Abstract
Inhaled nitric oxide is being evaluated as a preventative therapy for patients at risk for bronchopulmonary dysplasia (BPD). Nitric oxide (NO), in the presence of superoxide, forms peroxynitrite, which reacts with tyrosine residues on proteins to form 3-nitrotyrosine (3-NT). However, NO can also act as an antioxidant and was recently found to improve the oxidative balance in preterm infants. Thus, we tested the hypothesis that the addition of a therapeutically relevant concentration (10 ppm) of NO to a hyperoxic exposure would lead to decreased 3-NT formation in the lung. FVB mouse pups were exposed to either room air (21% O2) or >95% O2 with or without 10 ppm NO within 24 h of birth. In the first set of studies, body weights and survival were monitored for 7 days, and exposure to >95% O2 resulted in impaired weight gain and near 100% mortality by 7 days. However, the mortality occurred earlier in pups exposed to >95% O2 + NO than in pups exposed to >95% O2 alone. In a second set of studies, lungs were harvested at 72 h. Immunohistochemistry of the lungs at 72 h revealed that the addition of NO decreased alveolar, bronchial, and vascular 3-NT staining in pups exposed to both room air and hyperoxia. The lung nitrite levels were higher in animals exposed to >95% oxygen + NO than in animals exposed to >95% oxygen alone. The protein levels of myeloperoxidase, monocyte chemotactic protein-1, and intracellular adhesion molecule-1 were assessed after 72 h of exposure and found to be greatest in the lungs of pups exposed to >95% O2. This hyperoxia-induced protein expression was significantly attenuated by the addition of 10 ppm NO. We propose that in the presence of >95% O2, peroxynitrite formation results in protein nitration; however, adding excess NO to the >95% O2 exposure prevents 3-NT formation by NO reacting with peroxynitrite to produce nitrite and NO2. We speculate that the decreased protein nitration observed with the addition of NO may be a potential mechanism limiting hyperoxic lung injury.
Keywords: Protein nitration, Peroxynitrite, Lung injury, Oxygen toxicity
Introduction
Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator approved for the treatment of term and near-term neonates with hypoxic respiratory failure associated with evidence of pulmonary hypertension. The therapeutic use of iNO has expanded to older patients with congenital heart disease and acute respiratory distress syndrome (ARDS). In the NICU, iNO has also been used to treat premature infants with surfactant deficiency [1–4] and has been evaluated as a preventative therapy for neonatal chronic lung disease, termed bronchopulmonary dysplasia (BPD) [5–7].
Exposure to high levels of inspired oxygen increases the formation of superoxide [8]. Superoxide can react with NO to form peroxynitrite [9, 10]. Peroxynitrite is a strong oxidant capable of damaging the alveolar epithelium [11] as well as adversely affecting surfactant function [12]. Furthermore, peroxynitrite can react with tyrosine residues in proteins to form 3-nitrotyrosine. Nitrotyrosine formation can result in alterations of protein function, including redox cycling of proteins, post-translational incorporation of altered proteins into the cytoskeleton, loss of catalytic tyrosine residues, and inhibition of protein phosphorylation [13, 14]. Nitrotyrosine formation has been implicated in the pathogenesis of BPD; for example, Banks et al. [15] found that plasma 3-nitrotyrosine levels were elevated in the first month of life in patients who went on to develop BPD.
However, iNO can also act as an antioxidant. For example, iNO has been shown to decrease lipid peroxidation [9, 16]. Recently, Hamon et al. [17] found that iNO therapy in premature infants resulted in lower levels of malondialdehyde, a biomarker of oxidative stress. Lorch et al. [18] measured plasma 3-nitrotyrosine levels in patients with BPD before and after 72 h of iNO therapy and found that the patients who had a decrease in plasma 3-nitrotyrosine levels were more likely to wean off of mechanical ventilation. Thus, we hypothesized that the addition of a therapeutically relevant concentration (10 ppm) of NO to a lethal (>95%) hyperoxic exposure would lead to decreased 3-nitrotyrosine formation in the lung. To address our hypothesis, we studied neonatal mice who were exposed to either room air or >95% O2 within 24 h of delivery with or without added NO. To develop the animal model, the first studies done were growth and survival studies; we then exposed animals for 72 h based on these results. Lung wet weights were determined as a marker of severe lung injury. The formation of 3-NT in the lungs was assayed using immunohistochemistry. Finally, biomarkers of inflammation, including myeloperoxidase (MPO), monocyte chemotactic protein-1 (MCP-1), and intracellular adhesion molecule-1 (ICAM-1), were evaluated by Western blot of lung homogenate at 48 h.
Materials and Methods
Animals
Time-dated pregnant FVB mice were purchased from Charles River (Wilmington, MA) and were maintained in the animal care facility of the Research Institute at Nationwide Children’s Hospital. All animals were kept in polycarbonate cages with wire lids and 100% aspen wood chip bedding. The animals were allowed free access to food and water, and a 12-h day:12-h night cycle was maintained throughout the study. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children’s Hospital.
Oxygen/NO Exposure
Dams delivered naturally at term. Within 24 h of birth, the cage containing the mother and her pups was placed in a Plexiglas chamber designed specifically for oxygen exposures. The 21% O2 atmosphere was maintained using a flow of room air (21% oxygen) at approximately 10 l/min, and the >95% O2 atmosphere was maintained with a flow of 100% O2 at ~10 l/min through the chamber. For the NO-exposure groups, 800 ppm NO (Ikaria, Clinton, NJ) was delivered at approximately 125 ml/min using an AeroNOx® delivery system (Ikaria, Clinton, NJ). Soda lime (Fisher Scientific, Fair Lawn, NJ) was present in the chamber during all exposures to absorb CO2 and NO2. Each litter was exposed to either (1) 21% O2 (room air), (2) 21% O2 + 10 ppm NO, (3) >95% O2, or (4) >95% O2 + 10 ppm NO. O2 and CO2 levels in the exposure chamber were measured twice daily (AEI Technologies, Pittsburgh, PA). NO and NO2 levels in the exposure chamber were measured continuously using the AeroNOx®. For each exposure group, a room air control group was exposed concurrently. Dams were switched between the exposure group and the room air controls every 24 h to prevent oxygen toxicity to the dams.
Immunohistochemistry
Animals were sacrificed by an intraperitoneal injection of pentobarbital sodium (200 mg/kg). The tracheas were cannulated with a 25-gauge Silastic catheter, and 10% neutral buffered formalin was instilled at 25 cm H2O pressure over 5 min. After 5 min, the trachea was tied and lungs removed and fixed overnight in 10% neutral buffered formalin. The next day, lungs were washed five times in phosphate-buffered saline (PBS), transferred to PBS, and stored at 4°C. Both intact left and right lungs were serially dehydrated in increasing concentrations of ethanol and then paraffinized. For preparation of paraffin blocks, left lungs were cut transversely (perpendicular to longitudinal or craniocaudal axis of the animal) at the level of entry of the left main bronchus. The cut slices of lung tissue were identically oriented and made into paraffin blocks. Five-micron sections of lung tissues were deparaffinized for immunostaining. Tissues were immunostained with a polyclonal antibody against 3-nitrotyrosine (1:1000 dilution; 0.6 µg/ml antibody protein concentration, Millipore, Billerica, MA) as previously described [19]. Staining (isotypic) control tissues were exposed for the same duration to nonimmune rabbit IgG (0.6 µg/ml, Vector Labs, Burlingame, CA) in place of primary antibody. In preliminary studies under identical conditions, we demonstrated this antibody to be specific (preincubation of antibody with excess free 3-nitrotyrosine, but not iodotyrosine, caused complete quenching of detectable signal). Diaminobenzidine (0.06% w/v) was used to provide visualization of immunoreactivity followed by methyl green counterstaining. Identically oriented tissue sections were visualized with an Olympus Optical (New York, NY) BX-40 microscope (800× magnification) and captured under identical lighting conditions and optical settings using a Insight digital camera. Images were analyzed using digital image analysis software (Image Pro Plus 4.0, Media Cybernetics, Silver Spring, MD). Images were then segmented to eliminate background and nuclear counterstain from the analysis. Optical densities (OD) were determined for each image in three different regions (alveolar, airways, and large vessels). Using this procedure, we have found that the intraobserver and interobserver variabilities are less than 5% and less than 10%, respectively.
Lung-Wet-to-Dry Weights
After euthanasia with pentobarbital, both lungs were dissected, removed, and weighed to obtain the wet weight. Dry weights were obtained after drying the lungs in an oven at 70°C for 6 days.
Experimental Protocols
Growth Curves and Survival Studies
A litter of neonatal mice was exposed to either 21% O2 + 10 ppm NO (n = 17), >95% O2 (n = 30), or >95% O2 + 10 ppm NO (n = 19) as described above. For each exposure group, a room air control group (n = 61) was exposed concurrently. Dams were switched between the exposure group and the room air controls every 24 h to prevent oxygen toxicity to the dams. Each pup was weighed daily. To determine survival for each exposure group, the number surviving was recorded at least every 6 h.
Assessment of Pulmonary Edema
In a separate group of experiments, neonatal mice were exposed to either 21% O2 (n = 6), >95% O2 (n = 10), or >95% O2 + 10 ppm NO (n = 10) for 72 h after which the lungs were harvested and lung wet-to-dry weight ratios were determined.
Assessment of Protein Nitration
Neonatal mice were exposed to either 21% O2 (n = 6 animals, and 5–10 images per animal), 21% O2 + 10 ppm NO (n = 5 animals, and 5–10 images per animal), >95% O2 (n = 6, and 5–10 images per animal), or >95% O2 + 10 ppm NO (n = 6, and 5–10 images per animal) for 72 h. The lungs were fixed for immunohistochemistry for 3-nitrotyrosine as described above.
Protein Nitration of an Albumin Solution
To further evaluate the impact of nitric oxide on protein nitration due to hyperoxia, we conducted a benchtop experiment in which 100% O2 was bubbled into a beaker containing 200 ml of a 10% albumin solution and stirred continuously. The solution was sampled at 0, 1, and 2 h and assayed for protein concentration, 3-NT, and NO2− concentration. The experiment was repeated with 100% O2 and 800 ppm NO bubbled into the 10% albumin solution at 1.2 ml/min, and samples were taken at 0, 1, and 2 h for protein, 3-NT, and NO2− concentrations. Protein concentrations in the samples were determined using the Bradford method (Bio-Rad, Hercules, CA). The levels of 3-nitrotyrosine were assayed using Western blots as previously described [20]. The nitrite (NO2−) concentration was assayed using chemiluminescence (NOA, model 280i, GE, Boulder, CO) as previously described [21]. Briefly, 100 µl of sample was placed in a reaction chamber containing a mixture of NaI in glacial acetic acid to reduce NO2− to NO. The NO gas was carried into the NO analyzer using a constant flow of He gas. The analyzer was calibrated using a NaNO2 standard curve. The results for 3-NT and NO2− were normalized to protein concentration.
Western Blotting
The lung homogenate supernatants were assayed for MPO, MCP-1, and ICAM-1 immunoreactivity using Western blot analyses as previously described [20]. Reduced samples were prepared using 10 µg of lung homogenate and were separated on a 4–12% Bis-Tris gel (Invitrogen Life Technologies, Carlsbad, CA) before transfer to polyvinylidene difluoride (PVDF) membranes and blocked overnight with a 20% fish skin gelatin solution. Membranes were then incubated with the primary antibody overnight: MPO (1:1000, Cell Signaling Technology, Beverly, MA), MCP-1 (1:1000, Cell Signaling Technology), and ICAM-1 (1:1000, Cell Signaling Technology). Protein concentrations were corrected to β-actin (1:10,000, Abcam, Cambridge, MA) to ensure equal protein loading.
Nitrite Assay in Lung Tissue
Evaluation of the production of nitrite in the lungs required reduction of nitrate to nitrite prior to nitrite analysis as described above, since nitrite in the lungs can be rapidly oxidized to nitrate. The NO2− levels were determined in lung homogenate samples by adding 50 µl NADPH (β-nicotenamide adenine dinucleotide phosphate-reduced form [Sigma], 0.8 mg/ml, in 0.1 M KPO4 buffer) and 20 µl nitrate reductase (from Aspergillus species [Sigma], 100 mU/ml, in 0.1 M KPO4 buffer) to either 100 µl of thawed lung homogenate or 100 µl of sodium nitrate standards. These samples were then assayed in duplicate for nitrite using a chemiluminescence NO analyzer (model 280i, GE, Boulder, CO) as previously described above. The analyzer was calibrated using a NaNO2 standard curve.
Statistics
Results are given as the mean ± SEM. The correlation between body weight and day of life was assessed using linear regression (SigmaStat, Jandel Scientific, Carlsbad, CA). The percent animals that survived was compared among the four groups using the Fisher exact test (SigmaStat). In the physiologic studies and the benchtop studies, the groups were compared using one-way analysis of variance (ANOVA). Differences were identified using a Neuman–Keuls post hoc test (SigmaStat). Differences were considered significant when p < 0.05.
Results
Hyperoxia Decreases Growth in Neonatal Mice
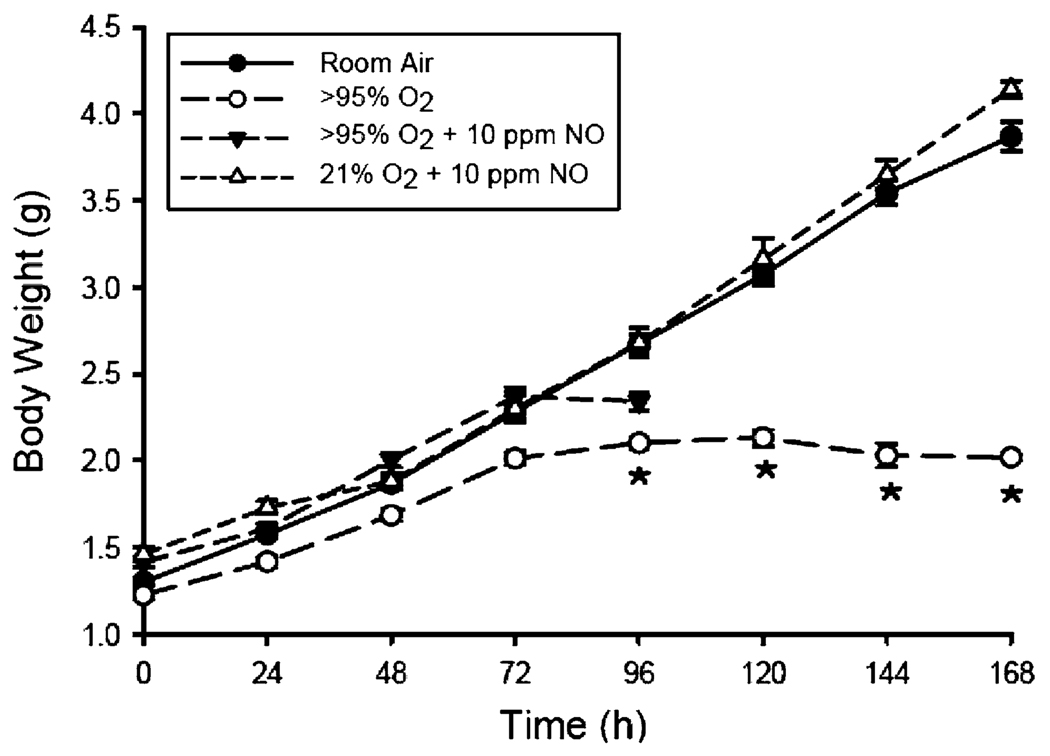
Pups exposed to room air had an essentially linear (r = 0.99, p < 0.001) weight gain over the 7-day exposure period, with an average weight gain of 0.38 g/day. The addition of 10 ppm NO to room air had no effect on weight gain through 1 week of age (Fig. 1). Hyperoxia with or without added NO impaired the weight gain of pups through 1 week of age (Fig. 1). At 96 h of exposure, the mean body weights were 2.10 ± 0.03 g in >95% O2, 2.34 ± 0.05 g in >95% O2 + NO, 2.68 ± 0.09 g in 21% O2 + NO, and 2.67 ± 0.05 g in the room air controls (p < 0.01). Those pups that survived past 96 h in >95% O2 did not gain weight after 72 h of exposure.
Fig. 1.
Daily body weight in grams for mouse pups exposed to room air (RA) ± NO or >95% O2 ± NO. The open circles represent the pups exposed to >95% O2 alone (n = 30); the closed triangles represent the pups exposed to >95% O2 + 10 ppm NO (n = 19); the open triangles represent the pups exposed to RA + 10 ppm NO (n = 17); and the closed circles represent pups exposed to RA (n = 61). * Different from 21% O2, p < 0.01
Hyperoxia Decreased Survival in Neonatal Mice
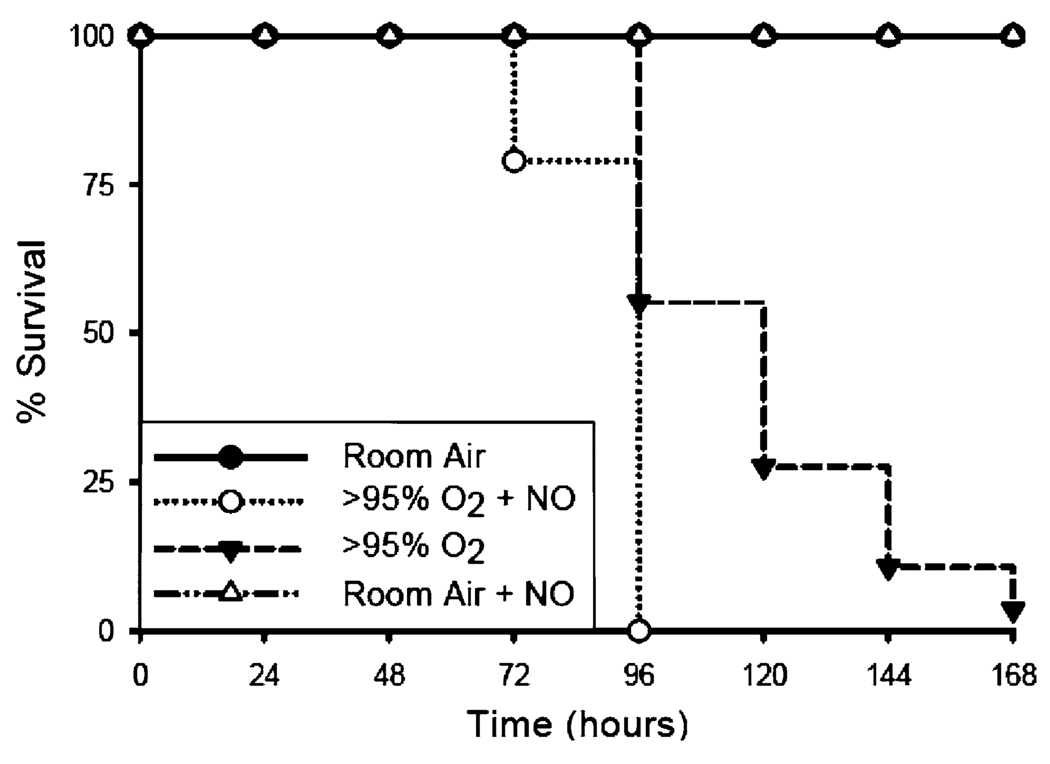
The results of the survival studies are presented in Fig. 2. There was no mortality observed in either the room air controls or the 21% O2 + 10 ppm NO groups through 1 week of exposure. Pups exposed to >95% O2 with or without NO were noticeably less active than room air-exposed pups after 72 h. The mortality curve for >95% O2 + NO was different from the mortality curve for >95% O2 alone by Kaplan-Meier analysis (p < 0.01), with mortality occurring sooner in the >95% O2 + NO group than in the >95% O2 alone group, although both groups experienced essentially 100% mortality by 168 h. Mortality was observed in the pups exposed to >95% O2 + 10 ppm NO group beginning at 72 h, whereas pups exposed to >95% O2 alone exhibited mortality beginning at 96 h, and by 168 h only 1 of 28 pups survived.
Fig. 2.
Survival for mouse pups exposed to room air (RA), RA + NO, and >95% O2 ± NO. The closed triangles connected by the dashed line represent the pups exposed to >95% O2 alone (n = 30); the open circles connected by the dotted line represent the pups exposed to >95% O2 + 10 ppm NO (n = 19); the open triangles connected by the dashed line represent the pups exposed to room air + 10 ppm NO (n = 17); and the closed circles connected by the solid line represent pups exposed to room air (n = 61)
Hyperoxia Did Not Effect Lung Weights
We examined lung tissue at 72 h, before the onset of most hyperoxia-induced mortality. To assess lung injury, we used lung weight-to-body weight ratios, and surprisingly they were not different after 72 h of exposure to >95% O2, >95% O2 + NO, or 21% O2 + NO compared to room air controls (19.2 ± 0.6 mg/g, >95% O2; 18.5 ± 0.4 mg/g, >95% O2 + NO; 16.9 ± 1.0 mg/g, 21% O2 + NO; and 18.5 ± 0.6 mg/g, room air controls). To assess whether other organs were affected, we compared liver weights. Similarly, we found no difference in liver weight-to-body weight ratios at 72 h between any of the four exposure groups (28.3 ± 1.8 mg/g, >95% O2; 27.0 ± 0.7 mg/g, >95% O2 + NO; 29.2 ± 0.8 mg/g, 21% O2 + NO; and 28.1 ± 1.4 mg/g, room air controls). As another measure of pulmonary edema formation, we determined wet-to-dry lung weight ratios in an additional 28 pups after drying the lungs at 70°C for 6 days. At 72 h, wet-to-dry lung weight ratios were not different between groups (5.65 ± 0.14, room air; 5.56 ± 0.21, >95% O2; and 5.39 ± 0.13, >95% O2 + NO, p = 0.58).
Hyperoxia Causes Protein Nitration and NO Prevents Protein Nitration
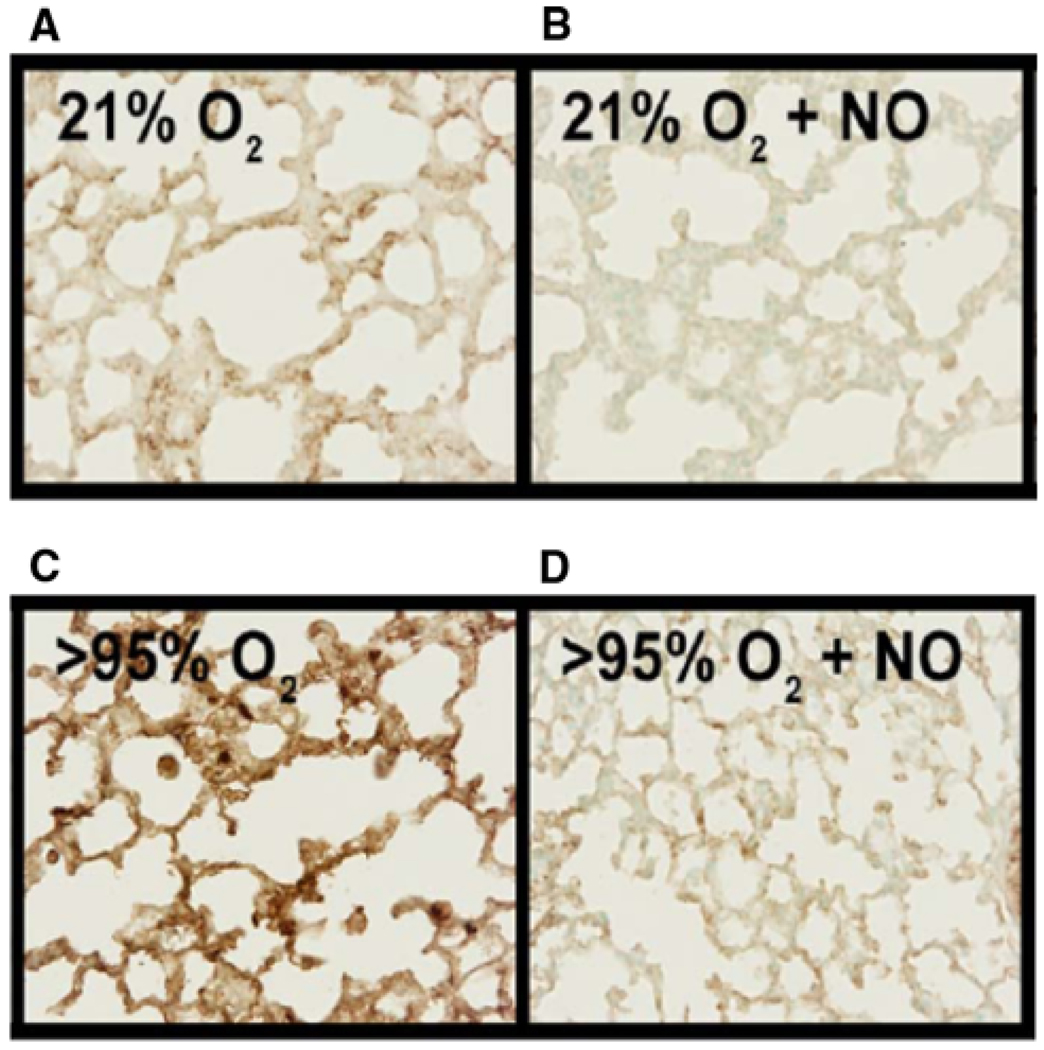
To evaluate protein nitration in the lungs of these neonatal mice, we performed immunohistochemistry for 3-NT on fixed lung tissue from mice exposed for 72 h to either 21% O2, 21% O2 + NO, >95% O2, or >95% O2 + NO. Representative sections are shown in Fig. 3. Animals exposed to 21% O2 for 72 h had some 3-NT staining (Fig. 3a). Interestingly, the addition of 10 ppm NO to the 21% O2 exposure decreased 3-NT in the lungs (Fig. 3b). We found the highest levels of 3-NT staining in sections from lungs of mice exposed to >95% O2 alone (Fig. 3c). The lungs from animals exposed to >95% O2 + NO had levels of 3-NT staining similar to that found in the lungs from the 21% O2-exposed controls (Fig. 3d).
Fig. 3.
Effect of hyperoxia ± NO on 3-NT immunolabeling. Representative images of fixed lung sections stained for 3-nitrotyrosine from pups exposed for 72 h to 21% O2 (A), 21% O2 + 10 ppm NO (B), >95% O2 (C), >95% O2 + 10 ppm NO (D)
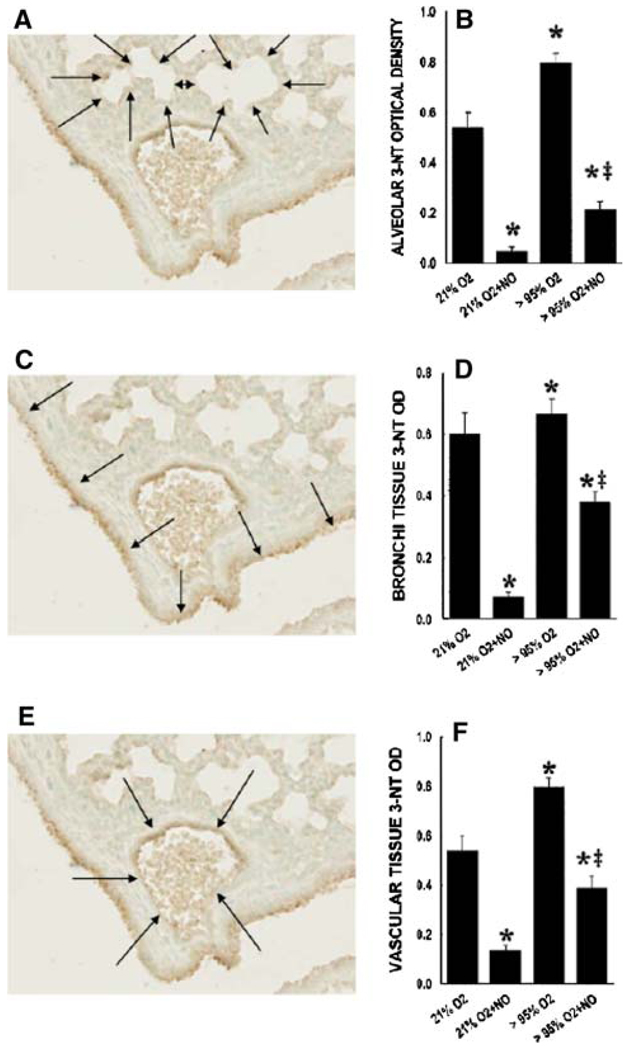
The 3-NT staining was quantified from the alveolar, vascular, and large airway regions of the lungs. Control animals exposed to 21% O2 had some basal levels of 3-NT staining in all lung regions (Fig. 4). Interestingly, the 3-NT staining was significantly less in all three lung regions studied in the animals exposed to 21% O2 + NO than it was in control animals (Fig. 4). As expected, exposure to >95% O2 resulted in significantly greater 3-NT staining in all of the lung regions studied than in the lungs from mice exposed to 21% O2 (Fig. 4). The 3-NT levels were significantly lower in the lungs from mice exposed to >95% O2 + 10 ppm NO than in the lungs from those animals exposed to >95% O2 alone (Fig. 4a). Indeed, the levels of 3-NT in the lungs of animals exposed to >95% O2 + NO were significantly lower in all of the lung regions studied than in the corresponding lung regions from those control animals exposed to 21% O2 (Fig. 4).
Fig. 4.
Effect of hyperoxia ± NO on 3-NT immunolabeling for alveolar, bronchial, and vascular lung sections. Optical density of 3-nitrotyrosine staining from lung tissue of mouse pups exposed to 21% O2, 21% O2 + 10 ppm NO, >95% O2, and >95% O2 + 10 ppm NO for specific areas of the lung tissue. a, c, e Representative lung section demonstrating how the lung sections were divided into alveolar (a), bronchial (c), and vascular (e) sections. b, d, f Data from all of the lungs studied. * Different from 21% O2, p < 0.01. ‡ 95% O2 + 10 ppm NO different from 95% O2 alone, p < 0.05
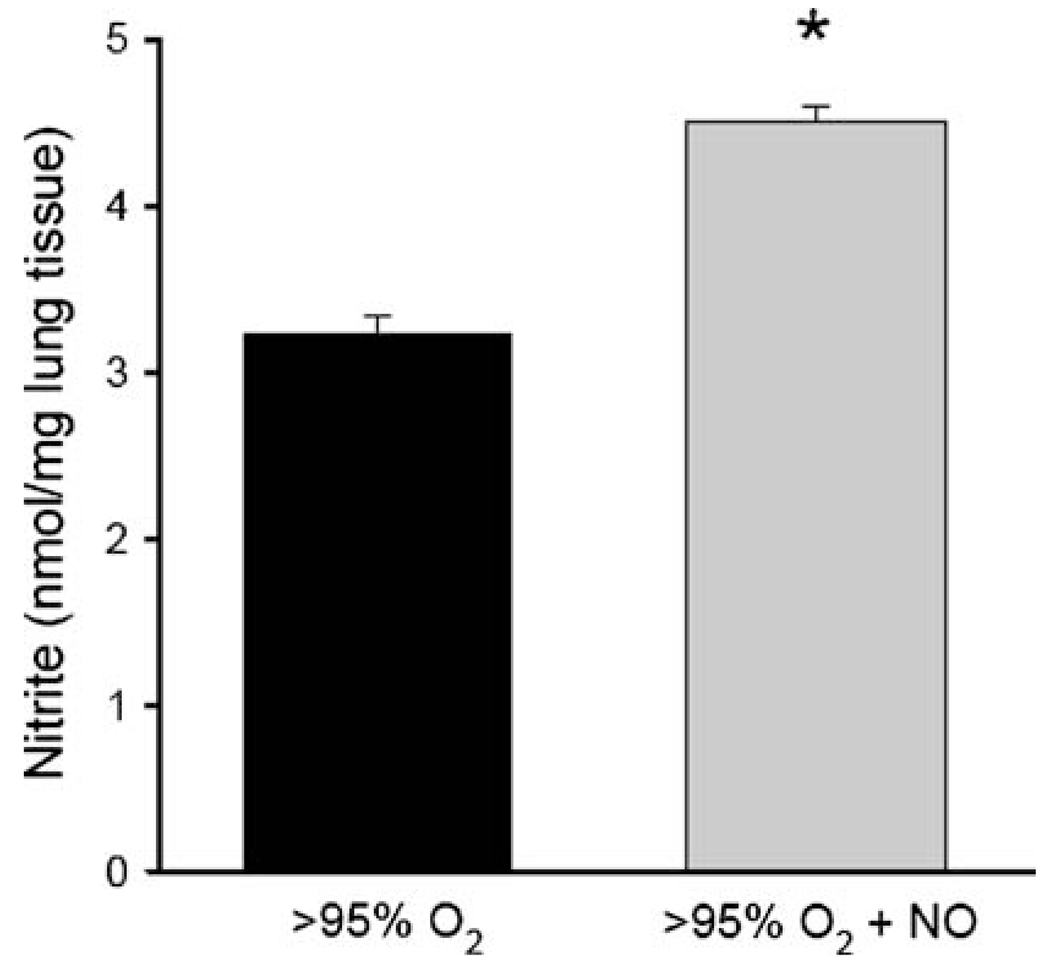
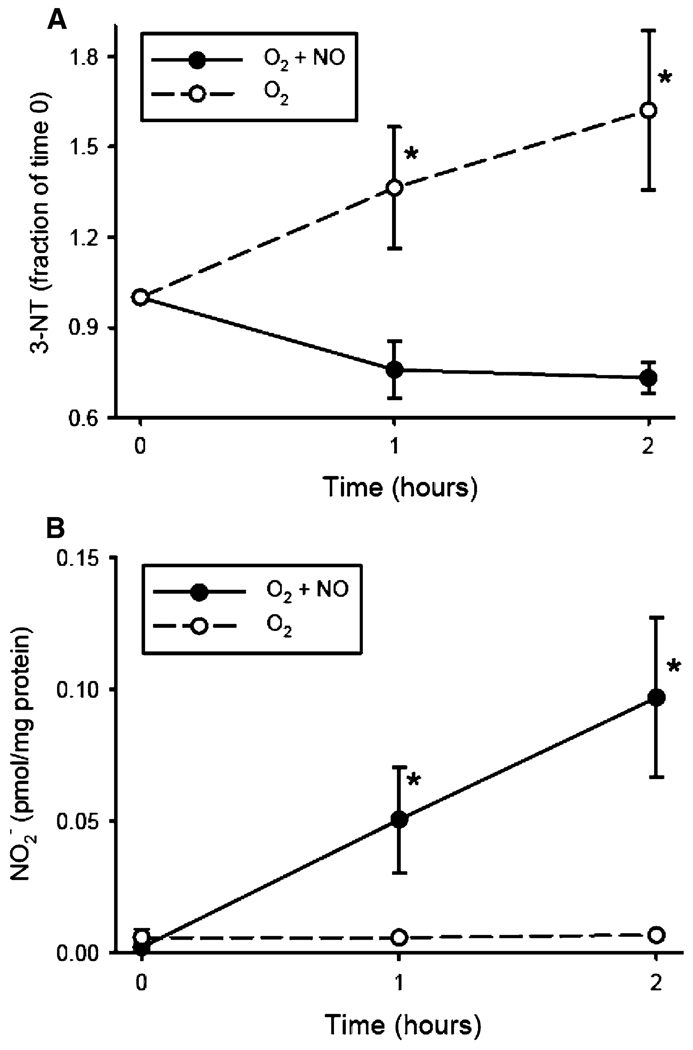
To evaluate a potential mechanism for the decreased protein nitration observed in the lungs of mouse pups exposed to >95% O2 + NO, we measured nitrite levels in the lung homogenate from animals sacrificed at 72 h. The hypothesis was that in animals exposed to pharmacologic levels of NO in their inhaled gas, the excess NO would scavenge radicals capable of protein nitration (i.e., ONOO−) before they could react with protein tyrosine residues to form 3-NT. As seen in Fig. 5, we found greater levels of nitrite in the lungs of pups exposed to >95% O2 + NO than in the lungs of pups exposed to >95% O2 alone (p < 0.05). To further evaluate the role of pharmacologic levels of NO gas in protein nitration, we performed a series of benchtop studies utilizing a 10% albumin solution and bubbling the solution with either O2 alone or O2 + NO. These cell-free experimental conditions clearly differ from a complicated in vivo system, yet the results were consistent with our animal data. We found that 3-NT formation, as assessed by Western blot analysis, increased over the 2-h study period in the albumin solution bubbled with O2 alone, while there was no increase in 3-NT formation in the albumin solution when bubbled with O2 + NO (Fig. 6a). The level of nitrite in the albumin solution at 1 and 2 h increased only in the albumin solutions bubbled with O2 + NO, and there was little nitrite formed over the 2-h experimental period in the albumin solution bubbled with O2 alone (Fig. 6b). Furthermore, the levels of 3-NT in the albumin solution bubbled with O2 + NO were less at each time point than those in the albumin solutions bubbled with O2 alone, while the levels of nitrite were greater at each time point from the albumin solutions bubbled with O2 + NO than in those bubbled with O2 alone (Fig. 6).
Fig. 5.
Effect of hyperoxia ± NO on nitrite levels. The lung nitrite levels are normalized to protein concentration in the lung tissue homogenate. n = 5 for each group, * >95% O2 + NO different from >95% O2, p < 0.05
Fig. 6.
Effect of adding NO on 3-NT and nitrite formation in albumin solution in hyperoxia. a 3-Nitrotyrosine formation in a 10% albumin solution bubbled with either O2 or O2 + NO. The 3-NT densities for each time point are corrected for the protein concentrations at each time point, and the fraction of normalized 3-NT levels are expressed as the fraction of 3-NT content at time 0. n = 4 for O2 and n = 3 for O2 + NO. * O2 alone different from O2 + NO, p < 0.05. b The amount of nitrite formed per milligram of protein in the 10% albumin solution after 0, 1, and 2 h of exposure to either O2 alone or O2 + NO. * O2 + NO different from O2 alone, p < 0.05
NO Attenuates the Inflammatory Response in the Lungs due to Hyperoxia
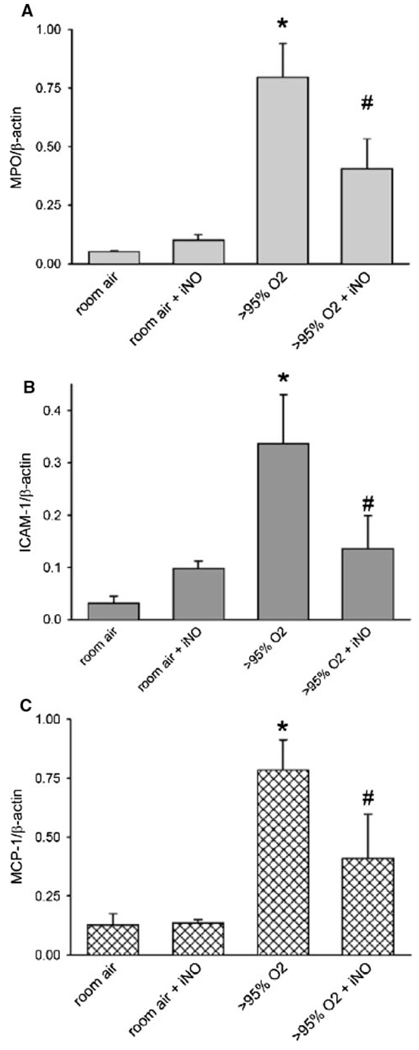
Inflammatory biomarkers measured in lung homogenates after 48 h of age were lower in neonatal mice exposed to hyperoxia plus nitric oxide versus hyperoxia alone. At 48 h, MPO levels increased significantly compared to animals exposed to room air + NO or room air controls (Fig. 7a). The addition of NO to the >95% O2 exposure significantly attenuated MPO protein levels after 48 h of exposure. ICAM-1 and MCP-1 protein levels were significantly greater in lungs of animals exposed to >95% O2 for 48 h than in lungs of animals exposed to either room air or room air with 10 ppm NO.
Fig. 7.
Effect of hyperoxia ± NO on MPO, MCP-1, and ICAM-1 protein levels in the lung. Protein levels from lung homogenate of pups after normalizing to β-actin are shown for a MPO after 48 h of exposure, b MCP-1 after 48 h of exposure, and c ICAM-1 after 48 h of exposure. * O2 different from RA, p < 0.05; # O2 + NO different from O2 alone, p < 0.05
Discussion
The main findings of this study in neonatal FVB mice were that (1) exposure to >95% O2 resulted in mortality, which was somewhat hastened when NO was added to the >95% O2 exposure; (2) there was little evidence of pulmonary edema in these lungs; (3) exposure to >95% O2 increased protein nitration; (4) the addition of NO to the >95% O2 exposure substantially decreased protein nitration in all lung regions studied; (5) the decrease in protein nitration seen with the addition of NO to the >95% O2 exposure is associated with an increase in nitrite levels; and (6) the addition of NO decreased protein levels of inflammatory markers in the lung at 48 h. These findings support our hypothesis that the addition of a therapeutically relevant concentration of NO to a lethal hyperoxic exposure decreases 3-nitrotyrosine formation in the lung. Indeed, our findings are also consistent with the concept that pharmacologic levels of NO in the hyperoxic exposure “scavenge” protein nitrating species to form nitrite.
Hyperoxia has been reported to cause pulmonary pathology and mortality in several species [22–24]. The addition of NO to >95% O2 has been found to either improve or to have no effect on survival. We previously found greater survival in adult rats exposed to >95% O2 + 100 ppm NO versus >95% O2 alone [22]. In newborn guinea pigs, Gries et al. [25] found that the addition of 20 ppm NO to >95% O2 delayed the onset of respiratory distress but did not improve lung compliance. Garat et al. [26] reported no effect of either 10 ppm NO or 100 ppm NO on survival of rats in >95% O2, but treatment with L-NAME or aminoguanidine (specific and nonspecific blockers of iNOS, respectively) reduced survival, suggesting that endogenous NO is protective in the presence of hyperoxia [27]. Taken together, these studies suggest that NO may reduce the harmful effects of hyperoxia. We chose to evaluate the effect of hyperoxia with or without a therapeutically relevant concentration of NO (10 ppm) in neonatal mice for two reasons. First, a newborn mouse has a relatively immature lung structure at birth that in some ways mimics the lung development of a premature human infant. Second, the availability of knockout animals should prove useful for future studies.
Pups exposed to >95% O2 with or without 10 ppm NO were noticeably less active and were dyspneic after 60 h. The decrease in weight gain starting at 72 h likely reflects this because the exposed pups had to work harder at breathing, which likely resulted in greater caloric expenditure without the ability to increase caloric intake. Nitric oxide alone does not affect weight gain, as pups exposed to 21% O2 + NO grew at the same rate as room air controls through 1 week of age. In this study we found that NO did not have a protective effect on survival in >95% O2− exposed mice; in fact, there was a somewhat more rapid mortality found in the neonatal mice when NO was added to >95% O2 exposure. The mortality appears to be related to >95% O2 since we observed no mortality in pups exposed to 21% O2 + 10 ppm NO for a week, and adult mice have breathed NO for up to 6 months without evidence of lung injury [28]. In the gas phase, NO and O2 spontaneously form NO2 [7], and inhaling NO2 causes lung damage. However, it is unlikely that NO2 was related to differences in the onset of mortality in this study since NO2 buildup in the exposure chamber was minimized by the use of a high rate of turnover (10 l/min) and the presence of soda lime in the chamber. Indeed, in these studies NO2 levels were measured and were always found to be less than 1.2 ppm, well below the current OSHA standard for peak exposure to NO2 of 5 ppm in an 8-h period [28]. Pulmonary edema does not seem to contribute to the morbidity of the pups exposed to >95% O2 with or without NO, at least at 72 h of age. No measurable amount of pleural effusion fluid was found for any of the exposure groups at 72 h, and neither the lung-to-body weight ratios nor the lung wet-to-dry weight ratios were different among groups. This differs from adult rats exposed to >95% O2 + 100 ppm NO who had increased lung weight and pleural effusions after 60 h of exposure [22]. While this could be due to differences in species or NO concentration, it may reflect the relative tolerance of newborn mice to hyperoxia compared to adult animals [24] or reflect differences between strains [29]. On the other hand, it has been found that exposure to hyperoxia can cause central nervous system (CNS) damage, including through alterations in blood flow caused by the elaboration of reactive oxygen and nitrogen species in the brain. Thus, it may be that the addition of NO to the hyperoxic exposure results in greater formation of free radicals in the brain and thereby has a greater effect on brain blood flow than does exposure to hyperoxia alone [30]. Further studies will need to be done to elucidate the exact mechanisms of hastened mortality in the hyperoxia + NO-exposed group, and attention should be paid to both pulmonary causes as well as CNS causes [31].
As we initially hypothesized, there was increased production of 3-NT in lung tissue from mouse pups exposed to >95% O2 compared to room air controls. Our findings are consistent with previous studies that found increased protein nitration in the lungs after exposure to hyperoxia for 48–72 h in adult mice [32, 33] and adult rats [34–36]. Human studies have also found elevated 3-NT levels in patients exposed to supplemental oxygen, including adult patients with ARDS [36] and premature infants who went on to develop bronchopulmonary dysplasia [15]. This increase in 3-NT could be due to a rise in superoxide production under hyperoxic conditions, with subsequent formation of peroxynitrite: •NO + O2•− → ONOO−. The formation of peroxynitrite occurs at a nearly diffusion-limited rate [37]. In situations where NO and/or O2•− production are increased, e.g., during exposure to hyperoxia, the amount of peroxynitrite is increased, which would be expected to lead to greater 3-NT formation. However, if NO is present in great excess (as would be expected when an animal is inhaling 10 ppm NO), then the excess NO can react with peroxynitrite to form nitrogen dioxide and nitrite: ONOO− + •NO → NO2 + NO2− [38]. This may lead to a decrease in 3-NT formation. The NO2− formed by the reaction of NO with peroxynitrite could then conceivably be metabolized to either •NO2 or NO2Cl by myeloperoxidase in activated neutrophils in the lung. However, although neutrophil activation in the lung is well described in hyperoxic exposure, we found a decrease in 3-NT formation in the lungs exposed to >95% O2 + NO. It may be that the conversion of NO2− by myeloperoxidase to protein-nitrating species does not account for a large portion of the 3-NT formed in the lung during exposure to >95% O2. Alternatively, it may be that the pharmacologic concentrations of NO scavenge •NO2 and/or NO2Cl through radical-radical interactions [39] before they can react with tyrosine residues to form 3-NT. This concept is consistent with a study using activated monocytes wherein high levels of NO flux resulted in diminished protein nitration [40]. Thus, we propose that in animals exposed to hyperoxia, iNO scavenges peroxynitrite (and perhaps other nitrating species) before it can react with tyrosine residues to form 3-nitrotyrosine.
The reported effects of iNO on lung 3-NT formation have been varied, and in some cases contradictory. In a rat model of acute lung injury induced by meconium, no change in lung 3-NT formation was found after the addition of 20 ppm NO [41]. The addition of 20 ppm NO to >95% O2 did not decrease 3-NT formation in either adult mice at 72 h [32] or in adult rats at 24–48 h [35]. These results are in contrast to our observed decrease in 3-NT formation in neonatal mice and may be related to differences in animal species or strain, dose of iNO employed, or the developmental stage of the lungs studied. We found similar 3-NT staining in the alveolar, bronchial, and vascular areas of the lung, indicating that changes were not confined to one area of the lung. This staining pattern suggests that the reactive nitrogen species produced during hyperoxia apparently were formed in parenchymal cell types and not confined to immune cell infiltrates. Lorch et al. [32] similarly found significant elevations in 3-NT staining in the airway epithelium, alveolar interstitium, and vasculature of adult rats exposed to hyperoxia.
The increased protein levels of MPO and MCP-1 in lung homogenate of pups exposed to hyperoxia have previously been shown to correlate with neutrophil activation and infiltration [42, 43]. NO appears to attenuate the inflammation from neutrophil accumulation in the lung due to hyperoxia as demonstrated by the decrease in MPO levels at 48 h (Fig. 7a) and previous work in preterm lambs and in isolated rat perfused lungs [44, 45]. ICAM-1 has previously been implicated in neutrophil-mediated hyperoxic lung injury in adult mice [46], and our finding that the addition of NO to the hyperoxic exposure attenuates this is consistent with previous reports in adult animals. For example, NO decreased ICAM-1 levels in the lungs of rats after 60 h in 100% oxygen [47] and after experimental pneumonia in 40 and 100% oxygen [48]. The decreased early inflammatory response observed with the addition of NO to hyperoxia could be beneficial clinically, as premature infants with elevated levels of MCP-1 or ICAM-1 in tracheal aspirates have an increased risk for developing BPD [49, 50].
In conclusion, the results of this study demonstrate that 3-NT is formed in the lungs of newborn mice and exposure to hyperoxia results in greater 3-NT formation. Consistent with our hypothesis, the addition of NO to either room air or >95% O2 results in decreased 3-NT formation. We propose that this decreased 3-NT formation is due to the reaction of excess NO with peroxynitrite before it can react with tyrosine residues to form 3-NT. Further studies are needed to determine the role of iNO on subsequent lung development when the inspired O2 concentration is weaned to sublethal levels.
Acknowledgments
We thank Dionna Hatch and T. J. Calvert for excellent technical assistance. We also thank Ikaria for providing the NO gas and the AeroNOx delivery system. This study was supported by National Heart Lung and Blood Institute Grant HL-075261.
Abbreviations
- 3-NT
3-Nitrotyrosine
- ARDS
Acute respiratory distress syndrome
- BPD
Bronchopulmonary dysplasia
- CO2
Carbon dioxide
- ICAM-1
Intracellular adhesion molecule-1
- iNOS
Inducible nitric oxide synthase
- L-NAME
Nitro-l-arginine methylester
- MCP-1
Monocyte chemotactic protein-1
- MPO
Myeloperoxidase
- NO
Nitric oxide
- NO2−
Nitrite
- NO2
Nitrogen dioxide
- O2
Oxygen
Superoxide
- Ppm
Parts per million
Contributor Information
Michael R. Stenger, Email: Michael.Stenger@nationwidechildrens.org, Pulmonary Hypertension Group, Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH 43205, USA; Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
Melissa J. Rose, Center for Cardiovascular and Pulmonary Research, The Research Institute at Nationwide Children’s Hospital, Columbus, OH 43205, USA Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
Mandar S. Joshi, Center for Cardiovascular and Pulmonary Research, The Research Institute at Nationwide Children’s Hospital, Columbus, OH 43205, USA Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
Lynette K. Rogers, Pulmonary Hypertension Group, Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH 43205, USA Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
Louis G. Chicoine, Pulmonary Hypertension Group, Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH 43205, USA Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
John Anthony Bauer, Center for Cardiovascular and Pulmonary Research, The Research Institute at Nationwide Children’s Hospital, Columbus, OH 43205, USA; Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
Leif D. Nelin, Pulmonary Hypertension Group, Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, OH 43205, USA Department of Pediatrics, The Ohio State University, Columbus, OH 43210, USA.
References
- 1.Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, Walsh-Sukys MC, McCaffrey MJ, Cornfield DN, Bhutani VK, Cutter GR, Baier M, Abman SH. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354:1061–1065. doi: 10.1016/s0140-6736(99)03558-8. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 3.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GM, Konduri GG, Aschner JL, Stoll BJ, D’Angio CT, Stevenson DK. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 5.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 6.Banks BA, Seri I, Ischiropoulos H, Merrill J, Rychik J, Ballard RA. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics. 1999;103:610–618. doi: 10.1542/peds.103.3.610. [DOI] [PubMed] [Google Scholar]
- 7.Clark PL, Ekekezie II, Kaftan HA, Castor CA, Truog WE. Safety and efficacy of nitric oxide in chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2002;86:F41–F45. doi: 10.1136/fn.86.1.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 9.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 10.Nelin LD, Morrisey JF, Effros RM, Dawson CA, Schapira RM. The effect of inhaled nitric oxide and oxygen on the hydroxylation of salicylate in rat lungs. Pediatr Res. 2003;54:337–343. doi: 10.1203/01.PDR.0000079183.85517.CE. [DOI] [PubMed] [Google Scholar]
- 11.Royall JA, Kooy NW, Beckman JS. Nitric oxide-related oxidants in acute lung injury. New Horiz. 1995;3:113–122. [PubMed] [Google Scholar]
- 12.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physio. 1993;265:L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 13.Ischiropoulos H. Biological tyrosine nitration: a patho-physiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 14.Eiserich JP, Patel RP, O’Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 15.Banks BA, Ischiropoulos H, McClelland M, Ballard PL, Ballard RA. Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics. 1998;101:870–874. doi: 10.1542/peds.101.5.870. [DOI] [PubMed] [Google Scholar]
- 16.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 17.Hamon I, Fresson J, Nicolas MB, Buchweiller MC, Franck P, Hascoet JM. Early inhaled nitric oxide improves oxidative balance in very preterm infants. Pediatr Res. 2005;57:637–643. doi: 10.1203/01.PDR.0000156507.03879.19. [DOI] [PubMed] [Google Scholar]
- 18.Lorch SA, Banks BA, Christie J, Merrill JD, Althaus J, Schmidt K, Ballard PL, Ischiropoulos H, Ballard RA. Plasma 3-nitrotyrosine and outcome in neonates with severe bronchopulmonary dysplasia after inhaled nitric oxide. Free Radic Biol Med. 2003;34:1146–1152. doi: 10.1016/s0891-5849(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 19.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol. 2002;135:581–588. doi: 10.1038/sj.bjp.0704495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley KP, Chicoine LG, Young TL, Reber KM, Lyons CR, Liu Y, Nelin LD. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L298–L306. doi: 10.1152/ajplung.00140.2005. [DOI] [PubMed] [Google Scholar]
- 21.Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2005;33:394–401. doi: 10.1165/rcmb.2005-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelin LD, Welty SE, Morrisey JF, Gotuaco C, Dawson CA. Nitric oxide increases the survival of rats with a high oxygen exposure. Pediatr Res. 1998;43:727–732. doi: 10.1203/00006450-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA. Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med. 1996;21:43–52. doi: 10.1016/0891-5849(95)02226-0. [DOI] [PubMed] [Google Scholar]
- 24.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol. 1978;45:699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- 25.Gries DM, Tam EK, Blaisdell JM, Iwamoto LM, Fujiwara N, Uyehara CF, Nakamura KT. Differential effects of inhaled nitric oxide and hyperoxia on pulmonary dysfunction in newborn guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1525–R1530. doi: 10.1152/ajpregu.2000.279.5.R1525. [DOI] [PubMed] [Google Scholar]
- 26.Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S. Effects of inhaled nitric oxide or inhibition of endogenous nitric oxide formation on hyperoxic lung injury. Am J Respir Crit Care Med. 1997;155:1957–1964. doi: 10.1164/ajrccm.155.6.9196102. [DOI] [PubMed] [Google Scholar]
- 27.Oda H, Kusumoto S, Nakajima T, Kurata A, Imai K. Long-term exposure to nitric oxide in mice. J Jpn Soc Air Pollut. 1976;11:150–160. [Google Scholar]
- 28.CDC National Institute for Occupational Safety and Health. Criteria for a recommended standard… occupational exposure to oxides of nitrogen (nitrogen dioxide and nitric oxide). NIOSH. 1976 Mar;:1–204. http://www.cdc.gov/niosh/76-149.html.
- 29.Wei R, Lin CM. Strain-dependent inflammatory responsiveness of rat microglial cells. J Neuroimmunol. 2009;211:23–38. doi: 10.1016/j.jneuroim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Sirinyan M, Sennlaub F, Dorfman A, Sapieha P, Gobeil F, Jr, Hardy P, Lachapelle P, Chemtob S. Hyperoxic exposure leads to nitrative stress and ensuing microvascular degeneration and diminished brain mass and function in the immature subject. Stroke. 2006;37(11):2807–2815. doi: 10.1161/01.STR.0000245082.19294.ff. [DOI] [PubMed] [Google Scholar]
- 31.Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol. 2009;106(2):662–667. doi: 10.1152/japplphysiol.91109.2008. [DOI] [PubMed] [Google Scholar]
- 32.Lorch SA, Foust R, Gow A, Arkovitz M, Salzman AL, Szabo C, Vayert B, Geffard M, Ischiropoulos H. Immunohistochemical localization of protein 3-nitrotyrosine and S-nitrosocysteine in a murine model of inhaled nitric oxide therapy. Pediatr Res. 2000;47:798–805. doi: 10.1203/00006450-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Hataishi R, Mitsufuji H, Tanaka M, Jacobson M, Tomita T, Zapol WM, Jones RC. Antiinflammatory properties of inducible nitric oxide synthase in acute hyperoxic lung injury. Am J Respir Cell Mol Biol. 2001;24:390–397. doi: 10.1165/ajrcmb.24.4.4218. [DOI] [PubMed] [Google Scholar]
- 34.Narasaraju TA, Jin N, Narendranath CR, Chen Z, Gou D, Liu L. Protein nitration in rat lungs during hyperoxia exposure: a possible role of myeloperoxidase. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1037–L1045. doi: 10.1152/ajplung.00008.2003. [DOI] [PubMed] [Google Scholar]
- 35.Lorch SA, Munson D, Lightfoot RT, Ischiropoulos H. Oxygen tension and inhaled nitric oxide modulate pulmonary levels of S-nitrosocysteine and 3-nitrotyrosine in rats. Pediatr Res. 2004;56:345–352. doi: 10.1203/01.PDR.0000134256.30519.9B. [DOI] [PubMed] [Google Scholar]
- 36.Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grisham MB. Reactive metabolites of oxygen and nitrogen in biology and medicine. Austin: R.G. Landes Co.; 1992. pp. 70–75. [Google Scholar]
- 39.Hogg N, Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochim Biophys Acta. 1999;1411:378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 40.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 41.Lu MP, Du LZ, Gu WZ, Chen XX. Nitric oxide inhalation inhibits inducible nitric oxide synthase but not nitrotyrosine formation and cell apoptosis in rat lungs with meconium-induced injury. Acta Pharmacol Sin. 2005;26:1123–1129. doi: 10.1111/j.1745-7254.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- 42.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59:1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 43.Puneet P, Hegde A, Ng SW, Lau HY, Lu J, Moochhala SM, Bhatia M. Preprotachykinin-A gene products are key mediators of lung injury in polymicrobial sepsis. J Immunol. 2006;176:3813–3820. doi: 10.4049/jimmunol.176.6.3813. [DOI] [PubMed] [Google Scholar]
- 44.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res. 1997;41:457–463. doi: 10.1203/00006450-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Guidot DM, Repine MJ, Hybertson BM, Repine JE. Inhaled nitric oxide prevents neutrophil-mediated, oxygen radical-dependent leak in isolated rat lungs. Am J Physiol. 1995;269:L2–L5. doi: 10.1152/ajplung.1995.269.1.L2. [DOI] [PubMed] [Google Scholar]
- 46.Wegner CD, Wolyniec WW, LaPlante AM, Marschman K, Lubbe K, Haynes N, Rothlein R, Letts LG. Intercellular adhesion molecule-1 contributes to pulmonary oxygen toxicity in mice: role of leukocytes revised. Lung. 1992;170:267–279. doi: 10.1007/BF00566679. [DOI] [PubMed] [Google Scholar]
- 47.Howlett CE, Hutchison JS, Veinot JP, Chiu A, Merchant P, Fliss H. Inhaled nitric oxide protects against hyperoxia-induced apoptosis in rat lungs. Am J Physiol. 1999;277:L596–L605. doi: 10.1152/ajplung.1999.277.3.L596. [DOI] [PubMed] [Google Scholar]
- 48.Sun Z, Sun B, Wang X, Wang W, Zhu L. Anti-inflammatory effects of inhaled nitric oxide are optimized at lower oxygen concentration in experimental Klebsiella pneumoniae pneumonia. Inflamm Res. 2006;55:430–440. doi: 10.1007/s00011-006-6029-7. [DOI] [PubMed] [Google Scholar]
- 49.Baier RJ, Loggins J, Kruger TE. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of Ureaplasma urealyticum. J Investig Med. 2001;49:362–369. doi: 10.2310/6650.2001.33902. [DOI] [PubMed] [Google Scholar]
- 50.Kojima T, Sasai M, Kobayashi Y. Increased soluble ICAM-1 in tracheal aspirates of infants with bronchopulmonary dysplasia. Lancet. 1993;342:1023–1024. doi: 10.1016/0140-6736(93)92880-3. [DOI] [PubMed] [Google Scholar]