Abstract
Monkeys can detect electrical stimulation delivered to striate cortex (area V1). We examined whether such stimulation is layer dependent. While remaining fixated on a spot of light, a rhesus monkey was required to detect a 100 ms train of electrical stimulation delivered to a site within area V1. A monkey signaled the delivery of stimulation by depressing a lever after which he was rewarded with a drop of apple juice. Control trials were interleaved during which time no stimulation was delivered and the monkey was rewarded for not depressing the lever. Biphasic pulses were delivered at 200 Hz and the current was typically at or less than 30 μA using 0.2 ms cathode-first biphasic pulses. For some experiments, the pulse duration was varied from 0.05 to 0.7 ms and anode-first pulses were used. The current threshold for detecting cathode-first pulses 50% of the time was the lowest (< 10 μA) when stimulation was delivered to the deepest layers of V1 (between 1.0 and 2.5 mm below the cortical surface). Also the shortest chronaxies (< 0.2 ms) and the shortest latencies (< 200 ms) for detecting the stimulation were observed at these depths. Finally, anode-first pulses were most effective at evoking a detection response in superficial V1 and cathode-first pulses were most effective at evoking a detection response in deep V1 (> 1.75 mm below the cortical surface). Accordingly, the deepest layers of V1 are the most sensitive for the induction of a detection response to electrical stimulation in monkeys.
Keywords: occipital cortex, electrical stimulation, current thresholds, chronaxies, manual responses, rhesus monkeys, phosphenes
Introduction
In the early to middle part of the last century, investigators established that animals could detect electrical currents delivered anywhere in the brain (Loucks, 1935-36; Konorski & Lubinska, 1939; Doty & Rutledge, 1959; Rutledge & Doty, 1962; Neilson et al., 1962; Doty, 1965, 1969; Nielson & Davis, 1966; Miller & Glickstein, 1967). To get an animal to respond to electrical stimulation, a reward or an absence of punishment had to follow a motor response (e.g. to release a lever) that was paired with the stimulation (e.g. Doty, 1965). More recently, such detection paradigms have been used to study how the brain mediates somatosensation, audition, and vision (Bartlett and Doty, 1980; Bartlett et al., 2005; DeYoe et al., 2005; Otto et al., 2005; Leal-Campanario et al., 2006; Butovas & Schwarz, 2007; Murphey & Maunsell, 2007).
The striate cortex (or area V1) in primates is the first station of the retino-geniculo-striate pathway that receives an integrated visual signal from the two eyes before relaying the signal to higher cortical areas (Hubel & Wiesel, 1977). V1 contains the highest density of neurons in neocortex (Rockel et al., 1980; O’Kusky & Colonnier, 1982) and has by far the highest number of neurons devoted to representing the visual field of any area along the retino-geniculo-striate pathway (Winters et al., 1969; Barlow, 1981; Perry & Cowey, 1985; Schein, 1988; Felleman & Van Essen, 1991). This ensures that visual stimuli can be decoded at resolutions of a fraction of a minute of visual angle (Levi et al., 1985).
Electrical stimulation of V1 in humans has been shown to produce a punctate visual percept called a phosphene (Brindley & Lewin, 1968; Dobelle & Mladejovsky, 1974; Schmidt et al., 1996). These results have lead to the notion that microstimulation of V1 in behaving monkeys could serve as a model for the implantation of a functional cortical visual prosthesis for blind patients (Troyk et al., 2003; Bartlett et al., 2005; Bradley et al., 2005; De Yoe et al., 2005; Tehovnik et al., 2005). In this paper, we investigate the excitability of the directly stimulated neuronal elements within the various layers of V1 that mediate the detection of electrical currents delivered to V1 of monkeys. For all experiments, we used a 100 ms train of either cathode-first (cathodal) or anode-first (anodal) pulses with a biphasic configuration. Four measures were studied across cortical depth: (1) current threshold, (2) response latency, (3) chronaxie, and (4) anode-cathode ratio. The last two measures require some introduction.
A chronaxie is an estimate of the time constant of a directly stimulated neuronal element (Ranck, 1975). It is a measure of neuronal excitability whereby axons have shorter chronaxies than cell bodies [axons: 0.03 to 7 ms; cell bodies: 7 to 31 ms (Ranck, 1975; Nowak & Bullier, 1998)], and large, myelinated axons have shorter chronaxies than small, non-myelinated axons [large: 0.03 to 0.7 ms; small: > 1.0 ms; (Ranck, 1975; Li & Bak, 1976; West & Wolstencroft, 1983)]. Chronaxies have been determined for individual neurons, as well as for neural elements that mediate neurotransmitter release, self-stimulation, saccadic eye movements, phosphene induction, and fMRI-signal generation (Brindley & Lewin, 1968; Dobelle & Mladejovsky, 1974; Matthews, 1977; Rushton & Brindley, 1978; Tehovnik & Lee, 1993; Tehovnik & Sommer, 1997; Farber et al., 1997; Tolias et al., 2005).
An anode-cathode ratio compares the response threshold of anodal pulses over cathodal pulses. A ratio of less than one indicates that anodal pulses are more effective at activating a neuronal element than are cathodal pulses; a ratio of greater then one indicates that cathodal pulses are more effective. Anodal pulses tend to activate cell bodies and terminals more effectively, whereas cathodal pulses activate axons more readily (Fritsch & Hitzig, 1879; Porter, 1963; Stoney et al., 1968; Clendenin et al., 1974; Ranck, 1975). Even though electrical stimulation is presumed to preferentially activate axons over cell bodies (Porter, 1963; Landau et al., 1965; Stoney et al., 1968; Gustaffson and Jankowska, 1976; Nowak & Bullier, 1998; Swadlow, 1992; Rattay, 1999; McIntyre & Grill, 2002), the mode of activation using cathodal and anodal pulses differs. It is the outward current at the initial segment or a node of Ranvier of an axon that triggers an action potential (Ranck 1975). An anodal pulse generates such a current at the initial segment or node of Ranvier if the electrode is positioned at the cell body, and a cathodal pulse generates such a current if the electrode is positioned at the axon.
We found that elements within the deepest layers of V1 were the most excitable for the elicitation of a detection response from monkeys. The excitability properties of elements subserving detection are compared to those of elements mediating the generation of saccadic eye movements in macaque V1 and to those of elements mediating the evocation of phosphenes in human V1.
Materials and methods
Subjects
Two adult rhesus monkeys (Macaca mulatta) C and H were used. Throughout this study food was freely available. A monkey’s access to water was restricted before each day of experimental testing. After testing, monkeys were allowed to drink until sated before being returned to the vivarium. The monkeys were provided for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Guidelines of the Massachusetts Institute of Technology Committee on Animal Care.
Surgery
Monkeys were anesthetized with pentobarbital intravenously (20 mg/kg) and prepared for aseptic surgery. A scleral search coil was implanted (Judge et al., 1980) and a stainless-steel post, to restrain the head, was secured to the skull with titanium head screws and acrylic cement. Subsequently, a recording chamber was implanted over the right V1.
Behavioural tasks
A monkey, with head fixed, faced a computer monitor (Sony Multiscan E210) positioned 57 cm away. All tasks started with an animal being required to fixate a central spot (0.1 degrees in diameter, 153 cd/m2) on a monitor screen with background luminance that could range from 7.7 to 76.7 cd/m2). During fixation, the animal had to keep his eyes within a 0.5 degree by 0.5 degree window; otherwise the trial was aborted. Each animal was trained to perform two tasks: one to map the visual receptive field of V1 cells (Fig. 1A, right) and a second to detect either a visual target (Fig. 1B) or electrical stimulation delivered to V1 (Fig. 1C). To map the visual receptive field, a monkey was required to remain fixated on the central spot for 2 to 4 seconds after which time he was rewarded (with a drop of apple juice) for generating a saccadic eye movement to a visual target positioned in one of four quadrants of the visual field away from the fixation spot. While the animal maintained fixation, a bar of light (153 cd/m2) was swept across a restricted region of the monitor screen. The orientation and size of the bar, as well as its direction of motion, were varied systematically until the location of the receptive field was determined (Fig. 1A, right).
Figure 1.
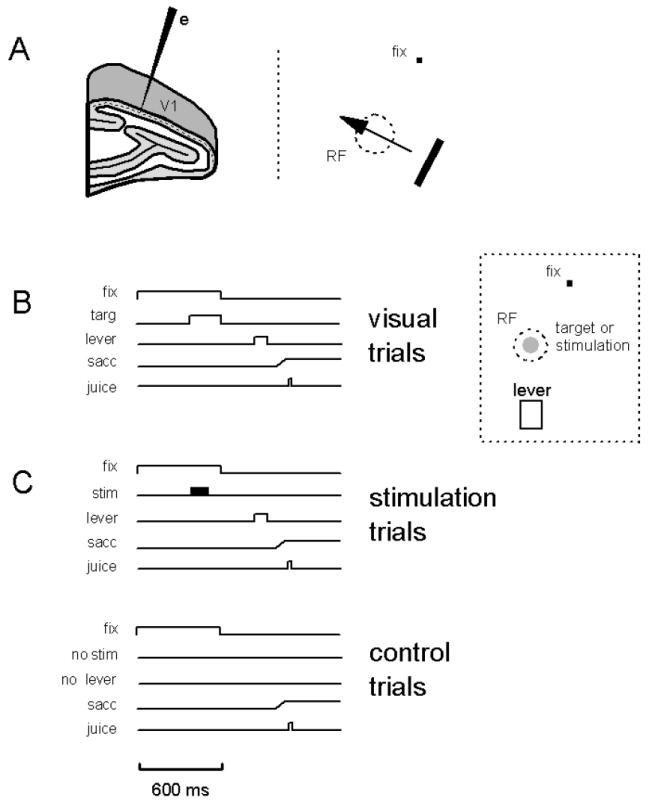
Methods. (A, left) All electrodes (e) were lowered into V1 such that they penetrated the cortex perpendicular to its surface. (A, right) Receptive fields (RF) were mapped by moving a bar of light at different orientations across the visual field, as the monkey remained fixated on a spot of light (fix). (B, C) For all trials, an animal fixated a spot for 600 ms (fix) otherwise the trial was aborted and no juice reward was delivered. Two types of trials were used as described in B and C: (B) For visual trials, a visual target (targ) was flashed for 200 ms at the end of the fixation period (fix). The target was set at positive contrast (33%, Michaelson; target luminance = 153 cd/mm2; background luminance = 76.7 cd/mm2) and at 0.25 degrees in size. It was centered in the receptive field of the neurons under study (see panel to the right). Following the termination of the fixation spot and visual target, the monkey was required to depress a lever (lever) with its left hand in order to obtain a juice reward (juice). The monkey was given 800 ms following fixation-spot and target offset to depress the lever. On 50% of trials, control trials during which no visual target was presented were interleaved with the visual trials. For such trials, the monkey was required not to depress the lever in order to obtain a juice reward. For both visual and control trials, the monkey was allowed to generate a saccadic eye movement (sacc) away from the fixation location at the termination of the fixation spot. (C) For stimulation trials, a 100 ms train of electrical stimulation (stim) was delivered to the neurons under study (also see panel to right of B). The stimulation commenced at 200 ms before the end of the fixation period (fix). Following termination of the fixation spot, the monkey was required to depress a lever (lever) with its left hand in order to obtain a juice reward (juice). The monkey was given 800 ms following fixation offset to depress the lever. Depressing the lever indicated that the monkey detected to electrical stimulation. On 50% of trials, control trials during which no stimulation was presented were interleaved with the stimulation trials. For such trials, the monkey was required not to depress the lever in order to obtain a juice reward. For both stimulation and control trials, the monkey was allowed to generate a saccadic eye movement (sacc) away from the fixation location at the termination of the fixation spot.
The second task required a monkey to remain on the fixation spot for 600 ms (Fig. 1B,C). This task had two forms. In the first form, a monkey had to detect a visual target presented in the receptive-field location of the cells under study. A visual target (0.25 degrees in diameter, 153 cd/m2) was presented for 200 ms at the end of the fixation period (Fig. 1B, visual trials). The background luminance of the monitor was set at 76.7 cd/m2 so that the contrast of the target was 33% (Michaelson). After extinction of the target and fixation spot, the monkey was required to depress a lever with its left hand in order to obtain a juice reward. The monkey was given 800 ms to initiate a response. On 50% of trials, no visual target was presented and the monkey had to refrain from depressing the lever in order to get a juice reward. After termination of the fixation spot the monkey was free to generate a saccade away from the fixation spot.
In the second form of the task, a monkey had to detect a 100 ms train of electrical stimulation delivered to the V1 site under study. The electrical stimulation commenced at 200 ms before the end of the fixation period (Fig. 1C, stimulation trials). After the extinction of the fixation spot, the monkey was required to depress a lever with its left hand in order to obtain a juice reward. The monkey was given 800 ms to initiate a response. A response indicated that the monkey had detected the electrical stimulation. On 50% of trials, no electrical stimulation was presented and the monkey had to refrain from depressing the lever in order to get a juice reward (Fig. 1C, control trials). After termination of the fixation spot the monkey was free to generate a saccade away from the fixation spot.
Within a block of trials, the visual or stimulation trials were interleaved randomly with control trials so that the monkey could not predict the sequence of trials (Fig. 1B,C). Monkeys performed all tasks in a dimly lit room.
Measuring the detection response using a lever
A monkey signaled the occurrence of a visual target or a train of electrical stimulation by pulling a small upright (1 cm wide by 3 cm tall) lever with its left hand. The hand of the monkey was positioned comfortably near the lever so that the amount of forelimb movement required to initiate a response was minimized. A circuit was broken by a slight pull of the lever; therefore, minimal force on the lever registered a response. A monkey could easily perform between 1000 and 2000 trials per day using this device.
Estimating electrode depth
For all electrode penetrations made into V1 (Fig. 1A, left), a standard method was used to deduce the electrode depth with respect to the top of V1 (Tehovnik et al., 2002, 2003a,b). Once single units were encountered, their visual receptive fields were mapped and the electrode was adjusted in depth until the unit activity was just detectable at the top of cortex. This point marked the initial depth at the top of V1. At the end of a test session the depth at the top of V1 was re-measured by noting the point at which the unit activity in response to visual stimuli became barely detectable as the electrode was slowly withdrawn. The estimated depth at the top of V1 was the average of the initial and final depth estimates.
Data collection and analysis
A PDP 11/73 computer controlled the presentation of visual stimuli, the delivery of electrical stimulation, the collection of eye position (sampled at 200 Hz) and the lever response, and the delivery of juice.
Electrical stimulation
Electrodes were introduced perpendicular to the dural surface with a hydraulic microdrive (Fig. 1A, left). Constant-current charge-balanced biphasic pulses were delivered to the brain via a monopolar glass-coated platinum-iridium electrode [@ 0.3 to 0.6 MΩ tested at 1 KHz] using a Grass S88 dual stimulator attached to a pair of constant-current stimulus isolation units (Grass PSIU6B, Quincy, MA, USA). For each biphasic pulse, a cathodal and anodal pulse followed in immediate succession to produce cathode-first (cathodal) pulses. Both pulses had the same amplitude and duration. For some experiments, the order of the cathodal and anode pulses was reversed to produce anode-first (anodal) pulses. Current was measured by the voltage drop across a 1000 Ω resistor. The current was monitored continuously using a Hitachi Oscilloscope (model V-212, differential amplifier, Japan) and was read as the amplitude of one pulse (cathodal or anodal) of a biphasic pair. For all experiments 20 pulses were delivered in a 100 ms train yielding a pulse rate of 200 Hz. For most experiments, the pulse duration (of a single phase) was fixed at 0.2 ms, but for some experiments the pulse duration was varied from 0.05 and 0.7 ms. The range of currents used was typically between 1 and 30 μA when using 0.2 ms pulses.
Unit recording was performed using the same electrode as was used to deliver the electrical stimulation to V1. The action potentials were amplified (Bak A-1B, Germantown, MD, USA) and filtered (Krohn-Hite 3750, Horsham, PA, USA) and displayed on an oscilloscope (Tektronix TDS-210). An audio-monitor (Grass AM8, Quincy, MA, USA) was used to listen to the unit response.
Results
Stimulation sites
All electrode penetrations were perpendicular to the cortical surface of V1 (Fig. 1A, left). A total of 20 penetrations were made into V1 of monkey C and a total of 13 penetrations were made into V1 of monkey H. For both monkeys, the penetration sites contained cells with visual receptive-field centers varying between 1.5 and 4.5 degrees from the fovea and between 205 and 270 degrees of meridian. Receptive-field mapping was done with a bright bar of light (Fig. 1A, right).
Threshold currents for evoking a detection response across cortical depth
While monkeys performed the task illustrated in Figure 1C, trials were conducted to determine the probability of evoking a detection response from V1 for currents ranging from 1 to 30 μA as an electrode was lowered into V1. The current level yielding a detection rate of 50% was used as the criterion. For the example shown (Fig. 2A, stim), the threshold current was estimated to be 8 μA (see arrow in figure) for a site situated 1.25 mm below the top of V1. For current levels greater than 10 μA the detection rate reached asymptote. For non-stimulation control trials, the monkey depressed the lever less than 10% of the time (Fig. 2B, cont). The latency of the detection response reached asymptote for currents at and beyond 10 μA (Fig. 2B). At these currents, the latency of the detection response was comparable to the latency of the detection response made to a visual target (i.e. 0.25 degrees in size at 33% positive contrast) presented in the receptive field of the neurons being activated with electrical stimulation (Fig. 2B, targ). For the stimulation experiments, 0.2 ms cathode-first pulses were delivered at 200 Hz within a 100 ms train that commenced 200 ms before the end of the fixation period (Fig. 1C).
Figure 2.
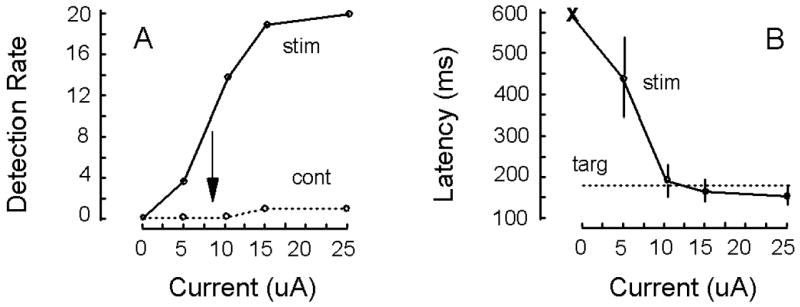
Measuring current threshold. (A) Detection rate is plotted as a function current for 20 stimulation trials (stim). The current threshold was defined as the amount of current required yielding a 50% detection rate that was 8 μA (arrow). Control trials indicate that the monkey depressed the lever less then 10% of the time when no stimulation was delivered (cont). (B) The latency to depress the lever following the termination of the fixation spot is shown for stimulation trials (stim) across different current levels. Standard errors of the mean are shown. The ‘x’ indicates that no latency measure was obtained for the zero current condition. The average latency to depress the lever for target trials is indicated by the dotted line (all latencies are with respect to the termination of the fixation spot). Data in A and B are from the same experiment in which a 100 ms train of 0.2 ms cathode-first pulses were delivered at 200 Hz to a V1 site at 1.25 mm below the cortical surface. Data are from monkey C. See Fig. 1 for other details.
The current threshold for evoking a detection response on 50% of stimulation trials is plotted as a function cortical depth for eleven penetrations made into V1 (Fig. 3). Stimulation tests were performed at depth increments of 250 μm spanning all the layers of V1 (Peters and Sethares, 1991). At the surface of V1, 30 μA was not sufficient to evoke a detection response. The lowest current threshold for eliciting this response occurred at 1.75 and 2.0 mm below the cortical surface. At these depths the average current threshold was 6 μA and the lowest threshold observed here for an individual site was 2 μA. The detection rate for the non-stimulation control trials was at or less than 10%.
Figure 3.
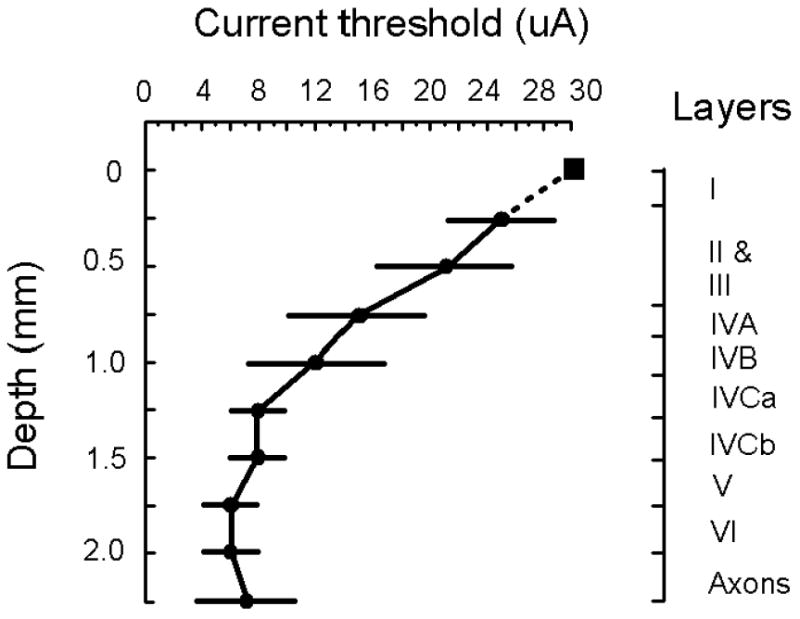
Current threshold. The current threshold to evoke a detection response on 50% of stimulation trials is plotted as a function of cortical depth. Each value is based on an average taken from 11 penetrations made into V1 (9 penetrations from monkey C and 2 penetrations from monkey H). A total of 110 sites were studied, ten for each penetration. The probability of evoking a detection response for a given site with a particular current was based on 10 stimulation trials (see Fig. 2A for details). The square marker indicates that a detection response was not readily elicited from the site using a maximal current of 30 μA. Standard deviations are shown. Illustrated to the right is the lamination of macaque V1 over a 2.25 mm depth starting from top of superficial V1 (Peters and Sethares, 1991). Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 200 Hz, and 100 ms, respectively. Cathode-first pulses were used for all experiments. All stimulation occurred 200 ms before the termination of the fixation spot. The detection rate for non-stimulation control trials was at 10% or less. All data were collected while a monkey performed the task illustrated in Figure 1C. Data are from monkeys C and H.
Chronaxie estimates as a function of cortical depth
To determine the excitability (or chronaxies) of the directly stimulated elements mediating the detection response, strength-duration functions were generated (Brindley & Lewin, 1968; Dobelle & Mladejovsky, 1974; Matthews, 1977; Rushton and Brindley, 1978; Tehovnik & Lee, 1993; Tehovnik & Sommer, 1997; Farber et al., 1997; Tolias et al., 2005). A function was produced for a single site of stimulation by measuring the current threshold to evoke a detection response on 50% of stimulation trials for pulse durations ranging from 0.05 ms to 0.7 ms (Fig. 4A). For all sites studied (n = 14), the current threshold to evoke a detection response decreased with increases in pulse duration, reaching asymptote for pulse durations beyond 0.5 ms. For the shortest pulse duration (i.e. 0.05 ms), the current threshold ranged from 26 and 155 μA; and for the longest pulse duration tested (i.e. 0.7 ms), the current threshold ranged from 5 to 20 μA. For these experiments, cathode-first pulses were delivered at 200 Hz within a 100 ms train that commenced 200 ms before the end of the fixation period (Fig. 1C).
Figure 4.
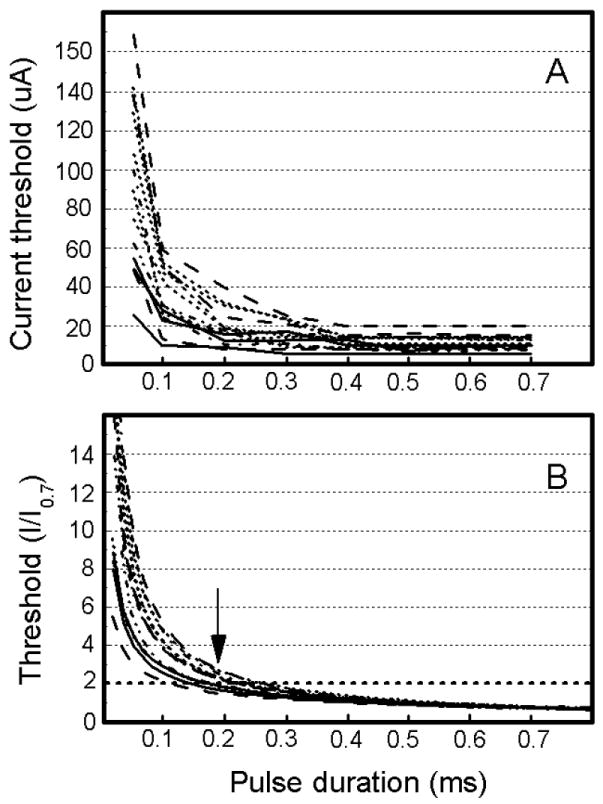
Excitability of neurons mediating the detection response. (A) Current threshold for evoking a detection response on 50% of stimulation trials is plotted as a function of pulse duration. Each data point for a curve is based on 10 stimulation trials. Each curve in a panel represents data from one stimulation site. A total of 14 stimulation sites situated between 0 and 2.5 mm below the cortical surface were studied (11 sites from monkey C and 3 sites from monkey H). Pulse frequency and train duration were fixated at 200 Hz and 100 ms, respectively. Cathode-first pulses were used for all experiments. The detection rate for non-stimulation control trials was at 10% or less. All data were collected while a monkey performed the task illustrated in Figure 1C. See Figure 2A for other details. (B) Normalized threshold current based on the data from ‘A’ is plotted as a function of pulse duration. For a pulse duration of 0.7 ms, the current required to evoke a detection response on 50% of stimulation trials is set to one and all other values are expressed as a multiple of the current used at the 0.7-ms pulse duration. Data are fitted using power functions for which the R2 values are greater than 0.8. The pulse duration at which a curve intersects two units of threshold (designated by the dotted horizontal line) indicates the chronaxie of the stimulated elements at the site of study. The arrow indicates that the average chronaxie for the 14 sites was 0.19 ms. Data are from monkeys C and H.
To determine the chronaxies of the directly stimulated elements mediating the detection response, the strength-duration functions from Figure 4A were normalized such that the current threshold to evoke a detection response was set to one for a pulse duration of 0.7 ms and all other thresholds were expressed as a multiple of this threshold (Fig. 4B). Power functions were fitted for every data set pertaining to a site. For all sites, the R2 values were always greater than 0.8. The chronaxie value for a site can be determined as the pulse duration at which a power function crossed two units of threshold (designated by the horizontal dashed line in Fig 4B). The chronaxie values ranged from 0.11 to 0.24 ms with an overall average of 0.19 ms (Fig. 4B, arrow). Finally, the chronaxie values decreased as a function of cortical depth (i.e. r = -0.76, n = 14, p < 0.01; Fig. 5) with the values in superficial V1 (i.e. from 0.3 to 1.6 mm below the cortical surface) ranging from 0.18 to 0.24 ms and with the values in deep V1 (i.e. > 1.6 mm below the cortical surface) ranging from 0.11 to 0.15 ms.
Figure 5.
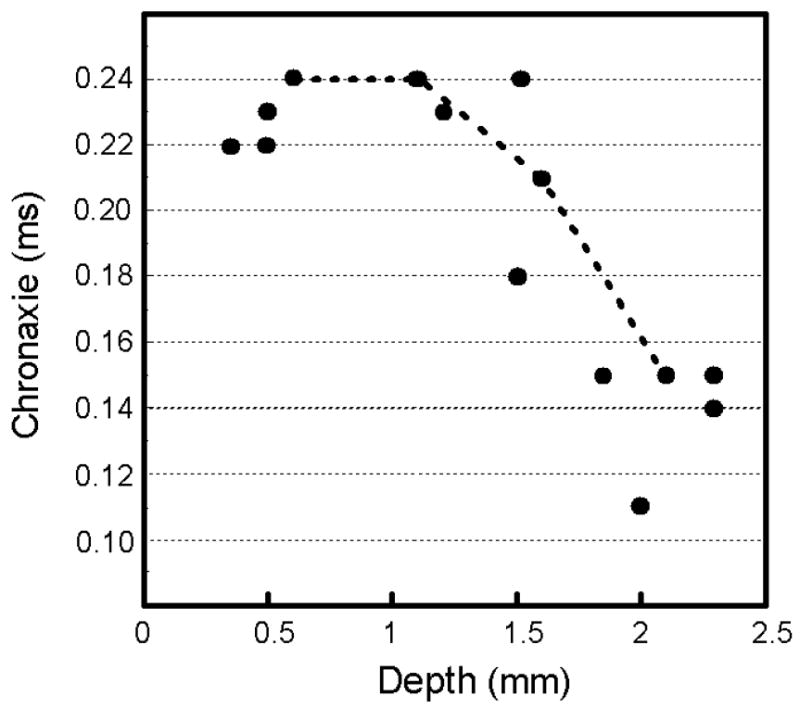
Chronaxies over cortical depth. The chronaxie values of Figure 4 are plotted as a function of cortical depth. The dotted line links chronaxie values collected from the same penetration made into V1. Overall the chronaxie values drop as a function of cortical depth (r = -0.76, p < 0.01, n = 14).
Effectiveness of current pulse polarity with changes in cortical depth
The effectiveness of cathode-first and anode-first pulses for the evocation of a detection response was studied as a function of cortical depth. Figure 6A shows the current threshold for eliciting a detection response on 50% of stimulation trials as a function of cortical depth for anode-first versus cathode-first pulses. The data in the figure are based on a single penetration made into V1. As the electrode was advanced into the cortex, for both pulse types the current threshold for evoking a detection response decreased reaching a minimal threshold from 1.0 to 2.25 mm below the cortical surface. For superficial sites of stimulation (i.e. between 0 and 1.5 mm below the cortical surface) anode-first pulses were the most effective, whereas for deep sites of stimulation (i.e. beyond 1.75 mm below the cortical surface) cathode-first pulses were the more effective. To establish the depth at which the effectiveness of pulse-type changed from anode-first pulses being superior to cathode-first pulses being superior, anode-cathode ratios were computed (Fig. 6B-E). A ratio was determined by dividing the threshold current using anode-first pulses by the threshold current using cathode-first pulses (Fig. 6B, inset). A ratio of less than one indicates that anode-first pulses were more effective than cathode-first pulses at eliciting at detection response, a ratio of one indicates that both pulse types were equally effective, and a ratio of more than one indicates that cathode-first pulses were more effective. Anode-cathode ratios are plotted as a function of cortical depth for four penetrations made into V1 (Fig. 6B-E, note that the plot in B is derived from Fig. 6A). Overall, the depth at which cathode-first and anode-first pulses were equally effective occurred at a depth between 1.75 and 2.1 mm below the cortical surface, and beyond this depth cathode-first pulse were best at evoking a detection response (Fig. 6B-E, arrow in each panel). For these experiments, pulses were delivered at 200 Hz within a 100 ms train that commenced 200 ms before the end of the fixation period (Fig. 1C).
Figure 6.
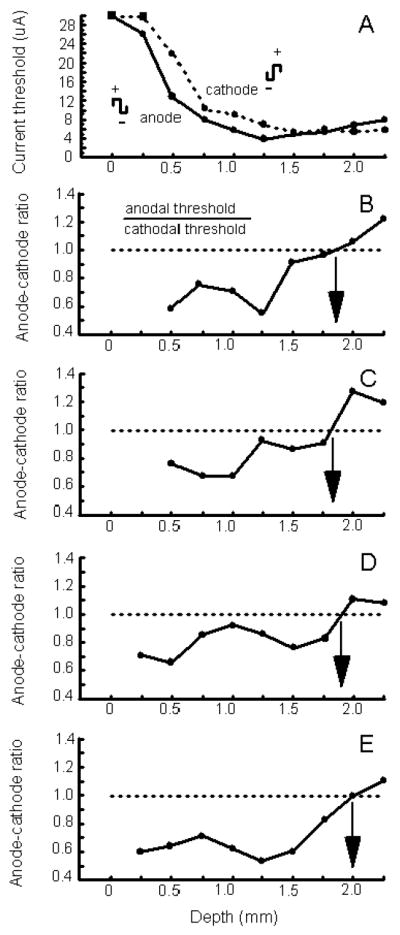
Effects of anode-first versus cathode-first stimulation. (A) The current threshold to evoke a detection response on 50% of stimulation trials is plotted as a function of cortical depth for anode-first (solid curve) versus cathode-first (dotted curve) pulses for one penetration made into V1. Each data point for a curve is based on 10 stimulation trials. The square marks indicate that a detection response could not be evoked using a maximal current of 30 μA. Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 200 Hz, and 100 ms, respectively. The detection rate for non-stimulation control trials was at 10% or less. All data were collected while a monkey performed the task illustrated in Figure 1C. See Figure 2A for other details. (B-E) Anode-cathode ratios are plotted as a function of cortical depth for three penetration sites made into V1. A ratio is determined by dividing the current threshold to evoke a detection response on 50% of stimulation trials using anode-first pulses by the current threshold to evoke a detection response on such trials using cathode-first pulses (see inset in B). The curve in B is based on data of ‘A’. For each curve (B-E), ratios were computed for sites between 0 and 2.5 mm below the surface of V1. Ratio values were not computed for the most superficial sites test (e.g. at a depth of 0 mm) since a detection response could not be evoked at these sites using a 30 μA current. The arrow within a panel indicates the depth at which the ratio equaled one. This is the depth beyond which cathode-first pulse became more effective than anode-first pulses. ‘A to D’ are from monkey C and ‘E’ is from monkey H.
Detection latency as a function of cortical depth
The latency at which a detection response was evoked from various depths of V1 was examined. Latency was defined with respect to the termination of the fixation spot (Fig. 7, lower right inset). For every site studied, the current threshold to elicit a detection response on 50% of stimulation trials was determined. The currents used to ascertain the latency of the detection response ranged from 1 to 4 times the threshold current (Fig. 7A-E, actual current used is listed at the top of each panel). As the multiple of threshold current was increased from 1 to 4, the latency at which a detection response was evoked dropped until a minimum latency was achieved (Fig. 7A-E, see arrow within a panel). This minimal latency approximated the latency of response made to a visual target (i.e. 0.25 degrees in size at 33% positive contrast) flashed in the receptive field of the stimulated neuronal elements (Fig. 7A-E, see dotted line within a panel, average latency of 225 ms with standard error of 3 ms). For these experiments, all stimulation (or onset of a visual target) commenced 100 ms before the end of the fixation period (Fig. 7, lower right panel plotting the time of events) and cathode-first pulses were delivered at 200 Hz within a 100 ms train.
Figure 7.
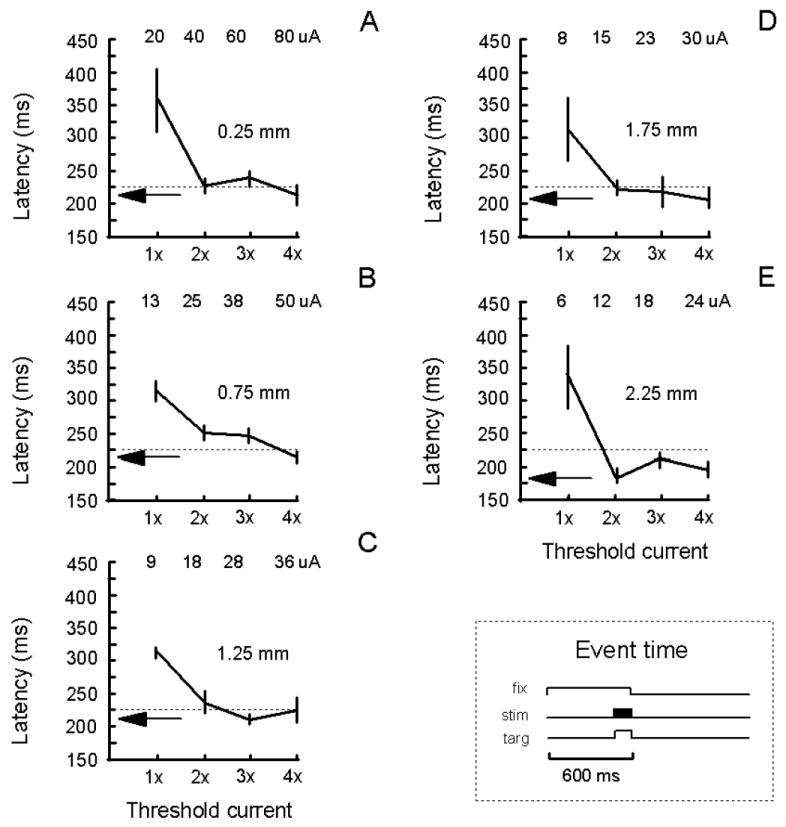
Latency to evoke a detection response. The latency to evoke a detection response is plotted as a function of a multiple of threshold current for five depths (i.e. 0.25, 0.75, 1.25, 1.75, and 2.25 mm) along one penetration (A-E). The latency was measured with respect to the termination of the fixation spot. The threshold current was the current required to evoke a detection response on 50% of stimulation trials. Each curve within a panel represents the saccadic latency for a given current threshold value. Each data point is based on 10 stimulation trials. Standard errors of the mean are shown. The actual currents used are listed at the top of each panel. The arrow within a panel indicates the average minimal latency to evoke a detection response. Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 200 Hz, and 100 ms, respectively. The detection rate for non-stimulation control trials was at 10% or less. The dotted line within a panel indicates the average latency for detecting a visual target presented in the receptive field of the stimulated neurons elements (standard error = 3 ms; N = 5 blocks of 10 trials each). All data were collected while a monkey performed the task illustrated in Figure 1B,C with a slight variation: both the electrical stimulation and visual target were presented 100 ms before the end of the fixation period [see lower-right panel: fixation time (fix); stimulation time (stim); target time (targ)] to reduce the time between the onset of these events and the termination of the fixation spot. All data are from monkey C. See Figure 2 for other details.
The latency of the detection response is plotted as a function of a multiple of threshold current for five depths along one penetration made into V1 (Fig. 7). At each depth (i.e. 0.25, 0.75, 1.25, 1.75, and 2.25 mm below the top of superficial V1), the latency of a detection response dropped with increases in current. The shortest latency (i.e. 177 ms) occurred at 2.25 mm below the cortical surface. The minimal latency across the five sites varied from 177 to 215 ms. For a total of nine sites tested from 0.25 to 2.25 mm below the cortical surface, the minimal latency was on average 208 ms with a standard error of 4 ms. For suprathreshold currents (Fig. 7, arrow), the latency for detecting the stimulation was typically less than the latency for detecting the visual stimulus.
From the data in Figure 7, we determined how latency varies as a function of depth for a fixed current of 20 μA current (Fig. 8). The shortest latency using such a current was 205 ms, and it occurred for stimulations of the deepest layers of V1 (i.e. 2.25 mm below the cortical surface).
Figure 8.
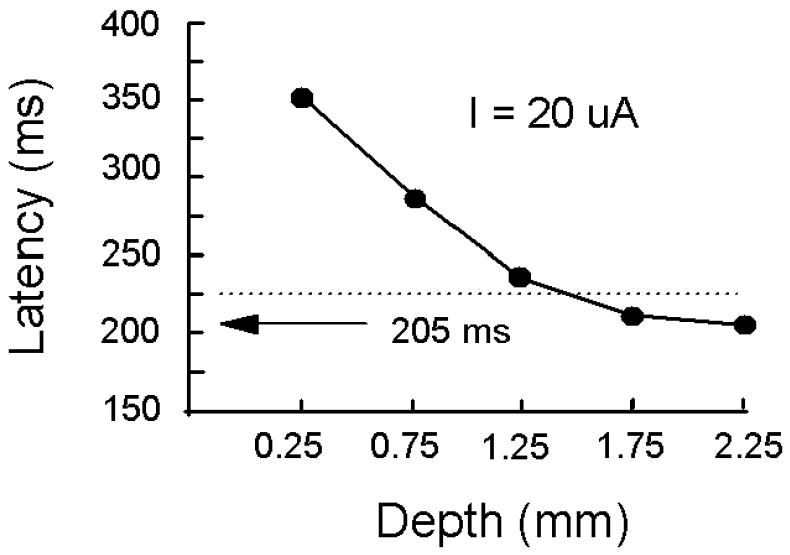
Latency over cortical depth. The latency to evoke a detection response is plotted as a function of the depth below the cortical surface using a 20 μA current. These data were derived from Figure 7 by interpolating between the data points to yield the latency values. The arrow indicates a minimal latency of 205 ms at the greatest cortical depth tested. The dotted curve indicates the average latency to detect the visual target (standard error = 3 ms; N = 5 blocks of 10 trials each). See Figure 7 for details.
Discussion
Our study establishes that the deepest layers of V1 (i.e. approximately > 1.5 mm below the cortical surface) are the most excitable for the elicitation of the detection response and suggests that such responses are mediated mainly by stimulation of axons rather than by stimulation of cell bodies and terminals. These conclusions are based on four observations. First, the lowest current thresholds for eliciting a detection response occurred when the electrode was positioned from 1.25 to 2.25 mm below the cortical surface. These thresholds could be as low as 2 μA, but the average minimal threshold was 6 μA. These threshold values agree with the lowest thresholds observed by others using a behavioural paradigm based on saccade generation (Murphey & Maunsell, 2007). Second, the shortest chronaxies of stimulated V1 neuronal elements mediating the detection response occurred between 1.6 and 2.5 mm below the cortical surface (i.e. ranging from 0.11 to 0.15 ms). Third, the shortest latency for the detection response—i.e. < 200 ms—occurred when the deepest layers of V1 (e.g. 2.25 mm below the cortical surface) were stimulated. Such latencies have been observed previously for stimulation of macaque V1 (Miller & Glickstein, 1967). Finally, for the deepest portions of V1 (i.e. > 1.75 mm below the cortical surface) cathode-first pulses were more effective at eliciting a detection response than were anode-first pulses. As mentioned in the introduction, cathodal pulses are superior to anodal pulses at activating axons over cell bodies and terminals (Fritsch & Hitzig, 1879; Porter, 1963; Stoney et al., 1968; Clendenin et al., 1974; Ranck, 1975). Accordingly, monkeys are most sensitive to the activation of the projections activated from the deepest layers of V1. This concurs with observations regarding V1 stimulation made by Doty and colleagues decades ago (Bartlett & Doty,1980; Rutledge & Doty, 1962).
In our experiments, the visual receptive fields of the stimulated V1 cells were identified to show that our stimulation sites contained neurons characteristic of V1. This, however, cannot rule out the possibility that our electrical stimulation was antidromically activating cell bodies that were located outside the field of stimulation but whose axons just happened to pass through this field.
Excitability of V1 neural elements across cortical depth for electrically evoked responses
Several studies have been conducted looking at how the current threshold to evoke a behavioural response from V1 varies with cortical depth (Bak et al., 1990; Tehovnik et al., 2003a; Tehovnik et al., 2004). Data from these studies are illustrated (Fig. 9, left panels). Tehovnik et al. (2003a) found that the current threshold for evoking saccadic eye movements by stimulation of V1 of monkeys was least for activations of V1 sites situated between 1.5 and 2.25 mm below the cortical surface (Fig. 9, top left panel). Here currents below 5 μA were effective at eliciting saccades into the receptive field of the stimulated neuronal elements. It is noteworthy that the parameters of electrical stimulation used in the study of Tehovnik et al. (2003a) were the same as those used in the present study to study the detection response (i.e. 0.2 ms cathode-first pulse delivered at 200 Hz within a 100 ms train, Fig. 3). Additionally, it was found that at depths beyond 1.75 mm below the cortical surface, cathode-first pulses were more effective than anode-first pulses at evoking saccadic eye movements. This again is similar to the results of the present study regarding the detection response (Fig. 6). This suggests that the neural elements that mediate the detection response from V1 are similar to those that mediate the electrical evocation of saccadic eye movements from this region.
Figure 9.
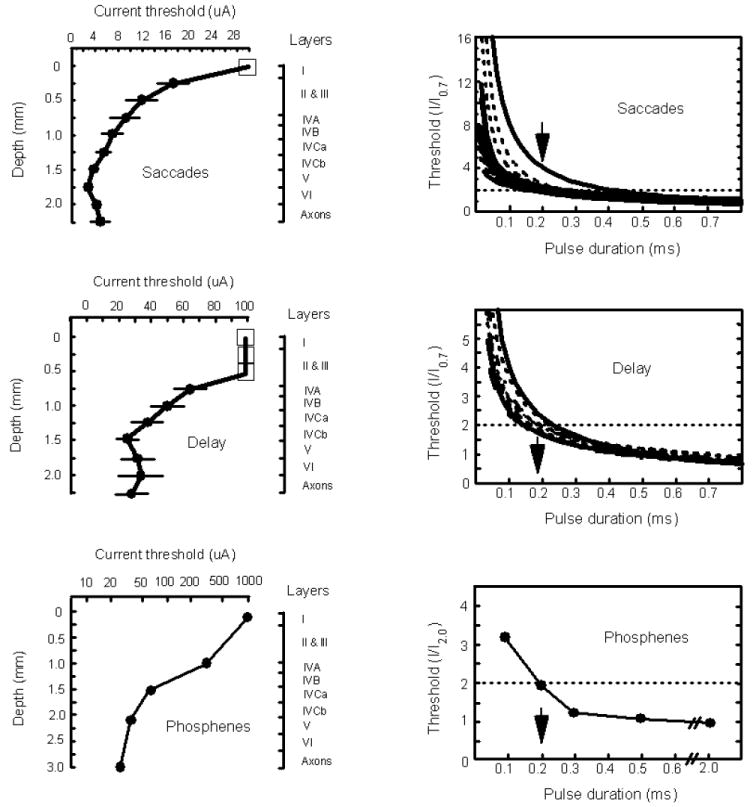
Current threshold and chronaxie functions for saccades, saccadic delays, and phoshenes induced from V1. Left panels illustrate current threshold functions: (top, left) The current threshold to evoke a saccadic eye movements on 50% of stimulation trials is plotted as a function of cortical depth for macaque V1. The square marker indicates that saccades were not elicited using a maximal current of 30 μA. Standard errors of the mean are shown. Illustrated to the right is the lamination of V1 over a 2.25 mm depth starting from the top of V1 (Peters and Sethares, 1991). Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 200 Hz, and 100 ms, respectively. Cathode-first pulses were used for all experiments. For other details see Tehovnik et al. (2003a). (middle, left) The current threshold to evoke a 20 ms saccadic delay of visually guided saccades made into the visual receptive field of the stimulated neuronal elements is plotted as a function of cortical depth for macaque V1. The square marker indicates that a delay was not elicited using a maximal current of 100 μA. Standard errors of the mean are shown. Illustrated to the right is the lamination of V1 over a 2.25 mm depth starting from top of V1 (Peters and Sethares, 1991). Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 200 Hz, and 100 ms, respectively. Anode-first pulses were used for all experiments. For other details see Tehovnik et al. (2004). (bottom, left) The current threshold to evoke a phosphene from human V1 is plotted as a function of cortical depth for one penetration. The current is represented on a log scale. Illustrated to the right is the approximate lamination of human V1 (Rockel et al., 1980). Pulse duration, pulse frequency, and train duration were fixated at 0.2 ms, 100 Hz, and 1000 ms, respectively. Anode-first pulses were used for all experiments. For other details see Bak et al. (1990). Right panels illustrate chronaxie functions: (top, right) Normalized threshold current for evoking a saccadic eye movement on 50% of stimulation trials is plotted as a function of pulse duration for macaque V1. For a pulse duration of 0.7 ms, the current required to evoke a saccade on 50% of stimulation trials is set to one and all other values are expressed as a multiple of the current used at the 0.7-ms pulse duration. The pulse duration at which a curve intersects two units of threshold (designated by the dotted horizontal line) indicates the chronaxie of the stimulated elements at the site of study. The arrow indicates an average chronaxie of 0.2 ms. Pulse frequency and train duration were fixated at 100 Hz and 100 ms, respectively. Cathode-first pulses were used for all experiments. For other details see Tehovnik et al. (2003a). (middle, right) Normalized threshold current for evoking a 20 ms delay of visually guided saccades made into the visual receptive field of the stimulated neuronal elements is plotted as a function of pulse duration for macaque V1. For a pulse duration of 0.7 ms, the current required to evoke a 20 ms delay is set to one and all other values are expressed as a multiple of the current used at the 0.7-ms pulse duration. The pulse duration at which a curve intersects two units of threshold (designated by the dotted horizontal line) indicates the chronaxie of the stimulated elements at the site of study. The arrow indicates an average chronaxie of 0.18 ms. Pulse frequency and train duration were fixated at 100 Hz and 100 ms, respectively. Anode-first pulses were used for all experiments. For other details see Tehovnik et al. (2004). (bottom, right) Normalized threshold current for evoking a phosphene is plotted as a function of pulse duration based on the average of five site studied over the surface of human V1. For a pulse duration of 2 ms, the current required to evoke a phosphene is set to one and all other values are expressed as a multiple of the current used at the 2-ms pulse duration. The pulse duration at which a curve intersects two units of threshold (designated by the dotted horizontal line) indicates the chronaxie of the stimulated elements. The arrow indicates a chronaxie of 0.2 ms. Pulse frequency and train duration was fixated at 100 Hz and 100 ms, respectively. Cathodal pulses were used for all experiments. For other details see Rushton & Brindley (1978).
Although electrical stimulation of macaque V1 can readily evoke saccadic eye movements using minute currents as long as the stimulation is delivered after the termination of the fixation spot (Tehovnik et al., 2003a,b), if the electrical stimulation is delivered during the fixation period immediately before an animal generates a visually guided saccade to a target in the receptive field of the stimulated neuronal elements the stimulation now delays saccade generation (Tehovnik et al., 2004). As with the detection response, the current threshold to evoke a saccadic delay was minimal from 1.5 to 2.25 mm below the cortical surface (cf. Fig. 9, middle left panel and Fig. 3).
Finally, it has been shown by Bak et al. (1990) that the current threshold for evoking phosphenes by stimulation of human V1 was least when the stimulation was delivered to the deepest layers of V1 (Fig. 9, bottom left panel). Furthermore, cathode-first pulses were more effective than anode-first pulses for stimulation of the deepest layers (Bak et al., 1990; Schmidt et al., 1996). These results agree with the present results for the detection response (Figs. 3 & 6). Therefore, every time a monkey detects a current delivered to V1 it may very well be experiencing a phosphene comparable to that experienced by humans as elicited by V1 stimulation.
Three independent studies—two in monkeys (Tehovnik et al., 2003a, 2004) and one in humans (Bak et al., 1990)—agree with the conclusion of present study that the deepest layers of V1 are the most excitable for the elicitation of behavioural responses. Furthermore, DeYeo et al. (2005) also found low threshold sites for generating the detection response in layer V of V1, which is in agreement with the present study, but they additionally reported such sites in layer III.
Chronaxies of V1 neuronal elements mediating electrically evoked responses
In the present study it was found that the chronaxies of the directly stimulated neuronal elements in V1 mediating the detection response had an overall average of 0.19 ms and ranged from 0.11 to 0.24 ms. This range overlaps with that previously report for macaque V1 using both micro- and macroelectrodes for the evocation of the detection response (Bartlett et al., 2005). Chronaxie values have been derived for saccadic eye movements evoked from macaque V1 (Fig. 9, top right panel; Tehovnik et al., 2003a), for the delay of visually guided saccades evoked from macaque V1 (Fig. 9, middle right panel; Tehovnik et al., 2004), and for phosphenes evoked from human V1 (Fig. 9, bottom right panel; Rushton and Brindley, 1978). In all cases, the average chronaxie values of the neuronal elements mediating these behaviours were comparable (i.e. about 0.2 ms) to those of neuronal elements subserving the detection response. A value of approximately 0.2 ms suggests that the directly stimulated neuronal elements in V1 mediating detection, saccadic eye movements, saccadic delays, and phosphenes are composed of similar elements and that these elements are made up of large pyramidal fibres (Stoney et al., 1968; Asanuma et al., 1976; Peters & Sethares, 1991). This conclusion is consistent with the observation that many simple and complex cells exhibit a pyramidal morphology and that these cells are readily activated by visual stimuli (Hubel and Wiesel, 1977; Gilbert & Wiesel, 1979).
Given that the chronaxies for detection, saccades, and phosphenes are similar, this further bolsters the idea that every time stimulation is delivered to V1, monkeys experience a visual percept. Moreover, this is true regardless of whether the behavioural response is a lever press or a saccadic eye movement. Thus V1 should not be regarded as a motor area per se, but rather a sensory area that can channel visual information through different motor systems.
Effect of anodal versus cathodal pulses on the detection response for V1 stimulation
As mentioned in the introduction, electrical stimulation preferentially activates axons over cell bodies (Porter, 1963; Landau et al., 1965; Stoney et al., 1968; Gustaffson and Jankowska, 1976; Nowak & Bullier, 1998; Swadlow, 1992; Rattay, 1999; McIntyre & Grill, 2002). It is the outward current at the initial segment or nodes of Ranvier that triggers an action potential (Ranck, 1975). An anodal pulse generates such a current at the initial segment or node of Ranvier of an axon if the electrode is positioned at the cell body, and a cathodal pulse generates such a current if the electrode is positioned at the axon. This means that anodal and cathodal pulses should have differential effects on the evocation of responses based on where the electrode is located with respect to the cell body. Porter (1963) found that when stimulation was delivered to the hypoglossal complex in the medulla, tongue motor responses were more readily evoked using an anodal pulse when the electrode was positioned in the nucleus of the complex and such responses were more readily evoked using a cathodal pulse when the electrode was positioned in the fibre tract of the complex. The results of our present study are consistent with this observation in that anodal pulses were more effective at evoking a detection response when stimulating superficial regions of V1—which contain a higher proportion of cell bodies to axons as compared to the deepest portions of V1 (Peters, 1994)—and cathodal pulses were more effective at evoking a detection response when stimulating the deepest regions of V1—which contain primarily output axons (Peters, 1994). This finding is also consistent with what we have previously observed for the electrical elicitation of saccades from V1 (Tehovnik et al., 2003a). As well, cathodal pulses were more effective than anodal pulses for the evocation of phosphenes from the deepest portions of human V1 (Bak et al., 1990; Schmidt et al., 1996).
One reason why electrical stimulation of the deepest layers of V1 are the most effective for evoking behavioural responses irrespective of the stimulation polarity (current study; Bak et al. 1990; Tehovnik et al. 2003a; Tehovnik et al. 2004) might be because the vertically-oriented myelinated axons in V1 are more prominent here (Peters and Sethares, 1996) and because the diameter of these fibres might be the highest here (Haug et al., 1976).
The latency of the detection response elicited electrically from V1
The shortest latency of the detection response occurred for stimulations of the deepest layers of V1 (Fig. 7E). When a fixed current of 20 μA was used, the shortest latency (< 225 ms) was evident at 1.75 and at 2.25 below the cortical surface (Fig. 8). Such a current is estimated to activate tissue directly within 172 μm from the electrode tip (estimate based on the current-distance relationship—r = (I/K)1/2—where r is the radial spread of current, K is the current-distance constant of 675 μA/mm2, and I is a current of 20 μA; see Tehovnik et al., 2006); therefore, this current was confined to the output layers of V1, which are roughly 500 μm thick (i.e. layers V & Vl; Peters & Sethares, 1991). A similar result was observed for the evocation of saccadic eye movements when a 20 μA current was delivered to the deepest layers of V1 (Tehovnik et al., 2003a). Stimulation of the deepest layers (i.e. 2.0 mm below the cortical surface) evoked saccadic eye movements at the shortest latencies (i.e. < 70 ms).
It has been suggested that saccadic eye movements elicited electrically from V1 are dependent on the corticotectal pathway (Tehovnik et al., 2003a). The deepest layers of V1 send direct projections to the superior colliculus (Spatz et al., 1970; Lund et al., 1975; Fries, 1984), and ablations of the superior colliculus abolish all saccades elicited electrically from V1 (Schiller, 1977; Keating et al., 1983; Keating & Gooley, 1988a). Some have argued that the superior colliculus also mediates forelimb movements (Werner et al., 1997ab). Whether the transection of projections between V1 and the superior colliculus or between V1 and some other subcortical site in monkeys (Spatz et al., 1970; Lund et al., 1975) can abolish the detection response is not known. Rutledge and Doty (1962) were able to largely abolish the detection response from cat visual cortex (areas V1 and V2) after cutting the output fibres from the deepest layers, but they could reinstate the response with additional training and by doubling the intensity of the electrical current. When they surgically isolated the visual cortex from other cortical areas, however, the detection response was spared.
Electrical stimulation of V1 to elicit phosphenes
Electrical stimulation delivered to the occipital cortex induces humans to report the appearance of phosphenes in their visual field (Penfield & Rasmussen, 1957). A phosphene has been described as a bright spot of light varying in size of up to roughly 2 degrees of visual angle (Dobelle & Mladejovsky, 1974; Schmidt et al., 1996). Stimulation parameters similar to those used in the current study (i.e. currents of 2 to 30 μA; pulse durations of 0.2 ms; train durations of 125 ms; and frequencies of 200 Hz; cathode-first biphasic pulses) have been effective at evoking phosphenes from V1 of humans when using microelectrodes (Schmidt et al., 1996).
As alluded to earlier, the chronaxies of elements that mediate phosphenes in humans are similar to those that mediate the detection response in monkeys (current study; Brindley & Lewin, 1968; Dobelle & Mladejovsky, 1974; Rushton & Brindley, 1978; Bartlett et al. 2005). This similarity is one reason why the detection response has been used to study phosphene induction in monkeys.
It is well established that the detection response can be evoked electrically from many regions of the brain including the lateral geniculate nucleus, the striate and extrastriate cortex, the pulvinar, and the superior colliculus (for a complete list see Doty, 1965). Electrical stimulation of all these areas in humans is known to evoke phosphenes (Marg & Dierssen, 1965; Brindley & Lewin, 1968; Nashold, 1970; Dobelle & Mladejovsky, 1974; Rushton & Brindley, 1978; Tasker et al., 1980; Bak et al., 1990; Schmidt et al., 1996; Lee et al., 2000). Stimulation of macaque V1 is believed to generate a qualitatively uniform percept irrespective of where in the V1 map the stimulation is applied, because the effect of stimulating one site in V1 to evoke a detection response is immediately generalized to another site in V1 (Doty, 1965; Schuckman et al., 1966; Doty, 1969; Schuckman et al., 1970; Bartlett et al., 2005). The same is not true when the detection response needs to be transferred between different anatomical structures such as between V1 and the lateral geniculate nucleus or between V1 and extrastriate cortex (Doty, 1965, 1969; Schuckman et al., 1970; Bartlett et al., 2005). Additional training is required to transfer the response between distinct anatomical structures (Doty, 1965, 1969; Schuckman et al., 1970; Bartlett et al., 2005). Poor transfer between sites has been used to infer that qualitatively different percepts are being generated by the stimulation of different anatomical structures (Doty, 1965; Schuckman et al., 1970).
For many of the ‘transfer’ experiments, the currents used were greater than 50 μA and up to 1000 μA (Doty, 1965; Schuckman et al., 1970; Bartlett et al., 2005). These currents activate up to an entire hypercolumn (Tehovnik et al., 2006). Hence it is not surprising that such stimulation of V1 induces a common percept irrespective of which hypercolumn is stimulated (Doty, 1965, 1969; Schuckman et al., 1970; Bartlett et al., 2005) given that a hypercolumn is presumed to be the functional unit of V1 (Hubel and Weisel, 1977). Transfer between various sites of stimulation within V1 might not occur as readily using extremely low currents that activate a minute portion of a hypercolumn, thereby eliciting phosphenes whose perceptual characteristics depend on the site of stimulation (Schmidt et al., 1996; Tehovnik and Slocum, 2007a,b). For instance, a site generating a red phosphene might be judged differently from a site generating a blue phosphene. Clearly, more experiments will need to be performed using low currents to address this issue.
Pathways utilized by V1 to transmit the phosphene signal
Two pathways have been proposed by which neocortex gains access to the saccade generator in the brainstem for the generation of visually-guided saccadic eye movements: one pathway that projects from striate and extrastriate cortex through the superior colliculus and a second pathway that projects from the frontal eye fields to the brainstem (Schiller and Tehovnik, 2001). Lesions of both the superior colliculus and the fontal eye fields abolish all visually guided saccades while lesions of either structure alone spare these saccades (Schiller et al., 1980; Keating and Gooley, 1988b). What needs to be determined is whether training monkeys on visual tasks can recover the saccadic eye movements evoked electrically from V1 that are lost following collicular lesions (Schiller, 1977; Keating et al., 1983; Keating & Gooley, 1988a). After collicular lesions the pathway to the saccade generator via the frontal eye fields is still intact and therefore possibly accessible to a phosphene signal generated electrically in V1. As previously mentioned, following transection of corticofugal fibres from visual cortex (areas V1 and V2) the detection response can be reinstated with training (Rutledge and Doty, 1962).
Conclusions
The detection response evoked electrically from the deepest layers of macaque V1 exhibit the lowest current threshold, the shortest chronaxies, and the shortest latencies. Also at these depths cathodal pulses are more effective at evoking a response than are anodal pulses, thereby suggesting that the output axons from V1 are being activated by the stimulation to generate the detection response. Finally, we believe that it is electrical stimulation experiments on animals of the sort described here that should eventually lead to the development of prosthetic devices for the restoration of sensory and motor functions in disabled individuals (Otto et al., 2005; Leal-Campanario et al., 2006; Butovas and Schwarz, 2007; Fitzsimmons et al., 2007; Pezaris and Reid, 2007; Cohen, 2008; Schiller and Tehovnik, 2008).
Acknowledgments
This work was supported by NIH EY014884 to Dr. P. H. Schiller. We thank Dr. P. H. Schiller for designing the levers.
Abbreviations
- V1
striate cortex
References
- Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tact cells by intracortical microstimulation. Exp Brain Res. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med Biol Eng Comput. 1990;28:257–259. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Critical limiting factors in the design of the eye and visual cortex. Proc R Soc Lond B Biol Sci. 1981;212:1–34. doi: 10.1098/rspb.1981.0022. [DOI] [PubMed] [Google Scholar]
- Bartlett JR, DeYoe EA, Doty RW, Lee BB, Lewine JD, Negrão N, Overman WH., Jr Psychophysics of electrical stimulation of striate cortex in macaques. J Neurophysiol. 2005;94:3430–3442. doi: 10.1152/jn.00406.2005. [DOI] [PubMed] [Google Scholar]
- Bartlett JR, Doty RW. An exploration of the ability of macaques to detect microstimulation of striate cortex. Acta Neurobiol Exp. 1980;40:713–728. [PubMed] [Google Scholar]
- Bradley DC, Troyk PR, Berg JA, Cogan M, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt EM, Towle VL, Xu H. Visuotopic mapping through a multichannel stimulating implant in primate V1. J Neurophysiol. 2005;93:1659–1670. doi: 10.1152/jn.01213.2003. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of visual cortex. J Physiol (Lond) 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovos S, Schwarz C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. Eur J Neurosci. 2007;25:2161–2169. doi: 10.1111/j.1460-9568.2007.05449.x. [DOI] [PubMed] [Google Scholar]
- Clendenin M, Ekerot C-F, Oscarsson O, Rosén I. The lateral reticular nucleus in the cat. I. Mossy fibre distribution in cerebellar cortex. Exp Brain Res. 1974;21:473–486. doi: 10.1007/BF00237166. [DOI] [PubMed] [Google Scholar]
- Cogan SF. Neural stimulation and recording electrodes. Ann Rev Neurosci. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Lewine JD, Doty RW. Laminar variation in threshold for detection of electrical stimulation of striate cortex by macaques. J Neurophysiol. 2005;94:2443–3450. doi: 10.1152/jn.00407.2005. [DOI] [PubMed] [Google Scholar]
- Dobelle WH, Mladejovsky MG. Phosphenes produced by electrical stimulation of humans occipital cortex, and their application to the development of a prosthesis for the blind. J Physiol (Lond) 1974;243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RW. Conditioned reflexes elicited by electrical stimulation of the brain in macaques. J Neurophysiol. 1965;28:623–640. doi: 10.1152/jn.1965.28.4.623. [DOI] [PubMed] [Google Scholar]
- Doty RW. Electrical stimulation of the brain in behavioral context. Ann Rev Psych. 1969;20:289–320. doi: 10.1146/annurev.ps.20.020169.001445. [DOI] [PubMed] [Google Scholar]
- Doty RW, Rutledge LT. “Generalization” between cortically and peripherally applied stimuli eliciting conditioned reflexes. J Neurophysiol. 1959;22:428–435. doi: 10.1152/jn.1959.22.4.428. [DOI] [PubMed] [Google Scholar]
- Farber SA, Bogdanov M, Marshall DL, Tehovnik EJ. Excitability of neural elements within rat corpus striatum. J Neurosci Meth. 1997;76:93–104. doi: 10.1016/s0165-0270(97)00090-3. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, Hanson TL, Lebedev MA, Nicolelis MAL. Primate reaching cued by multichannel spatiotemporal cortical microstimulation. J Neurosci. 2007;27:5593–5602. doi: 10.1523/JNEUROSCI.5297-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Fritsch G, Hitzig E. Über die electrische Erregbarkeit des Grosshirns. Arch Anat Physiol. 1879;37:300–332. [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterized neurons in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gustaffson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug H, Kölln M, Rast A. The postnatal development of myelinated nerve fibres in the visual cortex of the cat. Cell Tiss Res. 1976;167:265–288. doi: 10.1007/BF00224332. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–159. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Keating EG, Gooley SG. Disconnection of parietal and occipital access to the saccadic oculomotor system. Exp Brain Res. 1988a;70:385–398. doi: 10.1007/BF00248363. [DOI] [PubMed] [Google Scholar]
- Keating EG, Gooley SG. Saccadic disorders caused by cooling the superior colliculus or the frontal eye fields, or from combined lesions of both structures. Brain Res. 1988b;438:247–255. doi: 10.1016/0006-8993(88)91343-1. [DOI] [PubMed] [Google Scholar]
- Keating EG, Gooley SG, Pratt S, Kelsey JE. Removing the superior colliculus silences eye movements normally evoked from stimulation of the parietal and occipital eye fields. Brain Res. 1983;269:145–148. doi: 10.1016/0006-8993(83)90971-x. [DOI] [PubMed] [Google Scholar]
- Konorski J, Lubińska L. Sur un precede nouveau d’élaboration et reflexes conditionnels du II type et sur les changements d’excitabilité du center cortical moteur au cours de l’apprentissage. Acta Biol Exptl. 1939;13:143–152. [Google Scholar]
- Landau WM, Bishop GH, Clare MH. Site of excitation in stimulation of the motor cortex. J Neurophysiol. 1965;28:1206–1222. doi: 10.1152/jn.1965.28.6.1206. [DOI] [PubMed] [Google Scholar]
- Leal-Campanario R, Delgado-Garcia JM, Gruart A. Microstimulation of the somatosensory cortex can substitute for vibrissa stimulation during pavlovian conditioning. Proc Natl Acad Sci (USA) 2006;103:10052–10057. doi: 10.1073/pnas.0603584103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Hong SB, Seo DW, Tae TS, Hong SC. Mapping of functional organization in human visual cortex. Neurol. 2000;54:849–854. doi: 10.1212/wnl.54.4.849. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Verneir acuity, crowding and cortical magnification. Vision Res. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Li CL, Bak A. Excitability characteristics of the A- and C-fibres in the peripheral nerve. Exp Neurol. 1976;50:67–79. doi: 10.1016/0014-4886(76)90236-3. [DOI] [PubMed] [Google Scholar]
- Loucks RB. The experimental delimitation of neural structures essential for learning: the attempt to condition stripped muscle responses with faradization of the signmoid gyri. J Psychol. 1935-36;1:5–44. [Google Scholar]
- Lund JS, Lund RD, Hendrickson RD, Bunt AE, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975;164:287–304. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Marg E, Dierssen G. Reported visual percepts from stimulation of the human brain with microelectrodes during therapeutic surgery. Confin Neurol. 1965;26:57–75. [PubMed] [Google Scholar]
- Matthews G. Neural substrate for brain stimulation reward in the rat: cathodal and anodal strength duration properties. J Comp Physiol Psych. 1977;91:858–875. doi: 10.1037/h0077373. [DOI] [PubMed] [Google Scholar]
- McIntyre JT, Grill WM. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol. 2002;88:1592–1604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- Miller JM, Glickstein M. Neural circuits involved in visuomotor reaction time in monkeys. J Neurophysiol. 1967;30:399–414. [Google Scholar]
- Murphey DK, Maunsell JHR. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS., Jr Phosphenes resulting from stimulation of the midbrain in man. Arch Ophthal. 1970;84:433–435. doi: 10.1001/archopht.1970.00990040435006. [DOI] [PubMed] [Google Scholar]
- Nielson HC, Davis KD. Effect of frontal ablation upon conditioned responses. J Comp Physiol Psych. 1966;61:380–387. doi: 10.1037/h0023244. [DOI] [PubMed] [Google Scholar]
- Nielson HC, Knight JM, Porter PB. Subcortical conditioning, generalization, and transfer. J Comp Physiol Psych. 1962;55:168–173. doi: 10.1037/h0043129. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp Brain Res. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- O’Kusky J, Colonnier M. A laminar analysis of the number of neurons, glia, and synapses in the visual cortex (area 17) of adult macaque monkeys. J Comp Neurol. 1982;210:278–290. doi: 10.1002/cne.902100307. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Rousche PJ, Kipke DR. Microstimulation in auditory cortex provides a substrate for detailed behaviors. Hearing Res. 2005;210:112–117. doi: 10.1016/j.heares.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex in Man. The Macmillan Company; New York: 1957. pp. 1–241. [Google Scholar]
- Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey’s retina: implications for central magnification factors. Vision Res. 1985;25:1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Peters A. The organization of the primary visual cortex. In: Peters A, Rockland KS, editors. Cerebral Cortex. Vol. 6. Plenum Press; New York: 1994. pp. 1–35. [Google Scholar]
- Peters A, Sethares C. Myelinated axons and the pyramidal cell modules in monkey primary visual cortex. J Comp Neurol. 1991;365:232–255. doi: 10.1002/(SICI)1096-9861(19960205)365:2<232::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Organization of pyramidal neurons in area 17 of monkey visual cortex. J Comp Neurol. 1996;306:1–23. doi: 10.1002/cne.903060102. [DOI] [PubMed] [Google Scholar]
- Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci USA. 2007;104:7670–7675. doi: 10.1073/pnas.0608563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. Focal stimulation of hypoglossal neurons in the cat. J Physiol (Lond) 1963;169:630–640. doi: 10.1113/jphysiol.1963.sp007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Rattay F. The basic mechanisms for the electrical stimulation of the nervous system. Neurosci. 1999;89:335–346. doi: 10.1016/s0306-4522(98)00330-3. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hoirns RW, Powell TPS. The basic uniformity of structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Rushton DN, Brindley GS. Properties of cortical electrical phosphenes. In: Cools SJ, Smith EL, editors. Frontiers in Visual Science. Springer-Verlag; New York: 1978. pp. 574–593. [Google Scholar]
- Rutledge LT, Doty RW. Surgical interference with pathways mediating responses conditioned to cortical stimulation. Exp Neurol. 1962;6:478–492. doi: 10.1016/0014-4886(62)90073-0. [DOI] [PubMed] [Google Scholar]
- Schein SJ. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. J Comp Neurol. 1988;269:479–505. doi: 10.1002/cne.902690403. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The effect of superior colliculus ablation on saccades elicited by cortical stimulation. Brain Res. 1977;122:154–156. doi: 10.1016/0006-8993(77)90672-2. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Look and see: how the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Visual prosthesis. Perception. 2008;37:1529–1559. doi: 10.1068/p6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol. 1980;44:1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O’Rourke DK, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain. 1996;119:507–522. doi: 10.1093/brain/119.2.507. [DOI] [PubMed] [Google Scholar]
- Schuckman H, Battersby WS. Frequency specific mechanisms in learning. II. Discriminatory conditioning induced by intracortical stimulation. J Neurophysiol. 1966;29:31–43. doi: 10.1152/jn.1966.29.1.31. [DOI] [PubMed] [Google Scholar]
- Schuckman H, Kluger A, Frumkes TE. Stimulus generalization within the geniculostriate system of the monkey. J Comp Physiol Psych. 1970;73:494–500. doi: 10.1037/h0030212. [DOI] [PubMed] [Google Scholar]
- Spatz WB, Tigges J, Tigges M. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri) J Comp Neurol. 1970;140:155–173. doi: 10.1002/cne.901400203. [DOI] [PubMed] [Google Scholar]
- Stoney SD, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical stimulation: effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Monitoring the excitability of neocortical efferent neurons to direct activation by extracellular current pulses. J Neurophysiol. 1992;68:605–619. doi: 10.1152/jn.1992.68.2.605. [DOI] [PubMed] [Google Scholar]
- Tasker RR, Organ LW, Hawrylyshyn P. Visual phenomena evoked by electrical stimulation of the human brainstem. Applied Neurophysiol. 1980;43:89–95. doi: 10.1159/000102240. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Lee K-M. The dorsomedial frontal cortex of the rhesus monkey: topographic representation of saccades evoked by electrical stimulation. Exp Brain Res. 1993;96:430–442. doi: 10.1007/BF00234111. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM. What delay fields tell us about striate cortex. J Neurophysiol. 2007a;98:559–576. doi: 10.1152/jn.00285.2007. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM. Phosphene induction by microstimulation of macaque V1. Brain Res Rev. 2007b;53:337–343. doi: 10.1016/j.brainresrev.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Carvey CE, Schiller PH. Phosphene induction and the generation of saccadic eye movements by striate cortex. J Neurophysiol. 2005;93:1–19. doi: 10.1152/jn.00736.2004. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Differential effects of laminar stimulation of V1 on target selection by macaque monkeys. Eur J Neurosci. 2002;16:751–760. doi: 10.1046/j.1460-9568.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Saccadic eye movements evoked by microstimulation of striate cortex. Eur J Neurosci. 2003a;17:870–878. doi: 10.1046/j.1460-9568.2003.02489.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Carvey CE. Behavioural state affects saccadic eye movements evoked by microstimulation of striate cortex. Eur J Neurosci. 2003b;18:969–979. doi: 10.1046/j.1460-9568.2003.02798.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Microstimulation of V1 delays the execution of visually guided saccades. Eur J Neurosci. 2004;20:264–272. doi: 10.1111/j.1460-9568.2004.03480.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Electrically evoked saccades from the dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Exp Brain Res. 1997;117:369–378. doi: 10.1007/s002210050231. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol. 2006;97:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Sultan F, Augath M, Oeltermann A, Tehovnik EJ, Schiller PH, Logothetis NK. Mapping cortical activity elicited with electrical microstimulation using fMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Troyk P, Bak M, Berg J, Bradley D, Cogan S, Erickson R, Kufta C, McCreery D, Schmidt E, Towle V. A model for intracortical visual prosthesis research. Artif Organs. 2003;27:1005–1015. doi: 10.1046/j.1525-1594.2003.07308.x. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann K-P. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1977a;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]
- Werner W, Hoffmann K-P, Dannenberg S. Anatomical distribution of arm-movement-related neurons in the primate superior colliculus and underlying reticular formation in comparison with visual and saccadic cells. Exp Brain Res. 1977b;115:206–216. doi: 10.1007/pl00005691. [DOI] [PubMed] [Google Scholar]
- West DC, Wolstencroft JH. Strength-duration characteristics of myelinated and non-myelinated bulbospinal axons in the cat spinal cord. J Physiol (Lond) 1983;337:37–50. doi: 10.1113/jphysiol.1983.sp014610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters WD, Kado RT, Adey WR. A Stereotaxis Brain Atlas for Macaca Nemestrina. University of California Press; Los Angeles: 1969. pp. 1–93. [Google Scholar]


