Abstract
The testis is the main source of Fas ligand (FasL) mRNA in rodents; it is generally believed that this molecule, expressed on bordering somatic Sertoli cells, bestows an immune-privileged status in the testis by eliminating infiltrating inflammatory Fas-bearing leukocytes. Our results demonstrate that the attribution of testicular expression of FasL to Sertoli cells is erroneous and that FasL transcription instead occurs in meiotic and postmeiotic germ cells, whereas the protein is only displayed on mature spermatozoa. These findings point to a significant role of the Fas system in the biology of mammalian reproduction.
Keywords: testis, immunoprivilege, fertility
It is well known that the binding of the surface protein Fas ligand (FasL) to the Fas receptor triggers apoptosis in Fas-bearing cells (1). The Fas/FasL system has been implicated in the regulation of immune response because it initiates the Fas-induced apoptosis of activated lymphocytes (2, 3). This mechanism of immune down-regulation has been proposed as a way by which cells expressing FasL may establish a localized immune-privileged environment at a graft-site by inducing apoptosis in the host's infiltrating inflammatory cells (4). The testis has long been known to be an immune-privileged site because of its ability to support allogeneic and xenogeneic tissue transplants (5). Moreover, autoantigens expressed on differentiating germ cells elicit strong autoimmune reactions if injected elsewhere in the body, but are well tolerated in the testis (6). Local immune tolerance in the testis has been considered to result from the presence of two mechanisms: (i) segregation of most of the autoantigens (7) and (ii) local production of immunosuppressive molecules (8), both conditions being generated by Sertoli cells, the somatic cells of the seminiferous epithelium. Early studies on the expression of FasL mRNA in various mouse and rat tissues revealed that the testis is the main source of FasL mRNA in the body (9, 10), whereas no expression of Fas was detected in the testis (11). Subsequently, Bellgrau and colleagues published data (12) showing that mismatched grafts of mouse testis, which express FasL, survived, whereas grafts taken from gld donors, which lack functional FasL, were rejected. The authors suggested that immunoprivilege of the testis was due to FasL expressed on Sertoli cells.
The interaction of Fas with FasL also promotes the physiological deletion of potentially harmful or unnecessary cells in various tissues (13). In fact, the Fas system controls homeostasis in various tissues, such as the liver and the peripheral lymphoid organs (14), red blood cells (15), and the epithelium of the small intestine (16, 17). Thus, besides the presumptive immunoregulative functions of FasL in the testis, the Fas system has also been proposed as a regulator of physiological germ-cell apoptosis, essential for the development of functional spermatogenesis. In this model, FasL expressed by Sertoli cells initiates the apoptosis of germ cells expressing Fas (18, 19). However, an extensive study on the morphology of gld mice testis (which lacks functional FasL) and on the spontaneous rate of germ cell apoptosis in these mutants did not reveal any significant difference between wild-type and gld mice; on the contrary, there was, in the testes of gld mice, a paradoxical slight increase in the spontaneous incidence of germ cell apoptosis (20). These data suggest that FasL may not play a key role either in the control of germ cell apoptosis or in the establishment of immune privilege in the testis, because spermatogenesis was not perturbed and no infiltrating autoimmune leukocytes were observed in the gld testis.
It is generally accepted that FasL is expressed in Sertoli cells, whereas Fas antigen is expressed in the germ cells of rodents (18, 21) and humans (19, 22). However, direct evidence by Northern blot analysis or in situ hybridization of the presence of Fas or FasL mRNAs in specific subpopulations of testicular cells has never been reported. Similarly, the literature on the cellular location of Fas or FasL proteins in the testis is confusing: Two groups of researchers have described a different distribution of FasL protein by using two different, commercially available, antibodies (both from the same company) in immunohistochemical analyses of the testis (18, 23). Moreover, the specificity of several antibodies for FasL has been seriously questioned (24–26). Therefore, a systematic study of the cellular distribution and regulation of Fas and FasL is warranted to clarify the role of these molecules in the testis.
Our previous findings have indicated that, in mouse Sertoli cell cultures from prepuberal testis, Fas antigen is present both as mRNA and protein, whereas FasL mRNA is absent (27). In the present study, we extensively analyzed the expression of FasL in different testicular cell types, during testis development as well as in the various phases of spermatogenesis, in both mouse and rat. By using Northern blot analysis and in situ hybridization, we demonstrated that FasL mRNA is absent in Sertoli cells. By contrast, we observed a high expression of FasL mRNA and protein in purified populations of meiotic spermatocytes and of haploid spermatids. Moreover, we detected FasL protein also on the sperm cell surface. We confirmed our results by using in vivo models of germ cell deprived testes (28, 29).
The presence of FasL in mature spermatozoa may be related to a self-defense mechanism of gametes against antisperm-activated lymphocytes present in both male and female genital tracts.
Materials and Methods
Cell Lines, Probes, and Reagents.
Mock (neomycin-resistance cDNA)-transfected neuroblastoma Neuro-2a (N2a-Neo) and murine FasL cDNA-transfected Neuro-2a (N2a-FasL+) cell lines (kindly provided by Dr. A. Fontana, University Hospital of Zurich, Zurich) and murine plasmacytoma (P815-Fas) (kindly provided by Dr. P. Vassalli, University of Geneva, Geneva) were maintained in RPMI medium 1640 (Life Technologies, Paisley, Scotland) containing 5% FCS, 4.5 g/l glucose, 100 units/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) at 37°C under 5% CO2.
A plasmid pBL-MFLW4 carrying the full-length mouse FasL cDNA, a 0.94-kb XbaI fragment, has been described (2). The rat FasL cDNA was cloned as described below. DNase I and collagenase A were purchased from Roche Molecular Biochemicals, collagenase type XI from Sigma, and trypsin from Difco.
Cloning of Rat FasL Probe.
To produce the rat FasL probe corresponding to the amplified 285-bp DNA fragment (nucleotides 116–400) of rat FasL cDNA (9), we cloned the PCR product by using the standard molecular cloning technique (30). The fragment was blunt-ligated into pUC18 vector according to the manufacturer's instructions (Amersham Pharmacia).
Preparation of Testicular Cells.
Sertoli cells were prepared from 20-day-old Wistar rats as described (31). Testes were sequentially digested for 30 min, first with Hanks' balanced salt solution (HBSS) containing 0.25% trypsin + 10 μg/ml DNase and then with HBSS supplemented with 0.1% collagenase A + 10 μg/ml DNase to remove interstitial tissue and peritubular cells. Fragments of seminiferous epithelium, mainly composed of Sertoli cells, were cultured at 32°C in 95% air and 5% CO2 in serum-free Eagle's MEM supplemented with glutamine, nonessential amino acids, gentamicin, and streptomycin. After 3 days, Sertoli cell monolayers were incubated with 20 mM Tris⋅HCl buffer pH 7.4 for 2.5 min to remove residual germ cells. Sertoli cell cultures from CD1 mice were prepared as described above with minimal variations (32). Sertoli cell cultures were routinely checked for possible contamination by macrophages and peritubular myoid cells by means of indirect immunofluorescence with antimacrophage monoclonal antibody (Mac-1 antigen CD11/b, Roche Molecular Biochemicals) and by histochemical detection of alkaline phosphatase activity (33). On the fourth day of culture, Sertoli cell monolayers were analyzed for FasL expression by flow cytometric analysis or were lysed for Northern blotting experiments. To have freshly prepared rat Sertoli cells at our disposal, a highly purified Sertoli cell fraction was obtained by layering collagenase digestion supernatant onto Percoll density gradient, as described in ref. 34.
Germ cell suspensions enriched in the mitotic stages were collected by sequential collagenase A-hyaluronidase-trypsin treatment of testes from 7- to 8-day-old mice (35). Cell suspensions were preplated for 4–5 h on 10-cm tissue dishes with MEM supplemented with glutamine, nonessential amino acids, gentamicin, streptomycin, penicillin, 2 mM sodium lactate, 1 mM sodium pyruvate, and 10% FCS in a 5% CO2 atmosphere at 32°C. This step is required to allow adhesion of the somatic cells to the plastic. After this preincubation, floating mitotic germ cells were collected.
For germ cell preparation, seminiferous tubules from adult mice or rats were digested twice in HBSS containing 0.1% type XI-collagenase + 10 μg/ml DNase to free them of interstitial and peritubular tissues. Germ cells in the pachytene spermatocyte, round spermatid, and elongated spermatid phases were obtained by elutriation of the unfractionated single-cell suspension as described (36). Homogeneity of cell populations ranged from 80–85% (pachytene spermatocytes) or was 95% (round spermatids), and was routinely monitored morphologically. Germ cell fractions were centrifuged at 1,000 rpm for 10 min, washed twice in serum-free medium MEM supplemented with 2 mM sodium lactate and 1 mM sodium pyruvate, and immediately lysed for Northern blot analysis. For flow cytometric analysis, germ cells were incubated at 32°C for 18 h with matrix metalloproteinase inhibitor KB8301 (PharMingen), which blocks FasL cleavage (37).
Epididymal spermatozoa were collected from CD1 active breeder males. Epididymis were minced with sterile scissors in 1 ml of a modified Krebs–Ringer medium as described (38). Briefly, the spermatozoa were allowed to disperse for 5 min in medium supplemented with KB8301, and then centrifuged for 10 min at 800 g at 4°C. The sperm were resuspended in medium with 1% BSA, counted, and analyzed for FasL expression by flow cytometric analysis.
Irradiated and Cryptorchid Animals.
Sertoli cell-enriched testes (SCE) were obtained by X-irradiating pregnant mothers with a single dose of 120 rad at gestation day 21 (29). The male rats were killed at 15, 30, 41, and 63 days after birth and each testis was half fixed in 2.5% glutaraldehyde for morphological studies and half analyzed by Northern blot analysis.
Experimental cryptorchidism was induced in Wistar rats by translocation of one testis into the abdominal cavity, following procedures described in ref. 39. Surgery was performed when the males were 8 to 10 weeks of age. Rats were killed 15 days after surgery and both abdominal and scrotal testes were subjected to morphological studies and Northern blot analysis.
Morphological Studies.
Samples were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), postfixed in 1% OsO4 in Zetterquist buffer, dehydrated in ethanol, and embedded in epoxy resin. Semithin sections (2 μm) were stained with toluidine blue dye and photographed in a Zeiss compound microscope.
Northern Blotting.
Total RNA was extracted by using TRIZOL Reagent (Life Technologies). Samples were processed following the manufacturer's protocol. RNA was subjected to electrophoresis, through a 1% agarose gel containing formaldehyde, and was transferred onto a nylon membrane (Schleicher & Schuell). The filters were prehybridized in hybridization buffer containing 50% formamide (Sigma), 10% dextran sulfate, 1% SDS, 1.2 M NaCl (Sigma), and sonicated salmon sperm (Stratagene), at 42°C for 4 h. Hybridization was performed overnight in prehybridization buffer containing 1 × 106 cpm/ml of [32P]-labeled rat FasL cDNA. After washes, the blots were exposed to BIOMAX-MS film (Eastman Kodak).
In Situ Hybridization.
The mouse FasL cDNA, cloned into pSK+ Bluescript at XbaI site, was used to prepare sense and antisense probes. Radiolabeled cRNAs were synthesized by in vitro transcription in the presence of α-35S-labeled UTP (125 Ci/1 g cDNA; 1 Ci = 37 GBq). Cryostat sections (7 m) were mounted on poly-L-lysine (Sigma) coated microscope slides, fixed in 4% formaldehyde in PBS, and stored at −80°C until analyzed. For in situ hybridization, the sections were digested with 1 g/ml proteinase K in 10 mM Tris⋅HCl (pH 7.5) and 1 mM EDTA for 7 min at 37°C, acetylated with 0.25% (vol/vol) acetic anhydride in 0.1 M triethanolamine at room temperature for 10 min and incubated in 0.1 M Tris⋅HCl (pH 7.0) and 0.1 M glycine at room temperature for 30 min. A quantity amounting to 1.0 × 106 cpm of 35S-labeled RNAs was applied to each section in 10–15 μl of hybridization mixture and incubated overnight at 50°C. Sections were washed in 2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), 50% formamide, and 0.01 M DDT at 50°C for 20 min; and in 0.2× SSC, 50% formamide, and 0.01 M DDT at 50°C for 30 min. Unhybridized transcripts were digested with 10 μg/ml RNase A at 37°C for 30 min. The slides were washed again in 2× SSC, in 50% formamide at 50°C for 15 min, a further four times in 2× SSC at room temperature, and dehydrated in graded ethanol and air dried before immersion in ILFORD-K2 emulsion (Ilford). Slides were exposed for 1 to 3 weeks, developed in Kodak D-19 developer, fixed, and counterstained in carmallume. Specificity controls, which included the use of sense mRNA probe in each experiment, revealed the absence of significant labeling.
Flow Cytometry.
Sertoli cells, germ cells, and spermatozoa were washed once with cold HBSS + 1% BSA before incubation with antibodies. For detection of FasL expression on the cell surface, we used the (Biotin)-conjugated hamster IgG anti-mouse and rat CD95L monoclonal antibody (MFL4; PharMingen). A specific monoclonal antibody or the appropriate isotypic control mAb were used at 1 μg/106 cells for 30 min in ice. Thereafter, cells were washed twice with PBS + 1% BSA and incubated 30 min in ice with streptavidin-phycoerythrin conjugate (Sav-PE; PharMingen), washed twice with PBS + 1% BSA, and analyzed with a Coulter Epics XL flow cytometer (Beckman Coulter). Cells were gated by using forward versus side scatter to exclude dead cells and debris. Fluorescence of 104 cells/sample was acquired in logarithmic mode for visual inspection of the distributions, and in linear mode to quantify the expression of the relevant molecules by calculating the mean fluorescence intensity. To detect intracellular FasL protein, the cells were fixed with 1% cold paraformaldehyde for 20 min, whereas 0.1% saponin in HBSS was used for permeabilization (40).
Besides the morphological analysis, DNA was stained with propidium iodide for ploidy analysis to evaluate the purity of each germ cell fraction, as previously described (41).
Results
FasL Expression During Testis Development.
To study the expression of FasL during the development of the testis, we performed a Northern blot analysis of total testes RNA from rats of different ages.
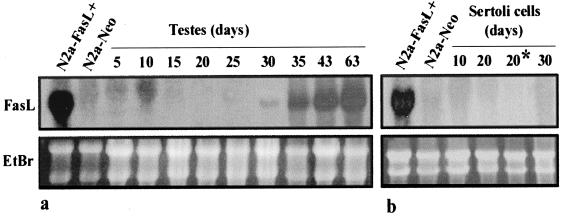
The FasL signal in the testis was detectable from the age of 30 days, subsequently increased, and remained constant in the adult testis (Fig. 1a). Similar results were obtained in the mouse, in which prepuberal testis was negative for FasL signal, whereas adult testis was strongly positive (data not shown).
Figure 1.
(a) Age-dependent expression of FasL in the rat testis. Total RNA (20 μg/lane) extracted from total testis from animals of various ages were analyzed by Northern blot. Nylon filter was hybridized with 32P-labeled rat FasL cDNA probe (b) Northern blot analysis of FasL expression in Sertoli cells at different ages. Total RNA (20 μg/lane) from cultured and crude (*) Sertoli cells were subjected to Northern blot analysis for detection of rat FasL. N2a-FasL+ and N2a-Neo cell lines were used as positive and negative controls for FasL, respectively. The integrity and equal loading of RNA was ascertained by ethidium bromide staining of the gel before transfer (Lower). Data are representative of three independent experiments.
Lack of FasL Expression in Cultured Sertoli Cells and in Sertoli Cell-Enriched Testes.
To study which cell types actually express FasL in the testis, we performed a Northern blot analysis on RNA from purified populations of single cell types present in the seminiferous epithelium. We did not detect any signal for FasL RNA in primary cultures of Sertoli cells from testes of rat at different ages (from 10 to 30 days), nor in freshly prepared Sertoli cells from prepuberal rats (Fig. 1b). The latter result demonstrates that fresh Sertoli cells, highly purified on Percoll gradient, are negative for FasL RNA, thereby ruling out the possible effect of culture conditions on FasL expression.
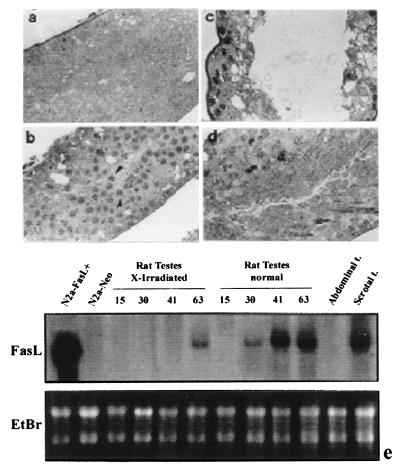
On the other hand, the adult rat testis expresses FasL. It is not, however, as yet possible to obtain sufficiently pure Sertoli cell preparations or in vitro cultures from adult rat or mouse testis because of the complex histological architecture of the mature seminiferous epithelium, in which Sertoli cells account for about only 5% of the total cellular population (42). Therefore, to verify whether the strong expression of FasL mRNA in the adult testis depends on Sertoli cells, we used two different in vivo experimental models to obtain adult rats with SCE. It is well known that rats irradiated in utero are born with “Sertoli-cell-only testes,” because of the selective depletion of the radiosensitive germ cells (29). Rats born from irradiated mothers were killed 15, 30, 41, and 63 days after birth, and the testes were used for histological and Northern blot analysis. As shown in Fig. 2a, testes from adult rat irradiated in utero completely lack differentiating germ cells; they contain Sertoli cells and germ stem cells alone until 41 days after birth; later on, spermatogenesis resumes and spermatocytes appear in the seminiferous tubules of the testes of 63-day-old rats (Fig. 2b). As regards FasL mRNA expression, we did not detect any signal for FasL in irradiated Sertoli-cell-only testes, whereas the signal for FasL mRNA was evident in the testes of 63-day-old rats containing meiotic germ cells (Fig. 2e). We performed a similar study using an alternative experimental model. When the adult normal testis is surgically fixed in the abdomen, mature stages of germ cells are lost; this experimental model is widely used to investigate testicular function. We therefore performed a series of experiments using adult unilateral cryptorchid rats, in which one testis is moved into the abdomen while the other is left in the scrotum. Cryptorchid rats were killed 15 days after surgery and the testes were resected, weighed, and examined by both morphological and Northern blot analysis. Histological analysis of cryptorchid testes showed total absence of differentiating germ cells in the seminiferous epithelium, which was composed exclusively of Sertoli cells and spermatogonia (Fig. 2c), whereas the morphology of the controlateral scrotal testis was normal (Fig. 2d). As shown in Fig. 2e, our results are comparable to those obtained in irradiated animal experiments, because FasL mRNA is present in the scrotal testis, whereas the signal in the abdominal cryptorchid testis is absent.
Figure 2.
Morphological study of seminiferous epithelium from SCE adult rats. The semithin sections show: (a) Aspermatogenic Sertoli-cell-only testis from 41-day-old rat irradiated in utero; (b) the recovery of spermatogenesis 63 days after birth (arrow heads indicate spermatocytes); (c) the abdominal testis, showing the absence of spermatogenesis; and (d) controlateral scrotal testis in unilaterally cryptorchid SCE rat (210× magnification for all). (e) Northern blot analysis of FasL expression in SCE rat testes. Total RNA from unilaterally cryptorchid SCE rat testis and Sertoli-cell-only testis from irradiated in utero rats of different ages were analyzed by Northern blot analysis. Each band was normalized by ethidium bromide staining of the gel before transfer (Lower). Results are representative of three independent experiments.
FasL Expression in Mouse and Rat Germ Cells.
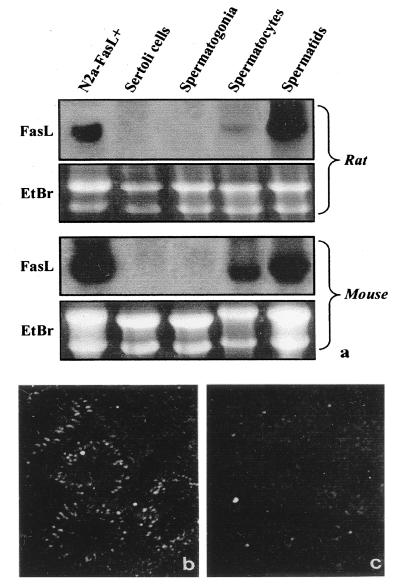
To further investigate which cells within the seminiferous epithelium express FasL, we purified single populations of germ cells that were subsequently analyzed by Northern blot analysis. As shown in Fig. 3a, mRNA for FasL is predominantly expressed in round spermatids and, to a lesser extent, in pachytene spermatocytes in mouse and rat testis.
Figure 3.
(a) Northern blot analysis of FasL expression in purified populations of rat and mouse germ cells. Results are representative of three experiments. (b) Expression of mRNA for FasL in adult rat testis by ISH analysis with antisense probe under dark-field illumination. The high density of silver grain indicates that the site of FasL expression is in the elongated spermatids. (c) ISH analysis with sense probe under dark-field illumination shows the probe specificity. (50× magnification)
To confirm FasL expression by germ cells, we applied the in situ hybridization (ISH) method to rat testes by using an antisense FasL riboprobe as described in Materials and Methods. We used ISH to study both prepuberal and adult rat testes. No signal was detected in prepuberal rat testis sections (data not shown). A distinct pattern of ISH was observed within the seminiferous epithelium of adult rats. Labeling for FasL mRNA was present only in postmeiotic germ cells, particularly in elongated spermatids, whereas Sertoli, peritubular, and interstitial cells were constantly negative (Fig. 3b). The labeling observed with the FasL probe is specific, because appropriate controls using the sense probe did not reveal any signal (Fig. 3c).
Protein Localization of FasL.
Several data regarding the detection of FasL protein by means of antibodies have been published. Some of these data have recently been criticized and the specificity of most of the antibodies used widely questioned (25). To investigate the possible role of FasL in vivo, we performed a series of experiments aimed at accurately locating this protein within the seminiferous tubules. We tested several antibodies [Santa Cruz Biotechnology polyclonal anti-FasL C20; Transduction Laboratories (Lexington, KY) mAb33; mAb H11, kindly provided by M. Hahne (Institute for Biochemistry, University of Lausanne, Epalinges, Switzerland)] by immunohistochemistry, immunofluorescence, and Western blot analysis, obtaining contradictory results.
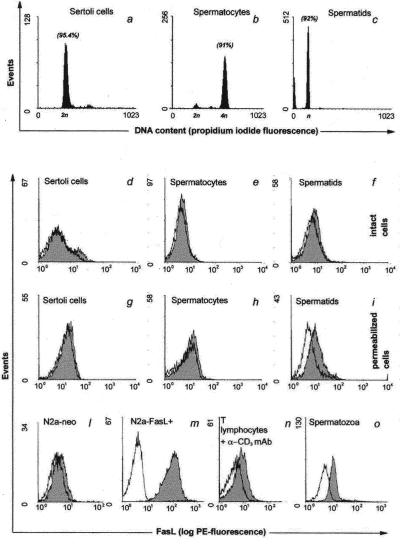
To estimate whether FasL protein colocalized with FasL mRNA expression, we performed a flow cytometric analysis by using an anti-rat and -mouse FasL antibody (MFL4) that fulfilled all of the specificity control requirements (Fig. 4 l and m). Because several findings suggest that FasL may be stored in specialized cytoplasmatic vesicles (43, 44), we performed the experiments both on intact and permeabilized cells, as described in Materials and Methods. Moreover, as FasL could be shed, at least in vivo (37), by a matrix metalloproteinase to yield soluble FasL, we performed all of the experiments in the presence of a metalloproteinase inhibitor—KB8301, which specifically blocks FasL cleavage.
Figure 4.
Evaluation of DNA content of different testicular cell types by propidium iodide incorporation. (a–c) The percentage of gated cells with indicated ploidy is shown in brackets. Flow cytometric analysis of FasL expression in the seminiferous epithelium and in activated T lymphocytes. The expression of FasL was determined by biotin-conjugated anti-FasL monoclonal antibody (MFL4). (d–o) The white histograms indicate isotype control and gray histograms indicate the staining with specific antibody. l and m show negative and positive controls for FasL, respectively. Results are representative of three independent experiments.
Both permeabilized and intact Sertoli cells were, according to Northern blot results, negative for the MFL4 antibody (Fig. 4 d and g). Although we detected mRNA for FasL both in spermatids and spermatocytes, we did not detect any significant expression of FasL protein on the surface of these cells by flow cytometric analysis (Fig. 4 e and f). By contrast, a significant level of FasL signal appears on spermatids after permeabilization (Fig. 4i).
We therefore investigated the presence of FasL on spermatozoa collected from the epididimis of rats and mice, detecting a high expression of FasL on the surface of these cells (Fig. 4o). To quantify the amount of FasL exposed on spermatozoa, we compared it with FasL protein present on splenocytes stimulated with anti-CD3 antibody for 48 h. We observed that the fluorescence intensity for FasL on the surface of spermatozoa was higher than that on stimulated splenocytes (Fig. 4 n and o).
To evaluate the purity of each cell population gated in flow cytometric analysis, DNA was stained with propidium iodide for ploidy analysis, as an additional marker to morphology, to differentiate between Sertoli cells (2n), pachytene spermatocytes (4n), and postmeiotic round spermatids (n). As shown in Fig. 4 a–c, the purity of all of the cell fractions analyzed was higher than 90%.
Discussion
In this study we demonstrate that high FasL expression in rodent testis is, contrary to claims in the literature, due to differentiating germ cells rather than to Sertoli cells. Our results were obtained both in vitro, on purified populations of testicular cells, and in vivo, by analysis of adult rat testes devoid of meiotic and postmeiotic germ cells by means of two different experimental methods (cryptorchidism and irradiation in utero). Northern blot analysis revealed the absence of FasL mRNA in the prepuberal testis as well as in purified populations of Sertoli and spermatogonial stem cells. Strong positivity was instead evident in adult testes and in highly purified populations of meiotic pachytene spermatocytes and haploid round spermatids. A total absence of FasL mRNA was observed in testes from irradiated rats and in cryptorchid testes lacking germ cells, whereas strong FasL mRNA expression was present in controlateral scrotal testes. Further evidence of FasL expression in mature stages of germ cells is the late appearance of FasL mRNA in adult testes from rats irradiated in utero, corresponding with the delayed onset of spermatogenesis following re-colonization by stem germ cells. In fact, in this model complete spermatogenesis was restored (after varying times depending on the amount of X-rad used), which indicates that stem cells remained functional and that the testis environment was normal.
Recently, other authors have shown the presence of FasL mRNA in mouse Sertoli cells by ISH on paraffin-embedded sections (45). Our in situ data contradict this finding, but are in accordance with our parallel Northern blot and cytometric analyses results. These contrasting results may be due to the fact that we performed the ISH experiments in cryosections of rat testis and consequently used different technical procedures.
By using ISH, we were able to confirm a strong signal for FasL in postmeiotic haploid cells (i.e., spermatids) but not in meiotic cells (spermatocytes), as was instead shown by Northern blot analysis. We believe that, because of technical problems and/or the characteristics of the probe used in our study, the ISH was not very sensitive, allowing the detection of only the strongest FasL expression in spermatids.
We did not detect FasL protein on the surface of maturing germ cells expressing high levels of FasL mRNA, finding it only within their cytoplasm. These results suggest the presence of a mechanism, yet to be explored, that regulates the display of the FasL protein on the cell surface.
By contrast, spermatozoa display on their surface a concentration of FasL that is as high as, if not higher than, that observed on the surface of activated lymphocytes. Considering that the transcription rate of condensed sperm chromatin is minimal, it seems reasonable to assume that mRNA for FasL is accumulated during earlier stages of spermatogenesis and spermiogenesis, and that the protein is partially accumulated to be displayed in mature spermatozoa.
In 1995, Bellgrau and colleagues (12) suggested that FasL is expressed on Sertoli cells and is responsible for immunoprivilege in the testis in a report based on a reverse transcription (RT)–PCR analysis for FasL on “freshly prepared” Sertoli cells from adult mice. Such preparations are known to contain significant amounts of germ cells and the use of RT-PCR may result in misleading interpretations. Several antibodies to FasL are available and most researchers and companies now use Sertoli cells as a positive control. Numerous papers in which these antibodies have been used have given rise to much speculation as to which Sertoli cells are assumed to be responsible for FasL production in the testis. Our results provide direct evidence based on Northern blot analysis and various other experimental approaches that the attribution of testicular expression of FasL to Sertoli cells is erroneous and that FasL transcription occurs, instead, in differentiating germ cells, the protein only being displayed on the surface of mature spermatozoa.
A recent commentary in Nature Medicine (25) pointed out “the continued proliferation of erroneous ideas about FasL” as a result of both technical problems (not highly specific antibodies) and the widely held misconception that FasL is the “enforcer” of immune privilege and tumor escape, a paradigm contradicted by several reports (46–48).
We agree with this commentary and believe that the roles so far proposed for the Fas system in the testis, such as maintenance of immunoprivilege (12) and regulation of physiological germ cell apoptosis (18), need to be reconsidered because both hypotheses are based on an erroneous cellular location of Fas and FasL in the seminiferous epithelium.
It is intriguing that the testis is the only organ that constitutively expresses abundant amounts of FasL mRNA, and it is hard to believe that the sole physiological function of this molecule is the elimination of potentially infiltrating leukocytes expressing Fas. However, it should be remembered that in normal conditions lymphocytes do not come into contact with cells of the seminiferous epithelium, and that physiological germ cell apoptosis is not affected in gld mice lacking functional FasL (20).
Our data demonstrate that FasL mRNA is strongly expressed in differentiating germ cells and that the protein is thereafter displayed on the surface only when the gamete is fully mature and, as spermatozoon, leaves the testis. These findings provide an intriguing area of investigation regarding the peculiar functions of FasL in the biology of mammalian reproduction. The presence of FasL on the sperm surface might represent one of the sophisticated ways developed by male gametes to escape both the autoimmune reaction during their journey through the male genital tract and the allogenic reaction in the host body of the opposite gender, thereby favoring outbreeding.
Acknowledgments
We thank Prof. G. Olivieri for his help in the X-irradiation experiments, Dr. G. Starace for his discussion of the cytometric data, Dr. M. Barchi for his advice on the germ cell preparation, and Ms. S. De Grossi for her technical assistance.
Abbreviations
- FasL
Fas ligand
- HBSS
Hanks' balanced salt solution
- SCE
Sertoli cell-enriched testes
- ISH
in situ hybridization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 3.Li J H, Rosen D, Ronen D, Behrens C K, Krammer P H, Clark W R, Berke G. J Immunol. 1998;161:3943–3949. [PubMed] [Google Scholar]
- 4.Abbas A K. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 5.Head J R, Neaves W B, Billingham R E. Transplantation. 1983;36:423–431. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Tung K S K. In: Immunological Aspects of Infertility and Fertility Regulation. Dhindsa D H, Shumacher G F B, editors. New York: Elsevier North–Holland; 1980. pp. 33–91. [Google Scholar]
- 7.Dym M, Fawcett D W. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 8.De Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G, Stefanini M, Ziparo E. Biochem Biophys Res Commun. 1992;186:1639–1646. doi: 10.1016/s0006-291x(05)81596-7. [DOI] [PubMed] [Google Scholar]
- 9.Suda T, Takahashi T, Goldstein P, Nagata S. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 10.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 11.Watanabe-Fukunaga R, Brannan C I, Itoh N, Yonehara S, Copeland N G, Jenkins N A, Nagata S. J Immunol. 1992;148:1274–1279. [PubMed] [Google Scholar]
- 12.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 14.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 15.De Maria R, Testa U, Luchetti L, Zeuner A, Stassi G, Pelosi E, Riccioni R, Felli N, Samoggia P, Peschle C. Blood. 1999;93:796–803. [PubMed] [Google Scholar]
- 16.Inagaki-Ohara K, Nishimura H, Sakai T, Lynch D H, Yoshikai Y. Lab Invest. 1997;77:421–429. [PubMed] [Google Scholar]
- 17.Bonfoco E, Stuart P M, Brunner T, Lin T, Griffith T S, Gao Y, Nakajima H, Henkart P A, Ferguson T A, Green D R. Immunity. 1998;9:711–720. doi: 10.1016/s1074-7613(00)80668-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Richburg J H, Younkin S C, Boekelheide K. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- 19.Pentikainen V, Erkkila K, Dunkel L. Am J Physiol. 1999;276:E310–E316. doi: 10.1152/ajpendo.1999.276.2.E310. [DOI] [PubMed] [Google Scholar]
- 20.Richburg J H, Nanez A, Williams L R, Embree M E, Boekelheide K. Endocrinology. 2000;141:787–793. doi: 10.1210/endo.141.2.7325. [DOI] [PubMed] [Google Scholar]
- 21.French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francavilla S, D'Abrizio P, Rucci N, Silvano G, Properzi G, Straface E, Cordeschi G, Necozione S, Gnessi L, Arizzi M, et al. J Clin Endocrinol Metab. 2000;85:2692–2700. doi: 10.1210/jcem.85.8.6723. [DOI] [PubMed] [Google Scholar]
- 23.Woolveridge I, de Boer-Brouwer M, Taylor M F, Teerds K J, Wu F C, Morris I D. Biol Reprod. 1999;60:461–470. doi: 10.1095/biolreprod60.2.461. [DOI] [PubMed] [Google Scholar]
- 24.Stokes T A, Rymaszewski M, Arscott P L, Wang S H, Bretz J D, Bartron J, Baker J R. Science. 1998;279:2015. , Technical Comments. [Google Scholar]
- 25.Restifo N P. Nat Med. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiedler P, Schaetzlein C E, Eibel H. Science. 1998;279:2015. , Technical Comments. [Google Scholar]
- 27.Riccioli A, Starace D, D'Alessio A, Starace G, Padula F, De Cesaris P, Filippini A, Ziparo E. J Immunol. 2000;165:743–749. doi: 10.4049/jimmunol.165.2.743. [DOI] [PubMed] [Google Scholar]
- 28.Van Demark N L, Free M J. In: The testis. Johnson A A, Gomez W R, Van Demark N L, editors. New York: Academic; 1970. pp. 233–312. [Google Scholar]
- 29.Beaumont H M. Int J Radiat Biol. 1960;2:247–256. doi: 10.1080/09553006014550291. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 31.Galdieri M, Ziparo E, Palombi F, Russo M A, Stefanini M. J Androl. 1981;5:249–254. [Google Scholar]
- 32.Kohno S, Ziparo E, Marek L F, Tung K S. J Reprod Immunol. 1983;5:339–350. doi: 10.1016/0165-0378(83)90243-7. [DOI] [PubMed] [Google Scholar]
- 33.Palombi F, Di Carlo C. Biol Reprod. 1988;39:1101–1108. doi: 10.1095/biolreprod39.5.1101. [DOI] [PubMed] [Google Scholar]
- 34.Palombi F, Farini D, De Cesaris P, Stefanini M. In: Molecular and Cellular Endocrinology of the Testis. Cooke B A, Sharpe R M, editors. New York: Raven; 1988. pp. 311–317. [Google Scholar]
- 35.Rossi P, Dolci S, Albanesi C, Grimaldi P, Ricca R, Geremia R. Dev Biol. 1993;155:68–74. doi: 10.1006/dbio.1993.1007. [DOI] [PubMed] [Google Scholar]
- 36.Meistrich M L. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- 37.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M A, Storey B T. Biol Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 39.Moore C. Endocrinology. 1924;8:493–508. [Google Scholar]
- 40.Sander B, Andersson J, Andersson U. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 41.Telford W G, King L E, Fraker P J. J Immunol Methods. 1994;172:1–16. doi: 10.1016/0022-1759(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 42.Steinberger A, Jakubowiak A. In: The Sertoli cell. Russell L D, Griswold M D, editors. Clearwater, FL: Cache River Press; 1993. pp. 155–179. [Google Scholar]
- 43.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 44.Martinez-Lorenzo M J, Anel A, Gamen S, Monle n I, Lasierra P, Larrad L, Pineiro A, Alava M A, Naval J. J Immunol. 1999;163:1274–1281. [PubMed] [Google Scholar]
- 45.Xu J P, Li X, Mori E, Guo M W, Matsuda I, Takaichi H, Amano T, Mori T. Am J Reprod Immunol. 1999;42:381–388. doi: 10.1111/j.1600-0897.1999.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 46.Allison J, Georgiou H M, Strasser A, Vaux D L. Proc Natl Acad Sci USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arai H, Gordon D, Nabel E G, Nabel G J. Proc Natl Acad Sci USA. 1997;94:13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang S M, Schneider D B, Lin Z, Hanahan D, Dichek D A, Stock P G, Baekkeskov S. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]