990 [DOI] [PubMed] [Google Scholar]
- 165. O'Brodovich H, Yang P, Gandhi S, Otulakowski G. Amiloride-insensitive Na+ and fluid absorption in the mammalian distal lung. Am J Physiol Lung Cell Mol Physiol 294: L401–L408, 2008 [DOI] [PubMed] [Google Scholar]
- 166. O'Grady SM, Jiang X, Ingbar DH. Cl− channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L239–L244, 2000 [DOI] [PubMed] [Google Scholar]
- 167. O'Grady SM, Lee SY. Chloride and potassium channel function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 284: L689–L700, 2003 [DOI] [PubMed] [Google Scholar]
- 168. Olivera W, Ridge K, Wood L, Sznajder J. Active sodium transport and alveolar epithelial Na-KATPase increase during subacute hyperoxia in rats. Am J Physiol Lung Cell Mol Physiol 266: L577–L584, 1994 [DOI] [PubMed] [Google Scholar]
- 169. Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. J Physiol 376: 321–340, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: beta2-Agonists and the acute respiratory distress syndrome. Crit Care 8: 25–32, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 172. Pitkanen O, Tanswell AK, Downey G, O'Brodovich H. Increased Po2 alters the bioelectric properties of fetal distal lung epithelium. Am J Physiol Lung Cell Mol Physiol 270: L1060–L1066, 1996 [DOI] [PubMed] [Google Scholar]
- 173. Pittet JF, Lu LN, Morris DG, Modelska K, Welch WJ, Carey HV, Roux J, Matthay MA. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol 166: 6301–6310, 2001 [DOI] [PubMed] [Google Scholar]
- 174. Planes C, Blot-Chabaud M, Matthay MA, Couette S, Uchida T, Clerici C. Hypoxia and beta 2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem 277: 47318–47324, 2002 [DOI] [PubMed] [Google Scholar]
- 175. Planes C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol 17: 508–518, 1997 [DOI] [PubMed] [Google Scholar]
- 176. Pruliere-Escabasse V, Fanen P, Dazy AC, Lechapt-Zalcman E, Rideau D, Edelman A, Escudier E, Coste A. TGF-β1 downregulates CFTR expression and function in nasal polyps of non-CF patients. Am J Physiol Lung Cell Mol Physiol 288: L77–L83, 2005 [DOI] [PubMed] [Google Scholar]
- 177. Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 27: 304–312, 1999 [DOI] [PubMed] [Google Scholar]
- 178. Randrianarison N, Clerici C, Ferreira C, Fontayne A, Pradervand S, Fowler-Jaeger N, Hummler E, Rossier BC, Planes C. Low expression of the β-ENaC subunit impairs lung fluid clearance in the mouse. Am J Physiol Lung Cell Mol Physiol 294: L409–L416, 2008 [DOI] [PubMed] [Google Scholar]
- 179. Rezaiguia-Delclaux S, Jayr C, Luo DF, Saidi NE, Meignan M, Duvaldestin P. Halothane and isoflurane decrease alveolar epithelial fluid clearance in rats. Anesthesiology 88: 751–760, 1998 [DOI] [PubMed] [Google Scholar]
- 180. Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, Matthay M. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha dependent mechanism. J Clin Invest 99: 325–335, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rezaiguia S, Garat C, Delclaux MM, Fleury J, Legrand P, Matthey MA, Jayr C. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest 99: 325–335, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the α2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res 92: 453–460, 2003 [DOI] [PubMed] [Google Scholar]
- 183. Roux J, Carles M, Koh H, Goolaerts A, Ganter MT, Chesebro BB, Howard M, Houseman BT, Finkbeiner W, Shokat KM, Paquet AC, Matthay MA, Pittet JF. Transforming growth factor β1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 285: 4278–4290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1β decreases expression of the epithelial sodium channel α-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005 [DOI] [PubMed] [Google Scholar]
- 185. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 186. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 187. Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med 155: 506–512, 1997 [DOI] [PubMed] [Google Scholar]
- 188. Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med 150: 305–310, 1994 [DOI] [PubMed] [Google Scholar]
- 189. Sakuma T, Suzuki S, Usuda K, Handa M, Okaniwa G, Nakada T, Fujimura S, Matthay MA. Preservation of alveolar epithelial fluid transport mechanisms in rewarmed human lung after severe hypothermia. J Appl Physiol 80: 1681–1686, 1996 [DOI] [PubMed] [Google Scholar]
- 190. Sakuma T, Takahashi K, Ohya N, Nakada T, Matthay M. Effects of ATP-sensitive potassium channel opener on potassium transport and alveolar fluid clearance in the resected human lung. Pharmacol Toxicol 83: 16–22, 1998 [DOI] [PubMed] [Google Scholar]
- 191. Sakuma T, Tuchihara C, Ishigaki M, Osanai K, Nambu Y, Toga H, Takahashi K, Ohya N, Kurihara T, Matthay MA. Denopamine, a β1-adrenergic agonist, increases alveolar fluid clearance in ex vivo rat and guinea pig lungs. J Appl Physiol 90: 10–16, 2001 [DOI] [PubMed] [Google Scholar]
- 192. Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, Hutter D, Turini P, Hugli O, Cook S, Nicod P, Scherrer U. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 346: 1631–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 193. Sartori C, Duplain H, Lepori M, Egli M, Maggiorini M, Nicod P, Scherrer U. High altitude impairs nasal transepithelial sodium transport in HAPE-prone subjects. Eur Respir J 23: 916–920, 2004 [DOI] [PubMed] [Google Scholar]
- 194. Sartori C, Matthay MA. Alveolar epithelial fluid transport in acute lung injury: new insights. Eur Respir J 20: 1299–1313, 2002 [DOI] [PubMed] [Google Scholar]
- 195. Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol Lung Cell Mol Physiol 262: L647–L661, 1992 [DOI] [PubMed] [Google Scholar]
- 196. Schwiebert EM, Potter ED, Hwang TH, Woo JS, Ding C, Qiu W, Guggino WB, Levine MA, Guggino SE. cGMP stimulates sodium and chloride currents in rat tracheal airway epithelia. Am J Physiol Cell Physiol 272: C911–C922, 1997 [DOI] [PubMed] [Google Scholar]
- 197. Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: 23–45, 1999 [DOI] [PubMed] [Google Scholar]
- 198. Skou JC. Nobel Lecture. The identification of the sodium pump. Biosci Rep 18: 155–169, 1998 [DOI] [PubMed] [Google Scholar]
- 199. Sloniewsky D, Ridge K, Adir Y, Fries F, Briva A, Sznajder J, Sporn P. Leukotriene D4 activates alveolar epithelial Na, K-ATPase and increases alveolar fluid clearance. Am J Respir Crit Care Med 169: 407–412, 2004 [DOI] [PubMed] [Google Scholar]
- 200. Smith JJ, Welsh MJ. Fluid and electrolyte transport by cultured human airway epithelia. J Clin Invest 91: 1590–1597, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic variation of the β2-adrenergic receptor is associated with differences in lung fluid accumulation in humans. J Appl Physiol 102: 2172–2178, 2007 [DOI] [PubMed] [Google Scholar]
- 202. Solomon GM, Konstan MW, Wilschanski M, Billings J, Sermet I, Accurso F, Vermeulen F, Levin E, Hathorne H, Reeves G, Sabbatini G, Hill A, Mayer-Hamblett N, Ashlock M, Clancy JP, Rowe SM. An international randomized multicenter comparison of nasal potential difference techniques. Chest 138: 919–928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Song Y, Fukuda N, Bai C, Ma T, Matthay M, Verkman A. Role of aquaporins in the alveolar fluid clearance in neonatal and adult lung, and in oedema formation following acute lung injury: studies in transgenic aquaporin null mice. J Physiol 525: 771–779, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J Gen Physiol 117: 573–582, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Song Y, Namkung W, Nielson DW, Lee JW, Finkbeiner WE, Verkman AS. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 297: L1131–L1140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 128: 525–532, 2005 [DOI] [PubMed] [Google Scholar]
- 207. Staub NC. The interdependence of pulmonary structure and function. Anesthesiology 24: 831–854, 1963 [DOI] [PubMed] [Google Scholar]
- 208. Staub NC. Pulmonary edema. Physiol Rev 54: 678–811, 1974 [DOI] [PubMed] [Google Scholar]
- 209. Strautnieks SS, Thompson RJ, Gardiner RM, Chung E. A novel splice-site mutation in the γ subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat Genet 13: 248–250, 1996 [DOI] [PubMed] [Google Scholar]
- 210. Sugita M, Ferraro P, Dagenais A, Clermont ME, Barbry P, Michel RP, Berthiaume Y. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lungs. Am J Respir Crit Care Med 167: 1440–1450, 2003 [DOI] [PubMed] [Google Scholar]
- 211. Suzuki S, Noda M, Sugita M, Ono S, Koike K, Fujimura S. Impairment of transalveolar fluid transport and lung Na+-K+-ATPase function by hypoxia in rats. J Appl Physiol 87: 962–968, 1999 [DOI] [PubMed] [Google Scholar]
- 212. Suzuki S, Zuege D, Berthiaume Y. Sodium-independent modulation of Na+-K+-ATPase activity by β-adrenergic agonist in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 268: L983–L990, 1995 [DOI] [PubMed] [Google Scholar]
- 213. Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. J Biol Chem 280: 35751–35759, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 118: 223–236, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tchepichev S, Ueda J, Canessa C, Rossier BC, O'Brodovich H. Lung epithelial Na channel subunits are differentially regulated during development and by steroids. Am J Physiol Cell Physiol 269: C805–C812, 1995 [DOI] [PubMed] [Google Scholar]
- 217. Thome U, Chen L, Factor P, Dumasius V, Freeman B, Iasha Sznajder J, Matalon S. Na,K-ATPase gene transfer mitigates an oxidant-induced decrease of active sodium transport in rat fetal ATII cells. Am J Respir Cell Mol Biol 24: 245–252, 2001 [DOI] [PubMed] [Google Scholar]
- 218. Towne JE, Harrod KS, Krane CM, Menon AG. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. Am J Respir Cell Mol Biol 22: 34–44, 2000 [DOI] [PubMed] [Google Scholar]
- 219. Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-α inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem 276: 18657–18664, 2001 [DOI] [PubMed] [Google Scholar]
- 220. Traylor ZP, Yu ENZ, Davis IC. Respiratory syncytial virus induces airway insensitivity to β-agonists in BALB/c mice. Am J Physiol Lung Cell Mol Physiol 298: L437–L445, 2010 [DOI] [PubMed] [Google Scholar]
- 221. Van Why SK, Mann AS, Ardito T, Siegel NJ, Kashgarian M. Expression and molecular regulation of Na+-K+-ATPase after renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 267: F75–F85, 1994 [DOI] [PubMed] [Google Scholar]
- 222. Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol 87: 1301–1312, 1999 [DOI] [PubMed] [Google Scholar]
- 223. Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol 159: 324–330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol 278: L867–L879, 2000 [DOI] [PubMed] [Google Scholar]
- 225. Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats. Reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol 25: 554–561, 2001 [DOI] [PubMed] [Google Scholar]
- 226. Voilley N, Lingueglia E, Champigny G, Mattei MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci USA 91: 247–251, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Wang S, Publicover S, Gu Y. An oxygen-sensitive mechanism in regulation of epithelial sodium channel. Proc Natl Acad Sci USA 106: 2957–2962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ware LB. Modulation of alveolar fluid clearance by acute inflammation: the plot thickens. Am J Respir Crit Care Med 169: 332–333, 2004 [DOI] [PubMed] [Google Scholar]
- 229. Ware LB, Matthay MA. Acute pulmonary edema. N Engl J Med 353: 2788–2796, 2005 [DOI] [PubMed] [Google Scholar]
- 230. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 231. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 232. Waters CM, Ridge KM, Sunio G, Venetsanou K, Sznajder JI. Mechanical stretching of alveolar epithelial cells increases Na+-K+-ATPase activity. J Appl Physiol 87: 715–721, 1999 [DOI] [PubMed] [Google Scholar]
- 233. Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol Rev 53: 419–495, 1973 [DOI] [PubMed] [Google Scholar]
- 234. Wigglesworth JS, Desai R, Hislop AA. Fetal lung growth in congenital laryngeal atresia. Pediatr Pathol 7: 515–525, 1987 [DOI] [PubMed] [Google Scholar]
- 235. Willis BC, Kim KJ, Li X, Liebler J, Crandall ED, Borok Z. Modulation of ion conductance and active transport by TGF-β1 in alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 285: L1192–L1200, 2003 [DOI] [PubMed] [Google Scholar]
- 236. Wilschanski M, Yahav Y, Yaacov Y, Blau H, Bentur L, Rivlin J, Aviram M, Bdolah-Abram T, Bebok Z, Shushi L, Kerem B, Kerem E. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med 349: 1433–1441, 2003 [DOI] [PubMed] [Google Scholar]
- 237. Wodopia R, Ko HS, Billian J, Wiesner R, Bartsch P, Mairbaurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1110–L1119, 2000 [DOI] [PubMed] [Google Scholar]
- 238. Yager D, Butler JP, Bastacky J, Israel E, Smith G, Drazen JM. Amplification of airway constriction due to liquid filling of airway interstices. J Appl Physiol 66: 2873–2884, 1989 [DOI] [PubMed] [Google Scholar]
- 239. Zemans R, Matthay M. Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit Care 8: 469–477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zhou Y, Dong Q, Louahed J, Dragwa C, Savio D, Huang M, Weiss C, Tomer Y, McLane MP, Nicolaides NC, Levitt RC. Characterization of a calcium-activated chloride channel as a shared target of Th2 cytokine pathways and its potential involvement in asthma. Am J Respir Cell Mol Biol 25: 486–491, 2001 [DOI] [PubMed] [Google Scholar]