Abstract
We report on an ‘unbiased’ molecular characterization of individual, adult neurons, active in a central, anterior hypothalamic neuronal circuit, by establishing cDNA libraries from each individual, electrophysiologically identified warm sensitive neuron (WSN). The cDNA libraries were analyzed by Affymetrix microarray. The presence and frequency of cDNAs was confirmed and enhanced with Illumina sequencing of each single cell cDNA library. cDNAs encoding the GABA biosynthetic enzyme. GAD1 and of adrenomedullin, galanin, prodynorphin, somatostatin, and tachykinin were found in the WSNs. The functional cellular and in vivo studies on dozens of the more than 500 neurotransmitter -, hormone- receptors and ion channels, whose cDNA was identified and sequence confirmed, suggest little or no discrepancy between the transcriptional and functional data in WSNs; whenever agonists were available for a receptor whose cDNA was identified, a functional response was found.. Sequencing single neuron libraries permitted identification of rarely expressed receptors like the insulin receptor, adiponectin receptor2 and of receptor heterodimers; information that is lost when pooling cells leads to dilution of signals and mixing signals. Despite the common electrophysiological phenotype and uniform GAD1 expression, WSN- transcriptomes show heterogenity, suggesting strong epigenetic influence on the transcriptome. Our study suggests that it is well-worth interrogating the cDNA libraries of single neurons by sequencing and chipping.
Keywords: Transcriptome single cell, microarray and sequencing, warm sensitive neuron, chemical neuroanatomy, epigenetics
1. Introduction
The goal of the discipline ‘chemical neuroanatomy’ was originally the chemical/molecular identification of neurons in a microscope, including visualization of signal substances released at their synapses and the receptors being activated (Bjorklund and Hokfelt, 1983; Emson, 1983). This approach was perhaps first taken using the formaldehyde fluorescence method to visualize monoamines (Carlsson et al., 1962; Falck et al., 1962; Dahlström and Fuxe, 1964) and subsequently with immunohistochemistry (Coons, 1958) and in situ hybridization. So far these morphological methods may, under fortunate circumstances, allow visualization of up to some six markers in a single neuron, if combining triple-labeling immunohistochemistry with the “mirror technique” (Kosaka et al.,1985) can be executed. This may be sufficient to characterize the transmitter molecules, the classical low molecular weight transmitters and neuropeptides of a neuron, but not the, very likely, much larger number of receptors a neuron express.. Apart from the first mapping of the localization of, for example, a novel protein/small molecule in the nervous system with a newly generated antibody, these approaches are inherently “biased”, because they rely on prior knowledge of the neuronal circuit for the choice of the ISH probe or the antibody to be tested.
In this review we describe the results of an unbiased, broad and in depth molecular characterization of a small group of adult brain neurons by means of single neuron chipping and sequencing of the cDNA library of each individual neuron to provide “single cell transcriptomics” data, that is single cell analysis without a microscope (Brady, 2000; Carter, 2006; Hinkle, et al., 2004; Kacharmina, et al., 1999). This unbiased approach (blind to the information on the location, function and earlier delineated transmitters and receptors of the neuron and of the circuit) to the molecular composition of individual neurons has produced many unexpected results and insights of molecular, physiological and pharmacological significance that we will review below.
This approach to prepare and amplify a cDNA library from a single neuron (Phillips and Eberwine, 1996) for chipping and sequencing is now becoming technically and economically feasible and has the potential to produce physiologically and pharmacologically important, highly relevant, and often unexpected, data., We therefore feel that it is worthwhile to review the methods and results obtained in the study of the warm sensitive neurons (WSNs). We here report on the unexpected degree of molecular heterogeneity revealed at the level of the entire transcriptome, in a group of adult neurons claimed “identical”, by electrophysiological criteria i.e. WSNs (cf. Boulant, 1981, 1998, 2000; Nakayama, et al., 1961; Nakayama, et al., 1963). These neurons, located in the preoptic area (POA) of the anterior hypothalamus, are also considered “identical” by the histochemical criterion of expressing the GABA synthetic enzyme GAD1 mRNA. These neurons are identical in terms of localization and connectivity as well ; all belong to the group of GABAergic projection neurons with soma in the POA and terminals in the rostral Raphe Pallidus (rRPa) and Dorsomedial hypothalamus (DMH).
We will describe a strong signaling asymmetry with only one to five neurotransmitters/neuropeptides synthesized and probably released by a single neuron, contrasting over 250 neurotransmitter receptors and ligand-gated ion channels that can sense neurotransmitters, neuropeptides, pyrogens reaching this single WSN. In addition to the more than 250 receptors for neuronal signals the WSNs express ca 250 hormone receptors. These warm sensitive neurons in the POA subserve integrative functions, and play a key role in regulating energy metabolism and core body temperature through their, finally, inhibitory control of adaptive thermogenesis in the Brown Adipose Tissue (BAT) (Dulloo, et al., 2004). These WSNs thus are key components of the thermoregulatory circuit which fine regulates the core body temperature, the largest energy expenditure in homeotherms (Bligh, 1973;H. C. Chen, et al., 2003;X. M. Chen, et al., 1998; Clark, et al., 1939). However, only the studies reviewed here, could conclusively demonstrate that these WSNs are GABAergic and peptidergic. Furthermore these studies revealed that they express receptors for metabolic signals like adiponectin, IGF-1 and insulin, besides the earlier identified receptors for pyrogens like the Interleukin-1 receptor type 1, that was detected by electrophysiological measurements (cf (Tabarean, et al., 2005). That the receptor repertoire of these neurons comprises several hundred receptors, with a third of them being orphan receptors, was neither researched nor would have been uncovered by any of the histochemical methods or global genetic mapping methods/approaches presently used, including the Allen Brain atlas (Bjorklund and Hökfelt, 1983; Emson, 1983; Gong, et al., 2003; Jones, et al., 2009; Lein, et al., 2007). These GABAergic warm sensitive projection neurons exert a robust inhibitory effect on the regulation of the activity of BAT, and thus on adaptive thermogenesis (Landsberg, 1986; Lowell and Spiegelman, 2000; Nedergaard, et al., 2007). This characteristic provided us with an in vivo experimental modality and opportunity to study the activity of these WSNs, and the modulation of this activity through their receptors, by measuring in vivo changes in the activity of BAT and in core body temperature. In this way the functionof many receptor cDNAs could be validated for the single cell transcriptome of a WSN.
The results from the present, single neuron transcriptomic study of WSNs, now open up for the study of other types of cells/neurons by the same approach, even in cases where unique functional characteristic are not experimentally as easy to establish as it was in our analysis.
The great differences in the composition of cDNA libraries of individual cells in the same warm sensitive, GABAergic cell group, and thus the epigenetic differences among warm sensitive GABAergic projection neurons of the anterior hypothalamus reported here may reflect the functional and anatomical position of the sampled neuron in the network, of developmental influences, of the prevailing neuronal firing patterns, and of neuroendocrine, metabolic and synaptic inputs at the time point, when we isolated mRNAs of a given warm sensitive neuron. The great transcriptome differences found also focus attention on the rich and individually unique expression of olfactory receptors on each of the studied warm sensitive neurons, whose role in siganlling in this brain region is not understood but which according to proposals may act as positional signaling (Dreyer, 1998; Feinstein, et al., 2004). This review summarizes the steps taken to establish the receptor and signal substance repertoire of individual (warm sensitive) neurons.
The data and the conclusions are, hopefully, of general interest for the physiology, chemical neuroanatomy and pharmacology of the central nervous system as they refer to a molecularly detailed dynamic chemical description of central neurons in a functioning network.
2. Warm sensitive neuron in the POA neuronal circuit; Roles in the fever response and in thermoregulation; Electrophysiology and morphology
The ability of neurons to alter their firing rate upon increased local temperature is a general phenomenon involving temperature-dependent changes in leakage currents (Barker and Carpenter, 1970; Carpenter, 1967; Hammel, 1965). There are, however, specialized warm sensitive neurons (Baldino and Geller, 1982; Boulant, 1981, 1998; Dean and Boulant, 1989; Nakayama, et al., 1961; Nelson and Prosser, 1981) in several central and peripheral locations that show an unusually high degree of increase in their firing rate upon relatively small changes of local temperature (1–3 °Centigrade); these warm sensitive neurons (WSNs) have been studied most in the anterior hypothalamic POA of homeotherms; rodents, rabbits, cats (Griffin, et al., 1996; Nakayama, et al., 1961; Nelson and Prosser, 1981; Shibata and Blatteis, 1991; Zhang, et al., 1995; Zhao and Boulant, 2005), and their involvement in changes of core body temperature has been first noted during the fever response (cf (Bartfai and Conti, 2010; Dinarello and Wolff, 1982; Saper and Breder, 1994; Welch, 1888), which is a stereotype stress response in homeotherms, with extremely well characterized rapid rise of core body temperature followed by a decline within 2–4 hours. This febrile response, i.e., the pyrogen, PGE2, IL-1b… evoked rise of core body temperature (cf (Coceani and Akarsu, 1998; Dinarello and Bunn, 1997; Feldberg and Saxena, 1971; Feleder, et al., 2004), is tightly controlled as it represents a very large energy expenditure for the body. When studying warm sensitive neurons, the change in core body temperature, hyper- or hypo-thermia response represents an easy to measure in vivo response that appears to be directly reflecting the changes in the activity of the warm sensitive neurons. All known pyrogens and centrally active hyperthermic agents that act at the warm sensitive neurons, were shown to reduce the firing rate of these inhibitory neurons (cf below), and thus, unexpectedly, or counter-intuitively, fever is not ‘switching on’ a thermogenic response, but rather it is disinhibiting a ‘normally inhibited’ adaptive thermogenesis in the Brown Adipose Tissue. The Brown Adipose Tissue, is a key tissue for heat production in rodents and infants, whose role in regulation of metabolism under physiological and pathophysiological (obese) conditions is being reassessed also in adults (Mattson, 2010; Zingaretti, et al., 2009).
Maintaining stable core body temperature is in itself the largest energy expenditure in mammals, the smaller the mammal (or more precisely the greater its surface to body mass ratio) the higher the metabolic rate that is required to maintain the 37°C that all mammals independent of body mass/size have settled for (were endowed with) during evolution. (Martin and Palumbi, 1993; Singer, 2007). Because of the large surface to mass ratio, rodents run at ca 20 times the metabolic rate of a human and these higher metabolic rates are resulting in shorter life span – thus the warm sensitive neurons controlling core body temperature and the metabolic rate are serving some very basic physiological functions that even affects life span (Conti, et al., 2006). Yet their chemical neuroanatomy and their pharmacology are not well studied as of now, prompting us to start a project reviewed here. (It should be added that, while there are temperature-sensitive neurons in the simpler model organisms (cf (McKemy, 2007), like C. elegans (cf (Mori, 1999; Ramot, et al., 2008), and the gene expression profiles of these thermosensory neurons involved in thermotaxis have been studied (Satterlee, et al., 2004), our focus on fever and thermoregulation in homeotherms, has made it mandatory for us that the studies are carried out on mammalian thermosensitive neurons rather than on thermosensitive neurons in non-homeotherms, regardless of the experimental advantages those systems might present).
Despite extensive electrophysiological studies of the warm sensitive anterior hypothalamic neurons in the preoptic area spanning over the past 45 years, these neurons do not have any specific morphological markers at present, in 2010, that would enable us to easily identify them in a slice preparation. The identification of the warm sensitive neurons that make up no more than 5–15% of the neurons of the POA, in vivo or in tissue slices from adult animals, is today achieved utilizing solely, electrophysiological methods (Griffin, et al., 1996; Nakayama, et al., 1961; Nelson and Prosser, 1981; Shibata and Blatteis, 1991; Tabarean, et al., 2005; Zhang, et al., 1995; Zhao and Boulant, 2005). Early on, mostly extracellular recording was used to study the change of firing rate of these, spontaneously firing warm sensitive neurons upon changing local temperature by termistors or upon applying any of the known pyrogenic substances - such as prostaglandins and interleukin -1 - the endogenous pyrogen, TNFalpha (Boulant, 1981; Hori, et al., 1988; Kelso and Boulant, 1982), etc. In the past decade, others and we have used whole cell patch recordings in most of our studies on PGE2 effects on the warm sensitive neurons (Tabarean, et al., 2004), and on the IL-1 mediated inhibition of the firing of warm sensitive neurons, respectively (Davis, et al., 2006; Tabarean, et al., 2005). These whole cell patch studies endowed us with the experimental, physical possibility to prepare cDNA library of individual neurons that have been characterized electrophysiologically in the POA slice and found to exhibit a greater than 0.8 Hz/C warm sensitivity upon stepping the temperature of the bath, by using the patch pipette to deliver the reverse transcriptase to transcribe the RNAs into cDNAs and to suck the cDNAs out (as in detail described by Eberwine and his colleagues in the past 14 years).
One unappreciated beauty of the core body temperature and fever studies is that changes in the firing rate of a WSN in the POA can be quantitatively correlated to changes in core body temperature measured with an exact but simple thermometer, or later by measuring changes in metabolic rate in BAT or by determining respiratory ratio (O2/Co2), or by measuring uncoupling of mitochondria in BAT by positron emission tomography (cf for example the discovery of the insulin receptor cDNA in a single warm sensitive neuron, a transcriptomics in a few warm sensitive neurons, that was extended to a study insulin effects on BAT, a study by PET and to a respiratory rate and heat production study (Sanchez-Alavez, et al., 2010). These in vivo measurements that utilize the powerful systemic amplifications at the tissue (BAT) or whole organism level of what happens at an individual warm sensitive neuron, or at a few warm sensitive POA neurons, are very helpful in determining that certain molecular components of these neurons such as receptors and ion-channels are important (or robustly important to leave whole body affecting changes) not only for the activity of the given neuron under recording, but also for large, acute in vivo physiological the possibility that these hypothalamic cells represent a unique category of cells as sensors of milieu interieur and hence rich in receptors is discussed changes in adaptive thermogenesis and in core body temperature. The caveats of validation of functional receptor expression on a WSN by use of measurement of BAT activation is that it involves many amplifications and that biological amplifications of this kind are nonlinear and can, and often, involve many redundant components: this remains an inherent weakness in the proof of functional expression of a cDNA in the WSNby measurement of BAT activity that it is essentially a correlative measure, but it does not negate the utility of the enormous gain in sensitivity that results from the reading of core body temperature change of degrees in a whole animal is represented after applying small amounts (femto-pico to nanomoles) of pyrogens on a few neurons.
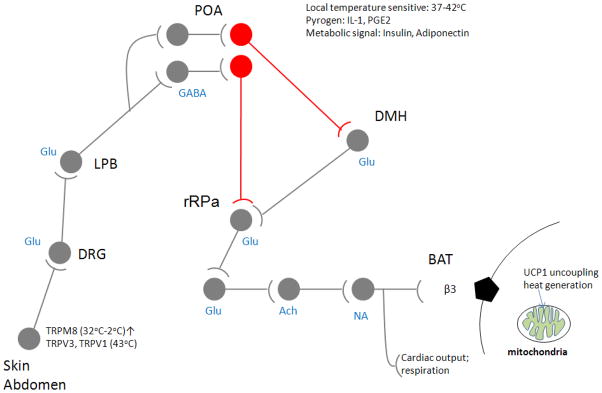
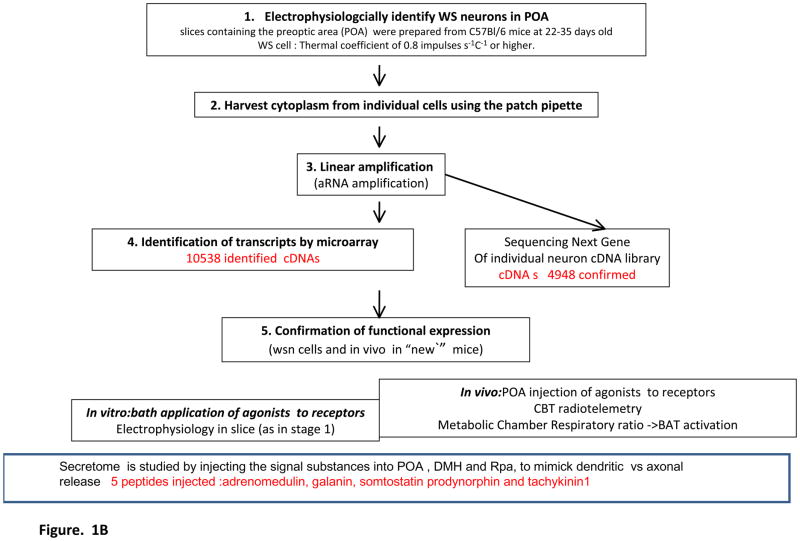
Figure 1A shows where the warm sensitive neurons of the POA are located in the proposed neuronal circuit that regulates BAT activity, utilizing/integrating afferent inputs from skin thermosensors and local heat input ( from circulation) in the POA itself, as suggested by several tracing and lesion studies of the skin sensor mediated effects on adaptive thermogenesis (cf (K. Nakamura and Morrison, 2008). We have focused on the adult, spontaneously firing warm sensitive neurons, that we have identified by patch clamp recording and changes of the temperature of the bath in which the anterior hypothalamic slice was suspended (Tabarean, et al., 2004; Tabarean, et al., 2005), and after the recording has been completed the patch pipette was used to extract the mRNA and reverse transcriptase was used to transcribe the RNAs into cDNAs. The experimental paradigm used to generate, amplify and determine the cDNA species present in the cDNA library from individual warm sensitive neurons is depicted in Figure 1B. In most of the similar transcriptional profiling studies on single cells, linear amplification is used to produce sufficient amounts of cDNA from a single cell for further study by PCR, microarray or as in our case for both microarray and sequencing.
Figure 1.
Figure 1A. Warm sensitive neurons in the Preoptic area that were subject of single cell transcriptomics study by chipping and sequencing. Thermoregulatory network is depicted showing the central integrative role of temperature sensitive GABAergic projection neurons (highlighted in red) in activation of thermogenesis in Brown Adipose Tissue, respiratory rate and cardiac output. It depicts data from multiple retrograde tracing studies that show that the inhibitory warm sensitive neurons project to DMH and or rRPA., and that the skin temperature and deep body temperature is affecting the activity of these neurons through Glutamatergic afferents while the local temperature is belived to exert its effect through circulation communicated heat affects. POA: Preoptic Area, DMH: Dorsomedial Hypothalamus, rRPA: rostral Raphe Pallidus, DRG: Dorsal Root Ganglion, BAT: Brown Adipose Tissue, LPB: Lateral parabrachial nucleus. Adapted from Nakamura, K. and S. F. Morrison (2008) and modified with our data.
Figure 1B. Flow chart of experimental design for single cell transcriptomics-based identification and in vitro and in vivo validation of the functional secretome and receptor repertoire of individual warm sensitive neurons. The numbers in red show the number of transcripts identified in individual neuron cDNA libraries by chipping using the Affymetrix mouse microarray, in the warm sensitive neurons, the number of cDNAs confirmed by NextGen sequencing that produces reads of 48–100 nucleotides, and accepting reads that are fully matching, in the functional validation experiments involved agonists that were tested electrophysiologically on spontaneously active warm sensitive neurons in slice (at least on ten to fifteen such neurons), 31of these agonist for recptors whose cDNA we identified, were found able to affect the behavior of the cells, i.e., have functional receptors expressed on warm sensitive cells, and the remaining ones may not havefunctional receptors expressed or the receptors do not affect the tested electrophysiological properties, or the number of cells tested was too low to find a cell with a functional receptor expressed, 21 recptor agonists were injected into the POA as cDNA encoding their receptor, or channel was found, 18 of these produced hypo- or hyperthermic response at the 0.01 to 10 nanomoles dose.
Most often the neuronal cDNA pool is studied by PCR examining the presence of a few or a dozen cDNAs that are of interest. It is more rarely that the cDNA is used in unbiased chipping studies, where the question is: which cDNAs are present and can be identified by the microarray hybridization at certain signal intensity? Again, PCR is the most common method, today, to confirm a subset of the chipping results. The sequencing of all cDNAs, as used in the studies by us, provides a second unbiased approach to the transcriptome of the cell, particularly as one is not forced to make a limiting selection of more or less important cDNAs identified by the microarray analysisand to be confirmed by PCR each of which requires design of several primer pairs and optimization of the reaction conditions, but rather all cDNAs present are sequenced. Furthermore the sequence reads provide a better confirmation of the microarray data than PCR, and they provide a confirmation of all of the array data simultaneously, providing a better coverage of the cellular mRNA composition than a few selected PCR reactions can do. Additionally, through the sequencing, information on abundance is also obtained in as much that the number of sequence reads is proportional to the number of cDNA copies, and thus likely proportional to the original copy number of mRNAs. The experimental details and the considerations of cut offs in the analysis of the microarray data and the considerations of sequencing accuracy are dealt with later in this review.
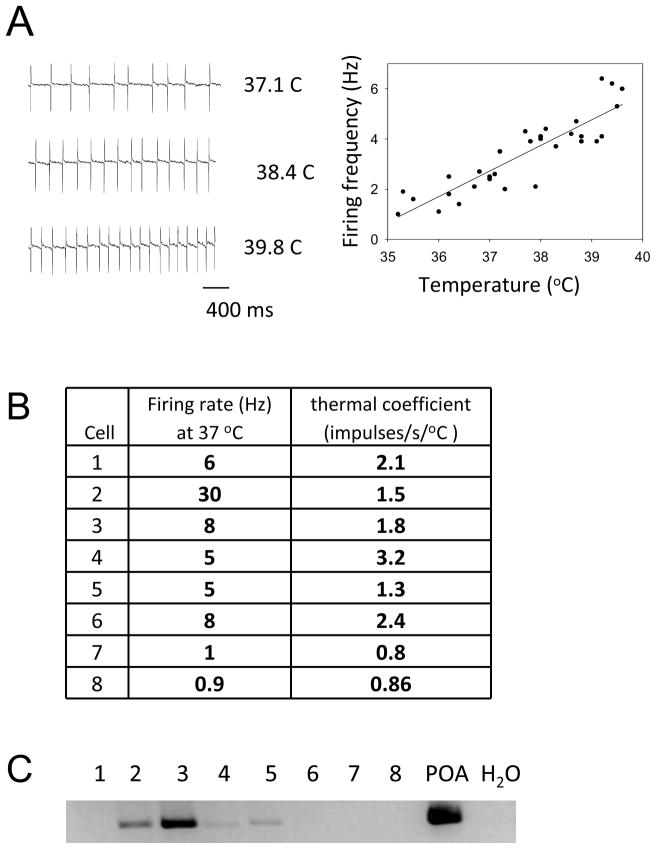
The firing pattern of a WSNused in our study is shown in Figure 2A. This figure demonstrates the robust effect of temperature changes, within a moderate range 33–41°C, on the firing rate of spontaneously active warm sensitive neurons, respectively. The thermal coefficient of all eight WSN was above 0.8/impulses/s/°C, Figure 2B, an arbitrary cut off thermo sensitivity measure, that is used in the literature (Boulant, 2006). This electrophysiologically determined phenotype, the ability to alter firing rate upon heating so dramatically, puts these neurons into a well-defined electrophysiological category, that encompass 5–15 % of POA neurons and this category posses no or extremely few neurons in other hypothalamic nuclei or in other brain regions.
Figure 2. Warm sensitive neurons (n=8) included into the transcriptomics study are all GABAergic.
A. Electrophysiological characterization of warm sensitive neurons: as the temperature of the bath in which the Preoptic area tissue slice is suspended increases, the firing rate of warm sensitive neurons increases. B. Firing rate and thermal coefficients of warm sensitive neurons whose transcriptome was examined by microarray analysis of their cDNA library. C. PCR confirmation of GAD1 in 4 out of 8 WS cells, it should however be noted that Affymetrix chipping and NextGen sequencing, both, showed that 8 of 8 of the warm sensitive neurons expressed the GAD1 cDNA.
All of these neurons are studied in the anterior hypothalamic POA, thus their localization is identical, and these neurons all originate from adult male mice of the same age and same strain. The microarray data has suggested that all eight warm sensitive neurons neurons chipped and sequenced express GAD1 mRNA, encoding the GABA biosynthetic enzyme. The PCR study that shows that the POA as a region has rich expression of GAD1 mRNAs, showed that four of these neurons have clear PCR product that upon sequencing corresponds to a GAD1 PCR product (Figure 2C). It is unclear why a PCR GAD1 mRNA product was not observed in all eight neurons, although it is likely due to primer selection for the GAD1-PCR as compared to the sequences that were spotted on the microarray; instead of generating dozens of additional PCR primers for GAD1 we turned to the sequencing data. The Illumina sequencing of the cDNAs confirmed that all studied warm sensitive neurons express GAD1 mRNA. Thus, these neurons are all GABAergic projection neurons in the POA, that project to the DMH or rostral Raphe Pallidus as retrograde tracing by Nakayama and Morrison, 2008 demonstrated.
When examining the transcriptomes of these neurons we can thus by today’s criteria claim that a homogenous set of GABAergic projection neurons is being compared.
An important validation of the experimental paradigm, as outlined in Figure 1B, is that it retrieves and reconfirms already known molecular components of the warm sensitive neuron. Since no unique marker is known, we could not ask as an internal validation of the methodology, that this particular unique morphological marker is found by transcriptomics, instead functional markers known from earlier studies were used to validate the methods The electrophysiological studies (Boulant, 1998; Tabarean, et al., 2004; Tabarean, et al., 2006) showed the presence of AMPA, NMDA, GABAA ligand-gated channels, but these are so commonly expressed in central neurons that they cannot serve as a marker validating the single neuron transcriptomics approach. The receptor for the endogenous pyrogen; IL-1R1 (Alheim and Bartfai, 1998; Tabarean, et al., 2006), a functional Toll type receptor (Dinarello, 1999,2004) was, however, well known to be expressed on warm sensitive neurons and its functioning was known to affect firing rate of these neurons (Hori, et al., 1984; Hori, et al., 1988; Tabarean, et al., 2006). Furthermore, studies on downstream signaling via IL-1R1 receptors have implicated the Src and ceramide pathways in IL-1 mediated non transcriptional effects on ion channels, and likely on firing rate of the IL-1R1 expressing warm sensitive neurons (Davis, et al., 2006). Thus, we have asked whether the single WSNcDNA library, after its amplification, microarray analysis and sequencing would indeed indicate the presence of cDNAs encoding the IL-1R1 and of some intracellular Toll signaling proteins, downstream from the IL-1R1 and thus could serve as validation paradigm for the whole methodology of single cell transcriptomics.
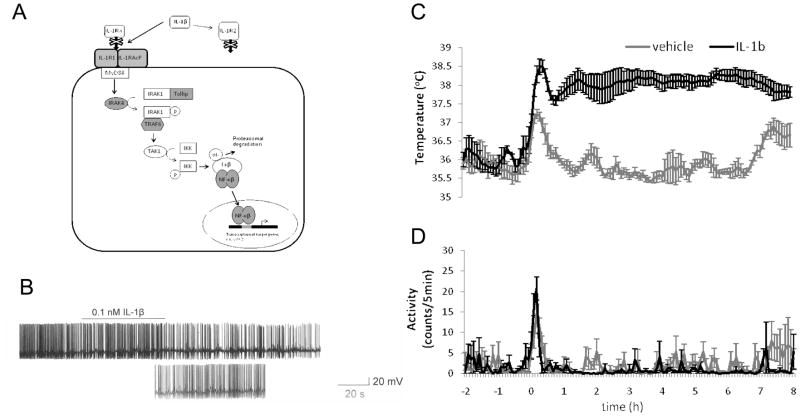
Figure 3 shows the endogenous pyrogen, interleukin-1-mediated inhibition of the firing of spontaneously active warm sensitive neurons. Figure 3A shows the IL-1R1 signaling pathway, the IL-1R1 and the downstream proteins for which we have identified the encoding cDNAs. Dozens of in vivo and in vitro studies show that pyrogens are inhibitory for the firing of spontaneously active warm sensitive neuronsin the POA. This is also true for the effects of IL-1, the endogenous pyrogen (Figure 3B). The pyrogen receptors (EP3R prostanoid receptor, IL-1R1, TNFa receptor) expressed by warm sensitive neurons also turned out to be widely expressed by other neuronal and non-neuronal ( microglia) cell types; therefore, despite the powerful effect they mediate on the firing rate of warm sensitive neurons, they are not representing a selective morphological marker for these neurons. The inhibition of the firing of warm sensitive neurons in POA slices or in vivo correlates with the hyperthermic response in vivo as shown in Figure 3C (the rise in core body temperature is not originating from increased motor activity, Figure 3D) but from activation of adaptive thermogenesis in Brown Adipose Tissue, as shown by us in the case of other hyperthermic and pyrogenic agents (cf (Sanchez-Alavez, et al., 2010). ( it is important to note that while IL-1R1 type receptors are expressed on other neurons in other brain areas like on cerbellar Purkinje cells where they mediate exciation( Motoki et al 2009) and no change in core body temperature, the coupling between IL-1R1 mediated inhibition of the activity and febrile response is unique to the POA warm sensitve neurons).
Figure 3. The experimental paradigm: Identification and functional validation of a Prototypic molecular component of the warm sensitive neuron receptor repertoire; the pyrogen receptor, IL-1R1, and its downstream signaling components.
Earlier electrophysiological and binding studies have shown IL-1R1 expression in the POA. These data thus serve as confirmation of the chipping, PCR, sequencing, electrophysiological and CBT measurement based functional identification strategy for receptors on warm sensitive neurons. A. Many components of the Interleukin 1 signaling pathway were identified by microarray analysis and NextGen sequencing of cDNA libraries from warm sensitive neurons. Transcripts found to be expressed with a significant p value of detection are shaded grey. B. Application of IL-1β (0.1nM) hyperpolarizes the cell and inhibits the spontaneous firing rate of POA/AH warm sensitive neurons. C. POA injection of IL-1β (150ng/0.5ul) elicited increases in core body temperature in wild type (wild type of what?) treated mice relative to vehicle treated mice. D. These changes in body temperature are not due to differences in motor activity of the IL-1β treated mice relative to the vehicle treated control mice.
Despite the excitement at the discovery of the temperature sensitive TRP channels (cf (Jordt, et al., 2003; Latorre, et al., 2007), and infrared sensitive TRP channels (Gracheva, et al., 2010), it became clear that these discoveries have, unfortunately, not explained central thermosensitivity as expressed by warm sensitive neurons in the POA. While the TRP channels are clearly important in peripheral heat sensing and nociception, they do not seem to be involved in central heat sensitivity of the warm sensitive anterior hypothalamic neurons, even though many TRP channel mRNAs are found in the WSN transcriptome (cf Supplemental data Table 1).
There have been many additional attempts to find morphological markers for warm sensitive neurons. In one attempt warm sensitive neurons after electrophysiological recording were filled with dyes and then found by slicing and their arborization and soma reconstructed. It was reported that in some cases they appear to be projecting interneurons with some authors elaborating the differences in the asymmetry of the arborization of processes close to the ventricles (Griffin, et al., 2001). These and other morphological studies, however, did not succeed in providing selective morphological marker(s) for the warm sensitive neurons, and thus these neurons are identified by electrophysiological recordings made at different temperatures of the buffer in which the tissue slices are kept under recording, or by heating, in vivo, the POA region by thermistors (cf Figure 2A).
The chemical neurotransmitters synthesized and released by the warm sensitive neurons were also not known conclusively until the cDNA chipping studies of the warm sensitive neurons, followed sequencing studies, have shown that each of the WSN has expressed mRNA encoding the GABA biosynthetic enzyme GAD1 (Bartfai, Eberwine and colleagues in preparation), although pharmacological suggestions were made earlier, that the warm sensitive neurons are likely to be GABAergic (Y. Nakamura, et al., 2005). These pharmacological suggestions originated from results obtained in sedated animals and were based on similar pharmacological actions seen by increased firing of WSNs, as achieved by e.g., local warming of the POA and by the actions by the GABA agonist, muscimol being injected into the two main projection areas of the POA warm sensitive neurons; the DMH and RPa, respectively (cf Figure 1A).
3. Neurotransmitter and neuropeptide repertoire of warm sensitive neurons
Below we will describe the identification of several neuropeptide preprohormone cDNAs in the cDNA libraries of individual WSNs and in this manner we define these, earlier, electrophysiologically identified warm sensitive neurons as GABAergic-peptidergic projection neurons; thus 50 years into their study, we provide classical, morphological markers for these warm sensitive neurons of the anterior hypothalamus.
We focused on the cDNAs encoding signal substances;neuropeptides and biosynthetic enzymes for neurotransmitters in the warm sensitive neurons, cf Table 1. The cDNA encoding GABA synthetic enzyme GAD1was found in all neurons studied by sequencing and microarray, while the PCR using one set of primers missed the GAD1 cDNA in 4 neurons (probably because the amplified cDNAs originating from GAD1 mRNA are representing somewhat different cDNA sequences in the different neurons). This finding, of the warm sensitive neurons being GABAergic, is in line with earlier pharmacological suggestions based upon injection of bicuculin, which could block the effects of the increased firing of these neurons and injection of muscimol that mimicked increased firing of warm sensitive neurons (K. Nakamura, et al., 2002; Y. Nakamura, et al., 2005). However, prior to this work there was no direct evidence of GAD1 expression and GABA synthesis in these neurons, even though it was known that many neurons are GABAergic in the anterior hypothalamus. In some ( 2of 8) WSNs the cDNA encoding choline acetytransferase was found by both microarray and sequence analysis. The presence of this enzyme if functionally expressed suggests that these neurons could in addition to GABA release acetylcholine, also. These findings at the level of detection need to be followed up, and the continuous improvements in technology will enable a more certain answer whether some WSNs express two classical transmitter or not.
Table 1.
The secretome of warm sensitive neurons in the POA : Neuropeptides and Biosynthetic enzymes for neurotransmitters identified with high expression signals from microarray analysis( cf AV signal intensity), PCR and NextGene sequencing ; thus likely to be expressed in WS neurons: in vivo studies with injection of these signal substances into POA, RPa, DMH, respectively are used to mimic the dendritic and/or axonal release of these signal substances
| SYMBOL | DEFINITION | sequence confirmed | Av | POA expression |
|---|---|---|---|---|
| Gad1 | glutamic acid decarboxylase 1 | yes | 134 | yes |
| Tac1 | tachykinin 1 | yes | 122 | yes |
| Adm | Adrenomedullin | yes | 118 | yes |
| Sst | Somatostatin | yes | 84 | yes |
| Pdyn | prodynorphin | yes | 259 | yes |
| Gal | galanin | yes | 96 | yes |
The presence of cDNAs encoding several preprohormones that give rise to the five neuropeptides; adrenomedullin, galanin, prodynorphin, somatostatin and tachykinin1. (Table 1) were found at the transcript level in the warm sensitive neurons by microarray and sequencing; The small number of neurons studied by both microarray and sequencing does not permit the determination of the peptide(s) that is the most commonly expressed neuropeptide(s) alongside GAD1, but in deeper analysis of much larger numbers of these warm sensitive neurons, one will establish how frequently a given neuropeptide is expressed by a warm sensitive neuron. So far we have used the information on the presence of the five cDNAs encoding for the precursors of the neuropeptides in some or several of the warm sensitive neurons as a qualitative finding, without aspiration to making statements about how large percentage of the GAD1 positive warm sensitive neurons in the POA does express these neuropeptides. To be able to estimate - with a high likelihood of being correct - which are ‘all’ the neuropeptides that the warm sensitive neurons may express, analysis of the transcriptome of several hundreds of warm sensitive neurons should have to be studied as there are over 70 endogenous peptides so far identified in the CNS (this number is further increased as we are looking at possible combinations of 2-3-4 of these neuropeptides, that could be co-expressed by the same GABAergic neuron as indeed found in this very limited analysis showing that any given WSN was GABAergic and had at least two neuropeptides expressed.
The functional importance of the neuropeptides in neurons is shown best under conditions of high frequency firing or burst type firing of the warm sensitive neurons that occurs with increasing local temperatures in the POA. Under these conditions of neuronal activity, we expect that the release of GABA that is already present at low frequencies is augmented by the release of these peptides from the axon terminals in the DMH and Ra Pa and in the somatodendritic area in the POA. To mimic such situation and peptide release, microinjections of all of the five peptides were thus carried out in these three brain areas and using radiotelemetric read out it was found that POA injections and DMH injections but not rR Pa injections of several peptides can alter core body temperature (Table 2), enabling the conclusion that functional receptors for several of these peptides are present in these regions. Taken together, the receptor presence and the presence of the mRNA encoding the endogenous ligand, neuropeptide strongly suggest that the neuropeptide is likely to be secreted from the warm sensitive neuron.
Table 2.
Effects of neuropeptides found in the cDNA library of the warm sensitive neuron, when injected into the somatodendritic area ( POA) or to the axonal projection areas DMH and rRPA
| Peptide in wsn | injected into | biological effect | ||
|---|---|---|---|---|
| POA | DMH | rRPa | ||
| Adrenomedulin | + | hyperthermia ++ | ||
| + | none | |||
| + | hyperthermia + | |||
| Galanin | + | hyperthermia ++ | ||
| + | none | |||
| + | none | |||
| Prodynorphin | + | hyperthermia + | ||
| + | none | |||
| + | hyperthermia + | |||
| Somatostatin 1–14 | + | hyperthermia +++ | ||
| + | none | |||
| + | none | |||
| Tachykinin1 | + | none | ||
| + | none | |||
| + | none | |||
It is important to emphasize that these five neuropeptides (their cDNAs) were identified thanks to: a) the sensitivity of the linear amplification methods used here, and b) because we decided to analyze single (electrophysiologically identified) neurons avoiding dilution of the rarely expressed cDNA to the extent that it is under the detection limit of even when these strong linear amplification techniques are used. c)Galanin and prodynorphin being expressed in the same WSN could also be concluded from the single neuron study whereas analyzing pooled cDNAs from laser capture or other methods providing cDNA from several neurons would not permit such conclusion).
Indeed, because of the low abundance and the low signal intensity in microarray analysis cf. Table 2, suggest that we most likely would not have identified these peptides in warm sensitive neurons had we used laser capture or other methods to pool neurons. Furthermore, The Allen Brain Atlas has not reported all of these peptides as being expressed in the anterior hypothalamus area, only two of them, and for the ones reported, it did not place them in GABAergic, or GABAergic warm sensitive projection neurons in the POA. Thus the advantages of the single cell transcriptomics in identifying rare signal substances and placing them in a selected cell are obvious and outweigh the effort and cost of this technique.
4. Receptor ion channel repertoire of the warm sensitive neurons; the pharmacological targets of these neurons
Cell surface receptors of different classes and ligand gated ion channels are the most common drug targets for drugs acting in the Central Nervous System. It is therefore important not only for understanding the physiology of a neuron but also for its pharmacological manipulation to know the receptor repertoire of a neuron through which its activity can be modified by non cell penetrating ligands. Our assumptions of how many kinds of receptors a neuron express diverge from the numbers found in this single cell transcriptomics analysis as we fund several hunded receptors types expressed while most neurobiologist assume this number being much lower, dozens, and indeed for the most pharmacologically targeted neurons in the CNS ( dopaminergic neurons in the substantia nigra and striatum, monoaminergic neurons in the dorsal Raphe Nucleus, and in Locus coeruleus) we utilize less than half a dozen receptors as drug targets. Yet as discussed below there are over 500 receptors found in a WSN, of which ca 250 encodes receptors for neurotransmitters and neuropeptides while the others are receptors for hormones, cytokines, metabolic signals.
Chipping and sequencing of eight randomly selected, electrophysiologically identified, adult warm sensitive neurons confirms that on average the studied warm sensitive neurons express 4948 mRNAs( cf Figure 4A for Go distribution, ) whose sequence( with no mismatch between cDNA and mouse genome) is confirmed and matches that in the mouse genome( cf Supplement for discussion on the much higher numbers of identified cDNAs when 1, or 2 mismatch is permitted).
Figure 4.
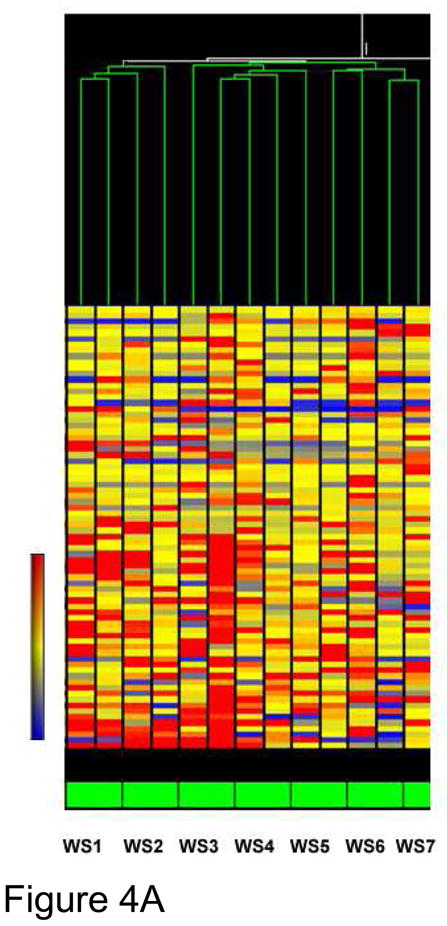
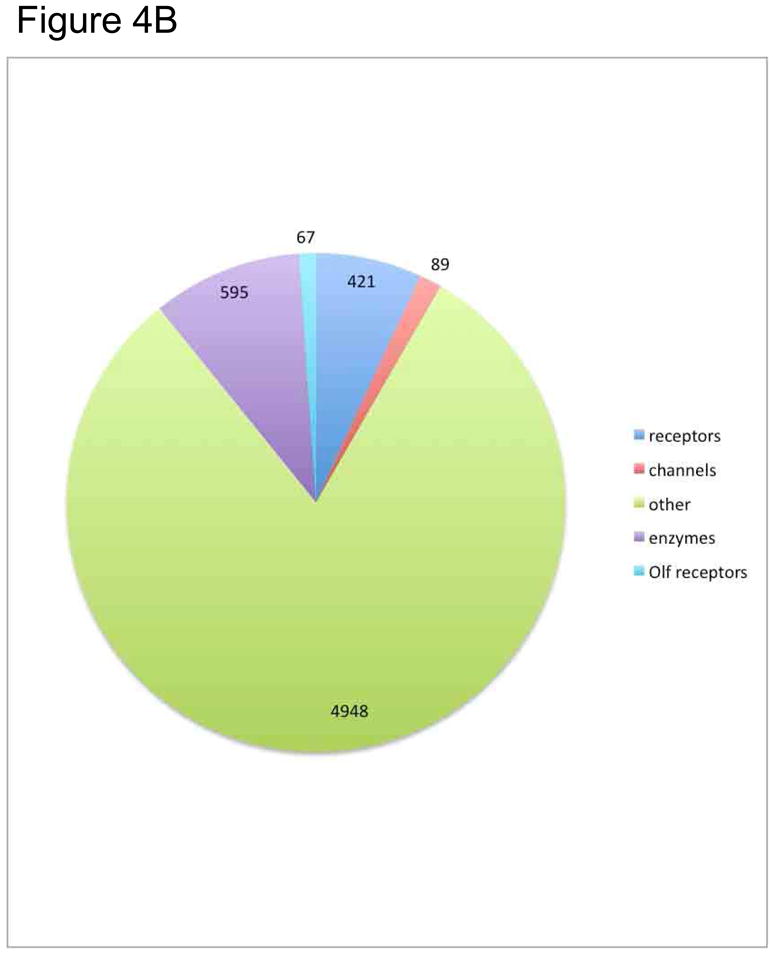
Figure 4A. Cluster analysis of warm sensitive neurons. The warm sensitivity of individual hypothalamic neurons was assessed electrophysiologically and the cytoplasmic contents of the cell harvested by aspiration. During the third round of aRNA amplification, the amplified single cell transcriptome was labeled with biotin for use as a probe for microarray analysis. The probes were analyzed on Illumina long oligonucleotide arrays and the resultant mRNA hybridization intensities quantified and the results were analyzed by GeneSpring (Agilent) analysis and displayed as a heatmap of normalized intensities. This analysis shows that the warm sensitive neurons do cluster together, but with some variation. Explain WS1–WS7
Figure 4B. Go - diagram on functional class distribution of the transcripts that were confirmed by NextGene sequencing of the cDNA libraries of 2 warm sensitive neurons, in separate experiments. This pie chart shows the fraction of extent cellular mRNAs that are present in GO designated functional classes. Approximately 8% of the cellular mRNA encodes receptors: ie cell membrane receptors, nuclear recptors.
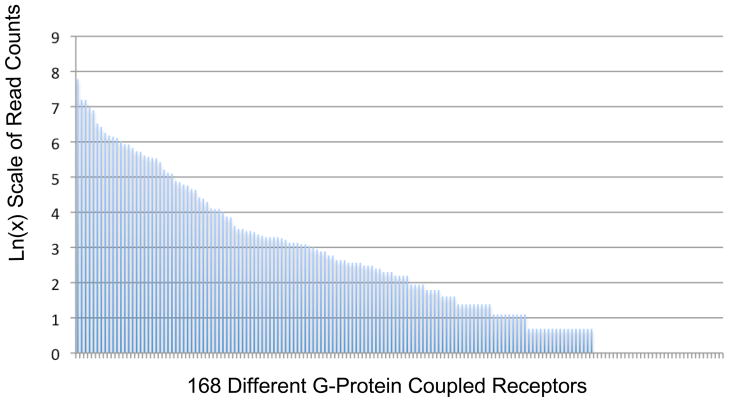
These sequence reads (cf below) match 421 receptors: GPCRs (168 non olfactory and 67 olfactory), single transmembrane domain –tyrosine kinase receptors, accounting for a very large portion of the sequence confirmed cDNAs (Figure 4B). Pharmacologically tractable, in neuronal signaling important cell surface proteins whose cDNA was confirmed by sequencing include the 89 ion channels, and 595 enzymes (Figure 4B). Figure 5 shows the relative abundances as deduced from read counts, of 168 GPCRs confirmed by sequencing in two warm sensitive neurons cDNA libraries.
Figure 5. Abundance distribution of G-protein coupled receptor cDNAs in warm sensitive neurons.
The Y-axis represents the read counts for the mRNAs encoding predicted G-protein coupled receptors that are present in these cells. The X-axis is the different genes that are expressed in these cells. The read counts at the far right of the graph appear to be 0 but are rather 1 or 2 which shows up as 0 on a Log(N) scale. There are receptors at very high levels such as Frizzled Homolog 3 (1858 reads) and the NPY receptor, Y1 (774 reads) and several others represented by only 2 or 3 read counts such as the orphan G-protein coupled receptor 133 or melanocortin 5 receptor. The large difference in RNA read counts for this class of molecules suggests a uniqueness to the receptor encoding repertoire of these cells and by analogy a functional uniqueness. The presence of (nearly) 35 receptor mRNAs at 1–2 read counts suggests that these mRNAs are either expressed at low levels or expressed in a subregion of the cell such as the dendrite where low levels of expression could have dramatic effects. Alternatively, the presence of these mRNAs shows that these genes are, epigenetically, able to be transcribed and with the proper stimulation may produce transcribed to higher abundances. In this model the low expressers may be an indicator of the additional epigenetic capacity of these warm sensitive neurons to respond to stimuli. Excluded from this analysis are the olfactory receptors.
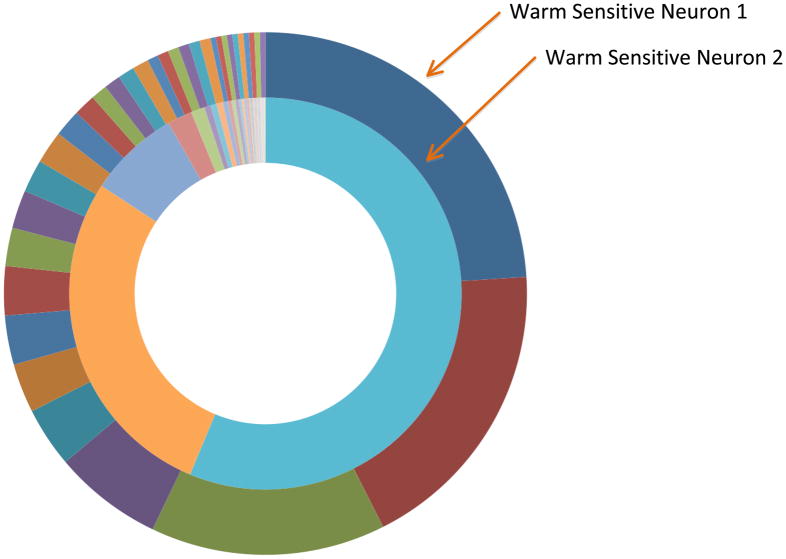
The GPCR class of receptors includes a surprising number of 67 olfactory receptors, The surprising finding of the very large number of non-overlapping olfactory receptors expressed by the two warm sensitive neurons whose cDNA libraries we sequenced (Figure 6) is presently not understood as we have no information about odorants reaching this hypothalamic nucleus or in other ways activating these receptors as agonist. This suggest, as speculated by Dreyer and a possible coding role of the olfactory receptor patterns in determining the network coordinates for the neuron that expresses them. Thus olfactory receptors may not participate in the signaling within the network but may be relevant to the organization of the network (Dreyer, 1998; Feinstein, et al., 2004).
Figure 6. Olfactory receptors in warm sensitive neurons.
These circle graphs show the distribution of predicted olfactory receptors in individual warm sensitive neurons as reflected in RNAseq read counts. One cell is represented by the outer circle and the other by the inner circle. The read counts for a particular receptor are represented by the arc around the circle that each colored box circumscribes. The range in expression of the genes in these cells of this class of receptors is ranging from 1 (in both cells) to 71 (warm sensitive neuron 1) and 541 (warm sensitive neuron 2). There are greater than 30 types of olfactory receptors expressed per cell with half of them expressed at greater than 2 read counts. The colors that are shared between the circles do not represent the same gene. None of the olfactory receptors found in one warm sensitive neuron were found in the other neuron suggesting exclusivity to the pattern of olfactory receptor expression in hypothalamic neurons.
Autoreceptors
There are several cognate receptor subtypes known for GABA expressed by WSN and for each of the five neuropeptides whose cDNA was identified in the WSN ; these receptors can mediate hyperpolarizing or depolarizing actions e.g., galanin has three known receptors of which GALR1 and GALR3 are mediating hyperpolarization and GALR2 mediating depolarization, respectively (cf (Lundstrom, et al., 2005; Mitsukawa, et al., 2008). Somatostatin can act at any of five receptor subtypes known to be expressed in the CNS (Siehler, et al., 2008, )and hypothalamic expression of sst1 subtype is proven ( Lanneau et al 2000). Which of the galanin receptor or somatostatin receptor subtypes are expressed on the warm sensitive neurons itself, i.e., which receptors could act as autoreceptors, for the peptides is known from the transcriptome study of the warm sensitive neurons. Table 3 shows the many different ‘auto receptors’ of the warm sensitive neurons. In the case of galanin, from the three known cognate receptors, the warm sensitive neurons express the GALR1 receptor subtype cDNA as identified by both chipping and confirmed by sequencing. This galanin receptor subtype is thus an ‘auto receptor’ on warm sensitive neurons, by definition. The sst3 receptor is similarly an auto receptor on warm sensitive neurons. Since we have identified the mRNA-cDNA for these auto receptors in the cDNA prepared from the soma of warm sensitive neurons in the POA we do not know whether the receptor within the neuron will be localized in the soma, or the receptor can be inserted into the membrane of the dendrites or expressed in the axon terminals in the DMH and or rRPa or in all of these sites.
Table 3.
Signal Substances and their (Auto) receptors expressed in warm sensitive neurons in the POA, identified by transcriptomics and validated by in vivo experiments on core body temperature
| SYMBOL | DEFINITION | Av WS |
|---|---|---|
| Adm | adrenomedullin (Adm), | 118 |
| Admr | adrenomedullin receptor (Admr),. | 136 |
| galanin | ||
| Galanin R1 | 111 | |
| Tac1 | tachykinin 1 (Tac1), | 122 |
| Tacr3 | tachykinin receptor 3 (Tacr3), | 109 |
| Sst | somatostatin (Sst). | 84 |
| Sstr1 | somatostatin receptor 1 (Sstr1). | 85 |
| Sstr2 | somatostatin receptor 2 (Sstr2). | 86 |
| Sstr3 | somatostatin receptor 3 (Sstr3). | 80 |
| Gad1 | glutamic acid decarboxylase 1 (Gad1), | 134 |
| Gabarapl1 | gamma-aminobutyric acid (GABA(A)) receptor-associated protein-like 1 (Gabarapl1),. | 129 |
| Gabrp | gamma-aminobutyric acid (GABA-A) receptor, pi (Gabrp),. | 105 |
| Gabra2 | gamma-aminobutyric acid (GABA-A) receptor, subunit alpha 2 (Gabra2),. | 114 |
| Gabra3 | gamma-aminobutyric acid (GABA-A) receptor, subunit alpha 3 (Gabra3),. | 147 |
| Gabrb2 | gamma-aminobutyric acid (GABA-A) receptor, subunit beta 2 (Gabrb2),. | 98 |
| Gabrd | gamma-aminobutyric acid (GABA-A) receptor, subunit delta (Gabrd),. | 101 |
| Gabrg2 | gamma-aminobutyric acid (GABA-A) receptor, subunit gamma 2 (Gabrg2),. | 121 |
| Gabrg3 | gamma-aminobutyric acid (GABA-A) receptor, subunit gamma 3 | 119 |
| Gabt4 | gamma-aminobutyric acid (GABA-A) transporter 4 (Gabt4), | 135 |
| Gabbr1 | gamma-aminobutyric acid (GABA-B) receptor, 1 (Gabbr1),. | 142 |
Which galanin, somatostatin, prodynorphin, adrenomedullin or tachykinin1 receptor subtype(s) are expressed on the postsynaptic target neurons in the DMH and rRPa remains also to be examined and serves as an example of the paradigm for molecular description of a whole circuit as opened up by single cell transcriptomics.
Functional validation of receptor cDNAs
Of the almost 5000 sequence confirmed cDNAs, we chose some dozens to ask the question, are these cDNAs represent mRNAs that are translated into functional gene products, and if so, can we identify the functional proteins? To identify the gene product at a single cell level is easiest if this gene product has a measurable function, preferably if this gene product initiates a cascade of amplification involving events. Receptors fulfill this requirement and thus we have focused our attention on receptor cDNAs and on the use of receptor agonists to examine whether functional receptors are expressed. Receptor mediated changes of firing rate of the WSNupon agonist exposure and receptor mediated changes in core body temperature upon injection of the agonist in the POA both represent high amplification involving test of the functional expression of the gene product of receptor cDNAs.
The first reason being that understanding of the physiology of these cells requires the identification of the signals that they can recognize, and the receptors expressed suggest which these signals may be. We could have asked :are there receptors for the signal substances known to be expressed by the afferents to these neurons, but after 50 years of study of these cells we know precious little about the chemical neuroanatomy of the afferents of WSN in POA despite breakthrough work by Nakamura and Morrison (K. Nakamura and Morrison, 2008) (cf Figure 1A). Turning this situation around, using the larger number of receptors we identified in the WSNcDNA library one can, often follow, retrogradely, the afferent neuron, and thus build a network around our well described WSNbased on its receptor repertoire. Hence, one truly well studied neuron in an important circuit can accelerate the mapping and understanding of signals that it receives and thereby the description of the whole neuronal circuit.
Secondly, the modification of the firing rate of the warm sensitive neurons has important consequences on activation of Brown Adipose Tissue, metabolic rate, core body temperature and aging, and thus knowing the receptor repertoire enables pharmacological targeting of these neurons with the aim to regulate core body temperature, metabolic rate and aging. (A proof of the importance of these neurons in regulating these variables was achieved by generating a transgenic mouse strain in which the local temperature is elevated in the anterior hypothalamus, causing these neurons to fire more and to inhibit BAT and lower core body temperature chronically over the life span of the animal: the result is significantly increased life span (Conti, et al., 2006).
Thirdly, the functional validation of gene products encoded by the sequence verified cDNAs is easiest in the case when these cDNAs encode receptors for which selective agonists are available. In these cases, we followed the paradigm shown to us by the studies of IL-1R1expression and IL-1R1 mediated effects in vitro (firing rate) and in vivo (core body temperature) (Figure 3A-D), namely, the receptor agonist can be used to test warm sensitive neurons in a tissue slice from the anterior hypothalamus; in most cases a response in modifying the firing rate of warm sensitive neurons is obtained within a study of 15–20 warm sensitive neurons. In cases, when application of a saturating concentration of a receptor agonist, is showing no response, no change in the electrophysiological parameters, firing rate, membrane resistance, etc., it may mean that many more warm sensitive cells would have to be studied, because in the few cells tested the receptor is not expressed as it is expressed only in e.g., 20% of the cells and we simply have not had the luck to test a cell belonging to this subgroup of WSN, or that the receptor is expressed but its occupancy is not causing an obvious easily recorded electrophysiological effect; such as an acute change in firing rate. When antibodies are available to the receptor, as is the case for several tyrosine kinase typereceptors like the Insulin receptor, IGF-1 receptor and Toll receptorslike the IL-1R1, but not for most GPCRs, then one can attempt the immunohistochemical localization of the gene product in POA neurons, now utilizing the information that the WSN are cells that are GAD1 and neuropeptide (adrenomedullin, galanin, prodynorphin, somatostatin or tachykinin1 expressing). We have done so on warm sensitive neurons expressing the insulin receptor (Sanchez-Alavez, et al., 2010); in this case we have also shown that these GABAergic projection neuronsin the POA can be retrogradely labeled from the DMH, a known major projection area of warm sensitive neurons (Figure 1A).
The micro injection of the receptor agonist into the POA provides a very sensitive, highly amplified, core body temperature response, confirming that functional receptors of the kind investigated are indeed present in the POA, and probably are expressed by warm sensitive neurons, in which their cDNA was also identified. Of course the receptors that are activated by microinjection into the POA may also be present on non-warm sensitive neurons in the POA, even if their cDNA was also present in warm sensitive neurons. Nevertheless pharmacologically important information was obtained, using the transcriptome data, namely that these receptors are present in the POA and that they mediate a potent effect on core body temperature and metabolic rate; an information that is of physiological and pharmacological value.
The several hundred receptors expressed at the mRNA/cDNA level include different classes of receptors: GPCRs, Tyrosine kinase type single transmembrane domain receptors for cytokines and growth factors (Tables 4–6). The number of receptors for which we had access to selective agonists was limited as by definition we had no agonists for the orphan receptors/( Table 7) which represent a group of 27 GPCR type receptors of the 168 non olfactory GPCRs;
Table 4. Functional validation studies on receptor cDNAs.
Known and orphan GPCRs cDNAs in the transcriptome of warm sensitive neurons that were studied to validate expression of functional receptors in vitro and in vivo
| Receptor | Ligand | Validation |
|---|---|---|
| CRF2 | Urocortin 2 | Yes |
| AdmR | Adr | Yes |
| Adcyap1r1 | PACAP | Yes |
| mGLuR6 | Homo-AMPA | Yes |
| GPR 119 | PF04713082 | Yes |
| GPR83/npy3-36 § | NPY 3-36 | Yes |
| Gpr17/P2y6 | UDP | NS |
| CCR4 | CCL22 | Yes |
| CCR5 | RANTES | Yes |
| Gpr40 | GW9508 | Yes |
| SSTR2 | Sst14 | Yes |
| CB1 | CP-55940 | Yes |
| 5HT4 | Cisapride | Yes |
| Gpr17 | LTD4 | Yes |
| NPY2R | PYY3-36 | NS |
siRNA mediated knock-down in the POA shows that GPR83 is involved in control of BAT activity
Table 6.
Receptors with known roles in regulation of metabolism identified with high expression signals from microarray analysis, PCR and sequencing confirmed and the proof of functional receptors as determined by radiotelemetry subsequent to POA injection of the receptor agonist
| SYMBOL | DEFINITION | functional proof | 1 | |
|---|---|---|---|---|
| Adipor2 | adiponectin receptor 2 | yes | hyperthermia | |
| Igf1r | insulin-like growth factor I receptor | yes | hyperthermia | |
| Igfbp4 | insulin-like growth factor binding protein 4 | ND | ||
| Brs3 | bombesin-like receptor 3 | yes | hyperthermia | |
| Gpr83 | G protein-coupled receptor 83 | yes | hyperthermia | |
| Insr | insulin receptor | yes | hyperthermia | |
| Insrr | insulin receptor-related receptor | ND |
Table 7.
Orphan receptors whose cDNA is found in warm sensitive neurons (several transgenic strains with null mutation of these GPCRs have metabolic phenotype)
| Gpr88 | GPR88 | ||
|---|---|---|---|
| S | orphan | ||
| Gpr108 | GPR108 | B | orphan |
| LOC381628 | GPR113 | B | orphan |
| Gpr143 | GPR143 | B | orphan |
| Gpr158 | GPR158 | B | orphan |
| Gpr3 | GPR3 | B | orphan |
| LOC384618 | GPR32 | B | orphan |
| Gpr37l1 | GPR37 LIKE 1 | B | orphan |
| Gpr45 | GPR45 | B | orphan |
| LOC209510 | GPR58 | B | orphan |
| GPR64, GPCR | GPR64 | B | orphan |
| Gpr74 | GPR74 | B | orphan |
| Gpr85 | |||
| Gpr83 | GPR83 | B | orphan |
| LOC233227 | MrgA4 RF-amide G protein-coupled receptor (LOC233227),. | B | orphan |
| LOC243978 | MrgB1 G protein-coupled receptor (LOC243978),. | B | orphan |
| P2ry10 | purinergic receptor P2Y, G-protein coupled 10 (P2ry10),. | B | orphan |
| LOC328820 | similar to G protein-coupled hepta-helical receptor Ig-Hepta (LOC328820),. | B | orphan |
| Fpr-rs2 | formyl peptide receptor, related sequence 2 (Fpr- rs2),. | C | orphan |
| Gpr22 | GPR22 | C | orphan |
| Gpr33 | GPR33 (pseudogene in man) | C | orphan |
| LOC209512 | GPR57 | C | orphan |
| Gpr61 | GPR61 | C | orphan |
| Celsr3 | cadherin EGF LAG seven-pass G-type receptor 3 (Celsr3). | S | orphan |
| Gpr111 | GPR111 | S | orphan |
| LOC381361 | GPR144 | S | orphan |
| Gpr21 | GPR21 | S | orphan |
In other cases, although the endogenous ligand is known we had no access to selective agonists to a receptor. The functional validation of receptors with selective agonists was a large and labor intensive task limiting the number of receptors whose functional expression we could test in a small laboratory, but yet showing the viability of the experimental paradigm in which one can mine the data generated by single cell transcriptomics. The in vitro testing included finding warm sensitive neurons in POA tissue slices by studying the firing rate changes of a neuron in a POA slice when the bath temperature is varied between 33–41C (only every 6th to 20th neuron is warm sensitive) and then applying increasing concentrations of the agonist to these neurons while keeping the whole cell patch recording. In vivo experiments were similarly labor intensive starting with implantation of radiotelemetric devices weeks prior to the implantation of POA-cannulae that was after days of recording diurnal changes in core body temperature and motor activity, used to deliver 0.01–1 nanomoles of the agonist and continue to record core body temperature, activity, respiratory exchange ratio: We have tested 21 agonists to receptors of all of receptor classes (GPCR, Toll receptors, TK receptors) in vivo and 33 in vitro in electrophysiological experiments, respectively (thanks to sustained efforts by Iustin Tabarean and Manuel Sanchez-Alavez).
A very important conclusion of our studies on identification of mRNAs for the different receptor types is that, in almost each case, when an agonist for the receptor in question is available, we could find a functional cellular response to this agonist by testing it on ca 10–15 warm sensitive neurons. In other experiments we can validate functional expression of a receptor whose cDNA was identified by injecting the agonist in vivo into the POA, in which case, again, the majority of injected agonists produced a hyperthermic or hypothermic response of different durations (Tables 4, 5, 6, Figure 7). This is demonstrated by injecting agonists to receptors in all cell membrane receptor classes that were expressed at mRNA level in the warm sensitive neurons: a) the Toll receptor type receptor class, IL-1R1 (Figure 3), b) the GPCRs; mGluR6, CCR5, CCR4, CRF R type2, Adrenomedullin receptor (Table 4), c) to the cytokine and chemokine receptors ( which are GPCRs) (Table 5), d) to the tyrosine kinase receptor family members: insulin receptor and IGF-1 receptor, e.) to the adipokine receptor Adiponectin receptor 1, respectively (Table 6). The cellular effects of the hypo - and hyperthermic agonists have, without exception, shown that when a receptor mediates inhibition of the spontaneous firing of warm sensitive neurons in vitro in slice or in vivo, it mediates upon POA injection hyperthermia, and when the receptor mediates increased firing of WSN, then the POA injection causes hypothermia (Figure 7). The list of receptors whose functional expression was validated by the POA injection and by cellular electrophysiological studies suggests that in each receptor class, the mRNA identification at cDNA level by chipping and sequencing can be followed up by demonstration of a functional response:, because the receptor-mRNA is translated into a functional receptor. These findings, covering many examples of several receptor classes, give greater weight to the transcriptome data concerning the functional receptor repertoire of central neurons, because by extension, the data suggest that when the mRNA is found for a receptor there is a high likelihood that the functional gene product of a receptor will also be expressed, and thus a target for pharmacological interventions is present and can be utilized.
Table 5.
Cytokine, chemokine and neuroimmune signal receptors with a role in temperature Regulation identified with high expression signals from microarray analysis, confirmed by sequencing; Functional proof of their expression and role in regulation of CBT was obtained by injection of the receptor agonists into the POA
| SYMBOL | DEFINITION | Av | POA expression | CBT change |
|---|---|---|---|---|
| Il1r1 | interleukin 1 receptor, type I | 155 | yes | yes |
| Crhr1 | corticotropin releasing hormone receptor 1 | 120 | yes | yes |
| Crhr2 | corticotropin releasing hormone receptor 2 | 131 | yes | yes |
| CCR4 | chemokine (C-C motif) receptor 4 | 97 | yes | yes |
| CCR5 | chemokine (C-C motif) receptor 5 | yes |
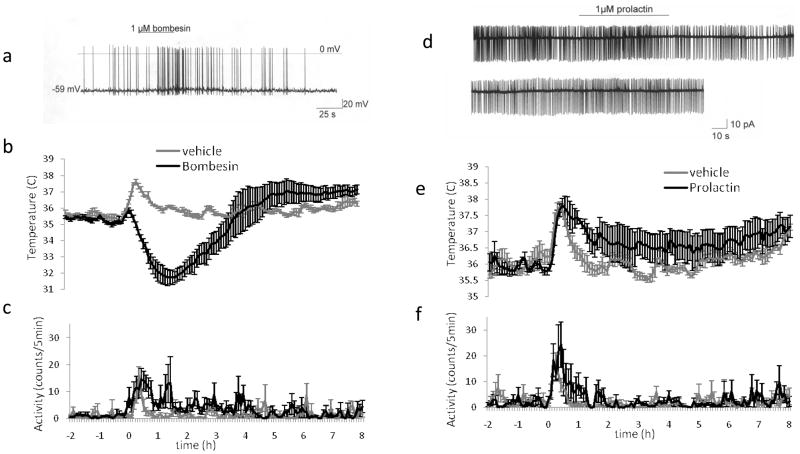
Figure 7. Functional validation in vitro and in vivo of the receptor transcripts identified in single warm sensitive neuron cDNA libraries: Hypothermic and hyperthermic effects can be exerted through receptors of warm sensitive neurons identified by single cell transcriptomics and validated by use of the recptor agonist injected into the POA and tested on the firing rate of WSN in POA slice.
Bombesin acting at BSR3 receptor activates firing of the warm sensitive neurons and causes hypothermia upon POA injection, while Prolactin inhibits firing of warm sensitive neurons and upon injection into the POA causes hyperthermia, respectively.
Validating orphan receptor functional expression in WSN
A large portion of receptor mRNAs found in the warm sensitive neurons encode orphan receptors that, by definition, today have no known endogenous ligand-agonist (Table 7). Thus, the straight forward pharmacological experiment in which an agonist to the orphan receptor whose mRNA was identified is applied onto a WSNin a tissue slice or injected into the POA to assess effects on firing and on core body temperature are not possible. In this situation it is of great importance that we have sufficient confidence in the assumption that orphan receptor-mRNA presence results in functional receptors, because the assessment of the involvement of the orphan GPCR, lacking pharmacological tools, is far more time and effort intensive than validation of the functional expression of receptors with known agonists.
We have proven for several orphan GPCRs that their mRNA/cDNA in warm sensitive neurons presence likely results in expression of functional receptors that have a sufficiently robust effect on warm sensitive neurons such that null mutation of this receptor affects the core body temperature. This was accomplished by testing core body temperature, thermogenesis, and metabolic parameters of transgenic animals with null mutation of the orphan GPCR in the WSN. The null mutation of a receptor, of course, is a blunt instrument; causing deletion of the receptor in all cell types in all anatomical sites, not just in the warm sensitive neurons in the POA, yet the finding of the cDNA in WSN cDNA library has directed attention towards possible involvement of the given orphan receptor in hypothalamically mediated metabolic regulation. The best example is the work of Dubbins et al, 2010 and in preparation showing the GPR83(Sah, et al., 2005) whose cDNA was found in WSN has an altered diurnal core body temperature profile and is resistant to high fat diet induced weight gain and glycemic changes. The knock down of GPR83with siRNA injected into the POA partly recapitulates the phenotype of the null mutation carrying mice.
A set of experiments, like this on GPR83, can render an orphan receptor from a relatively undefined, putative pharmacological target into a well-defined, highly interesting pharmacological target. In the case of this particular orphan receptor, the data now available localizes the receptor in an interesting neuronal population of warm sensitive neurons that not only controls core body temperature, and thus affects metabolic rate and body weight, but also shows that an antagonist (or an inverse agonist, if the receptor has constitutive activity) that acts centrally in the POA would be a desirable drug, because a local knock down of the receptor expression provides a sufficiently robust effect of desired kind on metabolic rate. Thus, the outlined paradigm starting with the transcriptome of a single neuron, and identification of the mRNA for an orphan receptor, can lead to validation of this receptor at this cell type as a drug target.
The mapping of receptor repertoires of important neuronal types is a prerequisite for successful pharmacological intervention, yet for most important neuronal classes no more than a dozen or more receptors are known to be functionally expressed by these neurons, it is hoped that the present example encourages and informs on the use of single cell transcriptomics.
There are many reasons for the receptor repertoire of central neurons being very poorly mapped. One of the reasons is that a large portion of the receptors expressed are GPCRs, and antibodies to these seven transmembrane proteins have been difficult to raise, preventing immunohistochemical localization of GPCRs. The IHC results achieved by using peptide antigens, whose sequences are derived from the GPCRs, have added hundreds of artifacts to the neurobiological literature as reviewed recently (Lu and Bartfai, 2009; Michel, et al., 2009).
The receptor numbers per cell are also low, often well below 300 copies per cell, and it is not always easy to identify in a given neuron the few dozens of tyrosine kinase receptors and cytokine receptors, even when good antibodies are available. Our experience with IGF1-receptor and insulin receptor expression in warm sensitive neurons illustrates these difficulties: Both receptor tyrosine kinase cDNAs have been found by chipping and verified by sequencing and also by PCR in the same individual warm sensitive cells (Sanchez-Alavez, et al., 2010) (Osborn et al. in preparation). That there are functional insulin receptors in the POA has been proven by electrophysiological studies in the case of the insulin receptor that mediates a strong inhibition of the firing of some spontaneously active warm sensitive neurons (Sanchez-Alavez, et al., 2010). The IGF1-R mediated effects on the firing rate of warm sensitive neurons were not obvious in the 20 WSNs studied. Yet, when insulin or IGF1 are micro injected in the POA, both cause robust hyperthermia through activation of adaptive thermogenesis in the BAT (Osborn et al. in preparation), suggesting that the both of the receptor cDNAs give rise to functional signaling receptors. The IGF-1 receptor being responsible for mediating some or all of the effects of IGF-1 was demonstrated by studies on IGF-1 mediated hyperthermia in the transgenic NIRKO mice(Bruning et al,2000) that lack neuronal expression of insulin receptors;
The IGF1 mediated hyperthermia is significantly more robust in the wild type mice expressing both the insulin receptor and IGF1receptorthan in the NIRO mice, suggesting that in the wild type mice, the two receptor types are not only present in the same cell but that they can form heterodimers (Osborn et al. in preparation)as suggested earlier by transfecting both receptor cDNAs or form analyzing in pooled cells by PCR the mRNAs for insulin receptor and IGF1-receptor..
Again, it needs to be emphasized that formation of heterodimers of receptors is strongly suggested by the analysis of the transcriptome because we have analyzed single cells, in transcriptome constructed from pools of cells we had not been served molecular evidence that the gene products coexists, and thus have the possibility to form a heterodimer.
Although we have produced chipping data, PCR-, sequencing data, and functional evidence for the expression of both the insulin receptor and IGF1R, in some cases in the very same warm sensitive neuron (permitting formation of hybrid (heterodimeric) IGF1R-IR receptor dimers, which from a pharmacological point of view are very important as they differ in their signal transduction from the two homodimers), we have not yet been able to demonstrate the IGF1R by IHC, while we have been able to show insulin receptor like immunoreactivity, by staining in the POA and also in neurons that were identified by retrograde tracing from the DMH (Sanchez-Alavez, et al., 2010). This is despite findings from NextGen sequencing, that the sequence call density, that is proportional to the mRNA, cDNA copy number, is higher for the IGF1R than it is for the IR. Clearly, many more cells would need to be analyzed by sequencing to be able to make solid conclusions about frequency of insulin and IGF1-receptor expression among all warm sensitive neurons.
The important conclusions from these experiments are that the often poor quality of the antibodies to receptors (particularly to GPCRs), the low copy numbers of the receptors per cell may make it difficult to demonstrate receptor proteins by IHC. Receptors not demonstrated by IHC, that nevertheless when activated by agonists in electrophysiological or in vivo experiments, mediate potent pharmacological effects such as altered firing rate and/or large hypo- or hyperthermia. Clearly, not knowing the expression of these receptors on key neuronal cell types in key brain areas deprives us from a host of possible pharmacological interventions; thus, single cell transcriptome analysis has been proven in this context useful.
The ISH atlases such as the Allen Brain Atlas (www.brain-map.org), for example, despite all their worthy information, sometimes do not have the sensitivity to inform on which cell types express the mRNA in question. In the above case of insulin receptor and IGF1receptor, the Allen Brain Atlas did not pick up IR or IGF1R in the anterior hypothalamus. An important conclusion from these data on “single cell transcriptome-in vivo and in vitro functional data” on receptor repertoire of the warm sensitive neurons is that the anatomical atlases at their present resolution would have not enabled anyone to establish that as in the example above: the IGF1R and insulin receptor can be and are expressed in the same neuron in many cases.
Considering the abundance of GPCRs in the transcriptome one must be aware of the possibility of formation of heterodimeric GPCRs ( cf for review Cascado et al 2007) and that the paradigm described can focus our attention on these receptor dimers that are being reported as interesting pharmacological targets in opiate signaling, etc. In this respect, the preparation and sequencing of cDNA library of single neurons and the analysis of single cell transcriptome is our most sensitive method today.
The focus in experiments on validation of translation of an mRNA on receptor cDNAs of all classes of cDNAs identified by chipping and sequencing (Figure 8) reflects our bias as neuropharmacologists and in no way suggests that physiologically this is the most interesting or important class of cDNAs. The large number of different receptor cDNAs( Tables 4–7) found is still surprising and we need to carry out similar single cell transcriptomics studies on several other neuronal types in different brain areas to be able to decide whether the warm sensitive neurons are typical with respect to the hundreds of receptors they express or that this richness of receptor repertoire is exceptional. Even if this would be the case, this study strongly suggests that we operate with extreme underestimates of how many receptors a central neuron express when we think in terms of dozens of diverse receptors per neuron only. This underestimation limits our ideas and efforts of pharmacological interventions. The use of Chipping –microarray data expands widely the number of different receptor cDNAs that are identified in a single cell transcriptome beyond what PCR reactions indicate, yet because the oligonucleotides displayed on the microarray and because the PCR primers often designed to cover some exon, chipping and PCR can miss the presence of a receptor cDNA that is recognized by an intron sequence or by a 3′UTR sequence. This was the case with the prolactin receptor cDNA for which we had identified a 3′UTR sequence but had no microarray or PCR data as those probes were not covering this region of the mRNA. Yet when functional studies in POA slices and in vivo by POA microinjections were carried out we couls verify functional expression of prolactin receptor in WSN and that the occupancy of this receptor robustly affects core body temperature ( Sanchez-Alavez et al in preparation). This instance suggests that the microarray-PCR data underestimates the number of cDNAs present in a single cell transcriptome and that even more receptors and other proteins are expressed from cDNAs tat we did not hybridize to microarray.
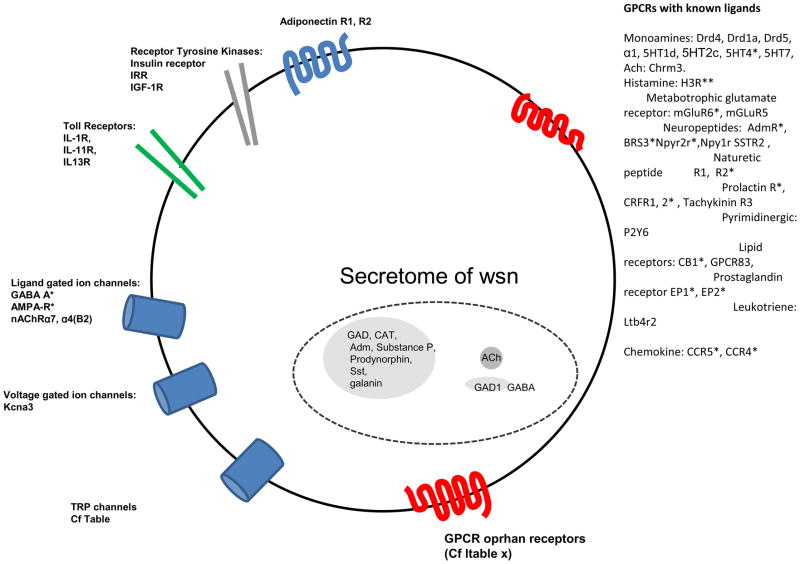
Figure 8. Receptor repertoire and secretome of warm sensitive neurons in POA identified by microarray and confirmed by NextGen sequencing of individual neuron cDNA libraries.
The mRNA was harvested from the soma, and it provides no information about where in the cell, dendrite, axon terminal, soma the translated proteins will be functional in these GABAergic peptidergic projection neurons. The figure depicts only such signal substances and receptors whose cDNA have been found by both microarray and confirmed by sequencing and in many cases by functional tests using recptor agonists.
5. Signaling asymmetry
The hypothalamic WSN play a key role in body homeostasis and regulation of Claude Bernard “milieu intérieur” and thus it is expected that these neurons are responsive to metabolic and endocrine signals. Indeed we have identified receptor cDNA and functionally validated it for receptors recognizing insulin, IGF1, adiponectin among other metabolic signals. We have found the prolactin receptor cDNA as an example of an endocrine receptor enabling the WSN to sense the hormonal signals as well ( cf above). The number of inflammatory mediators cytokines and chemokines that reach the POA is also high, both because the area is close to blood brain barrier and also because for several of these mediators, like for insulin there are dedicated transporters in the blood brain barrier. The number of metabolic, endocrine and inflammatory signals present and the receptors to sense them expressed by the WSN only beginning to be known.
The number of neurotransmitters and neuropeptides expressed and the number of receptors expressed for these neuronal signal substances is better known after the reviewed studies :The data so far in this review show that the warm sensitive neurons of the POA are GABAergic-peptidergic neurons, with 2–3 neuropeptides coexisting with GABA (Figure 8). The survey of the receptor cDNAs shows over 250 neurotransmitter receptor and ligand gated ion channel cDNAs present in each neuron, and we assume that most of these are expressed as functional receptors. Even when considering that fourteen serotonin receptor subtypes are known, (and twenty glutamate receptors) and five NPY receptor subtypes are known, and three galanin receptor subtypes are known, etc., we must assume that the 250 different neuronal signal receptor cDNAs encode receptors capable of recognizing and being activated by at least 40 or probably more different signal substances. This suggests that if all of the different receptors identified in warm sensitive neurons, or a large portion of the different receptors identified, are indeed occupied at one or another time by their cognate agonists, then there exists a large chemical-informational asymmetry in terms of neuronal signaling, involving warm sensitive neurons:
6 different chemical signals (GABA plus 5 neuropeptides, (Figure 8, Table 1) synthesized by the warm sensitive neurons to communicate with other neurons and non-neuronal cell types, and at the same time the same neuron is capable of responding to 40 or more chemically different signals that it can recognize selectively at its different dendrites and other postsynaptic sites that number tensof thousands ; if the spatial and temporal characteristics of the 40 signals permit it, the WSNcan integrate all of these signals, in terms of its receptor repertoire ( and downstream pathways). To these 40 or more synaptic chemical signal substances from which we have identified cell membrane bound receptors, one must add another uniquely important signal whose receptor has escaped identification so far - the key descriptor of these neurons - that these are warm sensitive neurons that react in a special manner to changes in local temperature; a physical signal: heat.
The presence of this signaling-informational asymmetry suggests that the warm sensitive neurons are capable of strongly integrative responses..
It would be important to delineate the receptor-signal substance asymmetries of important neurons in other brain areas to understand the informational multidimensionality of the brain, the parallel communications taking place and to know whether the warm sensitive neurons in the anterior hypothalamus are typical or atypical in terms of this asymmetry.
6. Heterogeneity of warm sensitive neurons: Epigenesis of warm sensitive neurons
An intriguing aspect of the analysis of hypothalamic single cell transcriptomes is the large and significant variability in gene expression profiles, as shown by heat map analysis, between these functionally similar, i.e., spontaneously firing GABAergic projection neurons that show warm sensitivity (cf Heat maps of the transcriptomes of WSNs Figure 4A). Each of the analyzed cells was localized within the preoptic area of the anterior hypothalamus and showed the characteristic warm sensitivity in electrophysiological experiments, as described earlier. The overlap in gene expression between these 8 cells (by microarray analysis) is approximately 40% and as expected this numbers grows as the number of cells compared decreases: at 7 cells the overlap becomes 43% while 6 cells show 49% overlap. While the quantification as provided here is not often presented in the literature, the variability in gene expression between these similarly warm sensitive neurons is to be expected, given the growing literature (Kim and Eberwine, ; Levin, et al., 2009; Sul, et al., 2009). One reason why past molecular analysis of tissue samples has failed to demonstrate this type of extensive cellular heterogeneity among ‘identical neurons ‘ is that a tissue sample analyzed was a collection of cells where unique cell expression profiles would be homogenized/ diluted with the other cells in the tissue sample. This is particularly apparent when assessing the transcriptome by NextGen sequencing where the range of transcript identification is much greater than exhibited by microarray analysis ( cf Supplemental Material for discussion on the technology and resolution of microarray versus NextGene sequencing and about discrepancies between microarray and sequencing data ). Further, there has been previous qualitative evidence suggesting this cell to cell variability as both immunocytochemistry and in situ hybridization studies have demonstrated cell to cell variability within small groups of similar cells in both their mRNA and protein signals. While there are several factors that could contribute to this variability, in retrospect, it seems clear that physiologically driven, individual expression profile variability, is one of the important (if not the most important) contributing factors to our observation.
An interesting aspect of cellular heterogeneity within the ‘identical warm sensitive neurons ‘ is apparent when examining the expression profiles of olfactory receptors, -independent of that we do not know if these receptors are occupied by ligands at this location ever or if they serve as molecular markers specifying spatial architecture, they are excellent examples of cellular heterogeneity: NextGene sequencing shows of two trasncriptomes of WSNs that 67 olfactory receptors mRNAs expressed between these two cells (Figure 6). One cell has 31 different olfactory receptor RNAs ranging in expression from 1 read count to 547 read counts and the other cell has 36 different olf-receptors ranging in expression from 1 to 71 read counts. Approximately 60% of these olf-receptors that are present have more than one read count in the cell in which they are expressed. Interestingly, while there are many of these receptors expressed in these two cells there is NO overlap of the expressed mRNAs: thus there is no olf-receptor shared between these two warm sensitive cells as the partition is distinct. In preliminary data this also is true for other neurons of the POA of the hypothalamus, suggesting that these olf-receptors perform a function that is unique to each cell. Others have previously speculated that the olfactory receptors could provide a spatial code during development so that cells find their way to their appropriate position (Dreyer, 1998; Feinstein, et al., 2004; Yoon, et al., 2005). It is intriguing to speculate that given the cellular heterogeneity of the hypothalamus as well as the precise synaptic wiring that a spatial, cell specific code is generated by expression of individual olf - receptor mRNAs (or perhaps by the composite of their expression within a cell). Such a model for cellular positioning has significant implications for understanding the building of networks and regulating hypothalamic function.
The warm sensitive neurons represent a very specialized small set of GABAergic–peptidergic projection neurons in the POA of the anterior hypothalamus. They have a robust effect on the core body temperature; pyrogens act by reducing their firing rate, as well as skin thermosensors mediating cold signals and metabolic signals like insulin, adiponectin are acting at these neurons to cause a potent reduction in firing rate and through these actions a robust hyperthermia. Glutamate and bombesin are acting at multiple receptor types on warm sensitive (GABAergic, inhibitory) neurons to increase their firing rate and thus to inhibit adaptive thermogenesis in the Brown Adipose Tissue (BAT). The stability of core body temperature, and its variations; diurnally and by pyrogens and feeding induced signals, show a dynamic regulation as judged from the in vivo output of these neurons.
The source of the cellular transcriptome variability, the epigenesis of the individual neurons expression profile, however is the most important and interesting topic of study if we want to understand the ability of central neurons and their networks to carry out complex tasks. The study of the epigenesis of neuronal phenotype is well served by the analysis of single cell transcriptomes as described here as it has the broadness to identify thousands of different transcripts and their splice variants and it has the ability through the number of read counts to inform on the abundance of the given mRNA.
In the literature discussing transcriptomes, the heterogeneity of similar cells seems to be the most common finding (Sul, et al., 2009). The heterogeneity observed of these adult neurons studied in a network partly may arise from their relative position in this neuronal network that has not been systematically studied here. In this sense the identified neurons of organism like C. elegans may have advantages being identifiable from organism to organism, but even there the study of the, for thermotaxis important, WSN transcriptomes showed large heterogeneity (Inada, et al., 2006). There are in fact “gene networks” that are linked at the level of transcription factors that account for morphogenic gradients formed (cf Kerszberg and Changeux 1994). The analysis of the transcriptome data here undoubtedly will reveal one as of the driving forces of heterogeneity the differential u-activation of transcription factors in the individual WSNs.
Many of the genes that give rise to receptor, ion channel and neuromodulator encoding mRNAs can be alternatively spliced to produce mRNA heterogeneity (Bell, et al., 2008; Benovic, et al., 1989; Yue, et al., 2008). The sequence differences between these splice variants can give rise to differentially functional peptides, channels and receptors. It is difficult to assess splice variants for mRNAs using traditional microarray analysis yet RNAseq promises to provide an unbiased approach for this analysis. From a pharmacological point of view it would be important to systematically study which receptors are expressed in all and which in only a subset of the warm sensitive neurons, but one must bear in mind that frequency of expression and copy number per cell in absence of functional data do not predict the functional importance of a receptor in these neurons. The example of the insulin receptor, discussed above, showed this; insulin receptor mRNA was expressed only in 2 of 8 of the studied cells, but when insulin is applied onto warm sensitive neurons it has robust inhibitory effect on their firing rate and when insulin is injected into POA, in vivo, it mediates a potent hyperthermic effect (Sanchez-Alavez, et al., 2010).
7. Chemical neuroanatomy beyond ICH and ISH; transcriptome as broad, high sensitivity complement
The 1958–1990 period brought a strong expansion of our understanding of the chemical signaling in the central nervous system with the mapping of monoaminergic circuits (Carlsson, et al., 1962; Dahlstrom and Fuxe, 1964; Falck and Torp, 1962; Hokfelt, 2010) and by extensive use of the immunohistochemical method of Coons (1958) and by the addition of ISH based identification of one, two and in some cases even three signal substances, coexisting in the same neuron (cf (Hokfelt, et al., 1987), with particular reference to the hypothalamic neurons (Hokfelt, et al., 1987). The concept of coexistence of classical neurotransmitters and of neuropeptides in the same neuron has been proven in invertebrate and vertebrate neurons (Schultzberg, et al., 1982). Additionally, the “frequency dependent differential release” of the low molecular weight classical neurotransmitter and of the coexisting peptide(s) has been studied in detail in peripheral nerves (cf (Bartfai, et al., 1988) and in a few cases even in CNS neurons, like the ACh–galanin expressing septal neurons (Consolo, et al., 1994), showing that at low frequency of firing the classical neurotransmitter was released and that at high frequencies of firing and or at bursting type firing the neuropeptide(s) were also released (cf (Lundberg, et al., 1981).
The limitation of simultaneous ISH and IHC for more than 2–3 mRNAs, and/or gene products in the same staining procedure has set a limit to 6 (Kosaka et al,1985) as to how many coexisting signal substances we can simultaneously detect with these Morphological Techniques: which form the methodological backbone of Chemical Neuroanatomy. We believe that the studies reviewed here on the transcriptomes of single warm sensitive neurons show that the sensitivity of this method coupled by functional read outs to validate that the transcript gives rise to a functional translated gene product (when possible), significantly complements the IHC-ISH provided description of individual neurons We have come to accept that a neuron can release several coexisting signal substances at its synaptic terminals and from its dendrites (cf (Bartfai, et al., 1988; Schultzberg, et al., 1982) while we have devoted too little study and discussion to the number and chemical diversity of signals that are received and can be recognized and responded to by a central neuron present in a network.
The surprisingly large number of different receptors which are expressed by and functional in neurons has not been determined in any systematic manner for central or peripheral neurons. When a receptor repertoire was determined, it was always guided by prior knowledge of specific innervations of the given neuron or given brain region that would present a specific signal i.e., noradrenaline, leading to a focused study of the presence of adrenoreceptors and of the subtypes of adrenoreceptors. If the presynaptic element was shown to express not only the noradrenaline-adrenaline biosynthetic enzyme Tyrosine hydroxylase, but also a coexisting peptide, often Neuropeptide Y, then the focused study added the five NPY receptor subtypes to be searched for, to understand or to predict the effects on the, by the noradrenaline-NPY neuron, innervated neuron, but none of these approaches would call for asking whether leptin, CCR4 or adiponectin receptors are expressed by the same postsynaptic neuron: Thus, using classical morphology -many interesting receptors - present on the postsynaptic neuron are neveridentified and examined. It is possible, and almost certain, that major physiological and pharmacological influences presented by signals for which receptors are expressed but not identified, have been missed. An unbiased method like single cell transcriptome, in which no prior knowledge of the innervations of the area in which the neuron is localized is a good complement to mapping which signals to which a neuron is likely to respond. Hence the receptor repertoire provides a fundamental basis for designing pharmacological interventions that modulate the activity of the neuron in question.
The receptor-mediated activation of intracellular processes that leave morphological and biochemical traces, like cFos induction or phosphorylation, are widely used to study changes in the activity of neurons upon binding of agonists to their receptors, expanding the range of methods to detect functional receptor expression on individual neurons. Nevertheless it is clear that today, on single neurons,; all receptors expressed are not detected by most methods routinely used in 2010 albeit knowing the full receptor repertoire would be highly desirable.
The transcriptional analysis of biological samples is today, in 2010, more sensitive than the proteomic analysis. ISH and single cell aRNA and PCR have long been utilized to detect mRNAs in individual cells and the sensitivity of these methods has been a great asset in working on the molecular composition and its changes of neurons as a result of neuronal activity. The caveat was and remains, that there is no clear understanding of which mRNAs, and under what conditions, are translated and thus how the gene product expression pattern detected by IHC and/or by functional studies correlates with the transcriptome of a cell.
In addition, we have to emphasize that RNA distribution in the neurons that are highly asymmetrical cells, is not even. Specific RNA binding proteins support the selective distribution of RNAs into different subcellular compartments ; dendrites and axon terminals, thus the RNA population analyzed from the soma of a neuron as in our case, presents a somewhat biased population of RNAs, and the frequency of RNAs in subcellular compartments is different( Myashiro et al, 2009). Nevertheless using a transcriptome analysis based on the cDNA library generated from the RNAs in the soma has been broadening our knowledge of the cellular components of a neuron dramatically compared to classical neuroanatomy. Our study of this small number of CNS neurons suggests that determining the cDNA libraries of single neurons, now that this becomes technically feasible and financial affordable, lays a foundation for functional studies that are unbiased, broader and deeper than the use of IHC or ISH of the tissue slice. In this sense, use of single cell transcriptomics based on microarray and sequencing, represents an extension of chemical neuroanatomy, in describing more of the chemical and molecular components of the neuron.
8. Future descriptors of individual neurons and the dynamics of chemical neurotransmission; Pharmacological consequences of these insights
One of the main conclusions that we may draw from these studies on transcriptomes of single warm sensitive neurons reviewed here, is that the depth and breadth of information about an individual adult cell in a network that can be gained by electrophysiologically identifying the cell and then preparing cDNA library from its soma for chipping and for sequencing, is worth the effort it takes. The use of this single cell transcriptomics utilizing both microarray and sequencing or similar paradigms will lead to a richer physiological and pharmacological view of neurons and to a broader and deeper pharmacological intervention potential.
After 50 years of study there were no molecular markers of the warm sensitive neurons, rather they were identified by a unique electrophysiologically determined phenotype. In the reviewed data we were led not by an experimental design but by the data produced and were to examine effects of signal substances (for which receptors were found by chipping and sequencing) that we would not have any reason to test on warm sensitive neurons of the POA. This is exemplified by our testing of chemokine CCL22, which we found to be a potent hyperthermic agent, or to study adiponectin or insulin, both shown to be potent hyperthermic agents following the lead provided by the transcriptome data on their receptors being expressed in warm sensitive neurons. This data-led search has significantly broadened our views on the pharmacology of warm sensitive cells and led in unexpected directions; like studies on hypothalamic insulin effects in thermoregulation. The sensitivity afforded by the molecular biological methods employed in studying the transcriptome of a single neuron is such that it has permitted us to identify cDNAs that are expressed in only a few of the studied neurons and would be diluted when a cDNA library is prepared from a large multitude of cells to avoid the need for amplifications. In the pooled cDNA many of the most interesting cDNAs are expressed in only a few of the studied cells and would escape detection. This was the case with the insulin receptor and adiponectin receptor 1, yet apparently the effects these few receptors on the few warm sensitive neurons is robust, when examined in vivo upon injecting the agonists into the POA where warm sensitive neurons are located (Sanchez-Alavez, et al., 2010).
Equally important to note is that we found the co-expression of several receptors of GPCR-class and tyrosine kinase receptor class that can form homodimers or heterodimers in the same neuron. Once the mRNAs were identified that encode GPCRs that are suspected to form heterodimers or the Tyrosine kinase receptors that can form homo and heterodimers, in the cDNA library from the same cell, there was an impetus to examine, whether indeed such heterodimers are formed by GPCRsor by tyrosine kinase type receptors expressed in the same cell. Prompted by the single cell transcriptomics data to examine whether the insulin receptor and the IGF1receptor that are expressed in the same cell, do form signaling heterodimers, we could by pharmacological tools and the neuronal insulin receptor knockout (NIRKO) mouse strain, come to the conclusion that IGF-1 mediated effects in the POA require insulin receptor presence and thus it is the insulin receptor–IGF1R heterodimer that mediates insulin and IGF1 effects on warm sensitive neurons (Osborn et al. in preparation).
The discovery of orphan receptor mRNAs in warm sensitive neurons has provided us with a long list of experiments for the time when these receptors are deorphanised and agonists became available for electrophysiological experiments on warm sensitive neurons. The presence of these orphan receptors has focused us on examining phenotypes and endotypes (that we otherwise would not have studied) in the transgenic strains with null mutation of these orphan receptors. For example, the null mutation carrying transgenics of three orphan-GPCRs(GPR21,83,85) found in the transcriptome of the warm sensitive neurons appear to have robust phenotypes not discovered when standard, immunologically focused ( and not metabolically focused ) phenotyping is carried out. The phenotype of thee orphan GPCR knock outs corresponding to some of the GPCR receptors present on three warm sensitive neuron demonstrate that they play key roles in regulating core body temperature and metabolic rate through this central neuron. The results clearly point to the orphan GPCR ; GPR83 playing a role in glucose metabolism(Dubbins et al, 2010). Thus focusing on just a few GPCRs, from the several dozen orphan receptors found in single warm sensitive neurons assisted us in defining these receptors as putative drug targets before their endogenous ligand has been identified and studied.
The recognition that we in most cases know only a few percent of the receptors expressed on important neuronal cell types is important for pharmacology and more so for future pharmacological studies. To provide a functionally complete the dynamic description of neurons, in molecular and cellular terms, requires study of the occupancy of its receptors, not only the receptor expression. This occupancy, for some receptors may be almost continuous, tonic and for others intermittent or a very rare event. These data will provide a dynamic understanding of the regulation of the neuronal activity of a network.
From a pharmacological point of view the presence of a receptor (occupied by endogenous ligand or not), presents an important target for the exogenous ligand/drug. The discovery of neuropeptide preprohormone mRNAs in the warm sensitive neurons suggests new experiments to determine if their synthesis and release occurs and under what conditions. The knowledge of some putative neuropeptide transmitters of POA neurons focuses us also on the identification of receptors for these signal substances in the dendritic and axonal projection areas of these neurons,( Cf Table 2) similarly to the logic of earlier chemical neuroanatomical identification of signal substances in a neuron. Thus the single neuron transcriptome is, in every sense, a useful complement to classical chemical neuroanatomy, expanding its scope in multiple ways: revealing more molecular components (thousands instead of a few), the complexity of these components (alternative splicing, editing, etc.), and providing information on absolute and relative abundances. As the fundamental unit of life is the single cell, elucidation of the rules for establishing and maintaining functional polarity of single cells, in context of their interacting environment, promises to provide unique and important information that underlies the organizational structure of biological systems.
The transcriptome results, with functional validation of some of the expressed receptors, indicate that a chemical snapshot of the neuronal circuit( Figure 1A) that includes the warm sensitive neurons will require a simultaneous and similar transcriptomics analysis of the afferent neurons to, and post synaptic neuronal targets of, the warm sensitive neurons. When we are able to analyze neuronal circuits in this molecular detail, including all receptors and all signal substances of each of the neurons of the circuit at play (i.e., being occupied by endogenous ligands, at a given time point), we will be able to provide a deeper and more dynamic chemical-molecular description of neuronal activity in context of its circuitry. Being able to assign receptor-agonist pairs at given synapses of a chosen neuron, will enable a dynamic chemical description of neuronal activity and organization and thus guide pharmacological intervention to be more precise with respect to target, time and degree of effect.
Supplementary Material
Acknowledgments
These studies involved many coworkers of ours over the past 5 years at Scripps (Iustin Tabarean, Manuel Sanchez-Alavez, Bruno Conti), University of Pennsylvania (Jeanine Jochems, Peter Buckley, Miler Lee and Junhyong Kim), Pfizer, and were supported from the Harold L. Dorris Neurological Research Institute, Skaggs Institute of Chemical Biology and NIH to TB and from NIH, NARSAD, and The Commonwealth of Pennsylvania to JE, respectively. Many colleagues have discussed these studies in depth with us; particular thanks are due to Jean-Pierre Changeux, Tomas Hökfelt, Gerald M. Edelman, Floyd Bloom, Paul Greengard and Lars Terenius.
List of Abbreviations
- BAT
Brown adipose tissue
- DRG
Dorsal root ganglion
- POA
Preoptic area
- rRPA
Rostral Raphe Pallidus
- DMH
Dorsomedial hypothalamus
- WSN
Warm sensitive neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheim K, Bartfai T. The interleukin-1 system: receptors, ligands, and ICE in the brain and their involvement in the fever response. Ann N Y Acad Sci. 1998;840:51–8. doi: 10.1111/j.1749-6632.1998.tb09548.x. [DOI] [PubMed] [Google Scholar]
- Baldino F, Jr, Geller HM. Electrophysiological analysis of neuronal thermosensitivity in rat preoptic and hypothalamic tissue cultures. J Physiol. 1982;327:173–84. doi: 10.1113/jphysiol.1982.sp014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JL, Carpenter DO. Thermosensitivity of neurons in the sensorimotor cortex of the cat. Science. 1970;169(945):597–8. doi: 10.1126/science.169.3945.597. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Conti B. Fever. ScientificWorldJournal. 2010;10:490–503. doi: 10.1100/tsw.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Miyashiro KY, Sul JY, McCullough R, Buckley PT, Jochems J, et al. Cytoplasmic BK(Ca) channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105(6):1901–6. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, DeBlasi A, Stone WC, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989;246(4927):235–40. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Hokfelt T. Methods in Chemical Neuroanatomy (Handbook of Chemical Neuroanatomy) (Vol. Hardcover) Amsterdam: Elsevier; 1983. Aug, [Google Scholar]
- Bjorklund A, Hokfelt T, Owman C. Handbook of Chemical Neuroanatomy. Elsevier; 1988. [Google Scholar]
- Bligh J. Temperature Regulation in Mammals and other Vertebrates. Amsterdam: North-Holland Publishing Company; 1973. [Google Scholar]
- Boulant JA. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981;40(14):2843–50. [PubMed] [Google Scholar]
- Boulant JA. Cellular mechanisms of temperature sensitivity in hypothalamic neurons. Prog Brain Res. 1998;115:3–8. doi: 10.1016/s0079-6123(08)62026-9. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–61. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol. 2006;100(4):1347–54. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Brady G. Expression profiling of single mammalian cells--small is beautiful. Yeast. 2000;17(3):211–7. doi: 10.1002/1097-0061(20000930)17:3<211::AID-YEA26>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–5. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand Suppl. 1962;56(196):1–28. [PubMed] [Google Scholar]
- Carpenter DO. Temperature effects on pacemaker generation, membrane potential, and critical firing threshold in Aplysia neurons. J Gen Physiol. 1967;50(6):1469–84. doi: 10.1085/jgp.50.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. Cellular transcriptomics -- the next phase of endocrine expression profiling. Trends Endocrinol Metab. 2006;17(5):192–8. doi: 10.1016/j.tem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Casadó FV, Cortés A, Ferrada C, Mallol J, Woods A, Lluis C, Canela EI, Ferré S. Basic concepts in G- protein-coupled receptor homo- and heterodimerization. ScientificWorldJournal. 2007;7:48–57. doi: 10.1100/tsw.2007.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Ladha Z, Smith SJ, Farese RV., Jr Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol Endocrinol Metab. 2003;284(1):E213–8. doi: 10.1152/ajpendo.00248.2002. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512(Pt 3):883–92. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Magoun HW, Ranson SW. Hypothalamic regulation of body temperature. Journal of Neurophysiology. 1939;2(1):61–80. [Google Scholar]
- Coceani F, Akarsu ES. Prostaglandin E2 in the pathogenesis of fever. An update. Ann N Y Acad Sci. 1998;856:76–82. doi: 10.1111/j.1749-6632.1998.tb08315.x. [DOI] [PubMed] [Google Scholar]
- Consolo S, Baldi G, Russi G, Civenni G, Bartfai T, Vezzani A. Impulse flow dependency of galanin release in vivo in the rat ventral hippocampus. Proc Natl Acad Sci U S A. 1994;91(17):8047–51. doi: 10.1073/pnas.91.17.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314(5800):825–8. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Coons AH. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20(7):398–9. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98(5):1379–89. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- Dean JB, Boulant JA. In vitro localization of thermosensitive neurons in the rat diencephalon. Am J Physiol. 1989;257(1 Pt 2):R57–64. doi: 10.1152/ajpregu.1989.257.1.R57. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179(Suppl 2):S294–304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10(4):201–22. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Bunn PA., Jr Fever. Semin Oncol. 1997;24(3):288–98. [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM. Molecular basis of fever in humans. Am J Med. 1982;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Dreyer WJ. The area code hypothesis revisited: olfactory receptors and other related transmembrane receptors may function as the last digits in a cell surface code for assembling embryos. Proc Natl Acad Sci U S A. 1998;95(16):9072–7. doi: 10.1073/pnas.95.16.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav. 2004;83(4):587–602. doi: 10.1016/j.physbeh.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Dubbins J, Hadcock J. GPR83 in warm sensitive neurons, KO and knockdown studies, Keystone Symposium; 2010. [Google Scholar]
- Emson P. Chemical Neuroanatomy (Vol Hardcover) New York: Raven Press; 1983. [Google Scholar]
- Falck B, Torp A. New evidence for the localization of noradrenalin in the adrenergic nerve terminals. Med Exp Int J Exp Med. 1962;6:169–72. doi: 10.1159/000135153. [DOI] [PubMed] [Google Scholar]
- Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117(6):833–46. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Saxena PN. Further studies on prostaglandin E 1 fever in cats. J Physiol. 1971;219(3):739–45. doi: 10.1113/jphysiol.1971.sp009686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder C, Perlik V, Blatteis CM. Preoptic alpha 1- and alpha 2-noradrenergic agonists induce, respectively, PGE2-independent and PGE2-dependent hyperthermic responses in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1156–66. doi: 10.1152/ajpregu.00486.2003. [DOI] [PubMed] [Google Scholar]
- Franco R, Casadó V, Cortés A, Ferrada C, Mallol J, Woods A, Lluis C, Canela EI, Ferré S. Basic concepts in G-protein-coupled receptor homo- and heterodimerization. ScientificWorldJournal. 2007;7:48–57. doi: 10.1100/tsw.2007.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464(7291):1006–11. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Kaple ML, Chow AR, Boulant JA. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J Physiol. 1996;492(Pt 1):231–42. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol. 2001;537(Pt 2):521–35. doi: 10.1111/j.1469-7793.2001.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT. Neurons and temperature regulation. In: Yamamoto WS, Brobeck JR, editors. Physiological controls and regulations. Philadelphia: Saunders; 1965. pp. 71–97. [Google Scholar]
- Harismendy O, Ng PC, Strausberg RL, Wang X, Stockwell TB, Beeson KY, et al. Evaluation of next generation sequencing platforms for population targeted sequencing studies. Genome Biol. 2009;10(3):R32. doi: 10.1186/gb-2009-10-3-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D, Glanzer J, Sarabi A, Pajunen T, Zielinski J, Belt B, et al. Single neurons as experimental systems in molecular biology. Prog Neurobiol. 2004;72(2):129–42. doi: 10.1016/j.pneurobio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hokfelt T. Looking at neurotransmitters in the microscope. Prog Neurobiol. 2010;90(2):101–18. doi: 10.1016/j.pneurobio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Tsuruo Y, Meister B, Melander T, Schalling M, Everitt B. Localization of neuroactive substances in the hypothalamus with special reference to coexistence of messenger molecules. Adv Exp Med Biol. 1987;219:21–45. doi: 10.1007/978-1-4684-5395-9_2. [DOI] [PubMed] [Google Scholar]
- Hori T, Nakashima T, Kiyohara T, Shibata M. Effects of leukocytic pyrogen and sodium salicylate on hypothalamic thermosensitive neurons in vitro. Neurosci Lett. 1984;49(3):313–8. doi: 10.1016/0304-3940(84)90308-2. [DOI] [PubMed] [Google Scholar]
- Hori T, Shibata M, Nakashima T, Yamasaki M, Asami A, Asami T, et al. Effects of interleukin-1 and arachidonate on the preoptic and anterior hypothalamic neurons. Brain Res Bull. 1988;20(1):75–82. doi: 10.1016/0361-9230(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006;350(3):762–7. doi: 10.1016/j.bbrc.2006.09.111. [DOI] [PubMed] [Google Scholar]
- Jones AR, Overly CC, Sunkin SM. The Allen Brain Atlas: 5 years and beyond. Nat Rev Neurosci. 2009;10(11):821–8. doi: 10.1038/nrn2722. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13(4):487–92. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Kacharmina JE, Crino PB, Eberwine J. Preparation of cDNA from single cells and subcellular regions. Methods Enzymol. 1999;303:3–18. doi: 10.1016/s0076-6879(99)03003-7. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Boulant JA. Effect of synaptic blockade on thermosensitive neurons in hypothalamic tissue slices. Am J Physiol. 1982;243(5):R480–90. doi: 10.1152/ajpregu.1982.243.5.R480. [DOI] [PubMed] [Google Scholar]
- Kim J, Eberwine J. RNA: state memory and mediator of cellular phenotype. Trends Cell Biol. 20(6):311–8. doi: 10.1016/j.tcb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu JY, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- Kerszberg M, Changeux JP. A model for reading morphogenetic gradients: autocatalysis and competition at the gene level. Proc Natl Acad Sci U S A. 1994;91(13):5823–7. doi: 10.1073/pnas.91.13.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61(236):1081–90. [PubMed] [Google Scholar]
- Lanneau C, Bluet-Pajot MT, Zizzari P, Csaba Z, Dournaud P, Helboe L, Hoyer D, Pellegrini E, Tannenbaum GS, Epelbaum J, Gardette R. Involvement of the Sst1 somatostatin receptor subtype in the intrahypothalamic neuronal network regulating growth hormone secretion: an in vitro and in vivo antisense study. Endocrinology. 2000;141:967–79. doi: 10.1210/endo.141.3.7349. [DOI] [PubMed] [Google Scholar]
- Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42(4–5):427–38. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levin MD, Lu MM, Petrenko NB, Hawkins BJ, Gupta TH, Lang D, et al. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J Clin Invest. 2009;119(11):3420–36. doi: 10.1172/JCI39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):417–20. doi: 10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Anggard A, Emson P, Fahrenkrug J, Hokfelt T. Vasoactive intestinal polypeptide and cholinergic mechanisms in cat nasal mucosa: studies on choline acetyltransferase and release of vasoactive intestinal polypeptide. Proc Natl Acad Sci U S A. 1981;78(8):5255–9. doi: 10.1073/pnas.78.8.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom L, Lu X, Langel U, Bartfai T. Important pharmacophores for binding to galanin receptor 2. Neuropeptides. 2005;39(3):169–71. doi: 10.1016/j.npep.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Mackler SA, Eberwine JH. Cellular adaptation to opiates alters ion-channel mRNA levels. Proc Natl Acad Sci U S A. 1994;91(1):385–9. doi: 10.1073/pnas.91.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AP, Palumbi SR. Body size, metabolic rate, generation time, and the molecular clock. Proc Natl Acad Sci U S A. 1993;90(9):4087–91. doi: 10.1073/pnas.90.9.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Perspective: Does brown fat protect against diseases of aging? Ageing Res Rev. 2010;9(1):69–76. doi: 10.1016/j.arr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD. Temperature sensing across species. Pflugers Arch. 2007;454(5):777–91. doi: 10.1007/s00424-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):385–8. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci. 2008;65(12):1796–805. doi: 10.1007/s00018-008-8153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet. 1999;33:399–422. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- Motoki K, Kishi H, Hori E, Tajiri K, Nishijo HM. The direct excitatory effect of IL-1beta on cerebellar Purkinje cell. Biochem Biophys Res Commun. 2009 Feb 13;379(3):665–8. doi: 10.1016/j.bbrc.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Bell TJ, Sul JY, Eberwine J. Subcellular neuropharmacology: the importance of intracellular targeting. Trends Pharmacol Sci. 2009;30:203–11. doi: 10.1016/j.tips.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22(11):4600–10. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11(1):62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22(12):3137–46. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–1. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hammel HT, Hardy JD, Eisenman JS. Thermal stimulation of electrical activity of single units of preoptic region. American Journal of Physiology. 1963;204(6):1122. [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nelson DO, Prosser CL. Intracellular recordings from thermosensitive preoptic neurons. Science. 1981;213(4509):787–9. doi: 10.1126/science.7256280. [DOI] [PubMed] [Google Scholar]
- Phillips J, Eberwine JH. Antisense RNA Amplification: A Linear Amplification Method for Analyzing the mRNA Population from Single Living Cells. Methods. 1996;10(3):283–8. doi: 10.1006/meth.1996.0104. [DOI] [PubMed] [Google Scholar]
- Ramot D, MacInnis BL, Goodman MB. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci. 2008;11(8):908–15. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Balasubramaniam A, Parker MS, Sallee F, Parker SL. Neuropeptide Y as a partial agonist of the Y1 receptor. Eur J Pharmacol. 2005;525(1–3):60–8. doi: 10.1016/j.ejphar.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230(4732):1350–4. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, et al. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59(1):43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330(26):1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- Satterlee JS, Ryu WS, Sengupta P. The CMK-1 CaMKI and the TAX-4 Cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr Biol. 2004;14(1):62–8. doi: 10.1016/j.cub.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Hokfelt T, Lundberg JM. Coexistence of classical transmitters and peptides in the central and peripheral nervous systems. Br Med Bull. 1982;38(3):309–13. doi: 10.1093/oxfordjournals.bmb.a071778. [DOI] [PubMed] [Google Scholar]
- Shibata M, Blatteis CM. Differential effects of cytokines on thermosensitive neurons in guinea pig preoptic area slices. Am J Physiol. 1991;261(5 Pt 2):R1096–103. doi: 10.1152/ajpregu.1991.261.5.R1096. [DOI] [PubMed] [Google Scholar]
- Siehler S, Nunn C, Hannon J, Feuerbach D, Hoyer D. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol. 2008;286(1–2):26–34. doi: 10.1016/j.mce.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Singer D. [Why 37 degrees C? Evolutionary fundamentals of thermoregulation] Anaesthesist. 2007;56(9):899–902. 4–6. doi: 10.1007/s00101-007-1220-y. [DOI] [PubMed] [Google Scholar]
- Sul JY, Wu CW, Zeng F, Jochems J, Lee MT, Kim TK, et al. Transcriptome transfer produces a predictable cellular phenotype. Proc Natl Acad Sci U S A. 2009;106(18):7624–9. doi: 10.1073/pnas.0902161106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Behrens MM, Bartfai T, Korn H. Prostaglandin E2-increased thermosensitivity of anterior hypothalamic neurons is associated with depressed inhibition. Proc Natl Acad Sci U S A. 2004;101(8):2590–5. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Conti B, Behrens M, Korn H, Bartfai T. Electrophysiological properties and thermosensitivity of mouse preoptic and anterior hypothalamic neurons in culture. Neuroscience. 2005;135(2):433–49. doi: 10.1016/j.neuroscience.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141(4):1685–95. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Welch CC. Fever The Cartwright Lectures. New York NY: 1888. [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669–82. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Yue C, Ponzio TA, Fields RL, Gainer H. Oxytocin and vasopressin gene expression and RNA splicing patterns in the rat supraoptic nucleus. Physiol Genomics. 2008;35(3):231–42. doi: 10.1152/physiolgenomics.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485(Pt 1):195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Boulant JA. Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. J Physiol. 2005;564(Pt 1):245–57. doi: 10.1113/jphysiol.2004.075473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. Faseb J. 2009;23(9):3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.