Abstract
Mucosal surfaces are colonized by large communities of commensal bacteria and represent the primary site of entry for pathogenic agents. To prevent microbial intrusion, mucosal B cells release large amounts of immunoglobulin (Ig) molecules through multiple follicular and extrafollicular pathways. IgA is the most abundant antibody isotype in mucosal secretions and owes its success in frontline immunity to its ability to undergo transcytosis across epithelial cells. In addition to translocating IgA onto the mucosal surface, epithelial cells educate the mucosal immune system as to the composition of the local microbiota and instruct B cells to initiate IgA responses that generate immune protection while preserving immune homeostasis. Here we review recent advances in our understanding of the cellular interactions and signaling pathways governing IgA production at mucosal surfaces and discuss new findings on the regulation and function of mucosal IgD, the most enigmatic isotype of our mucosal antibody repertoire.
Keywords: mucosal immunity, B cells, IgA, IgD, class switching
INTRODUCTION
Mucosal membranes provide a dynamic interface that separates the sterile milieu of our body from the external environment. A key component of this interface is the mucosal epithelium, which blocks invasion by pathogenic and commensal bacteria by forming multiple layers of physical and immune protection (1). Epithelial protection is particularly sophisticated in the intestine, which contains large communities of commensal bacteria that process otherwise indigestible polysaccharides, synthesize essential vitamins and isoprenoids, stimulate the maturation of the immune system, and form an ecological niche that prevents the growth of pathogenic species (2). Conversely, the intestine provides commensals with a stable habitat rich in energy derived from ingested food.
A fine line exists between the homeostatic balance required to maintain commensals and the destructive response required to repel pathogens (3). Such a balance involves an intimate dialogue between prokaryotic and eukaryotic cells of our body that ultimately generates finely tuned signaling programs that ensure a state of hyporesponsiveness against commensals and a state of active readiness against pathogens (4). In this dialogue, our epithelial cells function as interpreters that continuously translate prokaryotic messages to educate the mucosal immune system as to the composition of the local microbiota (5).
Although needed by the host, commensals represent a potential threat of infection and unrestrained inflammation (6). A major defensive mechanism that excludes commensals from the mucosal surface involves immunoglobulin A (IgA) (7). This antibody class works together with nonspecific protective factors such as mucus to block microbial adhesion to epithelial cells without causing a tissue-damaging inflammatory reaction (8). By doing so, IgA establishes a state of armed peace in the homeostatic interaction between the host and noninvasive commensal bacteria. When invasive pathogenic bacteria trespass the epithelial border, a state of open war breaks out and IgA receives help from IgG to repel the invaders. In this life-threatening situation, IgG provides a second line of defense that controls microbial dissemination by eliciting a robust inflammatory reaction (9).
Remarkably, different mucosal districts are characterized by distinct antibody signatures. The intestinal tract contains IgA and some IgM but virtually no IgG, whereas the respiratory and urogenital tracts contain equivalent amounts of IgA and IgG in addition to some IgM (10). In humans, the intestinal and urogenital tracts produce large amounts of an IgA subclass known as IgA2, whereas the respiratory tract contains IgD, the most enigmatic class of our mucosal antibody repertoire (10). This review discusses recent advances in our understanding of the regulation and function of mucosal IgA and IgD.
MUCOSA-ASSOCIATED LYMPHOID TISSUES
General Features
Mucosal surfaces comprise various lymphoid structures collectively referred to as mucosa-associated lymphoid tissue (MALT) (8). This secondary lymphoid organ can be further divided in functionally connected subregions, including the gut-associated lymphoid tissue (GALT), nasopharynx-associated lymphoid tissue (NALT), and bronchus-associated lymphoid tissue (BALT) (11–13). In the MALT, functionally distinct inductive and effector sites can be recognized. Intestinal Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) exemplify mucosal inductive sites, which contain T and B cells undergoing clonal expansion and differentiation upon activation by antigen (14). The intestinal lamina propria (LP) exemplifies mucosal effector sites, whose main function is to recruit effector T and B cells emerging from inductive sites (15).
Antibodies released by effector B cells, including plasma cells, provide the first line of protection at mucosal surfaces. In the intestinal tract and other mucosal districts, the vast majority of mucosal plasma cells secrete dimeric or oligomeric IgA and to a lesser extent pentameric IgM, both of which interact with the polymeric Ig receptor (pIgR) expressed on the basolateral surface of epithelial cells through a joining ( J) chain (16). This interaction is followed by endocytosis of IgA and IgM, pro-teolytic cleavage of pIgR, and basolateral-to-apical transcytosis of secretory IgA (SIgA) and SIgM, which comprise a pIg-derived secretory component (SC) providing mucophilic properties to the SIg complex (16). As for IgG, this antibody class gains access to respiratory and urogenital secretions through both nonspecific and specific mechanisms, the latter involving interaction with an antibody transporter known as neonatal Fc receptor (nFcR) (10, 17). The mechanism by which IgD enters respiratory secretions remains unknown (10, 18, 19).
GALT and NALT
Although equipped with a general architecture resembling that of systemic lymphoid organs, the MALT has several unique features, including absence of afferent lymphatics, which requires sampling of antigen directly from the epithelial surface (8). In the GALT, antigen sampling involves specialized microfold (M) cells lodged in the follicle-associated epithelium (20). M cells filter bacteria through a complex glycocalix and sample some of these bacteria via poorly defined receptors (21). IgA receptors allow M cells to sample IgA-coated commensals (22), whereas glycoprotein 2 receptor samples IgA-free commensals as well as pathogens (23). Sampled antigen is eventually transferred to dendritic cells (DCs), which occupy large basolateral invaginations of M cells (21). Alternatively, DCs directly sample antigen from the lumen through transepithelial projections (24). These antigen-loaded DCs migrate to the perifollicular area of PPs to present antigen to CD4+ T cells and initiate antigen-specific T and B cell responses (8).
The NALT has antigen-sampling strategies similar to those present in the GALT, including M cells (11). In humans, the NALT consists of a set of lymphoid aggregates known as Waldeyer’s ring that occupy strategic areas of the oropharynx and nasopharynx and include pharyngeal tonsils (or adenoids) as well as tubal, palatine, and lingual tonsils (10). NALT organogenesis markedly differs from GALT organogenesis both in terms of kinetics and cytokine requirements (11, 25). For example, PPs begin their development during embryonic life and require signals from interleukin-7 (IL-7) and lymphotoxin receptors, whereas tonsils initiate their development shortly after birth and require signals from environmental antigens (11, 25). Another important difference between GALT and NALT is that NALT-targeted immunization preferentially induces antigen-specific immunity in the respiratory and genital tracts, whereas GALT-targeted immunization mainly elicits protective responses in the gastrointestinal tract (11, 26).
NATURE AND FUNCTION OF MUCOSAL ANTIBODIES
Genesis and Function of Antibody Diversity
Diversification is essential for the mucosal immune system to mount protective responses. Antibodies diversify through three major DNA-modifying processes known as V(D)J recombination, class switch recombination (CSR), and somatic hypermutation (SHM). Bone marrow B cell precursors generate antigen recognition diversity through V(D)J recombination, an antigen-independent process mediated by recombination-activating gene (RAG) endonucleases that assemble antigen-binding Ig variable regions from individual V (variable), D (diversity), and J (joining) gene segments (27). Mature B cells emerging from the bone marrow colonize peripheral lymphoid organs, where they undergo a second wave of Ig gene remodeling through SHM and CSR, two antigen-dependent processes that require the DNA-editing enzyme activation-induced cytidine deaminase (AID) and mediate antibody affinity maturation and antibody class (or isotype) switching, respectively (28, 29).
SHM introduces point mutations within V(D)J exons, thereby providing the structural correlate for selection of high-affinity Ig mutants by antigen, whereas CSR replaces constant μ (Cμ ) and Cδ exons encoding IgM and IgD with Cγ, Cα, or Cε exons encoding IgG, IgA, or IgE, thereby providing antibodies with novel effector functions without changing their antigen-binding specificity (30, 31). In humans, a noncanonical form of CSR from Cμ to Cδ also exists and generates B cells specialized in IgD production (18, 32). As already discussed, IgA mediates its effector functions by interacting with pIgR on the basolateral surface of mucosal epithelial cells (16). In addition, IgA binds to a high-affinity IgA receptor known as FcαRI (or CD89), which is expressed on granulocytes, monocytes, macrophages, DCs, natural killer (NK) cells, and mast cells (33). These innate immune cells generate activating or regulatory signals upon FcαRI receptor engagement by IgA, depending on the degree of IgA oligomerization (34).
Additional IgA receptors include transferring receptor (CD71), Fcα/μR (which also binds IgM), and asyaloglycoprotein receptor, but their functions remain poorly understood (33). As for IgG and IgE, these antibody classes initiate multiple innate and adaptive immune responses by activating high- or low-affinity FcγR and FcεR on various innate immune cells (35). In addition, IgG undergoes bidirectional transcytosis across epithelial cells by binding to nFcR (17, 36). In IgE-mediated allergic disorders, IgE undergoes transcytoses across epithelial cells by utilizing a low-affinity IgE receptor known as FcεRII (or CD23) (37–39). The receptors mediating IgD effector functions and IgD transcytosis remain elusive. Although expressing abundant J chain, IgD-secreting plasma cells seem to release monomeric IgD only, which does not bind to pIgR (10). Considering that the hinge region of IgD has a heparin ligand–like site, heparin and other heparan sulphate proteoglycans may play an important role in at least some IgD effector functions (18).
Antibody Composition of Mucosal Sites
Differences in epithelial cell expression of specific antibody transporters and plasma cell–recruiting chemokines contribute to determining the antibody class composition of a given mucosal site. In general, IgA is the most abundant antibody isotype in mucosal secretions. Yet, IgA is somewhat less abundant than IgG in the urine, bile, and genital and bronchoalveolar secretions. IgD can be detected in nasal, salivary, lacrimal, and bronchoalveolar secretions (10, 18, 19), whereas IgE is measurable in nasal, bronchoalveolar, and intestinal secretions, at least when allergy is present (37).
Unlike mouse B cells, human B cells produce two subclasses, IgA1 and IgA2, which have a similar receptor-binding profile but different topography from each other (40). IgA1 is present in both systemic and mucosal districts, whereas IgA2 is mostly present in mucosal districts colonized by a large microbiota, including the distal intestinal tract and the urogenital tract (40). This circumstance could reflect the fact that IgA2 is more resistant than IgA1 to degradation by bacterial proteases because IgA2 has a shorter hinge region than IgA1 does (41, 42).
Function of Mucosal IgA
Mucosal IgA comprises antibodies that recognize antigen with high- and low-affinity binding modes (8). In general, high-affinity IgA neutralizes microbial toxins and invasive pathogens, whereas low-affinity IgA confines commensals in the intestinal lumen. Yet this distinction is not absolute, as growing evidence indicates an important role for high-affinity IgA in the control and regulation of the commensal microbiota (43). Conversely, additional evidence shows that low-affinity IgA protects against some pathogens (44, 45). High-affinity IgA is thought to emerge from follicular B cells stimulated via T cell–dependent (TD) pathways, whereas low-affinity IgA likely emerges from extrafollicular B cells stimulated via T cell–independent (TI) pathways (14). However, this view is rapidly changing. Indeed, recent findings document the existence of TI pathways of IgA production in follicular B cells (46).
IgA also promotes the maintenance of appropriate bacterial communities in specific intestinal segments (47). Indeed, IgA modulates the gene-expression profile of commensal bacteria, selecting species with less inflammatory activity on the host’s tissues (48). By preventing overstimulation of mucosal B cells, this IgA-mediated selection process may impede bacteria-induced expansion of autoreactive, inflammatory, proallergic, and neoplastic B cell clones (7, 14, 40). Furthermore, IgA facilitates the establishment of specific symbiotic relationships not only in the mucosal lumen, but also in PPs (49). As discussed above, IgA also facilitates sampling of luminal antigens by binding to poorly defined receptors on M cells (22, 50). Moreover, IgA neutralizes inflammatory microbial products inside epithelial cells (51). Finally, if bacteria trespass the epithelial barrier, IgA transports these bacteria back into the lumen via pIgR or promotes their phagocytosis through FcαRI (34, 52).
The in vivo relevance of IgA can be best seen in patients with SIgAD, common variable immunodeficiency (CVID), and hyper-IgM (HIGM) syndrome. In these primary immunodeficiencies, impaired IgA production is associated with recurrent respiratory and gastrointestinal infections as well as allergy and autoimmunity (53, 54). Some IgA-deficient patients also develop intestinal inflammation and small intestinal nodular lesions, possibly resulting from excessive B cell stimulation by aberrantly expanded commensals (14, 55). Yet SIgAD, CVID, and even some cases of HIGM syndrome can be asymptomatic or mildly symptomatic, possibly because of compensatory increases of unaffected antibodies such as IgM and IgD (10, 18, 53, 54).
Function of Mucosal IgD
The function of IgD has puzzled immunologists over the past several decades. Originally thought to be a recently evolved isotype, IgD is now recognized to be an ancestral molecule that has been conserved throughout evolution to complement the functions of IgM (18, 56). IgD would afford protection to the respiratory mucosa by binding to pathogenic bacteria such as Moraxella catarrhalis and Haemophilus influenzae as well as to their virulence factors (32, 57). In addition to crossing epithelial cells, IgD binds to circulating basophils, monocytes, and neutrophils as well as to mucosal mast cells through unknown receptors (18, 58).
Consistent with recently published data showing the important role of basophils in T helper type 2 (Th2) cell responses and antibody production (59–63), IgD cross-linking induces basophil release of B cell–activating cytokines such as interleukin (IL)-4 and IL-13, which in turn facilitate IgM as well as IgG and IgA production (32). Furthermore, IgD cross-linking triggers basophil release of antimicrobial peptides such as cathelicidin, inflammatory cytokines such as IL-1β and TNF, and various chemokines such as CXCL10 (32, 58). Therefore, IgD may contribute to mucosal immunity not only by neutralizing pathogens and excluding commensals, but also by recruiting basophils as well as other immune cells with antimicrobial and immune-augmenting functions (18).
PATHWAYS INDUCING MUCOSAL IgA RESPONSES
T Cell–Dependent Pathways
Most antigens initiate mucosal IgA responses through a TD reaction that takes place in mucosal lymphoid follicles (8, 15), such as intestinal PPs and MLNs (Figure 1a,b). These organized structures comprise a germinal center that fosters antibody diversification and affinity maturation, including SHM and CSR, through antigen-specific cognate interactions between B cells that express the CD40 receptor and CD4+ Th cells expressing CD40 ligand (CD40L) (13, 40). Together with cytokine receptors and B cell antigen receptor (BCR), CD40 is critical for the induction of AID expression and the initiation of SHM and CSR (40, 64). Engagement of CD40 by CD40L leads to (a) the recruitment of TNF receptor-associated factor (TRAF) adaptor proteins to the cytoplasmic tail of CD40 (65), followed by (b) activation of an NF-κB inhibitory protein (IκB) kinase (IKK) complex, which triggers (c) phosphorylation and degradation of IκB that retains NF-κB in an inactive state (66, 67). The resulting IκB-free NF-κB proteins translocate from the cytoplasm to the nucleus to initiate transcription of the AICDA gene promoter that encodes AID (40, 68). In contrast, NF-κB is not required for the activation of the intronic α (Iα) promoter upstream of the Cα gene and therefore has little or no role in germ-line Cα gene transcription (40, 69). This circumstance may explain why additional signals from cytokines such as transforming growth factor-β (TGF-β) are needed to elicit IgA CSR, at least in mice (13).
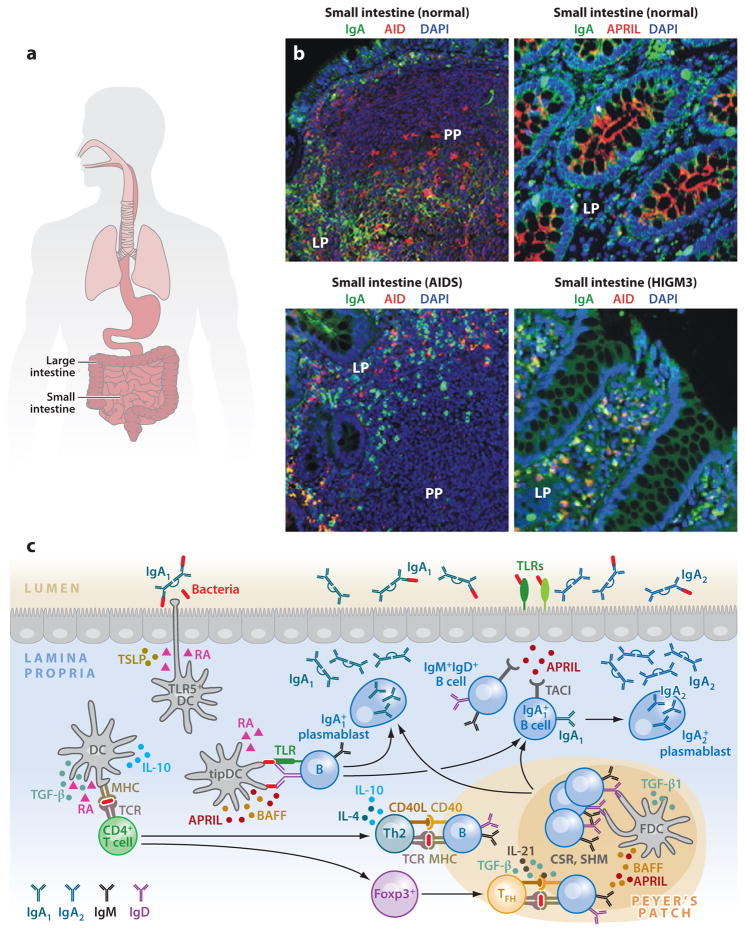
Figure 1.
IgA responses in the intestinal mucosa. (a) Scheme of human MALT, including intestinal mucosa. (b) Immunofluorescence analysis of gut mucosa from healthy, HIGM3, and AIDS donors stained for IgA ( green), AID or APRIL (red ), and nuclei (DAPI staining, blue). Left panels show Peyer’s patches (PPs) and lamina propria (LP); right panels show only LP. Original magnification, ×20. (c) Scheme of mucosal IgA responses. Antigen-sampling DCs receive conditioning signals from TLR-activated intestinal epithelial cells (IECs) via thymic stromal lymphopoietin (TSLP) and retinoic acid (RA). Various DC subsets releasing TGF-β, IL-10, RA, and nitric oxide initiate IgA responses in PPs by inducing Th2, Treg, and Treg-derived T follicular helper (TFH) cells that activate follicular B cells via CD40L, TGF-β, IL-4, IL-10, and IL-21. In addition, DCs activate some B cells in the LP via BAFF, APRIL, and RA. These molecules are also used by TLR-activated IECs to induce local IgA production, including sequential switching from IgA1 to IgA2, as well as plasma cell differentiation and survival. (Additional abbreviations used in figure: AID, activation-induced cytidine deaminase; APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor; CSR, class switch recombination; DC, dendritic cell; HIGM3, hyper-IgM 3; MALT, mucosa-associated lymphoid tissue; SHM, somatic hypermutation; TGF-β, transforming growth factor-β; TLR, Toll-like receptor.)
T Cell–Independent Pathways
The conventional TD pathway requires 5 to 7 days to initiate protective antibody responses in systemic lymphoid tissues (70, 71). Such kinetics may not be appropriate to afford optimal mucosal protection because mucosal surfaces are constantly exposed to dietary and bacterial antigens. In addition, the TD pathway is often associated with an inflammatory reaction that could disrupt the mucosal epithelial barrier. To compensate for these limitations, the intestinal mucosa has developed a faster TI pathway that generates IgA in response to highly conserved microbial signatures recognized by Toll-like receptors (TLRs) (14, 40), a family of germ-line gene-encoded antigen receptors involved in the activation of both innate and adaptive arms of the immune system (72, 73). In mice, TI IgA production involves B-1 cells from the peritoneal cavity and intestinal LP as well as conventional B-2 cells from isolated lymphoid follicles (ILFs) (13). These B cells release low-affinity IgA (and IgM) in the absence of help from CD4+ T cells via CD40L (74–76). The human counterpart of mouse B-1 cells remains unknown.
TLRs facilitate TI IgA responses either by activating B cells directly or by inducing release of the B cell–activating factor of the TNF family (BAFF, also known as BLyS) and its homolog a proliferation-inducing ligand (APRIL) from innate immune cells (7, 40). Engagement of TLRs by microbial ligands triggers activation of NF-κB (72, 73). This activation requires recruitment of the adaptor protein MyD88 to a cytoplasmic Toll-interleukin-1 receptor (TIR) domain that subsequently elicits formation of an IKK-activating signaling complex composed of IL-1 receptor–associated kinase (IRAK)1, IRAK4, TRAF6, and TGF-β-activated kinase (TAK)-1 (73).
In addition to inducing AID expression in B cells (77, 78), TLR signaling via NF-κB elicits BAFF and APRIL expression in DCs, monocytes, macrophages, granulocytes, and epithelial cells, including intestinal epithelial cells (IECs) (79–84). In the presence of appropriate cytokines, BAFF and APRIL initiate Cα germ-line expression and Cμ -to-Cα CSR by engaging a CD40-related receptor known as transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI) (84–88). One important property of this receptor is to establish a close cooperation with B cell–intrinsic signals from TLRs (85).
IgA RESPONSES IN MUCOSAL FOLLICLES
Role of CD4+ T Cells
Growing evidence indicates that TD IgA responses involve a heterogeneous population of CD4+ T cells (Figure 1c), including T follicular helper (TFH) cells, Th2 cells, and T regulatory (Treg) cells (13, 40). These CD4+ T cell subsets express CD40L and release large amounts of IgA-inducing cytokines (89–92). But how do IgA-inducing TFH, Th2, and Treg cells differentiate from naive CD4+ T cells? A key role is played by DCs strategically positioned in the subepithelial area (21). In addition to receiving antigen from M cells, these DCs directly sample antigen in the intestinal lumen by emanating transepithelial projections through a process controlled by signals from TLRs in IECs (24, 93). Overall, M cells and DCs capture only small amounts of bacteria because intestinal IgA responses have a much higher induction threshold (108–109 bacteria) than do systemic IgG responses (43).
Antigen sampling through transepithelial projections involves nonmigratory gut-resident DCs expressing the fractalkine receptor CX3CR (94) developmentally distinct from migratory DCs expressing the αEβ7 integrin CD103 (95, 96). As they sample antigen in the lumen, DCs receive powerful conditioning signals from IECs, thereby becoming primed for the induction of noninflammatory CD4+ T cells with an IgA-inducing function (7, 92, 97). Antigen-loaded DCs migrate from subepithelial to perifollicular areas, where they induce Treg and Th2 cell differentiation (95, 96, 98, 99). Although Th2 cells may elicit CD40-dependent IgA CSR and production by releasing IL-4, IL-5, IL-6, and IL-10 (89, 92, 100, 101), Treg cells would do so by releasing TGF-β (90, 91). Some Treg cells would further differentiate into TFH cells, which induce IgA CSR and production via IL-21 and TGF-β1 (91, 102). In general, CD4+ T cells that provide help to B cells in PPs are clearly functionally different from their counterparts that initiate IgG responses in systemic lymphoid follicles, which may explain why intestinal IgA responses have a slower onset than systemic IgG responses (>14 days versus 5–7 days) (43).
IgA-producing B cells generated via TD pathways further differentiate into IgA-secreting plasma blasts in the intestinal LP (7). Here, Th17 cells might facilitate transepithelial release of SIgA through an IL-17-dependent mechanism involving upregulation of pIgR expression (103), although thus far this mechanism has only been described in the respiratory mucosa. Despite requiring CD4+ T cells for germinal center formation, PPs are capable of producing IgA independently of canonical T-B cell interactions (104). Indeed, PPs retain TD IgA responses in the absence of BCR, which is instead required by systemic follicles to initiate TD IgG responses (104). Thus, PPs may generate IgA through an alternative TD pathway that involves activation of B cells by microbial TLR ligands (105). Supporting this model, lack of MyD88 impairs IgA production in intestinal PPs (46, 106).
Role of Conventional Dendritic Cells
Intestinal DCs maintain homeostasis not only by inducing noninflammatory IgA responses, but also by dampening inflammatory Th1 and Th17 cell responses (7, 13, 99, 107–109). Strikingly, both processes implicate DC-induced differentiation of antigen-specific Th2, Treg, and TFH cells (90–92, 98). Intestinal DCs are particularly skilled in eliciting these homeostatic CD4+ T cell responses (Figure 1c) because they receive conditioning signals from IECs (97, 107). One of these signals is provided by thymic stromal lymphopoietin (TSLP), an IL-7-like epithelial cytokine that shifts the Th1/Th2 balance toward Th2 polarization by attenuating DC production of IL-12 but not of IL-10 (92, 110).
Importantly, IECs release TSLP in response to TLR-mediated signals from bacteria (111). Accordingly, genetic disruption of TLR signaling via NF-κB reduces TSLP expression by IECs and augments IL-12 production by DCs (112). In addition to TSLP, IECs release TGF-β and retinoic acid, which stimulate the development of CD103+ DCs, at least in vitro (113). These DCs promote the formation of Treg cells via TGF-β and retinoic acid and suppress the development of inflammatory Th1 and Th17 cells (99, 114, 115). As discussed earlier, Treg cells emerging from this pathway could induce IgA CSR and production through TGF-β (90, 91).
It must be noted that thus far no DC subset has been formally assigned to the induction of IgA responses in PPs. One notable exception is a TNF-α-inducible nitric oxide synthase–producing DC (tipDC) subset that enhances IgA CSR and production by upregulating the expression of TGF-β receptor on B cells from PPs via nitric oxide (106). The ontogenetic and functional relationships of tipDCs with other intestinal DC subsets remain unclear.
Role of Follicular Dendritic Cells
Germinal centers from PPs and MLNs contain a meshwork of antigen-trapping follicular dendritic cells (FDCs) ontogenetically different from DCs. Indeed, FDCs originate from nonhematopoietic precursors that may include mesenchymal cells (116). One of the main functions of FDCs is to facilitate the positive selection of high-affinity follicular B cells by antigen (117). During this process, antigen arrays on the surface of FDCs activate B cells by extensively cross-linking BCR (118). As shown by recent studies (46), FDCs from PPs and MLNs efficiently induce IgA CSR and production (Figure 1c). This response occurs in a TI manner and involves TLR-mediated sensing of bacteria by FDCs, followed by FDC release of TGF-β, BAFF, and APRIL (46).
Role of Lymphoid Tissue Inducer Cells
Together with PPs and MLNs, IFLs represent another important site for IgA induction (14). These lymphoid structures are scattered throughout the intestine and consist of solitary B cell clusters built on a scaffold of stromal cells with interspersed CD4+ T cells and abundant perifollicular DCs (119). Unlike PPs, ILFs appear after birth in relationship to bacteria colonization (119). Recent findings indicate that TLR signals from commensal bacteria initiate a crosstalk centered on RORγt+ lymphoid tissue inducer (LTi) cells (74). These cells recruit DCs and B cells through various chemokines and stimulate release of active TGF-β, BAFF, and APRIL by activating DCs and stromal cells via a signaling loop involving lymphotoxin (74). Together with microbial TLR ligands, TGF-β, BAFF, and APRIL induce IgA CSR in the absence of help from CD4+ T cells (74). Of note, LTi cells also release the IEC-activating cytokine IL-22 and the B cell–attracting chemokine CXCL13, which may enhance IgA production in addition to promoting homeostasis (120).
Homing of IgA-Expressing B Cells
IgA-producing B cells generated in PPs and MLNs upregulate the expression of critical gut-homing receptors such as the α4β7 integrin as well as CCR9 and CCR10 chemokine receptors upon being primed by retinoic acid that is released by local DCs (121). These retinoic acid–primed B cells enter the general circulation via the thoracic duct and thereafter gain access to the LP by binding mucosal addressin-cell adhesion molecule-1 (MadCAM-1) expressed on LP-based high endothelial venules through α4β7 (122). Retinoic acid–primed B cells further respond to CCL25 and CCL29, two IEC chemokines that bind to CCR9 and CCR10, respectively (122). Once in the LP, IgA-expressing B cells terminally differentiate into IgA-secreting plasma cells, possibly through the help of local signals from IECs, DCs, and macrophages (40). Similar cells would also provide robust activation and survival signals to B cells emerging from PPs and MLNs, which may explain why intestinal IgA responses do not always correlate with a florid germinal center reaction in PPs and yet show a sustained half-life (>16 weeks) (43).
IgA RESPONSES IN MUCOSAL EXTRAFOLLICULAR AREAS
IgA CSR in the Intestinal Lamina Propria
The diffuse tissue of the LP is an additional site for IgA production and diversification (Figure 1c), although it is less important than PPs and MLNs (123). Consistent with this possibility, genetically engineered mice lacking PPs, MLNs, and even ILFs retain some antigen-specific IgA plasma cells, which are mostly located in the LP (124, 125). In both mice and humans, a fraction of B cells from the intestinal LP contain molecular hallmarks of ongoing IgA CSR, including AID, H2AX protein (a nuclear protein associated with double-strand DNA breaks generated by AID within S regions), excised Sα -Sμ switch circles, and switch circle Iα -Cμ transcripts (111, 126–128). Of note, AID and IgA remain detectable in LP B cells from mice and humans lacking CD4+ T cells or CD40L (74, 111, 129). In general, LP B cells are less activated and more scattered than PP B cells, and therefore LP IgA CSR can pass unrecognized unless highly sensitive and accurate methodologies are used (126, 130, 131). In this regard, a green fluorescent protein–AID reporter mouse model has proven very helpful (129). The mechanisms underlying IgA CSR in the LP remain poorly understood but likely involve DCs and IECs (111, 132).
Role of DCs
Abundant evidence demonstrates that DCs can initiate TI CSR and antibody production by activating B cells through antigen and cytokines, including BAFF and APRIL (84, 133–138). In the intestine, antigen-sampling CX3CR1+ DCs or CD103+ DCs may activate LP B cells through similar mechanisms (Figure 1c). A LP DC subset with clearer B cell–stimulating function is that of tipDCs (106). These DCs initiate TI IgA production by releasing BAFF and APRIL through a mechanism involving TLR-induced iNOS-mediated nitric oxide production (Figure 1c). In the LP, another DC subset with B cell–licensing functions is that of CD11chiCD11bhi DCs (139). These DCs induce TI IgA production upon sensing bacteria through TLR5, a process that elicits release of retinoic acid and IL-6 (Figure 1c).
Role of Intestinal Epithelial Cells
IECs and respiratory epithelial cells can deliver IgA-inducing signals to LP B cells by releasing BAFF, APRIL, and IL-10 in response to TLR signals (79, 111, 127, 140). Similar epithelial cells can also amplify DC production of BAFF, APRIL, and IL-10 by stimulating DCs through TSLP (79, 111). In humans, APRIL is particularly effective at inducing IgA2, an IgA subclass particularly abundant in the distal intestine (111). In addition to triggering direct IgM-to-IgA1 CSR (111), APRIL elicits sequential IgA1-to-IgA2 CSR in the LP of the distal intestine (Figure 1c). This process would allow B cells arriving from PPs to acquire an IgA2 subclass more resistant than IgA1 to degradation by bacterial proteases (42).
IgD RESPONSES IN THE RESPIRATORY MUCOSA
Geography of IgD Production
IgD constitutes a significant fraction of the antibodies produced in the upper segments of the human respiratory and digestive tracts (Figure 2a). The mucosal IgD class originates predominantly from IgD+IgM− B cells bearing morphologic and immunophenotypic features of plasmablasts (32). Indeed, IgD+IgM− B cells display canonical plasma cell traits such as an eccentric nucleus, a large basophilic cytoplasm filled with IgD (Figure 2b), and J chain as well as IgD-secreting activity, in addition to features typical of mature B cells such as expression of surface IgD and CD19 (32, 141–143). Of note, IgD+IgM− plasmablasts originate from a process of Cμ -to-Cδ CSR that leads to the loss of IgM expression (142). This process takes place in the aerodigestive mucosa because this site contains various molecular hallmarks of ongoing Cμ -to-Cδ CSR (32). In general, the respiratory mucosa expresses chemokines and vascular adhesion molecules capable of promoting the recruitment of IgD+IgM− plasmablasts from the periphery (144). In this regard, the peripheral blood contains some IgD+IgM− plasmablasts, which may be in transit to reach distant mucosal effector sites (32, 145). IgD+IgM− plasmablasts are rarely found in the GALT, probably because these B cells express little or no gut homing receptors such as α4β7 and CCR9 (144).
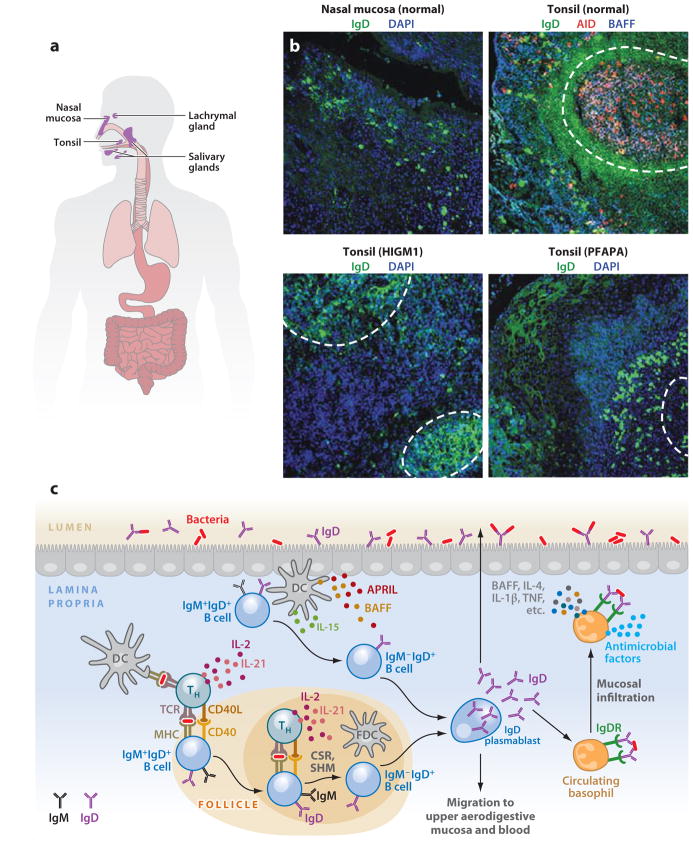
Figure 2.
IgD responses in the aerodigestive mucosa. (a) Scheme of human NALT, including tonsillar mucosa. (b) Immunofluorescence analysis of nasal and tonsillar mucosal surfaces from healthy, HIGM1, and PFAPA (periodic fever-aphthous stomatitis-pharyngitis-cervical adenitis) donors stained for IgD ( green), AID (red ), and BAFF or nuclei (DAPI staining, blue). Dashed lines demarcate follicles. Original magnification, ×10. (c) Scheme of mucosal IgD responses. Antigen-sampling DCs initiate IgD CSR by activating follicular or extrafollicular B cells through T cell–dependent (CD40L, IL-2, IL-15, IL-21) or T cell–independent (BAFF, APRIL, IL-15, IL-21) pathways, respectively. The resulting plasmablasts secrete IgD reactive against respiratory bacteria that exert protective functions either locally or systemically by interacting with an elusive IgD receptor (IgDR) on circulating basophils. In the presence of IgD-binding antigens, basophils migrate to systemic or mucosal lymphoid tissues, where they enhance immunity by releasing antimicrobial factors as well as B cell–stimulating and proinflammatory mediators such as BAFF, IL-4, IL-1β, and TNF. As compared to tonsil tissues of healthy subjects, there are decreased (and yet detectable) numbers of IgD class switched (IgD+IgM−) plasmablasts in follicular and extrafollicular areas in tonsils of patients with Hyper-IgM syndrome type 1 (HIGM1) caused by loss-of-function mutations in the CD40L gene. Increased numbers of IgD class switched (IgD+IgM−) plasmablasts are found in tonsils of a patient with PFAPA syndrome, with increased levels of IgD in tonsillar epithelium. (Additional abbreviations used in figure: APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor; CSR, class switch recombination; NALT, nasopharynx-associated lymphoid tissue; SHM, somatic hypermutation; TNF, tumor necrosis factor.)
Regulation of IgD Production
In humans and other higher mammals such as cows, a rudimentary S-like intronic DNA region known as σδ is present upstream of the Cδ exon (18). Like canonical S regions, σδ contains guanosine-cytosine repeats and serves as an acceptor DNA region for donor Sμ to mediate nonhomologous Cμ -to-Cδ CSR (18). Alternatively, virtually identical Iμ and Σμ intronic DNA regions located 5′ of Cμ and Cδ exons, respectively, could mediate homologous Cμ -to-Cδ CSR (18). Sμ -to-σδ CSR requires AID because HIGM2 patients with AID deficiency are completely devoid of IgD+IgM− plasmablasts (32). In addition, naive B cells from HIGM2 patients are unable to undergo Sμ -to-sδ CSR when stimulated with appropriate stimuli in vitro (32). HIGM1 and HIGM3 patients with deleterious substitutions of CD40L and CD40, respectively, and CVID patients with deleterious substitutions of TACI have a reduced and yet detectable fraction of IgD+IgM− plasmablasts in mucosal sites (32), suggesting that IgD CSR and production proceed through both TD and TI pathways (Figure 2c). Consistent with this possibility, CD40L, BAFF, or APRIL can induce IgD CSR and production when combined with IL-15 plus IL-21 or IL-2 plus IL-21 (32).
IgD+IgM− plasmablasts are biased toward the use of Ig light chain, harbor hypermutated V(D)J genes, and release polyreactive as well as monoreactive IgD (32, 142, 143). Secreted IgD would exert its protective function not only by binding to antigen, but also by interacting with innate immune cells, including basophils (18, 32). By arming basophils with IgD receptors highly reactive against respiratory bacteria, mucosal IgD+IgM− plasmablasts may educate our immune system as to the antigenic composition of the upper respiratory tract (18). Upon sensing respiratory antigen, IgD-activated basophils would initiate or enhance innate and adaptive immune responses both systemically and at mucosal sites of entry (18). This possibility is consistent with recent evidence showing that activated basophils can migrate to secondary lymphoid organs to initiate Th2 and B cell responses (59, 61, 63, 146).
COMPLEXITY OF MUCOSAL HUMORAL IMMUNITY
Despite a wealth of sensing and effector mechanisms capable of triggering inflammation in response to microbial sensing and intrusion, our mucosal immune system establishes homeostatic conditions based on a fine discrimination between commensals and pathogens (3). Epithelial cells play a key role in this process by sensing bacteria through a complex arsenal of pattern-recognition receptors, including TLRs (1). These innate antigen receptors educate the immune system as to the composition of the local microbiota and thereafter instruct the generation of effector and regulatory lymphocytes whose main function is to dampen inflammation and elicit massive IgA production (5).
A key aspect of this response relates to the intertwined nature of the signaling networks involved in the induction of mucosal IgA. In these networks, the adaptor protein MyD88 seems to form a critical hub because its deletion leads to a profound impairment of intestinal IgA responses, at least in mice (46, 106). This finding likely relates to the fact that TLRs are critical for the activation and differentiation of multiple immune and nonimmune cells involved in the production and release of IgA, including B cells, T cells, DCs, FDCs, and IECs. Yet some studies suggest that mucosal signaling pathways may be even more interconnected and integrated than currently thought.
As discussed earlier, TLR signaling via MyD88 induces IEC, DC, and FDC production of BAFF and APRIL, two innate factors that deliver IgG and IgA CSR signals by engaging TACI on B cells (40). In addition to generating TACI ligands, TLRs cooperate with TACI to optimize IgA and IgG CSR and production (79, 111, 147). Such cooperation involves upregulation of TACI expression on B cells (148). However, TLRs and TACI further cooperate at the signaling level (Figure 3). Indeed, TACI engagement triggers recruitment of MyD88 to a highly conserved cytoplasmic domain of TACI distinct from the cytoplasmic TIR domain of TLRs (85). Interaction of TACI with MyD88 is followed by activation of a TLR-like pathway that elicits AID expression and CSR via NF-κB (85). These findings could provide an alternative explanation to previously published in vivo data demonstrating an essential role of MyD88 in systemic TI IgG responses induced by BAFF (148) and, together with other studies (46, 106, 149, 150), suggest that TACI and TLRs may converge on MyD88 to generate mucosal IgA.
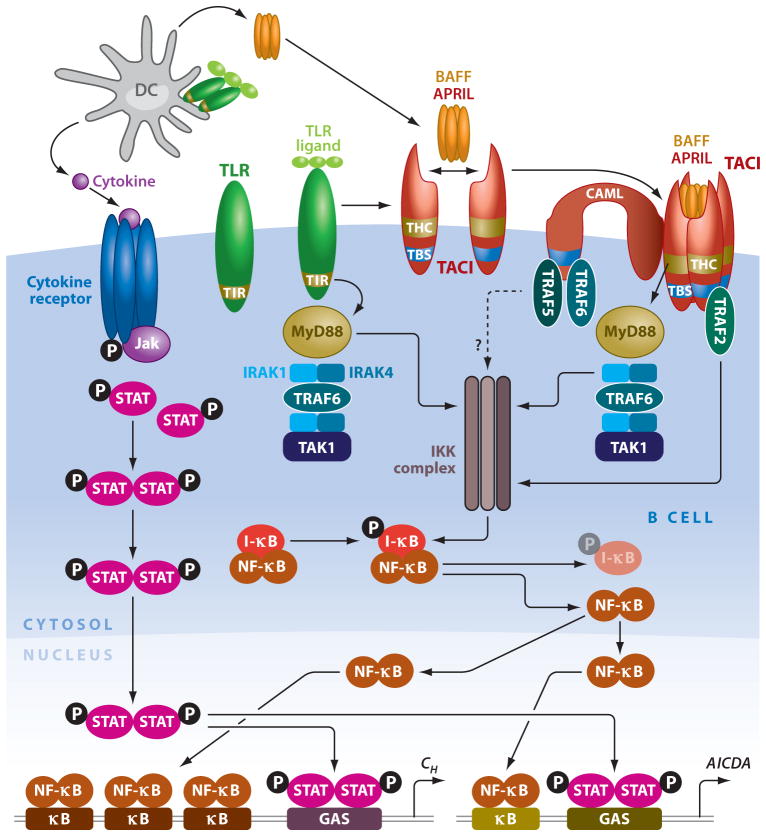
Figure 3.
Interconnectivity of signaling pathways emanating from TLRs and TACI. DCs activate B cells by releasing BAFF, APRIL, and cytokines upon sensing microbial TLR ligands. Engagement of TACI by BAFF and/or APRIL triggers association of the adaptor MyD88 to a TACI highly conserved (THC) domain that activates NF-κB via IRAK-1, IRAK-4, TAK-1, and IKK-mediated degradation of IκB. Additional NF-κB activation involves binding of TRAF2, TRAF5, and TRAF6 to a TRAF-binding site (TBS) in the cytoplasmic domain of TACI and calcium modulator and cyclophilin ligand (CAML), a transmembrane TACI-interacting protein. NF-κB initiates class switch recombination (CSR) by binding to κB motifs on AICDA and CH gene promoters. Engagement of TLRs by microbial ligands enhances CSR through a TIR-dependent pathway that shares MyD88 with the TIR-independent pathway emanating from TACI. Further CSR-inducing signals are provided by cytokines via signal transducer and activator of transcription (STAT) proteins that bind to γinterferon-activated site (GAS) motifs on AICDA and CH gene promoters. (Additional abbreviations used in figure: APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor; DC, dendritic cell; IKK, IκB kinase; IRAK, IL-1 receptor–associated kinase; TACI, transmembrane activator and calcium modulator and cyclophylin ligand interactor; TAK, TGF-β-activated kinase; TIR, Toll-interleukin-1 receptor; TLR, Toll-like receptor.)
CONCLUSIONS
The past decade has seen copious new discoveries regarding the regulation and function of mucosal antibodies and on the lineage, functional heterogeneity, and plasticity of mucosal cell types with B cell–modulating and antibody-inducing function. In addition, it is becoming increasingly clear that mucosal antibody responses follow dynamics quite different from those characterizing systemic IgG responses. For instance, intestinal IgA responses show additive increases after each antigenic challenge instead of prime-boost synergistic increases typical of systemic IgG responses (43). In addition, intestinal IgA responses to a given bacterial species are rapidly attenuated by colonization with a different species (43). Such IgA attrition could reflect the need of the gut immune system to rapidly adapt itself to the dominant microbial species present in the lumen at any given time (43). The lack of cardinal IgG memory characteristics in intestinal IgA responses has important implications with respect to the development of effective mucosal vaccines. One prediction is that induction of long-lasting IgA-mediated protection will require the development of creative vaccine delivery strategies capable of ensuring sustained stimulation of mucosal B cells, including the embedding of appropriate immunogens in stable components of our microbiota, edible probiotic bacteria, or genetically modified foods such as transgenic plants. Another critical step toward the generation of an effective mucosal vaccine is to acquire a more detailed knowledge of the multiple pathways involved in mucosal antibody responses. A better understanding of these pathways will be critical in devising mucosal vaccines that can provide rapid, robust, and sustained protection without causing excessive inflammation or inappropriate tolerance.
Hyper-IgD syndrome (HIDS) is an inherited autoinflammatory periodic fever syndrome caused by partial deficiency of mevalonate kinase (MVK), an enzyme of the cholesterol biosynthetic pathway. HIDS causes recurrent attacks of fever and inflammation that are often accompanied by cervical lymphadenopathy, abdominal pain, vomiting, and diarrhea. Hepatosplenomegaly, headache, arthralgias, arthritis, maculopapular rash, and purpura are also common together with continuously elevated IgD. Complete MVK deficiency causes mevalonic aciduria (MA), which is characterized by hyper-IgD production, periodic fever, and inflammation as well as developmental delay, failure to thrive, hypotonia, ataxia, myopathy, cataracts, uveitis, and blood disorders. Hyper-IgD production is also present in periodic fever-aphthous stomatitis-pharyngitis-adenitis (PFAPA) syndrome and a series of hereditary inflammasome defects, including familial Mediterranean fever (FMF) and cryopyrin-associated periodic syndromes (CAPS). This latter comprises neonatal onset multisystem inflammatory disease, Muckle-Wells syndrome, and familial cold autoinflammatory syndrome. Like HIDS and MA, PFAPA, FMF, and CAPS cause periodic antibiotic-resistant fever and inflammation that often targets the upper respiratory, urogenital and intestinal mucosae. The pathogenesis of hyper-IgD production and the role of IgD in periodic fever syndromes are unknown, but recent studies suggest that IgD may enhance fever and inflammation by triggering basophil release of IL-1β and IL-18 (32).
Acknowledgments
The authors are supported by U.S. National Institutes of Health grants R01 AI-05753 and R01 AI-074378 (to A. Cerutti), Ministerio de Ciencia e Innovación grant SAF 2008-02725 (to A. Cerutti), funds from Catalan Institute for Research and Advanced Studies (to A. Cerutti), funds from the Municipal Institute of Medical Research Foundation (to A. Cerutti), and a Sara Borrell fellowship (to A. Chorny).
- MALT
mucosa-associated lymphoid tissue
- Peyer’s patch (PP)
organized lymphoid aggregate of the small intestinal mucosa that functions as a major IgA inductive site (about 30 in humans)
- Mesenteric lymph node (MLN)
lymph node lying between two sheets of the peritoneal membrane connecting various segments of the small intestine to the abdominal cavity
- LP
lamina propria
- Microfold cell (M cell)
an intestinal epithelial cell type devoid of microvilli and specialized in antigen sampling
- CSR
class switch recombination
- Somatic hypermutation (SHM)
a process that introduces point mutations in the variable portion of Ig genes and provides a structural correlate for antibody affinity maturation
- Activation-induced cytidine deaminase (AID)4
a DNA-editing enzyme required for the induction of class switching and somatic hypermutation in B cells
- Class switching
an Ig gene–modifying process that occurs through class switch recombination and provides antibodies with novel effector functions
- TD
T cell–dependent
- TI
T cell–independent
- Germinal center
a specialized lymphoid structure fostering immunoglobulin class switching and somatic hypermutation
- B cell antigen receptor (BCR)
a term that refers to transmembrane immunoglobulin molecules on B cells
- Toll-like receptors (TLRs)
a family of innate antigen receptors that recognize highly conserved microbial patterns
- ILF
isolated lymphoid follicle
- S region
switch region, a highly repetitive intronic DNA sequence that guides recombination between actively transcribed CH genes
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 2.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 3.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 4.Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–42. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- 5.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 6.Sansonetti PJ, Medzhitov R. Learning tolerance while fighting ignorance. Cell. 2009;138:416–20. doi: 10.1016/j.cell.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–50. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti A. Immunology. IgA changes the rules of memory. Science. 2010;328:1646–47. doi: 10.1126/science.1192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–12. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–73. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 14.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–71. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- 16.Brandtzaeg P, Baekkevold ES, Morton HC. From B to A the mucosal way. Nat Immunol. 2001;2:1093–94. doi: 10.1038/ni1201-1093. [DOI] [PubMed] [Google Scholar]
- 17.Lencer WI, Blumberg RS. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 2005;15:5–9. doi: 10.1016/j.tcb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237:1–20. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preud’homme JL, Petit I, Barra A, Morel F, Lecron JC, Lelievre E. Structural and functional properties of membrane and secreted IgD. Mol Immunol. 2000;37:871–87. doi: 10.1016/s0161-5890(01)00006-2. [DOI] [PubMed] [Google Scholar]
- 20.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–9. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 21.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 22.Kadaoui KA, Corthesy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–57. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 23.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–30. doi: 10.1038/nature08529. Shows that M cells sample bacteria through a glycoprotein 2 receptor. [DOI] [PubMed] [Google Scholar]
- 24.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–67. doi: 10.1038/86373. Shows that DCs sample bacteria through interepithelial projections. [DOI] [PubMed] [Google Scholar]
- 25.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–50. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 27.Schlissel MS. Regulating antigen-receptor gene assembly. Nat Rev Immunol. 2003;3:890–99. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 29.Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–96. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–52. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 31.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Xu W, Wilson M, He B, Miller NW, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–98. doi: 10.1038/ni.1748. Shows that IgD enhances immunity by linking upper respiratory mucosa B cells with circulating basophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 34.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Investig. 2006;116:2142–51. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, et al. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 39.Montagnac G, Yu LC, Bevilacqua C, Heyman M, Conrad DH, et al. Differential role for CD23 splice forms in apical to basolateral transcytosis of IgE/allergen complexes. Traffic. 2005;6:230–42. doi: 10.1111/j.1600-0854.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 40.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–34. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flanagan JG, Lefranc MP, Rabbitts TH. Mechanisms of divergence and convergence of the human immunoglobulin α1 and α2 constant region gene sequences. Cell. 1984;36:681–88. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- 42.Plaut AG, Wistar R, Jr, Capra JD. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Investig. 1974;54:1295–300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–9. doi: 10.1126/science.1188454. Elucidates the dynamics of intestinal IgA production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–79. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 45.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. Shows that FDCs from Peyer’s patches induce TI IgA production after sensing bacteria. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–86. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–39. doi: 10.1016/j.chom.2007.09.013. Shows that IgA enhances intestinal homeostasis by modulating gene expression in commensal bacteria. [DOI] [PubMed] [Google Scholar]
- 49.Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci USA. 2010;107:7419–24. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–51. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A. Anti-inflammatory role for intracellular dimeric immunoglobulin A by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003;18:739–49. doi: 10.1016/s1074-7613(03)00122-5. [DOI] [PubMed] [Google Scholar]
- 52.Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham-Rundles C, Knight AK. Common variable immune deficiency: reviews, continued puzzles, and a new registry. Immunol Res. 2007;38:78–86. doi: 10.1007/s12026-007-0024-0. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5:880–92. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc Natl Acad Sci USA. 2006;103:10723–28. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forsgren A, Brant M, Karamehmedovic M, Riesbeck K. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect Immun. 2003;71:3302–9. doi: 10.1128/IAI.71.6.3302-3309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drenth JP, Goertz J, Daha MR, van der Meer JW. Immunoglobulin D enhances the release of tumor necrosis factor-α, and interleukin-1β as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology. 1996;88:355–62. doi: 10.1046/j.1365-2567.1996.d01-672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 62.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 63.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 64.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 65.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–86. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 66.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-κB. Nat Rev Immunol. 2005;5:435–45. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 67.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 68.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFκB. Int Immunol. 2004;16:395–404. doi: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 69.Schultheiss U, Puschner S, Kremmer E, Mak TW, Engelmann H, et al. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 2001;20:5678–91. doi: 10.1093/emboj/20.20.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fagarasan S, Honjo T. T-independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 71.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–86. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 72.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 73.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 74.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. doi: 10.1016/j.immuni.2008.05.014. Documents TI IgA production in ILFs. [DOI] [PubMed] [Google Scholar]
- 75.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–26. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 76.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–65. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 77.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 78.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–87. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu W, He B, Chiu A, Chadburn A, Shan M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 80.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell–dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–71. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 81.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 82.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huard B, McKee T, Bosshard C, Durual S, Matthes T, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Investig. 2008;118:2887–95. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–29. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He B, Santamaria R, Xu W, Cols M, Chen K, et al. TACI triggers immunoglobulin class switching by activating B cells through the adaptor protein MyD88. Nat Immunol. 2010;11:836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 88.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–28. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 89.Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, et al. Helper T cell subsets for immunoglobulin A responses: Oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–20. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA. 2009;106:19256–61. doi: 10.1073/pnas.0812681106. Demonstrate that intestinal Treg cells induce IgA production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. Demonstrate that intestinal Treg cells induce IgA production. [DOI] [PubMed] [Google Scholar]
- 92.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 93.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niess JH, Brand S, Gu X, Landsman L, Jung S, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–58. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 95.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rescigno M, Lopatin U, Chieppa M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr Opin Immunol. 2008;20:669–75. doi: 10.1016/j.coi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–26. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 101.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center-like phenotype differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 102.Dullaers M, Li D, Xue Y, Ni L, Gayet I, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–29. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: Lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182:4507–11. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–27. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 105.Casola S, Rajewsky K. B cell recruitment and selection in mouse GALT germinal centers. Curr Top Microbiol Immunol. 2006;308:155–71. doi: 10.1007/3-540-30657-9_7. [DOI] [PubMed] [Google Scholar]
- 106.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–33. doi: 10.1038/nature06033. Shows that a subset of intestinal DCs promotes IgA production in Peyer’s patches and lamina propria through nitric oxide. [DOI] [PubMed] [Google Scholar]
- 107.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Investig. 2009;119:2441–50. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 109.Kelsall BL, Rescigno M. Mucosal dendritic cells in immunity and inflammation. Nat Immunol. 2004;5:1091–95. doi: 10.1038/ni1104-1091. [DOI] [PubMed] [Google Scholar]
- 110.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 111.He B, Xu W, Santini PA, Polydorides AD, Chiu A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–26. doi: 10.1016/j.immuni.2007.04.014. Shows TI IgA2 production in the human intestinal lamina propria. [DOI] [PubMed] [Google Scholar]
- 112.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–56. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 113.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–89. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 114.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–57. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 118.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. 2009;182:3482–91. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 119.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 120.Marchesi F, Martin AP, Thirunarayanan N, Devany E, Mayer L, et al. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2:486–94. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 121.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 122.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take center stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cerutti A. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 2008;1:8–10. doi: 10.1038/mi.2007.8. [DOI] [PubMed] [Google Scholar]
- 124.Kang HS, Chin RK, Wang Y, Yu P, Wang J, et al. Signaling via LTβR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576–82. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- 125.Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 126.He B, Xu W, Cerutti A. Comment on “Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination”. Mucosal Immunol. 2010;3:92–94. doi: 10.1038/mi.2009.125. author reply 94–95. [DOI] [PubMed] [Google Scholar]
- 127.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–38. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 129.Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204:1145–56. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boursier L, Gordon JN, Thiagamoorthy S, Edgeworth JD, Spencer J. Human intestinal IgA response is generated in the organized gut-associated lymphoid tissue but not in the lamina propria. Gastroenterology. 2005;128:1879–89. doi: 10.1053/j.gastro.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 131.Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J Immunol. 2010;184:3545–53. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]
- 132.Uematsu S, Akira S. Immune responses of TLR5+ lamina propria dendritic cells in enterobacterial infection. J Gastroenterol. 2009;44:803–11. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 133.Fayette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, et al. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J Exp Med. 1997;185:1909–18. doi: 10.1084/jem.185.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–19. [PubMed] [Google Scholar]
- 135.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 136.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 137.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 138.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 139.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 140.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-β-dependent mechanism. J Immunol. 2006;177:7164–72. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu YJ, de Bouteiller O, Arpin C, Briere F, Galibert L, et al. Normal human IgD+IgM− germinal center B cells can express up to 80 mutations in the variable region of their IgD transcripts. Immunity. 1996;4:603–13. doi: 10.1016/s1074-7613(00)80486-0. [DOI] [PubMed] [Google Scholar]
- 142.Arpin C, de Bouteiller O, Razanajaona D, Fugier-Vivier I, Briere F, et al. The normal counterpart of IgD myeloma cells in germinal center displays extensively mutated IgVH gene, Cmu-Cδ switch, and λ light chain expression. J Exp Med. 1998;187:1169–78. doi: 10.1084/jem.187.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng NY, Wilson K, Wang X, Boston A, Kolar G, et al. Human immunoglobulin selection associated with class switch and possible tolerogenic origins for Cδ class-switched B cells. J Clin Investig. 2004;113:1188–201. doi: 10.1172/JCI20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 145.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–18. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–95. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 148.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–71. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 150.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA. 2004;101:3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]