Summary
Sensory synapses of the visual and auditory systems must faithfully encode a wide dynamic range of graded signals, and must be capable of sustained transmitter release over long periods of time. Functionally and morphologically, these sensory synapses are unique: their active zones are specialized in several ways for sustained, rapid vesicle exocytosis, but their most striking feature is an organelle called the synaptic ribbon, which is a proteinaceous structure that extends into the cytoplasm at the active zone and tethers a large pool of releasable vesicles. But precisely how does the ribbon function to support tonic release at these synapses? Recent genetic and biophysical advances have begun to open the ‘black box’ of the synaptic ribbon with some surprising findings, and promise to resolve its function in vision and hearing.
Introduction
Changes in our external environment are detected by sensory receptor cells, which transduce sensory stimuli into an electrical signal that is graded depending on the stimulus intensity. In vision, balance, and hearing, synapses of the receptor cells are unusual because they function tonically, that is, they transmit graded information with high fidelity across a broad range of stimulus intensity and for long periods of time, which places stringent demands on their release machinery. Rod photoreceptor cells of the retina can detect single photons but are nevertheless able to signal changes in light intensity over a range of ~105. Auditory hair cells are also exquisitely sensitive to small mechanical displacement, and yet can signal sound intensity over six orders of magnitude. To these requirements must be added the remarkably high, sustained rates of release provided by some ribbon synapses, driving several hundred action potentials per second in cochlear afferent neurons. Contrast this with neurotransmission at conventional synapses, which is tightly phase-locked to the occurrence of presynaptic action potentials, allowing the resulting highly episodic release to be supported by relatively small pools of readily releasable synaptic vesicles.
To cover this wide dynamic range, and sustained release, the synaptic outputs of these sensory neurons have evolved specialized machinery — the synaptic ribbon — at the active zones, where synaptic vesicle exocytosis occurs. Besides auditory hair cells and retinal photoreceptor cells, other types of primary sensory neurons have ribbon synapses as well, including pineal photoreceptors, electroreceptors, and hair cells of vestibular organs and the lateral line system. In the visual system, ribbons are also found at the output synapses of the second-order retinal bipolar neurons, which signal by means of graded changes in membrane potential, similar to photoreceptors and hair cells. Indeed, synaptic ribbons are found wherever transmission requires graded modulation of ongoing vesicle exocytosis.
Ribbons vary considerably in size, shape, and number in different cell types, but they share the common characteristic of having numerous glutamate-containing synaptic vesicles attached to their surface by filamentous tethers. Because ribbons seem to be exclusive to synapses where graded depolarization drives sustained neurotransmitter release, it is generally thought that ribbons increase the pool of vesicles available to support continuous transmission, by serving as a storehouse for release-ready vesicles and perhaps as a transport and/or timing system for delivering those vesicles to the plasma membrane. Consistent with this view, the vesicles associated with ribbons do not exchange with cytoplasmic vesicle pools in unstimulated synapses, but do turn over rapidly when calcium channels open. As we will see, ribbons may have the additional role — and possibly primary role in some instances — of synchronizing the release of multiple vesicles at stimulus onset. In this review we focus first on the structure of ribbon synapses, including both presynaptic and postsynaptic features, then discuss what is known about the functional roles of ribbons at retinal synapses and in hair cells of the inner ear, and end by briefly describing some of the remaining questions about how ribbon synapses work, as well as approaches to providing answers.
The structure of ribbon synapses
Ribbon synapses confer a capacity for sustained, graded vesicle release in sensory receptor cells. However, the diverse types of stimuli and wide dynamic range over which they need to operate are reflected in the wide variation in the size, shape and functionality of ribbons across cell types as well as the number of tethered vesicles. The study of retinal and auditory synapses has revealed that ribbon morphology is tailored to the needs of particular synapses for particular rates of ongoing vesicle exocytosis.
Retinal photoreceptor ribbons
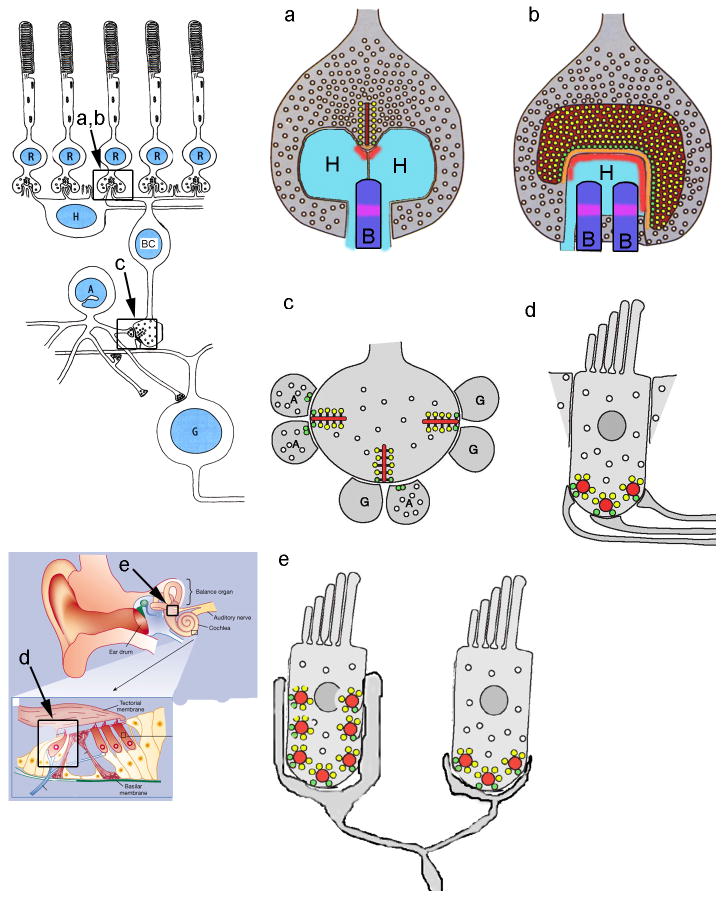
In photoreceptors, ribbons are planar structures, which when cut transversely appear in electron microscopy (EM) images as an electron-dense bar, ~30 nm thick, extending vertically from the plasma membrane into the cytoplasm (Figure 1a and Box 1). This long, narrow appearance in single transverse sections gives ribbons their name, but it can be misleading about their true 3-dimensional shape (Figure 1b). In reality, photoreceptor ribbons are plate-like structures that in rods can run several microns parallel to the plasma membrane, curving around the deep invaginating pocket that contains the dendrites of postsynaptic horizontal and bipolar cells. The ribbon attaches at its base to the presynaptic membrane via an electron-dense structure called the arciform density (Figure 1a), which keeps the ribbon on a leash, hovering just above the plasma membrane. The cytomatrix protein Bassoon (Box 1) is thought to be an important component of the leash, and ribbons float free in the cytoplasm of the photoreceptor terminal when Bassoon is functionally disrupted1.
Figure 1. Diversity of ribbon morphology and postsynaptic architecture in different cell types.
a, b: Schematic diagram of transverse (a) and en-face (b) views of a mammalian photoreceptor ribbon synapse117, whose context in the retina is illustrated in the diagram to the left. c: Synaptic arrangement at bipolar cell ribbons. The location of this synapse in retinal circuitry is illustrated in the diagram to the left. d: Synaptic arrangement at ribbons of cochlear inner hair cells. e: Vestibular afferents receive synaptic inputs from multiple ribbons of multiple hair cells. The locations of cochlear inner hair cells and the vestibular apparatus are shown schematically in the diagram to the left. Dark red areas, synaptic ribbons; bright red areas, AMPA receptors; orange areas, arciform density; yellow circles, vesicles attached to ribbon; green circles, docked vesicles; light blue areas, horizontal cell dendrites (H); dark blue areas, ON bipolar cell dendrites (B); magenta areas, mGluR6 receptors; G: ganglion cell dendrite; A: amacrine cell process.
Box 1. Molecular composition of ribbon synapses.
An increasing array of proteins have been identified at ribbon active zones (see Figure), including many that are also present at conventional, non-ribbon active zones. Among the latter is the cytomatrix protein Bassoon and various proteins that are involved in the synaptic vesicle cycle50. Bassoon is thought to be an important component of the arciform density, because Bassoon immunoreactivity is located at the boundary between the ribbon and the plasma membrane79–81. Bassoon binds to the main structural protein of the ribbon, RIBEYE80, and ribbons fail to anchor in photoreceptors and hair cells when Bassoon is functionally disrupted1,13. The ribbon protein RIBEYE consists of an A-domain that is unique to ribbons and a C- terminal B-domain that is mostly identical to the transcriptional co-repressor C-terminal binding protein 2 (CtBP2)82. RIBEYE self-assembles via interaction sites in both the A- and B-domains to form complexes that resemble rudimentary ribbons83. Based on known protein interactions of CtBP2, the identity between RIBEYE B-domain and CtBP2 has been used to design a fluorescent peptide that binds RIBEYE and renders ribbons visible in living cells29. This has proved to be quite useful for studies of ribbon function, as well as for photoinactivation of ribbons39. To date, RIBEYE is the only protein known to be unique to the ribbon itself, but in freeze-fracture studies the surface of the ribbon is studded with particles of various sizes84, which suggests that a number of different proteins are part of the ribbon. Among those identified so far are Rab3 interacting protein 1 (Rim1)85 (involved in priming vesicles for exocytosis), Kinesin-like protein KIF3A86 (a component of kinesin motor complexes), Piccolo87 (a component of the cytomatrix at the active zone), CtBP180 (a transcriptional regulator that might bind to the B-domain of RIBEYE), and guanylyl cyclase-activating protein 2 (GCAP2)88 (a calcium-binding protein). In addition to proteins associated with the ribbon, ribbon synapses express specific isoforms of the SNARE protein Syntaxin (Syntaxin3b89,90) and SNARE-associated complexins (Complexin3/491). In cochlear hair cells a C2-domain transmembrane protein, otoferlin, is required for normal transmitter release, and is mutated in a form of human deafness92. The precise functions of these ribbon-specific components are not yet certain (see REF. 50 for a recent review). The calcium sensor for release is unknown. Although otoferlin92 and a variant of synaptotagmin59 have been suggested to act in cochlear hair cells, at least for otoferlin this is not certain12, and it seems likely that different ribbon synapses have differently composed sensors, because the calcium sensitivity of release varies across cell types. In photoreceptors6,33,93, the calcium sensor has high affinity and low cooperativity, which would allow random openings of individual calcium channels to trigger release at a rate sufficient for tonic signaling to postsynaptic targets during steady depolarization. Contrast this with cochlear hair cells94, where the sensor has high cooperativity and low affinity — features that are suited for highly synchronous release.
The large ribbons of photoreceptor cells can harbor thousands of synaptic vesicles, tethered to both surfaces of the plate via fine filaments. Unfortunately, the molecular composition of these filaments is not yet known, although this information would be very useful in deciphering ribbon function. The large size of the tethered pool of vesicles might be required because photoreceptor synapses have release rates of up to hundreds of vesicles per second2, sustained for long periods. In addition to the vesicles attached to the face of the ribbon at a distance from the plasma membrane, a subset of tethered vesicles at the base of the ribbon — coloured green in Fig. 1 — also touch the plasma membrane, much like the ‘docked’ vesicles of conventional active zones. The calcium channels that drive exocytosis (Box 2) are arrayed linearly parallel to the long axis of the ribbon, just beside the arciform density, which means that these docked vesicles are poised for very rapid release when the channels open and admit a puff of calcium. The molecular machinery of release itself, such as SNARE complexes, is for the most part similar at ribbon-type and conventional active zones, although there are ribbon-specific isoforms of some components (Box 1).
Box 2. Specific subtypes of L-type calcium channels drive release at ribbon synapses.
Ribbon synapses require calcium channels that remain active during prolonged depolarization and respond rapidly to changes in membrane potential. Accordingly, calcium currents recorded from ribbon synapses exhibit relatively little inactivation53, 95, activate rapidly upon depolarization, and deactivate rapidly upon repolarization96,97. Various lines of evidence indicate that either Cav1.3 or Cav1.4 L-type calcium channels drive release at ribbon synapses, depending on cell type. First, the presynaptic calcium current at ribbon synapses is dihydropyridine-sensitive, indicating that the channels are L-type96,98,99, and blocking this current with dihydropyridines reduces glutamate release in bipolar cells98, photoreceptors100, and hair cells101,102. Second, mutations in Cav1.4 cause rod and cone dysfunction and visual impairment in humans103,104 and mice105, immunostaining for Cav1.4 is tightly colocalized with synaptic ribbons in photoreceptor terminals106,107, and calcium influx is reduced by 90% in photoreceptor synaptic terminals of Cav1.4 knockout mice105. Third, interaction with calcium-binding protein 4 (CaBP4) shifts the activation range of Cav1.4108 to better align with the resting potential of photoreceptor cells in darkness (about −40 mV), and loss of CaBP4 in mice or humans strongly impairs photoreceptor neurotransmission108,109. This reinforces the central role of Cav1.4 in driving synaptic vesicle exocytosis at photoreceptor ribbon synapses. Fourth, cochlear hair cells strongly express Cav1.3110, and Cav1.3 knockout mice are deaf51 because of greatly reduced calcium currents and calcium-triggered exocytosis in cochlear hair cells111,112. Although vestibular function has been reported to be normal in Cav1.3-null mice, zebrafish with mutation in an ortholog of Cav1.3 have both auditory and vestibular deficits. In general, the evidence indicates that Cav1.3 channels are important for neurotransmitter release at auditory hair cell synapses, while Cav1.4 is primary at photoreceptor synapses. However, Cav1.3 subunits have also been identified at retinal ribbon synapses, in both photoreceptors and bipolar cells, and it is possible that this subtype may play a role in the retina as well as in the cochlea. The conventional wisdom is that members of the Cav2 (P/Q, N) subgroup of calcium channels drive neurotransmitter release at synapses, but it is clear that Cav1, L-type subunit s can also support neurotransmitter release, when the demands of the release process require them.
The postsynaptic architecture of photoreceptor ribbon synapses is quite complex, because the output of a single photoreceptor cell must be distributed to multiple parallel pathways that simultaneously process different types of visual information. To achieve this, glutamate released at ribbon active zones reaches several different postsynaptic targets, including dendrites of horizontal cells and several types of bipolar neurons, that are located at progressively greater distances from the ribbon and express ionotropic or metabotropic glutamate receptors with appropriately tuned kinetics and affinity3. This ‘engineered spillover’ of glutamate to multiple targets at different distances introduces variable temporal filtering, so that targets near the site of vesicle fusion respond rapidly and transiently, whereas those farther away provide slower, integrated signals. As a result, different postsynaptic cells carry different temporal bandwidths of visual information4, providing the basis for separate visual pathways that are concerned with sustained vs. transient aspects of visual stimuli.
Retinal bipolar cell ribbons
The ribbons of retinal bipolar neurons (Figure 1c) are typically much smaller than the microns-long rod photoreceptor ribbons. For instance, planar ribbons of the giant synaptic terminal of goldfish bipolar neurons are ~150 nm tall and ~400 nm long5. Although bipolar-cell ribbons are small, there are many in a single cell, ranging from 30 to >100 ribbons depending on species and subtype of bipolar neuron. As a result of their small size, single bipolar-cell ribbons tether at most ~100 vesicles, of which ~20 are docked. This smaller vesicle pool translates into a more transient synaptic output of bipolar neurons, which serves to accentuate changes in light intensity. Interestingly, cone ribbons are also smaller than those of rods, and they may function similarly to provide a transient burst of release when illumination dims6. Each bipolar cell ribbon typically communicates with a pair of postsynaptic processes, which are symmetrically placed in close contact with the active zone (Figure 1c). A given ribbon may contact a pair of a pair of amacrine cell processes, a ganglion cell dendrite and an amacrine cell process, or more rarely, a pair of ganglion cell dendrites7. This dyad arrangement is considerably simpler than the complicated architecture of the postsynaptic elements at photoreceptor synapses, but it nevertheless also serves to distribute the output of a single ribbon to more than one functional target.
Hair cell ribbons
In hair cells, synaptic ribbons (also called dense bodies) are morphologically diverse: they can be spherical, planar, or oblong in shape and variable in size, tethering from 20–400 vesicles8. Even within one hair cell, individual ribbons can vary in size and shape. Although those in nonmammalian hair cells can be somewhat larger (200–400 nm in diameter), ribbons in mammalian hair cells are ≤200 nm width, closer in size to bipolar cell ribbons than to photoreceptor ribbons. Mammalian cochlear ribbons have 100 to 200 tethered synaptic vesicles, and associated voltage-gated calcium channels that are estimated to range from ~100 to 200 per ribbon9–11. As with retinal ribbons, a subset of vesicles (~12 estimated by electron tomography12) attached to ribbons are docked at the plasma membrane in hair cells, but in the case of spherical ribbons, the docked vesicles can be sandwiched between the ribbon and the plasma membrane (Figure 1d). Even though hair cells have no visible arciform density like that of photoreceptors, ribbons float free in hair cells when Bassoon is functionally knocked out13, indicating that the anchoring mechanism is similar in both cell types. Calcium entry through voltage-gated channels occurs near ribbons in hair cells14,15, so it is likely that the docked vesicles fuse rapidly at the onset of a depolarizing stimulus. The number of vesicles in this ‘readily releasable pool’ estimated from physiological measurements never differs by more than a factor of 2 from the morphologically defined number of docked vesicles in hair cells of various vertebrates16. Increasing attention is being paid to differences in structure and function among hair cells located at different points along the cochlea. Ribbons in the chicken basilar papilla (hearing organ) vary in size and vesicle capacity along the tonotopic axis, with hair cells in the high-frequency region having larger ribbons and correspondingly larger calcium currents17. Growing evidence suggests that calcium channel numbers vary significantly among the ribbons within a single mammalian cochlear hair cell10,15, which could contribute to marked differences in sensitivity of individual postsynaptic afferent fibres to sound stimuli. There is also considerable variety in afferent innervation of hair cells in different end-organs and species8. Vestibular afferents can branch to contact several presynaptic hair cells (Figure 1e), and may receive transmitter released from dozens or even hundreds of synaptic ribbons. At the other extreme, the solitary dendrite of a type I afferent usually is postsynaptic to a single ribbon of a single inner hair cell in the mammalian cochlea (Figure 1d).
Functional Roles of Retinal Ribbon Synapses
Ribbons play several parts in neurotransmission, whose relative importance varies in different cell types, mirroring the diversity in ribbon structure. Like conventional synapses, ribbon synapses release vesicles immediately when calcium channels open, but they also support resupply of vesicles so that release continues during sustained depolarization. In addition, increasing evidence indicates that ribbons coordinate simultaneous fusion of multiple vesicles, which is essential when precise timing relationships must be maintained, such as in audition. Recent work provides new insights into how ribbons might accomplish these varied tasks.
Synaptic vesicles on ribbons contribute to neurotransmission
Vesicles in the cytoplasmic pool at ribbon synapses are highly mobile18–20, but those tethered to ribbons are not18,21, raising the question of whether the ribbon-tethered vesicles have a role in neurotransmitter release. It seems that vesicles attached to ribbons do participate in the synaptic vesicle cycle upon depolarization, because vesicles labeled with activity-dependent extracellular markers are observed among the ribbon- attached pool in EM18,22–25. Also, turnover of ribbon-associated vesicles has been observed directly using fluorescence imaging in living bipolar neurons18,21. In addition, uptake of fluorescent dye into newly endocytosed vesicles after stimulation occurs preferentially near ribbons26, demonstrating selective fusion and retrieval of vesicles at ribbons. Furthermore, EM shows fewer synaptic vesicles associated with ribbons in stimulated synapses6,20,27,28. In some cases, the depletion of vesicles at stimulated ribbons is strongest near the plasma membrane6,20,28, consistent with selective release of vesicles that are nearest to calcium channels. Calcium influx through voltage-gated channels in bipolar cell terminals occurs at ‘hot spots’ that coincide with ribbons14,29, and when calcium channels open, capacitance measurements indicate immediate exocytosis of a subset of vesicles within ~1 ms30, followed by slower release (within ~200 ms) of a larger vesicle pool. Postsynaptic recordings at retinal ribbon synapses also reveal two kinetically distinct components of release31–34. The size of the fast component correlates with the total number of vesicles docked at the plasma membrane at ribbon active zones, defined by EM. Also, there is good agreement between the size of the total releasable pool and the number of vesicles tethered to all of the ribbons in goldfish bipolar cell terminals5, as well as in salamander rods33. Taken together, the evidence is consistent with a model in which fast, transient release at ribbons is supported by the lowest row of docked vesicles, followed by slower, sustained release of vesicles tethered in higher rows on the ribbon. In this view, ribbons could be considered a more elaborate version of the cytomatrix at the active zone of conventional synapses (Box 3), which also serves to bring releasable vesicles into close proximity with calcium channels at the release site.
Box 3. Insights into ribbon synapse function from conventional synapses.
Active zones of conventional, non-ribbon synapses are also thought to have mechanisms to capture and orient synaptic vesicles at release sites. This is perhaps most striking at the frog neuromuscular junction, where two rows of synaptic vesicles are held at the presynaptic membrane just across from high-density clusters of postsynaptic acetylcholine receptors. Electron tomography shows that this precise geometry is maintained by a network of ‘beams’ that run parallel to the active zone and ‘ribs’ that extend to the side from the beams and contact synaptic vesicles113. This vesicle-tethering network is reminiscent of the arrangement of vesicles on ribbons, except that the tethering machinery runs parallel to the membrane instead of perpendicular to it, as it does at ribbon synapses. At cortical synapses, electron tomography also reveals an elaborate polyhedral matrix of active zone material, extending from the plasma membrane into the cytoplasm and contacting both docked and undocked synaptic vesicles114, whereas similar experiments on cryopreserved hippocampal synapses show a more extended mesh of filaments that extend from the active zone and interconnect synaptic vesicles in the terminal115. Therefore, synaptic ribbons could be viewed as an extreme example of the more general need for presynaptic machinery to facilitate the supply of release- ready vesicles to the active zone116.
Although the correspondence between vesicle pools as defined by capacitance measurements and anatomically defined populations of ribbon-associated vesicles is suggestive, it is by no means conclusive. Measurements of membrane capacitance reflect total surface area and so may not accurately report vesicle fusion specifically at active zones. For instance, prolonged depolarization can elevate calcium levels throughout the cell and trigger exocytosis of synaptic vesicles or other structures at sites distant from the ribbon21,35,36. However, the exocytosis reported by capacitance changes results in glutamate release within striking distance of postsynaptic receptors, because direct comparison of presynaptic capacitance changes and simultaneously recorded postsynaptic responses31,33,37 reveals good correspondence between the two estimates for the number of vesicles contributing to the fast and slow components of release. Furthermore, experiments based on analysis of postsynaptic responses rather than presynaptic capacitance at cone–horizontal cell synapses indicated that the fast component corresponds to docked vesicles at ribbons and the sustained component to the rest of the ribbon-tethered population34. Despite these indications that the ribbon contributes both the fast and slow components of release, disruption of ribbons by knockout of Bassoon13 or by natural circadian regulation38 leaves the sustained component largely intact, whereas it eliminates the fast component. This suggests that something other than ribbons accounts for sustained release. To resolve these conflicting conclusions, it would be useful to knock out ribbon function acutely at a single synapse while monitoring the effect on the rapid and sustained components of release in paired recordings from synaptically coupled neurons. For instance, photoablation of fluorescently labeled ribbons39 is a promising way to resolve the issue.
Models of vesicle fusion at ribbons
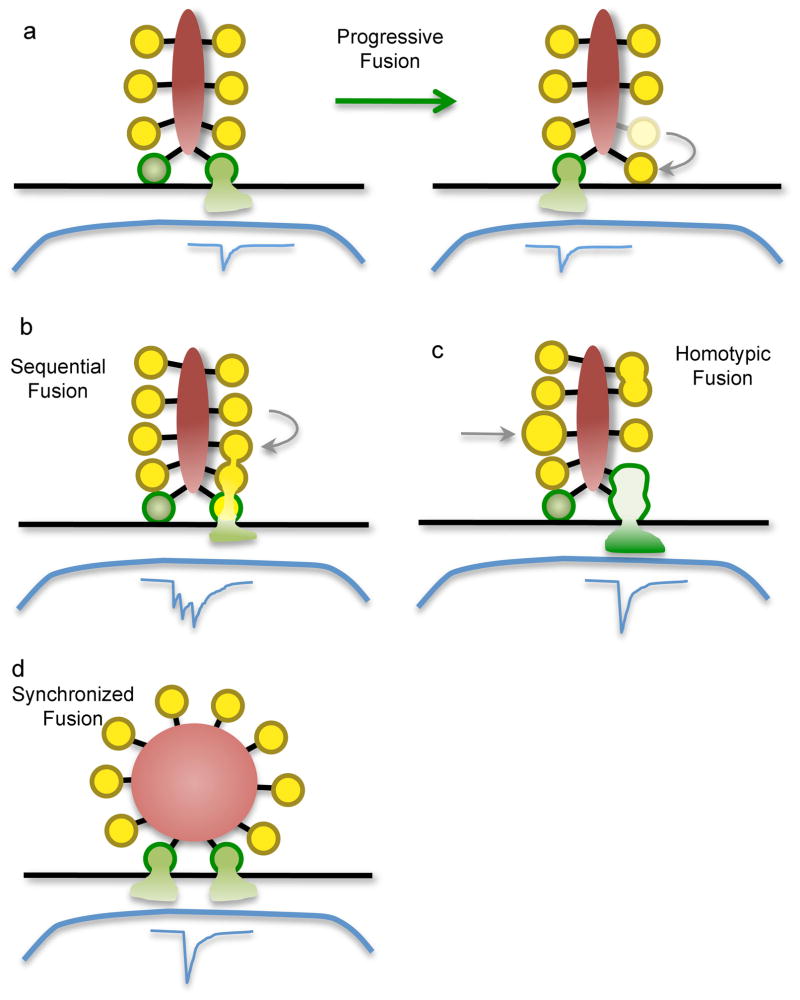
Assuming for the moment that the vesicles that are attached in higher rows on ribbons account for sustained release, how do they fuse when calcium channels open? A commonly held notion is that the ribbon provides a surface along which vesicles move towards the plasma membrane, either actively or passively, to replace those that fused previously at the base of the ribbon (Figure 2a, progressive fusion). If so, then the movement is evidently calcium dependent, because the sustained component of exocytosis is blocked by millimolar levels of the calcium buffer ethylene glycol tetraacetic acid (EGTA)30,40–43; by contrast, EGTA does not affect the fast component, which is consistent with the fast component being due to fusion of docked vesicles that are located very near calcium channels. In the progressive fusion model, newly arrived vesicles would require some time to dock and assemble fusion complexes before that particular release site would again be release-competent. In the meantime, other docked vesicles could take up the slack and release their neurotransmitter (Figure 2a) to sustain glutamate signaling to the postsynaptic elements. This could account for the fact that rod photoreceptor ribbons extend a large distance along the active zone: their size provides a sufficiently large number of release sites for docked, primed vesicles to maintain the requisite rate of ongoing vesicle fusion.
Figure 2. Modes of synaptic vesicle fusion at ribbon synapses.
Green vesicles represent vesicles that are docked at the plasma membrane (black line) at release sites. Blue traces represent the postsynaptic current. a: Progressive fusion, in which primed vesicles docked at different release sites fuse progressively during maintained depolarization, each producing an independent unitary postsynaptic current. Vesicles in higher rows move along the ribbon to replenish empty release sites. b,c: Two forms of compound fusion. In sequential fusion (b), vesicles fuse in sequence up the face of the ribbon, starting with the vesicles docked at the plasma membrane. This produces a burst of quantal postsynaptic currents in rapid sequence. Vesicles attached to the ribbon might fuse with each other (homotypic fusion; c) prior to undergoing exocytosis. The resulting larger bolus of glutamate produces a larger postsynaptic current. d: Synchronized fusion of multiple docked vesicles, which results in a multiquantal postsynaptic current.
Alternatively, the ribbon might serve as a substrate for compound fusion, in which vesicles attached to the ribbon fuse with each other. This could occur in two ways, which are not mutually exclusive. In one scenario, vesicles at the base of the ribbon release their contents first — accounting for the fast component of exocytosis — but do not collapse fully into the plasma membrane. Then, vesicles in higher rows fuse successively, releasing their neurotransmitter through the previously fused vesicles (Figure 2b, sequential fusion). The filaments that tether vesicles would serve to hold vesicles in the proper configuration, so that a wave of calcium-triggered exocytosis sweeps up the ribbon, generating the slower component of exocytosis. In frog saccular hair cells, sequential fusion has been proposed to account for the fact that brief depolarization triggers exocytosis of many more vesicles than are docked at the plasma membrane44. In general, then, sequential fusion of this type might allow single release sites to be reused more rapidly than in the progressive fusion model, which could be an advantage for smaller ribbons that lack the large pool of docked vesicles found in photoreceptor synapses. In another scenario for compound fusion, ribbon-attached vesicles fuse among themselves, but prior to fusion with the plasma membrane. This form of compound fusion results in larger vesicles attached to the ribbon (Figure 2c, homotypic fusion), which then release a larger bolus of glutamate when they fuse at the plasma membrane during depolarization. EM studies of bipolar cell ribbons have indeed revealed a subset of larger vesicles attached to ribbons after stimulation27, consistent with vesicle–vesicle fusion on the ribbon during calcium influx. In addition, large pleiomorphic infoldings of the plasma membrane are attached to the base of the ribbon in repetitively stimulated bipolar cell ribbons, as is expected for compound exocytosis27. Definitive evidence for compound exocytosis in its various forms is still lacking, and more direct studies at living ribbon synapses are required. Of course, it is also possible that the multiple modes of vesicle fusion co-exist, in different proportions depending on stimulation conditions.
Postsynaptic recordings at ribbon synapses of hair cells and retinal bipolar neurons suggest that another function of ribbons is to coordinate the simultaneous fusion of multiple vesicles at the active zone. For instance, in the retina, large synaptic events consistent with synchronous release of multiple quanta were observed in simultaneous pre- and postsynaptic recordings from pairs of bipolar neurons and amacrine cells45. These were interpreted to reflect synchronized fusion of multiple synaptic vesicles (Figure 2d, synchronized fusion), but such large events could also occur from homotypic pre-fusion of vesicles (Figure 2c). Recordings from afferent fibres that receive inputs from hair cells also show that ‘unitary’ postsynaptic currents correspond to simultaneous fusion of several synaptic vesicles, as discussed below. How ribbons might coordinate the fusion of independent vesicles (if that is indeed the case) remains mysterious, but in the auditory system, such synchronization is likely a major functional role of ribbons, because it promotes the precisely timed activation of afferent neurons that is required for encoding speech patterns and for sound localization.
Functional Roles of Hair Cell Ribbons
As in the retina, ribbon synapses provide tonic transmitter release from sensory cells of the inner ear. Most notably, each type I afferent neuron in the mammalian cochlea is usually postsynaptic to a single ribbon in a single inner hair cell. It is remarkable that the timing, intensity and frequency content of sound is transmitted to the brain through these solitary, submicroscopic contacts. Little wonder that considerable attention has been paid to hair cell synapses in the cochlea and elsewhere. These efforts over the past decade have provided surprising insights into ribbon function, including the variable reliance on calcium influx at different synapses, and the ability to spontaneously release multiple vesicles. Provocative as these findings are, our understanding of hair cell ribbon function remains far from complete. Fortunately the endpoint is certain — to explain the signaling of afferent neurons that is driven by transmitter release from the ribbon. The following sections briefly summarize the requirements for afferent signaling, the evidence for our emerging picture of ribbon function, and point to what a future synthesis should include.
Afferent activity driven by hair cell ribbons
Mechanotransduction by sensory hair cells occurs with minimal delay, allowing the cochlea to signal stimulus frequencies exceeding 100 kHz in some mammals. The hair cell’s membrane potential cannot vary at these ultrasonic frequencies; however, phase-locked transmitter release from hair cells can drive afferent firing at frequencies up to 5 kHz. At the other extreme, vestibular epithelia signal continuously to provide information on head position and movements that occur over the span of seconds. In fact, all hair cells drive continuous ‘spontaneous’ afferent activity that is modulated by a stimulus. These synaptic transfer functions arise from intimate, but still unresolved relations between voltage-gated calcium channels and the vesicular release machinery, evidence for which will be summarized in the following sections.
Afferent activation in the mammalian cochlea presents additional challenges. Strikingly, the 10 to 30 individual afferents that contact a single inner hair cell can have different spontaneous rates, acoustic thresholds and dynamic ranges46. Although postsynaptic membrane properties47 and modulation by lateral efferents also must be considered, it is likely that functional variations among ribbons contribute to differential afferent signaling for a given change in hair cell membrane potential. Certainly, individual ribbons within individual hair cells differ in size, shape and the number of associated vesicles48. Also, the number of voltage-gated calcium channels may vary between hair cell ribbons8,17,49. Confocal measurement with fluorescent calcium indicators has shown that peak influx varies substantially between different ‘hotspots’ in one cochlear hair cell during depolarizing voltage commands, suggesting that the maximum number of open channels varied15. The calcium hotspots were co-localized with a fluorescent ‘ribbon-binding’ peptide and ribbon size correlated with hotspot amplitude. Variations in calcium channel number per active zone could account for differences in spontaneous rate, as well as the acoustic threshold (depolarization threshold) of individual afferent neurons contacting an inner hair cell.
Calcium channel gating at hair cell ribbons
Although our understanding of most ribbon proteins remains in progress50 (Box 1), it is certain that transmitter release from cochlear hair cells begins with the voltage gating of Cav1.3 calcium channels51. These have been studied in detail using voltage-clamp recording. Whole-cell and single channel measurements have shown that these channels activate near the resting membrane potential of the hair cell, ~−60 mV (V1/2 −35 to −40 mV), have open times of a fraction of a millisecond, and show only modest, slow inactivation52,53. These gating properties meet the dual demands of precise temporal coding and continuous, steady-state activity. Single channel open probability in mammalian inner hair cells was reported to reach a maximum of 0.15, so that only a small number of the 100–200 channels at an active zone may be open for any release event11. Given this limitation, a variation in total channel number is still more likely to significantly alter the release statistics for each active zone.
Ribbon function assayed by presynaptic membrane capacitance
The presynaptic function of hair cells has been studied most extensively using voltage-evoked changes in membrane capacitance54, serving as a proxy for transmitter release. Capacitance measurements have established basic parameters, including estimates of the number and rates of vesicular release. In vertebrate hair cells, the size of the earliest component of release, the ‘readily releasable pool’, lies somewhere between that of the membrane-docked13 and ribbon-tethered vesicle pool42,44, and variance analysis of capacitance changes in cochlear hair cells have suggested that the majority of release events in mouse inner hair cells involved single vesicles55. Capacitance measurements have also been employed to describe differences in synaptic release by hair cells from different positions along the cochlea’s tonotopic axis56, as a function of development57,58 and as a test of molecular mechanisms59,60 (see ref. 16 for additional examples). These studies have revealed interesting variations in the relationship between calcium influx and membrane capacitance. For example, with the onset of hearing during the second postnatal week in rodents, calcium currents of inner hair cells become smaller but produce relatively larger increases in capacitance43,61. This increase in release efficiency may result from a spatially close association between calcium channels and synaptic vesicles, the postulated ‘nanodomain’9.
Ribbon function assayed by postsynaptic currents
Physiologists depend heavily on intracellular recording from postsynaptic cells to understand transmitter release. Such recordings recently have become prevalent for the study of hair cell transmission, although pioneering efforts in fish62, reptiles63 and mammals64 provided important early signposts. Whole-cell patch-clamp from afferent terminals at the base of inner hair cells in the immature cochlea (up to the 12th postnatal day) revealed randomly-timed, rapid, glutamatergic excitatory postsynaptic currents (EPSCs) and provided early evidence for multi-vesicular release65, as did recordings from a frog auditory organ, the amphibian papilla66. In both cases these ‘spontaneous’ (in the sense that presynaptic membrane potential was constant) individual EPSCs were much larger and more variable than expected for the release of single vesicles — ranging more than ten-fold in amplitude, with a coefficient of variation up to 0.95 (ref. 65) — and some had irregular, inflected (‘multiphasic’) waveforms as though they were composed of smaller units. The hypothesis for multivesicular release was based on these observations and on a correspondence between the smallest responses and glutamatergic ‘minis’ in the CNS, as well as consistency with estimates from capacitance recordings43,61. These assumptions have been strengthened by observations of classical ‘minis’ that are thought to form larger, multivesicular EPSCs at frog hair cell synapses67 (see below). Multivesicular release has also been proposed to explain the similarly large and variably-sized EPSCs in retinal bipolar cell synapses45,68, that are composed from univesicular ‘minis’69. If larger EPSCs indeed result from the synchronized release of multiple vesicles, then this occurs without a coordinating presynaptic action potential to evoke release, in contrast to what occurs at ‘phasic’ synapses. The mechanism for multi-vesicular release is unknown and remains under active investigation. Likewise, it is still uncertain as to whether multi-vesicular release operates at all ribbon synapses under all conditions. Both frog hair cells67 and mouse retinal bipolar cells69 regularly release single vesicles when the membrane potential is at or negative to the resting membrane potential. And, as previously noted, variance analysis of membrane capacitance fits with the assumption that mostly single vesicle release occurs in mammalian cochlear hair cells55.
Paired recordings from hair cells and afferents in both the frog66 and the mammalian cochlea70 further defined the synaptic transfer function. At solitary mammalian synapses, release rates were low enough to resolve individual excitatory postsynaptic currents (EPSCs), and the low release rates indicated that the transfer function resulted entirely from changes in EPSC frequency without changes in average amplitude70. The somewhat surprising implication, based on the assumption that release of single vesicles produced the smallest EPSCs, is that the number of vesicles that make up a multivesicular EPSC is independent of calcium influx70. This conclusion was further supported by the observation that EPSC frequency (presynaptic hair cell membrane potential at −29 mV), but not amplitude, varied as a function of calcium buffer concentration70, with equally large EPSCs under all conditions. Strikingly, this held true even when EGTA was substituted with BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid), whose more rapid binding normally interrupts calcium-dependent activities. To date however, this finding has not been reported for other ribbon synapses. Small — presumably univesicular — events were observed in adult frog hair cell afferents when the presynaptic hair cell was held at −80 or −90 mV. These had a Gaussian distribution with a coefficient of variation (0.29), as expected for univesicular events67. As the hair cell was depolarized to membrane potentials between −75 and −63 mV, the average EPSC amplitude grew larger, leading to the conclusion that both frequency and amplitude of EPSCs are calcium-dependent at this synapse. Similarly, the retinal bipolar to amacrine cell synapse seems to switch from univesicular to multivesicular release as the calcium channel open probability rises69.
Initial descriptions of multivesicular release from hair cells were made in immature cochlear tissue (that is, tissue taken before hearing onset at postnatal 12), prompting concerns that this is a transient feature of ribbon function67. Studies on cochleas from older rats (postnatal days 19–60) have extended the original observations71. Mature EPSCs were faster and larger, with more symmetrical amplitude distributions in some recordings. Using the foregoing assumptions for the response to a single vesicle, the average mature EPSC was estimated to result from the release of 7–9 vesicles. As in young synapses, the mean and distribution of EPSC amplitudes did not vary with release probability. Continued study of hair cells with differing synaptic transfer functions will help further to define the range of mechanisms operated by the ribbons. For example, numerous outer hair cells contact the extended dendritic arbor of type II afferents in the cochlea to produce small, apparently monophasic EPSCs72. Although the effects of cable loss on the spread of signals along the extended type II dendrite remain to be determined, the overall rate of release seems to be much lower than that from ribbons of inner hair cells. It will be of interest to quantify ultrastructural details that may underlie these functional differences.
Implications for hair cell ribbon function
Our current view of hair cell ribbon function is based largely on two types of experiment: voltage-driven changes in presynaptic membrane capacitance of the hair cell, and recordings from afferent dendrites of EPSCs. The chief distinction is that capacitance measurements report the summed behaviour of all the ribbons in that cell, whereas EPSCs can result from release at a single ribbon. Postsynaptic EPSCs provide evidence suggesting that individual hair cell ribbon synapses often release multiple vesicles simultaneously, as reconfirmed recently12,67,71. By contrast, capacitance measurements suggest that cochlear hair cell ribbons most commonly release single vesicles55. These different insights regarding vesicle release in mammalian cochlear hair cell ribbons eventually must be merged, as has recently been achieved for hair cells of the frog amphibian papilla67. In particular it will be useful to establish conditions for quantifying univesicular EPSCs from cochlear hair cell ribbons.
Both presynaptic capacitance and postsynaptic EPSCs have revealed that transmitter release (in some cases) rises linearly with increasing calcium influx. Combined with the effects of varied calcium buffering this has led to the postulate of a ‘nanodomain’ for vesicular release in which calcium flux through a single channel can drive the fusion of a closely-associated synaptic vesicle9,66,67,70. This postulate has received recent support at the retinal bipolar — amacrine cell synapse, where single-vesicle EPSCs were produced under conditions in which only a single calcium channel was likely to be open at any ribbon69. It is less certain, however, how multiple vesicles would be coordinately released at single ribbons as calcium channel gating probability rises. More challenging still is the task of coupling a ‘nanodomain’ mechanism of gating to the observation that EPSC amplitude is independent of release probability at rat cochlear hair cell synapses65,70,71, as has also been observed in the mouse12.
The detection of ‘univesicular’ EPSCs at low release probability in ribbon synapses of bipolar cells and hair cells may provide a way forward to new hypotheses. Perhaps the ribbon operates in the ‘nanodomain’ mode at very low calcium channel open probabilities, but switches into an as-yet-undefined multivesicular release mode as the calcium channel open probability rises. Conceivably, this higher probability release mode could then depend on a ‘cloud’ of calcium, as has been proposed for the gating of calcium-sensitive potassium channels in hair cells73. The frequency at which that condition is met will depend on the mean open time of calcium channels and local calcium buffering, but fundamentally is a binomial prediction based on the number of channels and their mean open probability. Mean open time seems to be invariant with open probability11, and so will not by itself alter the extent of calcium ‘overlap’. Thus, for a given ribbon with a fixed number of calcium channels, EPSC frequency will rise with open probability, and fall with stronger calcium buffering, as has been observed at hair cell synapses in rats70 and frogs67.
A satisfying corollary of this proposal is that synaptic strength should vary with the number of calcium channels that are clustered at each ribbon10,15, (Figure 3) in keeping with substantially different activity patterns of afferents contacting an individual inner hair cell46. With more channels, the ‘threshold’ calcium for fusion would be reached at lower open probabilities (that is, more-negative membrane potentials). Intrinsic variations in release efficiency71 could add additional range to these synapses. Continued refinements in imaging technology, coupled with postsynaptic recordings, could provide much needed information on the relationship between stochastic calcium channel gating and EPSC amplitude at individual ribbon synapses.
Figure 3. Variable ribbon function within one cochlear inner hair cell.
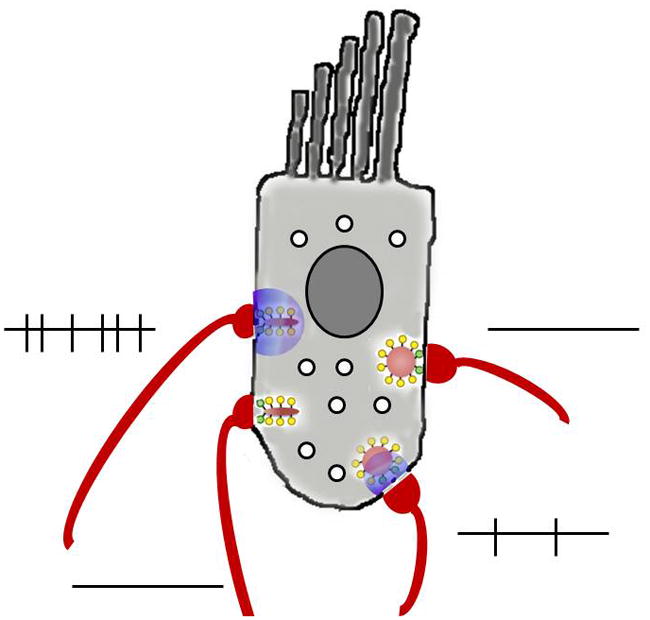
‘Low threshold’ ribbons may have greater numbers of calcium channels, release transmitter more frequently at a given membrane potential, and so trigger more afferent action potentials. ‘High threshold’ ribbons may have fewer calcium channels and therefore require greater depolarization to cause equivalent levels of afferent activity. Alternatively, variations in vesicular fusion (e.g., different sensitivities to calcium or coordination of multivesicular release) could produce similar outcomes. Overall probability of transmission is relatively low, so not every synapse will be active at a given point in time. The purple cloud indicates calcium influx; lines indicate action potentials in afferent fibres.
The role of ribbon function in afferent firing patterns
Does the literature on afferent recordings help to refine hypotheses of ribbon function? Spontaneous activity in cochlear afferent fibres ranges from ~1 to over 100 Hz, requiring that some ribbons produce EPSCs that are large enough to exceed the threshold at these same frequencies. Grant et al.71 proposed that ribbons with only large monophasic EPSCs could drive higher frequency activity — most EPSPs being suprathreshold. On the other hand, ribbons releasing a large fraction of smaller-amplitude, multiphasic EPSCs would produce more subthreshold responses and so drive lower levels of afferent activity. On this basis, calcium channel numbers and gating could be identical at each ribbon, but the spontaneous rate and acoustic threshold of afferent fibres would be determined by the ratio of mono- to multi-phasic events at each synapse.
Another relevant aspect of afferent function is the relatively low cycle-by-cycle probability of acoustically driven activity. For example, during phase-locking to a 1 kHz tone (the best frequency for the afferent), the probability of firing on a cycle-by-cycle basis ranged from only 0.04 to a maximum of 0.1574. This is reminiscent of the low gating probability described for hair cell calcium channels11. If the probability of release arises largely from the gating probability of calcium channels, an additional implication is that vesicle docking and priming do not further lower release probability. Indeed, vesicle recruitment at ribbons seems to be fast and inexhaustible75. Multi-vesicular release may be an adaptation to ensure precise timing76, and indeed, first spike latency to acoustic stimuli was degraded in mice with reduced numbers of ribbons77. Finally, as discussed earlier78, the phase-constancy of afferent firing for different sound intensities74 is nicely consistent with the observation that average EPSC amplitude is invariant during hair cell depolarization65,70,71. That is, the afferent fibre is depolarized to threshold at the same rate (that is, the same average synaptic amplitude) regardless of the acoustic intensity (that is, regardless of hair cell depolarization).
Conclusion
Although we know a great deal about ribbon synapses, mysteries still abound. Do ribbons account for the rapid burst of release at the onset of depolarization, for sustained release during prolonged depolarization, or for both? If synaptic vesicles attached to the ribbon participate in transmitter release, how do the vesicles in higher rows reach the plasma membrane in order to fuse? Do vesicles move on the surface of the ribbon, and if so, is this an active process or simple diffusion? Do vesicles fuse with other vesicles on the ribbon? How are ribbons able to coordinate nearly simultaneous fusion of multiple vesicles? All of these fundamental questions remain to be answered, and new approaches are needed to answer them, such as high-resolution optical imaging in living cells or ultrastructural methods to catch the vesicles in the act, as well as ways to alter ribbon function in real time during electrophysiological experiments.
It should also be kept in mind that not all ribbons may operate in the same way, and as studies advance, it will be important to discriminate between general features and unique specializations. After all, ribbons vary enormously in size and postsynaptic architecture, and so the relative importance of fast synchronous vs. slow sustained release is likely to vary as well, depending on cell type and on the functional context of the synapse. In this regard, ribbon synapses are no different from conventional synapses, which also vary widely in their functional properties.
Box Figure.
Acknowledgments
Support: NIH grants NEI R01EY003821 (GM) and NIDCD R01DC000276, R01DC001508, P30 DC005211 (PF)
Glossary
- tonotopic
The mapping of tones of different frequencies onto space along the receptive surface, such as the mammalian cochlea, or onto different spatial locations within a brain nucleus that processes auditory information
- capacitance
Electrical measure of charge-storing capacity. Cell membranes behave as electrical capacitors, because the insulating lipid separates two electrically conductive salt solutions. The capacitance of a cell is proportional to the cell’s surface area, and thus serves as an index of membrane addition and retrieval
- cytomatrix at the active zone
The complex of membrane-associated and cytoplasmic proteins that provide structural organization for the many components required to dock, prime, and fuse synaptic vesicles at presynaptic active zones
- pleiomorphic
Varied in shape
- voltage-clamp
A fundamental electrophysiological technique for measuring ionic currents in cells, while ‘clamping’ the membrane potential to prevent changes caused by those currents
- ‘minis’
Shorthand for miniature postsynaptic current, which is the current produced by spontaneous or evoked exocytosis of a single synaptic vesicle
- cable loss
The decrement of ‘passive’ voltage signals along neuronal processes
- phase-locking
Precise timing between two signals. In the auditory system phase-locking refers to coordination between action potentials in auditory neurons, and the cycles of a tonal stimulus
- freeze-fracture
A technique for ‘three-dimensional’ imaging of cellular ultrastructure by coating the surface of fractured tissue with electron dense material
References
- 1.Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 2.Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal ribbon synapses. Prog Retina Eye Res. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 5.von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- 6.Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light response. Nature Neurosci. 2009;12:303–310. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- 8.Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006;326:347–59. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt A, Khimich D, Moser T. Few Cav1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer AC, Frank T, Khimich D, Hoch G, Riedel D, Chapochnikov NM, et al. Tuning of synapse number, structure and function in the cochlea. Nature Neurosci. 2009;12:444–453. doi: 10.1038/nn.2293. [DOI] [PubMed] [Google Scholar]
- 11.Zampini V, Johnson SL, Franz C, Lawrence ND, Munkner S, Engel J, et al. Elementary properties of Cav1.3 Ca2+ channels expressed in mouse cochlear inner hair cells. J Physiol. 2010;588:187–199. doi: 10.1113/jphysiol.2009.181917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pangrsic T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, et al. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nature Neurosci. 2010;13:869–876. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- 13.Khimich D, Pujol R, tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- 14.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci USA. 2009;106:4483–4488. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J Mem Biol. 2006;209:153–165. doi: 10.1007/s00232-005-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chick’s cochlea. J Neurosci. 1997;17:9133–9144. doi: 10.1523/JNEUROSCI.17-23-09133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoGiudice L, Sterling P, Matthews G. Mobility and turnover of vesicles at the synaptic ribbon. J Neurosci. 2008;28:3150–3158. doi: 10.1523/JNEUROSCI.5753-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41:755–766. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 20.Holt M, Cooke A, Neef A, Lagnado L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr Biol. 2004;14:173–183. doi: 10.1016/j.cub.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci USA. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaeffer SF, Raviola E. Membrane recycling in the cone cell endings of the turtle retina. J Cell Biol. 1978;79:802–825. doi: 10.1083/jcb.79.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina, ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985;100:175–188. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel JH, Brownell WE. Synaptic and Golgi membrane recycling in cochlear hair cells. J Neurocytol. 1986;15:311–328. doi: 10.1007/BF01611434. [DOI] [PubMed] [Google Scholar]
- 25.Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logiudice L, Sterling P, Matthews G. Vesicle recycling at ribbon synapses in the finely branched axon terminals of mouse retinal bipolar neurons. Neuroscience. 2009;164:1546–1556. doi: 10.1016/j.neuroscience.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews G, Sterling P. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J Neurosci. 2008;28:5403–5411. doi: 10.1523/JNEUROSCI.0935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenzi D, Crum J, Ellisman MH, Roberts WM. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron. 2002;36:649–659. doi: 10.1016/s0896-6273(02)01025-5. [DOI] [PubMed] [Google Scholar]
- 29.Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24:9752–9759. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 31.von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- 32.Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron. 2004;42:595–605. doi: 10.1016/s0896-6273(04)00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartoletti TM, Babai N, Thoreson WB. Vesicle pool size at the salamander cone ribbon synapse. J Neurophysiol. 2010;103:419–423. doi: 10.1152/jn.00718.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, Tachibana M. Different roles of ribbon-associated and ribbonfree active zones in retinal bipolar cells. Nature Neurosci. 2007;10:1268–1276. doi: 10.1038/nn1963. [DOI] [PubMed] [Google Scholar]
- 36.Coggins MR, Grabner CP, Almers W, Zenisek D. Stimulated exocytosis of endosomes in goldfish retinal bipolar neurons. J Physiol. 2007;584:853–865. doi: 10.1113/jphysiol.2007.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer MJ. Characterisation of bipolar cell synaptic transmission in goldfish retina using paired recordings. J Physiol. 2010;588:1489–1498. doi: 10.1113/jphysiol.2009.185850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snellman J, Zenisek D. Program No. 522.7. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Photodamaging the ribbon disrupts coordination of multivesicular release and blocks vesicle replenishment at the rod bipolar cell synapse in mouse. Online. [Google Scholar]
- 40.Innocenti B, Heidelberger R. Mechanisms contributing to tonic release at the cone photoreceptor ribbon synapse. J Neurophysiol. 2008;99:25–36. doi: 10.1152/jn.00737.2007. [DOI] [PubMed] [Google Scholar]
- 41.Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 2004;5:376–390. doi: 10.1007/s10162-004-5003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmonds BW, Gregory FD, Schweizer FE. Evidence that fast exocytosis can be predominantly mediated by vesicles not docked at active zones in frog saccular hair cells. J Physiol. 2004;560:439–450. doi: 10.1113/jphysiol.2004.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nature Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- 46.Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- 47.Yi E, Roux I, Glowatzki E. Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea. J Neurophysiol. 2010;103:2532–2543. doi: 10.1152/jn.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea, quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- 49.Wu YC, Tucker T, Fettiplace R. A theoretical study of calcium microdomains in turtle hair cells. Biophys J. 1996;71:2256–2275. doi: 10.1016/S0006-3495(96)79429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Molecular Neurobiology. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoartrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 52.Nouvian R. Temperature enhances exocytosis efficiency at the mouse inner hair cell ribbon synapse. J Physiol. 2007;584:535–542. doi: 10.1113/jphysiol.2007.139675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grant L, Fuchs P. Calcium, calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99:2183–2193. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- 54.Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell, capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 55.Neef A, Khimich D, Pirih P, Riedel D, Wolf F, Moser T. Probing the mechanism of exocytosis at the hair cell ribbon synapse. J Neurosci. 2007;27:12933–12944. doi: 10.1523/JNEUROSCI.1996-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson SL, Forge A, Knipper M, Munkner S, Marcotti W. Tonotopic variation in the calcium dependence of neurotransmitter release and vesicle pool replenishment at mammalian auditory ribbon synapses. J Neurosci. 2008;28:7670–7678. doi: 10.1523/JNEUROSCI.0785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci. 2008;28:1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson SL, Franz C, Knipper M, Marcotti W. Functional maturation of the exocytotic machinery at gerbil hair cell ribbon synapses. J Physiol. 2009;587:1715–1726. doi: 10.1113/jphysiol.2009.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson SL, Franz C, Kuhn S, Furness DN, Ruttiger L, Munkner S, et al. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nature Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci. 2009;29:10474–10487. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furukawa T, Hayashida Y, Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. J Physiol. 1978;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crawford AC, Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel JH. Spontaneous synaptic potentials from afferent terminals in the guinea pig cochlea. Hear Res. 1992;59:85–92. doi: 10.1016/0378-5955(92)90105-v. [DOI] [PubMed] [Google Scholar]
- 65.Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nature Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- 66.Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell's afferent synapse. Proc Natl Acad Sci USA. 2006;103:5537–5542. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li GL, Keen E, Andor-Ardo D, Hudspeth AJ, von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse. J Neurosci. 2009;29:7558–7568. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maple BR, Werblin FS, Wu SM. Miniature excitatory postsynaptic currents in bipolar cells of the tiger salamander retina. Vision Res. 1994;34:2357–2362. doi: 10.1016/0042-6989(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 69.Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci USA. 2007;104:16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grant L, Yi E, Glowatzki E. Two modes of release shape the postsynaptic response at the inner hair cell ribbon synapse. J Neurosci. 2010;30:4210–4220. doi: 10.1523/JNEUROSCI.4439-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994;14:3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- 75.Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;434:212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- 76.Wittig JH, Jr, Parsons TD. Synaptic ribbon enables temporal precision of hair cell afferent synapse by increasing the number of readily releasable vesicles: a modeling study. J Neurophysiol. 2008;100:1724–1739. doi: 10.1152/jn.90322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci. 2010;30:7587–7597. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuchs PA. Time and intensity coding at the hair cell's ribbon synapse. J Physiol. 2005;566:7–12. doi: 10.1113/jphysiol.2004.082214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- 80.tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, et al. Molecular dissection of the photoreceptor ribbon synapse, physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Kitajima I, Ohtsuka T, Takai Y. Active zone protein CAST is a component of conventional and ribbon synapses in mouse retina. J Comp Neurol. 2006;495:480–496. doi: 10.1002/cne.20893. [DOI] [PubMed] [Google Scholar]
- 82.Schmitz FA, Konigstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons, a protein's journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 83.Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Usukura J, Yamada E. Ultrastructure of the synaptic ribbons in photoreceptor cells of Rana catesbeiana revealed by freeze-etching and freeze-substitution. Cell Tiss Res. 1987;247:483–488. doi: 10.1007/BF00215740. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 86.Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstätter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina, comparison with Bassoon. J Comp Neurol. 2001;439:224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- 88.Venkatesan JK, Natarajan S, Schwarz K, Mayer SI, Alpadi K, Magupalli VG, et al. Nicotinamide adenine dinucleotide-dependent binding of the neuronal Ca2+ sensor protein GCAP2 to photoreceptor synaptic ribbons. J Neurosci. 2010;30:6559–6576. doi: 10.1523/JNEUROSCI.3701-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J Comp Neurol. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curtis L, Datta P, Liu X, Bogdanova N, Heidelberger R, Janz R. Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience. 2010;166:832–841. doi: 10.1016/j.neuroscience.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reim K, Wegmeyer H, Brandstätter JH, Xue M, Rosenmund C, Dresbach T, et al. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;169:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 93.Duncan G, Rabl K, Gemp I, Heidelberger R, Thoreson WB. Quantitative analysis of synaptic release at the photoreceptor synapse. Biophys J. 2010;98:2102–2110. doi: 10.1016/j.bpj.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 95.von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zidanic M, Fuchs PA. Kinetic analysis of barium currents in chick cochlear hair cells. Biophys J. 1995;68:1323–1336. doi: 10.1016/S0006-3495(95)80305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mennerick S, Matthews G. Rapid calcium-current kinetics in synaptic terminals of goldfish retinal bipolar neurons. Vis Neurosci. 1998;15:1051–1056. doi: 10.1017/s0952523898156043. [DOI] [PubMed] [Google Scholar]
- 98.Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci. 1993;13:2898–2909. doi: 10.1523/JNEUROSCI.13-07-02898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmitz Y, Witkovsky P. Glutamate release by the intact light-responsive photoreceptor layer of the Xenopus retina. J Neurosci Meth. 1996;68:55–60. doi: 10.1016/0165-0270(96)00070-2. [DOI] [PubMed] [Google Scholar]
- 101.Robertson D, Paki B. Role of L-type Ca2+ channels in transmitter release from mammalian inner hair cells. II. Single-neuron activity. J Neurophysiol. 2002;87:2734–2740. doi: 10.1152/jn.2002.87.6.2734. [DOI] [PubMed] [Google Scholar]
- 102.Zhang SY, Robertson D, Yates G, Everett A. Role of L-type Ca(2+) channels in transmitter release from mammalian inner hair cells I. Gross sound-evoked potentials. J Neurophysiol. 1999;82:3307–3315. doi: 10.1152/jn.1999.82.6.3307. [DOI] [PubMed] [Google Scholar]
- 103.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, et al. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 104.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 105.Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, et al. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Gen. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 106.Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 2001;42:2414–2418. [PubMed] [Google Scholar]
- 107.Wässle H, Haverkamp S, Grünert U, Morgans CW. The cone pedicle, the first synapse in the retina. In: Kaneko A, editor. The Neural Basis of Early Vision. Springer Verlag; Tokyo: 2003. pp. 19–38. [Google Scholar]
- 108.Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, et al. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nature Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, et al. Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Gen. 2006;79:657–667. doi: 10.1086/508067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc Natl Acad Sci USA. 1997;94:14883–14888. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, et al. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. J Physiol. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brandt A, Striessnig J, Moser T. Cav1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- 114.Zampighi GA, Fain N, Zampighi LM, Cantele F, Lanzavecchia S, Wright EM. Conical electron tomography of a chemical synapse, polyhedral cages dock vesicles to the active zone. J Neurosci. 2008;28:4151–4160. doi: 10.1523/JNEUROSCI.4639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtová A, et al. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology. 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- 117.Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal, architecture of a binary synapse. Neuron. 1995;14:561–569. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]