Abstract
To date the only point mutations demonstrated to cause hemophilia are C to T transitions in TaqI sites. These were detected by screening Southern blots with cloned factor VIII probes. During the development of improved methods for detecting and analyzing mutations in genomic DNA, a novel G to C transversion mutation has been identified. This rare transversion results in a missense mutation, with proline being substituted for arginine in one of the active domains of the factor VIII molecule. The results suggest that the improved methods will be useful for detecting mutations in hemophilia as well as in other genetic disorders. In this method, specific DNA sequences in genomic DNA are amplified using oligonucleotide primers and a heat-resistant DNA polymerase. Mutations are detected and localized in the amplified samples by RNase A cleavage, and the altered region is then sequenced.
Full text
PDF

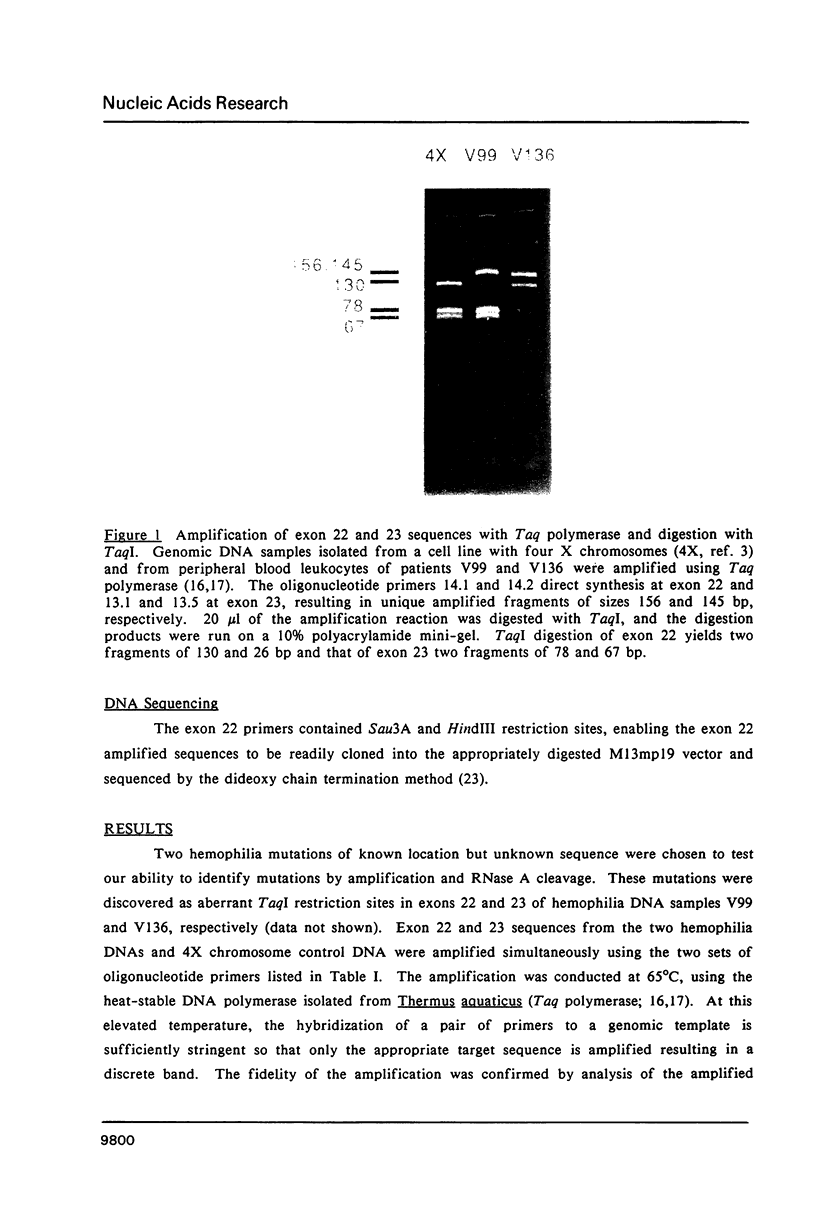




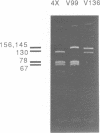
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Waber P. G., Kittur S. D., Patel A. S., Kazazian H. H., Jr, Mellis M. A., Counts R. B., Stamatoyannopoulos G., Bowie E. J., Fass D. N. Hemophilia A. Detection of molecular defects and of carriers by DNA analysis. N Engl J Med. 1985 Oct 3;313(14):842–848. doi: 10.1056/NEJM198510033131402. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Miller J. H. Mutagenic deamination of cytosine residues in DNA. Nature. 1980 Oct 9;287(5782):560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Caskey C. T. Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science. 1987 Apr 17;236(4799):303–305. doi: 10.1126/science.3563511. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Goralka T. M., Wion K. L., Chen E. Y., Eaton D. H., Vehar G. A., Capon D. J., Lawn R. M. Characterization of the human factor VIII gene. Nature. 1984 Nov 22;312(5992):326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Shuman M. A., Lawn R. M. Identification of a missense mutation in the factor VIII gene of a mild hemophiliac. Science. 1986 Jun 13;232(4756):1415–1416. doi: 10.1126/science.3012775. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Tuddenham E. G., Shuman M. A., Goralka T. M., Chen E. Y., Lawn R. M. Detection and sequence of mutations in the factor VIII gene of haemophiliacs. 1985 May 30-Jun 5Nature. 315(6018):427–430. doi: 10.1038/315427a0. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Winter E., Yamamoto F., Almoguera C., Perucho M. A method to detect and characterize point mutations in transcribed genes: amplification and overexpression of the mutant c-Ki-ras allele in human tumor cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7575–7579. doi: 10.1073/pnas.82.22.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wion K. L., Kelly D., Summerfield J. A., Tuddenham E. G., Lawn R. M. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985 Oct 24;317(6039):726–729. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Antonarakis S. E., Aronis S., Tsiftis G., Phillips D. G., Kazazian H. H., Jr Characterization of five partial deletions of the factor VIII gene. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3772–3776. doi: 10.1073/pnas.84.11.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Kazazian H. H., Jr, Phillips D. G., Aronis S., Tsiftis G., Brown V. A., Antonarakis S. E. Recurrent mutations in haemophilia A give evidence for CpG mutation hotspots. 1986 Nov 27-Dec 3Nature. 324(6095):380–382. doi: 10.1038/324380a0. [DOI] [PubMed] [Google Scholar]