Abstract
IL-18 can be considered a proinflammatory cytokine mediating disease as well as an immunostimulatory cytokine that is important for host defense against infection and cancer. The high-affinity, constitutively expressed, and circulating IL-18 binding protein (IL-18BP), which competes with cell surface receptors for IL-18 and neutralizes IL-18 activity, may act as a natural antiinflammatory as well as immunosuppressive molecule. In the present studies, the IL-18 precursor caspase-1 cleavage site was changed to a factor Xa site, and, after expression in Escherichia coli, mature IL-18 was generated by factor Xa cleavage. Mature IL-18 generated by factor Xa cleavage was fully active. Single point mutations in the mature IL-18 peptide were made, and the biological activities of the wild-type (WT) IL-18 were compared with those of the mutants. Mutants E42A and K89A exhibited 2-fold increased activity compared with WT IL-18. A double mutant, E42A plus K89A, exhibited 4-fold greater activity. Unexpectedly, IL-18BP failed to neutralize the double mutant E42A plus K89A compared with WT IL-18. The K89A mutant was intermediate in being neutralized by IL-18BP, whereas neutralization of the E42A mutant was comparable to that in the WT IL-18. The identification of E42 and K89 in the mature IL-18 peptide is consistent with previous modeling studies of IL-18 binding to IL-18BP and explains the unusually high affinity of IL-18BP for IL-18.
Immune responses to tumors are primarily the function of T helper type 1 (Th1) lymphocytes. Th1 responses include the secretion of cytokines IL-2, IL-12, IL-18, and IFN-γ, as well as the generation of specific cytotoxic T lymphocytes recognizing specific tumor antigens. The Th1 response is also a vital arm of host defense against many microorganisms; however, the Th1 response also can be associated with several autoimmune diseases as well as organ transplant rejection.
IL-18 alone or together with IL-12 can be a potent adjuvant for reducing tumor growth and metastasis in models of immunogenic tumors (1, 2). In these experiments, the role of IL-18 appears to be the activation of natural killer (NK) cells and production of IFN-γ. However, using mouse models of cytokine adjuvants to increase the immunogenicity of tumor cells in human cancer patients has produced mixed results. Limitations due to systemic toxicity may explain the poor results. However, the use of graft versus leukemia or graft versus tumor has been expanded in recent studies. The use of the patient's own tumors fused to allogeneic dendritic cells and used as an immunogen (3) or the use of peripheral blood stem cell transplantation (4) has met with apparent success in inducing tumor regression in a majority of patients. These human trials will require confirmation, but these studies in human cancer immunotherapy are increasingly applied as a nonmyeloablative “minitransplant” (5, 6).
The method provides a basis for further exploitation of cytokine-based adjuvant effects in human immunotherapy for cancer. However, the perennial problem in using cytokines as adjuvants in inducing tumor immunogenicity remains the systemic toxicities of the administered cytokine. In the case of IL-18 and IL-12, the toxicities are likely to limit their use systemically. However, surface expression of cytokines by tumor cells or antigen-presenting cells provides a method for achieving local but not systemic activity. In the case of IL-18, there is the additional problem of overcoming neutralization by constitutively expressed and circulating IL-18 binding protein (IL-18BP) (7). The high affinity (400 pM) (8) and the 20-fold molar excess¶ likely function to keep the Th1 response low after infections and to avoid autoimmune disease in genetically predisposed individuals. The other side of the IL-18BP coin is that it may suppress the host's immune response to tumors. For example, some tumors produce IL-18BP constitutively.
In the present study, mutations in the IL-18 mature peptide were made and studied for their biological activities as well as the ability of IL-18BP to neutralize their effects. The precursor of IL-18 requires cleavage by the IL-1 converting enzyme (ICE, caspase-1) to yield an active, mature cytokine (9, 10). To maintain the structural integrity of the natural processing of IL-18, the precursor of human IL-18 was first expressed and purified, and then the mature IL-18 peptide was generated. However, the ICE site was replaced with a factor Xa recognition sequence, and mature IL-18 was generated by factor Xa rather than ICE cleavage (11).
Materials and Methods
Reagents and Cytokines.
RPMI 1640 culture medium was purchased from GIBCO/BRL (Grand Island, NY) and supplemented with 10 mM l-glutamine, 24 mM NaHCO3, 10 mM Hepes, 100 units/ml penicillin, and 100 μg/ml streptomycin (Cellgro, Waukesha, WI). FBS was obtained from GIBCO/BRL. Polymyxin B and histopaque-1077 were purchased from Sigma. Recombinant human IL-18 was generated by cleavage of proIL-18 with ICE (9) and was a kind gift from Vertex Pharmaceuticals (Cambridge, MA) as described previously (12). IL-2 was purchased from R & D Systems. Recombinant human IL-12 was a gift provided by PeproTech (Rocky Hill, NJ). The “a” isoform of human IL-18BP was expressed in Chinese hamster ovary cells as a his-6-tagged carboxy-terminal recombinant protein as previously described (8). IL-18BP was supplied by Dr. Ron Pincus (Interpharm Laboratories, Nes Ziona, Israel). Talon affinity columns were obtained from CLONTECH and used as recommended. For Western blot analysis, goat anti-mouse or donkey anti-rabbit peroxidase-conjugated antibodies were purchased from Jackson ImmunoResearch and developed with the use of enhanced chemiluminescence (New England Nuclear Life Science Products). The ELISA for human IL-18 was purchased from R & D Systems.
Mutagenesis and Expression Plasmid.
The human IL-18 cDNA was isolated as described (11). The cDNA of the human IL-18 precursor (proIL-18) was used for generating a mutation of IL-1β converting enzyme (ICE, caspase-1) cleavage site into a factor Xa site. Introduction of mutation in proIL-18 was performed by a two-step PCR reaction. The propiece of IL-18 cDNA was generated by using the sense primer containing the EcoRI site before the ORF, 5′-ATATGAATTCATGGCTGCTGAACCAGTAG, and a reverse primer was designed for the ICE site (33-LESD-36) to the factor Xa site (33-IEGR-36), 5′-AAAGTAACGTCCTTCGATGTTTTC. The mature piece of IL-18 cDNA was generated by using the sense primer that is complementary to the ICE site mutated into factor Xa, 5′-GAAAACATCGAAGGACGTTACTTT, and the reverse primer containing the BamHI site after the ORF of IL-18, 5′- ATATGGATCCTAGTCTTCGTTTTGAACAGTG. The pro and mature pieces of the IL-18 cDNAs were resolved by electrophoresis in 1% agarose, and the pro 150-bp and mature 450-bp bands were eluted by a gel extraction system (GIBCO/BRL). These two cDNA pieces were mixed at a 1:1 ratio and used for the template for the second PCR step to generate a complete human IL-18 cDNA in which the ICE site is mutated into the factor Xa site (ICE/Fx). The IL-18 (ICE/Fx) cDNA was ligated into a BlueScript vector (Stratagene) with the use of EcoRI and BamHI (GIBCO/BRL) restriction sites.
Three mutants, E42A, K89A, and E42A + K89A, were generated in the same manner with the use of the primers described below. The primer of E42A was 5′-GGCAAGCTTGCATCTAAATTA; the primer of K89A was 5′-AGTATGTATGCAGATAGCCAG. For the double mutation, E42A + K89A, the primer was formed with the use of the E42A mutation at the base of the K89A cDNA template. The wild type (WT) and each of the three IL-18 mutants were ligated into the Bluescript vector for sequence confirmation. For Escherichia coli expression, each of four IL-18 cDNA inserts were religated into the pPROEX HTa vector (GIBCO/BRL) with the use of EcoRI and XbaI sites.
Protein Expression and Purification.
Each of the four pPROEX HTa/IL-18 plasmids was transformed into the competent E. coli strain DHα (GIBCO/BRL) and expressed as described (11). An overnight culture of 25 ml was added to 450 ml of LB medium containing 100 μg/ml ampicillin and grown until it reached a density of 0.6–1 OD600. Protein expression was induced by adding isopropylthiogalactoside (0.3 mM), and incubation continued at 37°C with shaking for 3 h. The cultured bacteria cells were harvested by centrifugation (5,000 × g for 15 min at 4°C), and the pellet was suspended in 30 ml of Talon buffer (50 mM NaH2PO4/20 mM Tris⋅HCl/100 mM NaCl, pH 8). Cells were lysed by sonication (2 × 30-s bursts) on ice. The soluble protein was obtained by centrifugation (4,000 × g for 30 min at 4°C) and applied to a 3-ml mini-Talon column. The Talon column then was washed with 30 bed volumes of Talon buffer and then eluted with 6 ml of 100 mM imidazole in Talon buffer. The eluant was dialyzed against factor Xa buffer (20 mM Tris⋅HCl/150 mM NaCl/2 mM CaCl2) at 4°C for 20 h.
The 0.2 ml of Talon affinity-purified N-terminus His-6 fusion proIL-18 was incubated with 4 μg of factor Xa enzyme (New England Biolabs) for 4 h at room temperature in the presence of 2 mM phenylmethylsulfonyl fluoride (GIBCO/BRL). The cleaved mature WT and three mutants were resolved by 10% reducing SDS/PAGE followed by Coomassie blue staining.
Isolation and Culture of Peripheral Blood Mononuclear Cells (PBMCs).
These studies were approved by the Colorado Combined Investigational Review Board. Residual leukocytes from platelet pheresis of healthy human donors were rinsed from blood tubing and subjected to centrifugation over Histopaque. PBMCs were aspirated from the interface, washed three times in pyrogen-free saline (Baxter Health Care, Mundelein, IL), and resuspended at 5 × 106 cells per ml in RPMI 1640 medium. The cells were cultured in flat-bottomed 96-well plates (Becton Dickinson) with RPMI 1640 medium (control), varying concentrations of recombinant human IL-18, and WT IL-18 (ICE/Fx) or the three mutants, in the presence of 1 ng/ml IL-12. In some experiments, IL-18 preparations were first mixed with polymyxin B (1 μg/ml) before being added to the cells. Cells were incubated for 16–20 h at 37°C in humidified air with 5% CO2, and then the culture supernatant was collected for IFN-γ measurement.
NKO Cell Line.
The original NK92 cell line was obtained from Hans Klingerman (Rush Medical Center, Chicago). The human NKO cell line used in the present studies was a subclone of that cell line. NKO cell line (NKO) cells were maintained in supplemented RPMI 1640 medium containing 10% FBS and 50 pg/ml of IL-2 and 200 pg/ml of IL-15 (PeproTech). For assays, NKO cells were suspended at 0.5 × 106 cells per ml in RPMI 1640 medium and stimulated in 0.2-ml volumes in 96-well plates with 0.5 ng/ml of IL-12 and different concentrations of recombinant human IL-18, WT IL-18 (ICE/Fx), or the three mutants. After 16–20 h at 37°C in humidified air with 5% CO2, the culture supernatant was collected for IFN-γ measurement.
Analysis of Cytokines.
The liquid-phase electrochemiluminescence (ECL) method was used to measure IFN-γ (13) and IL-8 (12) in cell culture media. The amount of ECL was determined with the use of an Origen Analyzer (Igen, Gaithersburg, MD). The limit of detection of IFN-γ and IL-8 was 62 pg/ml and 40 pg/ml, respectively.
Immunoblotting.
The rabbit polyclonal antibody anti-human IL-18 was developed to the recombinant mature form of human IL-18 as described (14). This antiserum recognized precursor and mature human IL-18. The monoclonal anti-human IL-18 antibody clone 8-31-4 (IgG2a) was generated against the recombinant mature form of human IL-18 and characterized as described (15). The clone 8-31-4 recognizes both pro and mature forms of IL-18. Immunoblotting was performed with the use of a 1:500 dilution of the ascitic fluid of the monoclonal antibody; the polyclonal antibody was used at a 1:500 dilution of the antiserum. Human IL-18 preparations containing the IL-18 WT and mutants were resolved by SDS/PAGE (10% acrylamide) under reducing conditions. Gels were transferred to nitrocellulose membranes and then incubated with primary antibodies described above. After 24 h, goat anti-mouse and donkey anti-rabbit IgG peroxidase was added and developed by ECL (New England Nuclear).
Statistical Analysis.
Data are expressed as the mean ± SEM. Group means were compared by ANOVA, with the use of Fisher's least significant difference. Statistical significance was accepted within 95% confidence limits. ANOVA and correlation analyses were performed with the statistical packages statview 512 + (Brain Power, Calabasas, CA).
Results
Construction of WT and Mutants of proIL-18 Fusion Proteins for Cleavage by Factor Xa.
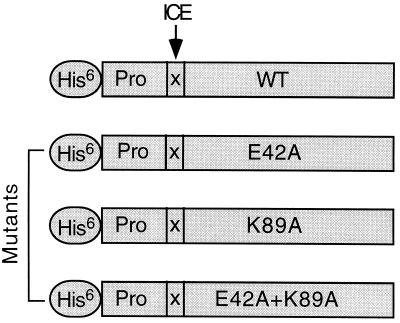
As shown in Fig. 1, the His-6 residue tag was expressed on the N terminus of the proIL-18, allowing for purification on Talon. The ICE cleavage site (LESD) of the WT proIL-18 molecule was replaced with the factor Xa site (IEGR) (indicated by the arrow). After purification on Talon, the proIL-18 was cleaved into a mature molecule by factor Xa. This WT IL-18/ICE/Fx was active in inducing IFN-γ from NKO cells and comparable in activity to that of recombinant human IL-18 generated by ICE cleavage of proIL-18 (9, 10) (data not shown). Also indicated in Fig. 1 are the locations of the amino acid exchanges for each of the three mutant IL-18.
Figure 1.
Mutated human IL-18 fusion proteins schema. The His-6 tag indicates the location of the six histidines fused in the N terminus of the IL-18 precursor propiece. The arrow indicates the ICE cleavage site as replaced by the factor Xa cleavage site (X). WT indicates wild-type mature IL-18. E42A indicates Glu-42 to Ala, K89A indicates Lys-89 to Ala, and E42A + K89A indicates the double mutation. On the basis of WT, three IL-18 mutants (E42A, K89A, and E42A + K89A) were generated by two-step PCR.
Antibody Recognition of WT and That of Mutant IL-18 Differ.
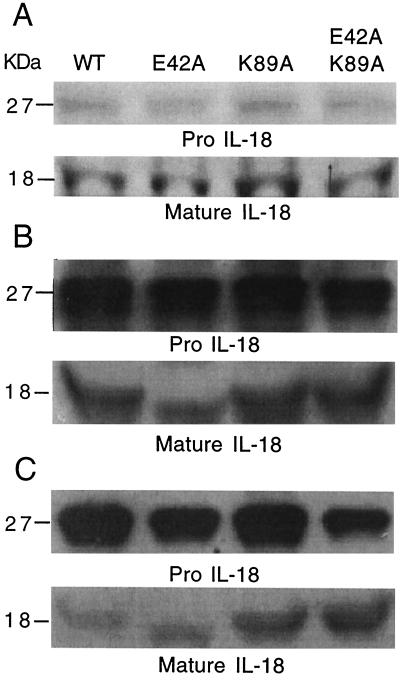
The WT and mutant IL-18 precursor forms, after Talon affinity purification, stained with comparable intensity by Coomassie blue (Fig. 2A Upper). After cleavage by factor Xa, the mature forms also stained with comparable intensity with Coomassie blue (Fig. 2A Lower). Therefore, one can conclude that any differences in antibody recognition of the WT and mutant forms is not due to an unequal presence of IL-18 protein, because these were the same preparations used for immunoblotting.
Figure 2.
Purified human IL-18 by Coomassie blue staining and Western blot. (A) E. coli expressing the His-6 fusion IL-18 was subjected to 10% SDS/PAGE and stained with Coomassie blue. The numbers on the left indicate molecular masses in kDa. Lanes are identified as WT, E42A, K89A, and E42A/K89A. (Upper) ProIL-18; (Lower) mature IL-18 after factor Xa cleavage. (B) Western blot of preparations shown in A. (Upper) ProIL-18 probed with rabbit anti-human IL-18 (1:500 dilution); (Lower) mature IL-18 probed with the same antibody. (C) Western blot of same preparations shown in A, probed with monoclonal anti-human IL-18 (1:500 dilution of ascitic fluid). (Upper) ProIL-18; (Lower) mature IL-18 probed with the same monoclonal antibody.
In Fig. 2B Upper, the staining of proIL-18 by polyclonal rabbit anti-human IL-18 was of near equal intensity for the WT and each of the three mutants. Similarly, the staining of the mature forms of WT IL-18 and each of the three mutants by the polyclonal antiserum was not significantly different (Fig. 2B Lower). In contrast, the staining of these forms of pro and mature IL-18 were different when the monoclonal antibody generated against mature human IL-18 was used (Fig. 2C). The two mutants, K89A and E42A + K89A, appear to stain more intensely than the WT or E42A, suggesting that the affinity of the monoclonal antibody is greater for these mutants. According to an ELISA for human IL-18, these same mutants yielded higher concentrations compared with the WT and E42A.
Increased Biological Activity of IL-18 Mutants.
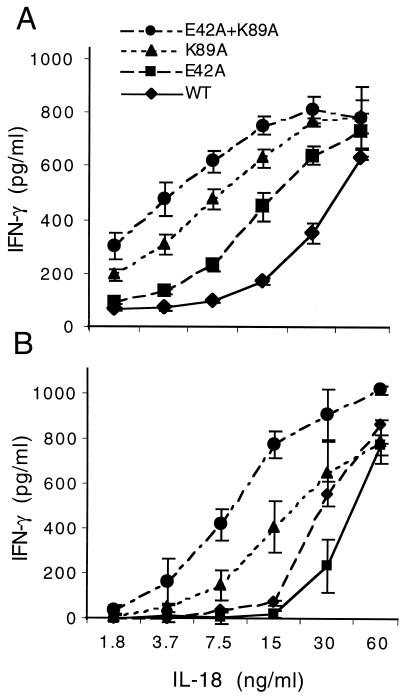
The purified mature forms of IL-18 ICE/Fx were assessed for the coinduction of IFN-γ in NKO cells. In general, IL-18 does not induce IFN-γ in these cells unless IL-12 (or IL-15) is used as a costimulator. Low concentrations of IL-12 (1–2 ng/ml) induce small amounts of IFN-γ; however, in the presence of such low concentrations of IL-12, IL-18 greatly augments IFN-γ production. As shown in Fig. 3A, the WT IL-18 was active as a coinducer of IFN-γ, beginning at 7.5 ng/ml and increasing progressively to 60 ng/ml (the highest concentration tested). However, each of the three mutant IL-18 mature forms exhibited biological activity greater than that of WT in these cells. For example, the single point mutation mutant of Glu-42 to Ala (E42A) was twice as active as the WT at each of the concentrations. The single point mutation of Lys-89 to Ala (K89A) at 15 ng/ml was four times more active than the WT. The double mutation of Glu-42 and Lys-89 (E42A + K89A) each to an Ala residue resulted in the most active IL-18. As shown in Fig. 3A, this mutant exhibited maximal activity in NKO cells at a concentration of 15 ng/ml, one-fourth the concentration of the WT and twice as active as the single point mutation K89A.
Figure 3.
Biological activity of different human IL-18 analogues. (A) NKO cells were stimulated with each of the different IL-18 analogues at concentrations shown under the horizontal axis in the presence of IL-12 (0.5 ng/ml). (B) PBMCs were stimulated with each of the different IL-18 analogues at concentrations shown under the horizontal axis in the presence of IL-12 (1.0 ng/ml). After 24 h, IFN-γ was measured; it is shown on the vertical axis in pg/ml (mean ± SEM). n = 3.
Similar findings were observed with IFN-γ production from freshly isolated human PBMCs. In these cells, the costimulation of IL-12 plus IL-18 results in IFN-γ, whereas neither cytokine induces IFN-γ alone. The double mutant (E42A + K89A) was the most active (Fig. 3B). In fact, the double mutant (E42A + K89A) induced significant IFN-γ production at a concentration of 3.7 ng/ml in the presence of 1 ng/ml of IL-12. Using both assays, we conclude that exchange of two charged amino acids (Glu and Lys) for Ala consistently increases the biological activity of IL-18, despite the differences in the cells used in these two assays.
Mutants of IL-18 Exhibit Decreased Neutralization by IL-18BP.
One can assume that the increased biological activity of the IL-18 mutants is due to increased binding of IL-18 to the IL-18Rα chain and that the IL-18α and IL-18Rβ chain complex with IL-18 results in a greater high-affinity complex. As a result, the mutants trigger the IL-18R complex to a greater extent than does the WT IL-18, with a resultant greater production of IFN-γ. In these two assays, the role for IL-18 binding to the naturally occurring IL-18BP was not specifically assessed but may contribute to the observations. Therefore, the ability of IL-18BP to neutralize the biological activity of IL-18 was specifically assessed. To assess neutralization of IL-18 biological activity by IL-18BP, IL-18BP was incubated with WT IL-18 or its mutants before being added to cell cultures.
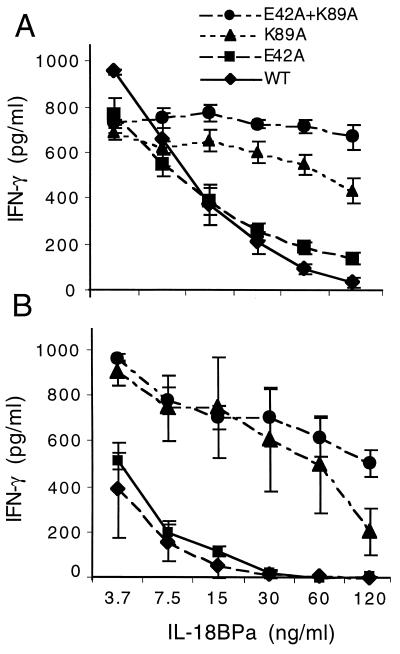
As shown in Fig. 4A, the 50% inhibitory concentration of IL-18BP for coinduction of IFN-γ by WT IL-18 from NKO cells was approximately 15 ng/ml. The single mutation of E42A resulted in a similar dose-inhibitory concentration by IL-18BP. However, when the mutant K89A was incubated with IL-18BP, the ability to act as a coinducer of IFN-γ in NKO cells was hardly neutralized (Fig. 4A). Only at a concentration of 120 ng/ml was there a statistically significant reduction. In contrast, there was near total failure of IL-18BP to neutralize the double IL-18 mutant E42A + K89A.
Figure 4.
Neutralization of four different human IL-18 analogues by IL-18BP. (A) Each of the four IL-18 analogues was mixed with IL-18BP at the concentrations indicated under the horizontal axis for 1 h at room temperature and then added to NKOs that were stimulated with IL-12 at 0.5 ng/ml. (B) Each of the four IL-18 analogues was mixed with IL-18BP at the concentrations indicated under the horizontal axis for 1 h at room temperature and then added to PBMCs that were stimulated with IL-12 at 1.0 ng/ml. After 24 h, IFN-γ was measured at the concentration shown to the left of the vertical axes. The data represent mean ± SEM. n = 3.
As shown in Fig. 4B, PBMCs are particularly sensitive to the neutralizing ability of IL-18 compared with NKO cells. The amount of IL-18BP needed to neutralize WT IL-18 was observed at 3.7 ng/ml, the lowest concentration tested. The single point mutation of E42A behaved similarly to that of WT IL-18 in that similar low concentrations of IL-18BP neutralized biological activity in PBMCs. In contrast, the single point mutation of K89A was neutralized at 120 ng/ml. Similar to the observations of neutralization of IL-18 mutants by IL-18BP in NKO cells, the double mutant E42A + K89A was only slightly affected by IL-18BP.
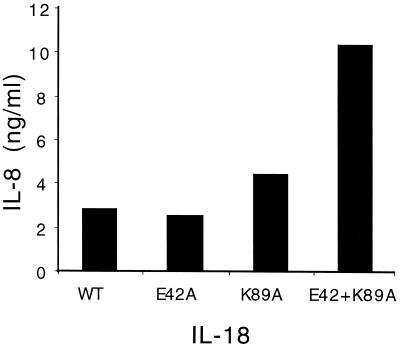
IL-18 Mutants with Increased Ability to Induce IL-8 Production from PBMCs.
As shown in Fig. 5, the ability of IL-18 to induce IL-8 was assessed. As with previous studies (12), the production of IL-8 from human CD14+ cells in the PBMC preparation requires higher concentrations of IL-18 than does induction of IFN-γ from T cells and NK cells; moreover, IL-18 induces IL-8 without a requirement for IL-12. Although the two single point mutations were comparable to the WT in the induction of IL-8, the double mutant IL-18 induced significantly more (2-fold) IL-8 (Fig. 5).
Figure 5.
Induction of IL-8 by IL-18 mutants. PBMCs were incubated with 30 ng/ml of either WT IL-18 or mutants. Polymyxin B (1 μg/ml) was mixed with IL-18 for 30 min before being added to the PBMCs. After 24 h, the supernatants were removed and assayed for IL-8 concentrations by ECL. One of three experiments is shown.
Discussion
In the present study, mutants were constructed in the IL-18 mature protein and assessed for biological activity for the induction of IFN-γ and IL-8 in the NKO cell line and in PBMCs. The replacement of charged amino acids Glu and Lys with Ala resulted in IL-18 mutants with enhanced biological activities compared with the WT IL-18. At first, one must consider that the mutations may have resulted in molecules of greater stability compared with the WT. However, the observation suggests that mutants with enhanced activity bind to the cell-surface IL-18R with a higher affinity compared with the WT IL-18 and that greater binding results in greater signal transduction. Alternatively, the binding of IL-18 mutants to the cells may not be significantly different from WT binding, but signal transduction may be greater. The dissociation of binding affinities with biological activities of IL-1β mutants is well described (16, 17). In the case of IL-1 mutants, receptor affinities are either unchanged or somewhat lower, but biological activity is decreased by 100-fold. In the case of IL-18, we have focused on the consequence of these mutations on biological activity rather than affinities for cell receptors.
Similar to other members of the IL-1 family of receptors, the signal-tranducing receptor complex of IL-18 is composed of a heterodimer of two structurally related but distinct Ig-like chains: the IL-18Rα chain (18), the primary ligand binding chain, and the IL-18Rβ chain (19), an essential signaling chain (8, 20). Soluble cytokine receptors found in the extracellular compartment are thought to down-regulate the biological activity of their respective cytokines. For IL-18, the soluble receptor is the IL-18Rα chain; for IL-1, the soluble IL-1 receptor types I and II bind each of the IL-1 family members (IL-1α, IL-1β, and IL-1 receptor antagonist). A similar situation exists for the two soluble receptors of tumor necrosis factor (21). In the case of IL-18, however, the dominant molecule that is found in the extracellular compartment that binds and neutralizes the activity of IL-18 is not the soluble form of the cell-bound IL-18Rα chain but rather the IL-18BP. The high affinity (400 pM) of IL-18BP for IL-18 (8) suggests that this molecule and not the soluble IL-18Rα chain regulates IL-18 activity in vivo. Although the likely sites for IL-18 binding to IL-18BP have been reported (8), there are no crystallographic analyses or cocrystallization of the ligand with the binding protein. Although one can assume that IL-18 binding to specific sites on the cell surface IL-18Rα chain and binding to the sites on IL-18BP are similar, it remains possible that additional and distinct binding sites exist.
Whatever the explanation for the increase in biological activity of the IL-18 mutants, the unexpected finding was the decrease in the ability of IL-18BP to neutralize the K89A mutant and the near total lack of neutralization of IL-18BP for the double mutant E42A + K89A. This observation was made in both NKO cells and PBMCs. The interaction of IL-18 with IL-18BP has been described through the use of computer modeling (8) and based on the interaction of IL-1β with the IL-1R type I (22). In the model for IL-18 binding to the IL-18BP, Glu at IL-18 position 42 and Lys at IL-18 position 89 have been proposed to bind to Lys-130 and Glu-114 in IL-18BP, respectively (8). The present data suggest that indeed these interactions may be correct.
The high affinity as well as the high concentration of IL-18BP in the circulation of healthy subjects (20-fold molar excess)¶ is a unique situation in cytokine biology. IL-18 is constitutively present in many cells (14) and circulates in healthy humans (23). Therefore, most, if not all, of the IL-18 circulating in healthy or unhealthy subjects is bound to the IL-18BP. For insulin-like growth factor, six binding proteins exist that can either inhibit or potentate insulin-like growth factor activity in vivo. Recently, mutations in insulin-like growth factor-I created by replacing Glu residues with Ala resulted in a 100-fold decrease in binding to insulin-like growth factor binding proteins-1 and 3 (24).
The concept that a constitutive natural inhibitor of a cytokine has a role in the development of disease is not new. Molluscum contagiosum viral proteins MC53 and MC54 share a significant homology to mammalian IL-18BP (7). M. contagiosum proteins MC53 and MC54 possess the ability to bind and neutralize human IL-18 in a fashion similar to that of IL-18BP (25). The ectromelia poxvirus p13 protein, which is homologous to IL-18BP, binds human IL-18 and inhibits its activity in vitro. Mice infected with a p13 deletion mutant virus exhibited decreased levels of infectivity (26).
The high concentration of IL-18BP in the circulation of healthy subjects (20-fold molar excess) is higher in patients with infection.¶ High levels of constitutive IL-18BP may be a natural defense against a runaway Th1 response to infection and the development of autoimmune diseases. However, by the same rationale, if IL-18 contributes to the Th1 response in host defense against tumors, then the high-affinity circulating IL-18BP contributes to the failure of the host to develop cytotoxic T cells directed against tumor cells. In fact, there is evidence that IL-18 promotes host defense against tumors in mice. For example, in syngeneic mice, murine mammary carcinoma cells expressing murine IL-12 or murine IL-18 were less tumorigenic and formed tumors more slowly than did control cells (2). Antibody neutralization studies revealed that the antitumor effects required IFN-γ. Systemically administered IL-18 in combination with B7–1 (CD80) expressed on B16 melanoma resulted in dramatic suppression of melanoma formation, tumor growth, and a significant improvement in survival (1).
In experiments described above, the amount of systemically administered IL-18 may be greater than needed for IL-18 to act as an immunostimulant because binding to constitutive IL-18BP will reduce the efficacy of IL-18. One important consequence of immunotherapy in general is that the adjuvant effect of cytokines in cancer, such as IL-2 for renal cell carcinoma or melanoma, is associated with severe systemic toxicity profiles. Therefore, increasing the dose of a systemic cytokine like IL-18 will be needed to overcome neutralization by the IL-18BP. Using locally expressed cytokines, particularly those expressed on the patients' own tumor cells or dendritic cells, is a logical solution to the problem. If cytokines such as IL-18 are to be used as adjuvants in tumor immunotherapies, then the ability of constitutive levels of IL-18BP to neutralize IL-18 in the local environment would still exist. However, if the IL-18 double mutant E42A + K89A is expressed on tumor cells, one could expect a greater adjuvant effect with less or no neutralization by IL-18BP.
The use of nonmyeloablative allogeneic transplants, the so-called minitransplants, to treat leukemia and solid tumors is increasingly successful in inducing graft-versus-leukemia and graft-versus-tumor reactions (5, 6). Two studies that used either peripheral blood stem cells (4) or dendritic cells (3) to treat patients with metastatic renal cell carcinoma have met with remarkable success. Although these studies need to be extended and confirmed, the concept that an ongoing graft-versus-tumor reaction is exploitable for immunotherapy in cancer is gaining acceptance (5). If IL-18 plays a role in the development of graft versus tumor, these mutants with enhanced biological activity and resistance to neutralization by constitutive IL-18BP may be useful. On the other hand, in severe graft-versus-host disease, IL-18BP may also be useful as a therapy to reduce the intensity of the immunostimulation.
Acknowledgments
We authors thank Dr. Ron Pincus of InterPharm Laboratories (Nes Ziona, Israel) for providing the purified Chinese hamster ovary-derived IL-18BPa-His-6 tag and Dr. Giamila Fantuzzi for help with the ECL assays. This study was supported by National Institutes of Health Grant A-15614 (to C.A.D.) and Colorado Cancer Center Grant CA 46934.
Abbreviations
- BP
binding protein
- Th
T helper cell
- IL-1R
IL-1 receptor
- His-6 tag
six histidines
- ECL
electrochemiluminescence
- PBMCs
peripheral blood mononuclear cells
- WT
wild type
- ICE
IL-1 converting enzyme
- NK
natural killer
- NKO
NKO cell line
Footnotes
Novick, D., Kim, S.-H., Shtoegger, Z., Dinarello, C. A. & Rubinstein, M. (2000) Eur. Cytokine Network 11, 104 (abstr.).
References
- 1.Cho D, Kim T G, Lee W, Hwang Y I, Cho H I, Han H, Kwon O, Kim D, Park H, Houh D. J Invest Dermatol. 2000;114:928–934. doi: 10.1038/sj.jid.5600685. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin C M, Salhany K E, Wysocka M, Aruga E, Kurzawa H, Chang A E, Hunter C A, Fox J C, Trinchieri G, Lee W M F. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller C A, Becker V, et al. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 4.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read E J, Tisdale J, Dunbar C, Linehan W M, et al. N Engl J Med. 2000;343:77550–77558. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 5.Slavin S. N Engl J Med. 2000;343:802–803. doi: 10.1056/NEJM200009143431109. [DOI] [PubMed] [Google Scholar]
- 6.Slavin S, Or R, Prighozina T, Gurevitch O, Aker M, Panighari S, Shapira M, Nagler A. Bone Marrow Transplant. 2000;25, Suppl. 2:S54–S57. doi: 10.1038/sj.bmt.1702356. [DOI] [PubMed] [Google Scholar]
- 7.Novick D, Kim S-H, Fantuzzi G, Reznikov L, Dinarello C A, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim S-H, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello C A. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 10.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Nature (London) 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Novick D, Kim S H, Rubinstein M. Cytokine. 2000;12:1519–1525. doi: 10.1006/cyto.2000.0749. [DOI] [PubMed] [Google Scholar]
- 12.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. J Clin Invest. 1998;101:711–724. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puren A J, Razeghi P, Fantuzzi G, Dinarello C A. J Infect Dis. 1998;178:1830–1834. doi: 10.1086/314481. [DOI] [PubMed] [Google Scholar]
- 14.Puren A J, Fantuzzi G, Dinarello C A. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho, Y.-S., Cho, M.-C., Chung, M.-K., Cho, J.-W., Kang, Y.-H., Lee, H.-G., Choe, Y.-K., Choi, I.-C., Kim, S.-H., Dinarello, C. A., et al. (2001) J. Biochem. Mol. Biol.34, in press.
- 16.Gehrke L, Jobling S A, Paik L S, McDonald B, Rosenwasser L J, Auron P E. J Biol Chem. 1990;265:5922–5925. [PubMed] [Google Scholar]
- 17.Evans R J, Bray J, Childs J D, Vigers G P A, Brandhuber B J, Skalicky J J, Thompson R C, Eisenberg S P. J Biol Chem. 1994;270:11477–11483. doi: 10.1074/jbc.270.19.11477. [DOI] [PubMed] [Google Scholar]
- 18.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikate T, Murakami T, Sanou O, Kojima H, Fuji M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 19.Born T L, Thomassen E, Bird T A, Sims J E. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 20.Debets R, Timans J C, Churakowa T, Zurawski S, de Waal Malefyt R, Moore K W, Abrams J S, O'Garra A, Bazan J F, Kastelein R A. J Immunol. 2000;165:4950–4956. doi: 10.4049/jimmunol.165.9.4950. [DOI] [PubMed] [Google Scholar]
- 21.Engelmann H, Novick D, Wallach D. J Biol Chem. 1990;265:1531–1536. [PubMed] [Google Scholar]
- 22.Vigers G P A, Anderson L J, Caffes P, Brandhuber B J. Nature (London) 1997;386:190–194. doi: 10.1038/386190a0. [DOI] [PubMed] [Google Scholar]
- 23.Urushihara N, Iwagaki H, Yagi T, Kohka H, Kobashi K, Morimoto Y, Yoshino T, Tanimoto T, Kurimoto M, Tanaka N. J Pediatr Surg. 2000;35:446–449. doi: 10.1016/s0022-3468(00)90211-2. [DOI] [PubMed] [Google Scholar]
- 24.Dubaquie Y, Lowman H B. Biochemistry. 1999;38:6386–6396. doi: 10.1021/bi990089p. [DOI] [PubMed] [Google Scholar]
- 25.Xiang Y, Moss B. Proc Natl Acad Sci USA. 1999;96:11537–11542. doi: 10.1073/pnas.96.20.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Born T L, Morrison L A, Esteban D J, VandenBos T, Thebeau L G, Chen N, Spriggs M K, Sims J E, Buller R M. J Immunol. 2000;164:3246–3254. doi: 10.4049/jimmunol.164.6.3246. [DOI] [PubMed] [Google Scholar]