Abstract
The tumor suppressor gene FHIT spans a common fragile site and is highly susceptible to environmental carcinogens. FHIT inactivation and loss of expression is found in a large fraction of premaligant and malignant lesions. In this study, we were able to inhibit tumor development by oral gene transfer, using adenoviral or adenoassociated viral vectors expressing the human FHIT gene, in heterozygous Fhit+/− knockout mice, that are prone to tumor development after carcinogen exposure. We therefore suggest that FHIT gene therapy could be a novel clinical approach not only in treatment of early stages of cancer, but also in prevention of human cancer.
Human chromosomal fragile sites map to chromosome bands that are nonrandomly altered by translocations or deletions in human neoplasms (1). The recombinant nature of fragile sites, possibly enhanced by environmental carcinogens, has been linked to altered expression of oncogenes or tumor suppressor genes at fragile sites (reviewed in ref. 2). This finding implies that alteration of expression of genes at fragile sites could trigger clonal expansion of preneoplastic and neoplastic cells.
FHIT, spanning the most inducible human common fragile site, FRA3B, at chromosome 3p14.2, is thus far the only example of a frequently altered gene at a constitutive fragile region and shows hallmarks of a tumor suppressor gene (3). The FHIT gene is altered by deletion in a large fraction of many types of cancer, including lung, breast, head and neck, cervical, bladder, esophageal, gastric and pancreatic cancer (2, 4). FHIT is also interrupted by a translocation in a family with predisposition to the development of renal carcinomas. Fhit protein is lost or reduced in the majority of these cancers, in a large fraction of other cancer types, and preneoplastic lesions in the esophagus and lung (reviewed in ref. 4).
Although the precise mechanism of Fhit action remains unclear, the role of FHIT as a tumor suppressor gene has been experimentally verified in cultured human cancer cells (5). At the cellular level, Fhit has been shown to induce apoptosis and retard tumor cell proliferation in vitro and in vivo (6–8). The murine Fhit locus, which resembles its human homolog, encompasses a common fragile site and is altered in murine cancer cell lines (9, 10).
To further clarify the role of Fhit protein in cancer development, we inactivated one Fhit allele in mouse embryonic stem cells and established Fhit+/− heterozygous and Fhit−/− homozygous mice. We previously demonstrated that Fhit+/− mice are susceptible to carcinogen-induced tumor development in the esophagus and forestomach. One hundred percent of the heterozygous mice (Fhit+/−) developed multiple tumors in the forestomach and at the squamocolumnar junction (SCJ) after exposure to the carcinogen N-nitrosomethylbenzylamine (NMBA), compared with 25% of mice with intact Fhit alleles (Fhit+/+ mice) (11). Fhit−/− mice are even more sensitive to carcinogen than Fhit+/− mice, and both heterozygous and homozygous knockout mice exhibit increased frequencies of spontaneous tumors (N.Z., V.F., L.Y.Y.F., T. Druck, R.M., C.M.C., P. A. McCue, and K.H., unpublished work), suggesting Fhit knockout mice as models for tumor treatment and prevention. NMBA induces morphologically similar esophageal lesions in rodents and human (12). In analogy to the human distal esophagus, the mouse forestomach has an epithelial lining. The mouse SCJ, the transition zone between epithelial and glandular tissue, corresponds to the human esophago-gastric junction. These structures are commonly studied as a model system for the distal esophagus in humans. Both regions have a predilection to cancer development and the incidence of cancer in the distal esophagus is rising (13).
Methods
Construction of the Recombinant Vectors.
cDNAs for green fluorescent protein (GFP) and lacZ were obtained from expression vectors (CLONTECH). Full-length FHIT cDNA was isolated from human normal placental tissue by reverse transcription–PCR strategy (3).
Adenovirus (Ad).
The recombinant adenoviral vector was constructed as described (6). In summary, the cDNA were ligated into an adenoviral backbone vector DNA (Quantum, Durham, NC). The adenoviral vector was transfected with human fetal kidney 293 cells (Microbix, Toronto) with plaque isolation and vector purification after homologous recombination in 293 cells.
Adenoassociated Virus (AAV).
For the AAV-GFP plasmid, the GFP cDNA was linked to the promoter EF and was cloned into the KpnI and HindIII restriction sites of the multiple cloning site of pAM/pL-WPRE-BGH poly(A), creating pAM/pL-EF-GFP-WPRE-BGH poly(A). For the AAV-FHIT plasmid, the FHIT gene fragment was cloned into the BamHI and HindIII sites of pAM/pL-EF-GFP-WPRE-BGH poly(A), replacing GFP with FHIT to generate pAM/pL-EF-FHIT-WPRE-BGH poly(A). The packaging plasmid pDG was cotransfected with the corresponding vector plasmids to generate recombinant AAV-GFP and AAV-FHIT. Virus purification and titration was performed as described (14–16).
Transgene Expression.
For Ad-LacZ 100 μl of virus (1011 plaque forming units/ml) and for AAV-GFP 100 μl of virus (1011 viral particles/ml) was administered via an orogastric tube into the stomachs of a group of healthy mice (n = 6). At 3, 7, and 14 days postviral administration mice were overdosed with pentobarbital and perfused transcardially with saline followed by 2% paraformaldehyde containing 2 mM MgCl2 and 1.25 mM EGTA in 0.1 M phosphate buffer (pH 8.0) to inhibit endogenous β-galactosidase. The esophagus and stomach then were fixed briefly before cryoprotection in a 30% sucrose solution in PBS. Sections 12 μm in thickness were cut on a cryostat and thaw-mounted onto slides. Sections were immersed briefly in 4% paraformaldehyde, washed extensively with PBS, and immersed in a solution containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-d-galactopyranoside, 2 mM MgCl2, 50 mM K3Fe(CN)6, and 50 mM K4Fe(CN)6 in PBS overnight at 37°C.
Carcinogenicity Study.
(C57BL/6J x129/SvJ) F1 mice (B6129F1s) that were Fhit +/− were produced in the Kimmel Cancer Center animal facility. Thirty six Fhit+/− mice (20–32 wk) were given six intragastric doses of NMBA (Ash Stevens, Detroit) over the course of 3 weeks at 2 mg/kg body weight. At 4 weeks, one group of eight animals received a single dose of Ad-FHIT (1011 plaque-forming units/ml), a second group of eight animals received a single dose of AAV-FHIT (1011 viral particles/ml, 100 μl), and the third treatment group received the same dose of Ad5-FHIT and AAV-FHIT combined, necessitating a double oral volume. Twelve control animals did not receive any recombinant virus. All mice were killed 13 weeks after the initial NMBA dose.
Immunohistochemistry.
After antigen retrieval, endogenous peroxidase was inhibited with 3% hydrogen peroxide, and nonspecific binding sites were blocked with normal goat serum. Slides were incubated with primary rabbit anti-human Fhit antibody against the C terminus of the human Fhit protein (1:1,000 dilution, overnight, Zymed), followed by incubation with biotinylated goat anti-rabbit antibody. Slides then were incubated with streptavidin horseradish peroxidase (Dako; 1:1,000 dilution). For proliferating cell nuclear antigen (PCNA) we used mouse mAb (1:500, Santa Cruz Biotechnology).
Results
To examine the therapeutic approach we used two viral gene delivery systems for the FHIT gene, to determine whether FHIT gene delivery to the esophagus and forestomach could prevent tumor formation in Fhit+/− mice after carcinogen exposure. Ad and AAV vectors were constructed for human FHIT, lacZ encoding β-galactosidase, and GFP. The AAV-FHIT and Ad-FHIT were tested in vitro, and Fhit bioactivity conferred by the vectors was assessed by in vitro induction of apoptosis and in vivo suppression of tumor growth in nude mice, as described (6).
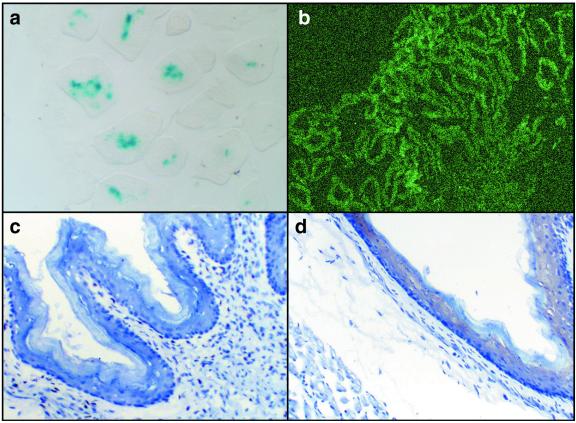
The stability of infection in murine esophageal and gastric tissue and levels of transgene expression for both viral vectors were investigated by using the control vectors, Ad-LacZ and AAV-GFP. Comparable doses of recombinant Ad virus expressing β-galactosidase (Ad-lacZ) and AAV virus expressing GFP (AAV-GFP) were administered via an intragastric tube, into the stomachs of a group of six healthy mice. Mice were killed at 3, 7, and 14 days after viral administration with cryopreservation of esophagus and forestomach. As expected, 5-bromo-4-chloro-3-indolyl-d-galactopyranoside staining of mice injected with Ad-lacZ revealed blue nuclei in the epithelial layers of the forestomach 3 days postadministration (Fig. 1). After AAV-GFP administration, GFP immunohistochemistry failed to detect transgene expression at 3 days, with clear transgene expression 2 weeks after viral administration in lamina propria and epithelial cell layers of esophagus and forestomach, confirmed by confocal microscopy imaging of expression in approximately 80% of the cells. Microscopic sections did not reveal any cytopathic effect attributable to the Ad-lacZ or AAV-GFP viral vectors (Fig. 1).
Figure 1.
Transgene expression with control vectors in normal murine tissue (a) section of glandular stomach with β-galactosidase staining 3 days after inoculation with Ad-lacZ. (b) Confocal microscopy image of glandular stomach 14 days after AAV-GFP inoculation. GFP immunohistochemistry of the forestomach region 3 days (c) and 14 days (d) after AAV-GFP infection. Brown chromogen in d indicates presence of GFP. [Magnifications: (a) ×400, (b) ×100, and (c and d) ×200.]
The efficacy of Ad-FHIT and AAV-FHIT in protecting the forestomach region from the effect of carcinogen exposure was assessed in vivo with the NMBA mouse model of forestomach and esophageal cancer in Fhit+/− mice (11). On bioactivation, NMBA produces benzaldehyde and an electrophilic agent that methylates DNA, resulting in the formation of the promutagenic adducts, O6-methylguanine (17). NMBA induced both esophageal and forestomach tumors in mice when administered by gavage at low doses (18). To our knowledge, NMBA is by far the most extensively used animal model for esophageal carcinogen induction in rodents. This in vivo model is relevant to human cancer, because epidemiological studies link the exposure to carcinogenic nitrosamines, NMBA in particular, to the high incidence of esophageal cancer in northern China and parts of Iran (19–22).
Fhit+/− mice (age 20–32 weeks) received six doses of NMBA through oral gavage at intervals of 3 or 4 days. After 4 weeks, mice were divided into four groups: one group of 12 mice remained untreated, two groups of eight mice each received a single intragastric administration of comparable doses of Ad-FHIT or AAV-FHIT, and an additional group of eight mice received a combined administration of AAV-FHIT and Ad-FHIT. During the 10-week observation period, two of eight mice in the combined treatment group died, due to a fatal infection with aspiration pneumonia, as determined by autopsy. This was probably related to the larger volume of the oral viral dose used in this treatment group. No other side effects were observed in the remaining 34 animals.
At 14 weeks after the first NMBA dose, corresponding to 10 weeks after administration of viral recombinant FHIT, mice were euthanized; the esophagus, forestomach and other organs, including digestive tract, spleen, liver and brain were removed, and were gross anatomically evaluated by four investigators. In addition, organs were fixed in 10% formalin and embedded in paraffin. Serial cross sections (4 μm) were prepared for histology and immunohistochemistry.
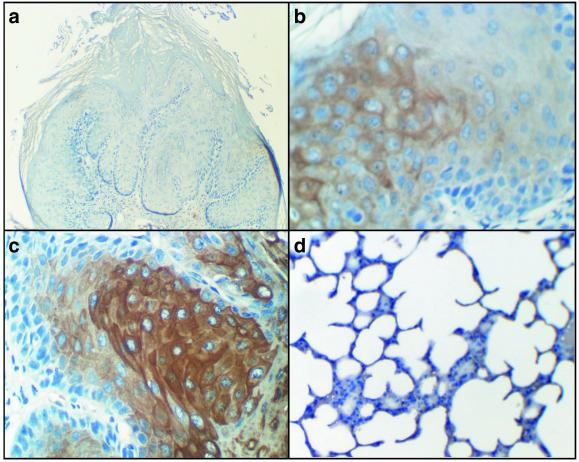
In mice treated with oral AAV-FHIT or Ad-FHIT vectors, as well as in the combined treatment group, transduction of intestinal cells was confirmed by human Fhit protein expression in murine esophagus and forestomach sections. The human Fhit protein was detected in AAV-FHIT- and Ad-FHIT-infected mouse esophagus and forestomach, but not in sections from the intestine of untreated mice, or in the liver, lung, or brain of any AAV-FHIT- or Ad-FHIT-infected mice (Fig. 2).
Figure 2.
Fhit expression in murine forestomach epithelium 10 weeks after virus administration (magnification ×200). Fhit immunohistochemistry using methods as described. (a) Control. (b) AAV-FHIT. (c) Ad-FHIT. (d) Murine lung tissue of AAV-FHIT- and Ad-FHIT-treated mouse.
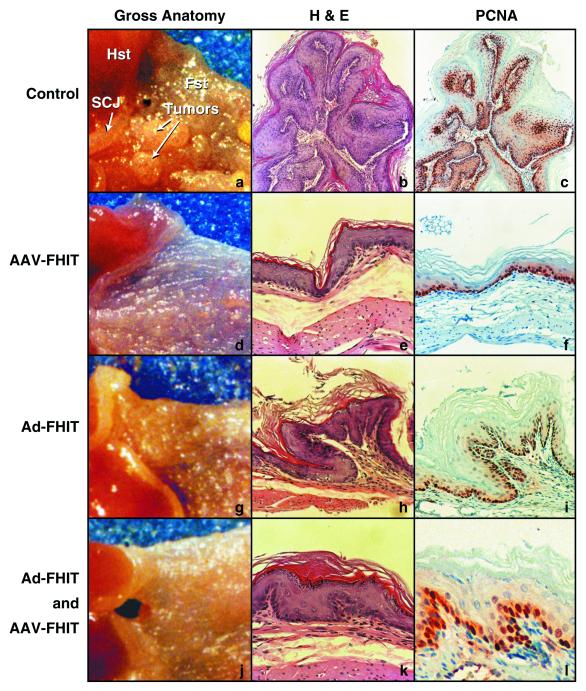
On gross examination, there was a marked difference between the three treatment groups and mice left untreated after carcinogen exposure. In the forestomach, large visible tumors, usually multiple, were seen in mice exposed to NMBA without transgene administration (Fig. 3a), whereas in AAV-FHIT (Fig. 3d) and Ad-FHIT-treated mice (Fig. 3g), as well as in the combined treatment group (Fig. 3j) there was a substantial reduction both in number and size of tumors. Also, on inspection of the SCJ, a clear difference between the control group and the three treatment groups was noted.
Figure 3.
Gross anatomy and histopathology of murine forestomach after FHIT-gene therapy. Typical aspects of NMBA-induced pathology in the control group (a–c) are compared with the three treatment groups: AAV-FHIT (d–f), Ad-FHIT (g–i), and the combined treatment group Ad-FHIT with AAV-FHIT (j–l). (a, d, g, and j) Gross anatomy of the forestomach and SCJ (magnification ×5). b (magnification ×50) and e, h, and k (magnification ×200) are hematoxylin and eosin (H&E)-stained forestomach sections. c (magnification ×50) and f, i, and l (magnification ×200) depict PCNA immunohistochemistry in forestomach sections adjacent to the corresponding H&E sections. PCNA immunohistochemistry shows abundant, intensely stained cells in S phase in a control forestomach showing a papilloma with hyperplastic epithelium (b and c). In AAV-FHIT (e and f), Ad-FHIT (h and i) and the combined treatment group (k and l), PCNA-positive cells are found mostly in basal cells of the near normal epithelium.
The statistical significance of the observed differences in tumor incidence between the control and treatment groups was tested by the Fisher's exact test (Table 1). At both predilection regions of tumor development (the SCJ and forestomach), there was a significant difference between the three treatment groups combined and the control group. When treatment groups were considered separately, statistically significant differences were achieved in forestomach between AAV-FHIT versus control, as well as in SCJ between Ad-FHIT versus control and AAV-FHIT versus control.
Table 1.
Effect of FHIT gene therapy on NMBA-induced forestomach carcinogenesis in Fhit+/− mice
| Fhit+/− animal group (no. of animals) | Tumor incidence (%)
|
||
|---|---|---|---|
| Esophagus | Forestomach | SCJ | |
| Control (12) | 1/12 (8) | 11/12 (92) | 8/12 (67) |
| Ad-FHIT (8) | 0/8 (0) | 4/8 (50) | 0/8 (0) |
| AAV-FHIT (8) | 0/8 (0) | 3/8 (38) | 0/8 (0) |
| Ad-FHIT and AAV-FHIT (6) | 0/6 (0) | 3/6 (50) | 1/6 (17) |
Forestomach: Ad-FHIT vs. control, P = 0.11; AAV-FHIT vs. control, P = 0.02; Ad-FHIT + AAV-FHIT vs. control, P = 0.08. SCJ: Ad-FHIT vs. control, P = 0.005; AAV-FHIT vs. control, P = 0.005; Ad-FHIT + AAV-FHIT vs. control, P = 0.13. Overall FHIT-treatment is effective in forestomach, P = 0.01 and in SCJ, P = 0.0002. For each animal, total NMBA dose was 12 mg/kg (6 × 2 mg/kg, twice weekly); gene therapy was administered 10 days after the last NMBA dose. Statistical significance was analyzed by Fisher's exact test, two-tailed test.
We also performed histological examination of the esophagus and forestomach of all animals to screen for signs of tumor development. In addition to the hematoxylin and eosin staining, immunohistochemistry with the endogenous cell proliferation marker, PCNA (23), was performed in adjacent sections to evaluate the effect of FHIT gene therapy on carcinogen-induced proliferation associated with NMBA exposure.
As summarized in Table 2, the pathology found in sections from control animals included papillomas (Fig. 3b), focal hyperplastic lesions (FHL) and invasive carcinomas with predominance of tumors in the forestomach and at the SCJ. In contrast, in 22 of 32 sections of AAV-FHIT-treated mice (Fig. 3e) and in 14 of 34 sections of the combined treatment group (Ad-FHIT and AAV-FHIT) (Fig. 3k), near normal epithelia were seen.
Table 2.
NMBA-induced phenotypes in Fhit+/− mice with and without FHIT gene therapy
| Fhit+/− mice (no. of animals) | Phenotype
|
Histology of forestomach sections | ||
|---|---|---|---|---|
| Esophagus | Forestomach | SCJ | ||
| Control (12) | 1 tumor in 12 | ≫6 tumors/mouse; some large | Multiple tumors in 8; thickened in 4 | FHL in all sections of all mice; papillomas in 11 mice, dys in 3, ca in 1 |
| Ad-FHIT (8) | No tumors | 1.7 tumors/mouse | No tumors; thickened in 2 | FHL in average of 75% of sections; near normal epithelium in average of 25% of sections |
| AAV-FHIT (8) | No tumors | <1.4 tumors/mouse; 1 large | No tumors; thickened in 1 | FHL in average of 44% of sections; near normal in 56% |
| Ad-FHIT and AAV-FHIT (6) | No tumors | 3.7 tumors/mouse; most small | Tumors in 1; thickened in 1 | FHL in 60% of sections; near normal in 40%, ca in section of 1 mouse |
At autopsy, whole esophagi and stomachs were removed and opened longitudinally. Esophageal and forestomach tumors with diameters > 0.5 mm were mapped and counted. ca, squamous cell carcinoma; dys, dysplasia. Tumor sizes were graded as follows: large tumors (diameter > 2 mm), medium tumors (diameter 1–2 mm), and small (diameter < 1 mm). Sections, microtome sections cut across SCJ and forestomach. For each forestomach 4–7 sections were examined, in most cases five sections. Near normal epithelium denotes an epithelium that is 3–5 cells thick with mild folding at places and a thin keratin. All control sections show abundant keratin production.
In some animals, carcinogen-induced changes were found after FHIT transgene administration, but overall these changes were less extensive and significantly less frequent compared with the control group. An example of such a lesion, a small papilloma found in the forestomach of an Ad-FHIT-treated mouse, is depicted in Fig. 3h. The only animal that exhibited invasive carcinoma, as well as FHL, after combined treatment (Ad-FHIT and AAV-FHIT) did not show murine or human Fhit expression in the tumor (not shown).
In the control group, PCNA staining revealed a substantial increase in cellular proliferation in pathologic lesions such as FHL (not shown) and papillomas (Fig. 3c), whereas in the three treatment groups proliferation was typically confined to the basal layer (Fig. 3 f, i, and l).
Besides the carcinogen-induced pathology found in some animals of the three treatment groups, no additional pathology or in vivo cytotoxic effects could be related to FHIT transgene administration, which confirms the previous in vitro observation that viral Fhit (Ad-FHIT) administration is not toxic to normal cells (6, 7).
Discussion
Although two different recombinant viral vectors were used for FHIT administration, macroscopic and histologic examination revealed the same biological effect with both vectors: FHIT transgene resulted in a significant protection of the forestomach and SCJ against NMBA-induced tumor development in Fhit+/− mice. Some studies with adenoviral vectors have described virus-related effects such as cellular immune responses triggered by viral proteins (24), innate immune mechanisms (25), and direct cytotoxicity caused by expression of viral genes (26). In contrast, recombinant AAV vectors, which are devoid of all viral genes, minimize the possibility of recombination and viral gene expression (14). Also, in vivo host immune responses to AAV thus far described are minimal (14). Because the same biological effect was seen with both vector systems, it is unlikely that immunological responses or direct viral effects contributed to the protective effect seen with both Ad and AAV vectors in this study.
Consistent with the two-hit model, where a somatic mutation superimposed on a germ-line mutation of a tumor suppressor gene leads to tumor development, our previous in vivo study in Fhit knockout mice demonstrated that carcinogen-induced loss of expression of the second Fhit allele leads to tumor development (11). Here we report that the tumor development can be inhibited through recombinant viral FHIT gene delivery, which opens the window to therapeutic applications.
Using different transgenes, adenoviral systems have been used previously in human clinical cancer gene therapy trials. In addition, a recent human phase II clinical trial reported that intratumoral injection of selectively replicating Ad in combination with chemotherapy resulted in objective response (27). Because the efficacy of selectively replicating viruses can be increased by the expression of therapeutic transgenes from the virus itself (28), FHIT would be a good candidate.
Also, the prominent in vivo biological response seen with AAV-FHIT in this trial is probably a reflection of its high efficiency in transducing gut lamina propria cells. This confirms previous studies in which high efficiency of AAV in transducing endoluminal cells in the intestine was reported (15, 16). The human gut and the respiratory tract were found to be the normal host tissues for AAV (29). Although not widely explored in cancer gene therapy, its nonpathogenic nature in humans and other species (29) render AAV vectors attractive for clinical use.
On the basis of our findings it will be important to explore whether local FHIT gene delivery will be effective in treating human premalignant lesions and cancers in which loss of Fhit has been implicated. Alterations of FHIT transcripts were observed in 86% of Barrett's metaplasia, a premalignant lesion of the esophagus, and in 93% of esophageal adenocarcinomas (30, 31). This finding is in accordance with the high proportion of premalignant and malignant esophageal lesions lacking Fhit expression (31). It is therefore tempting to relate the observed effect in the well-established rodent NMBA model of esophageal and gastric cancer to the human condition. In humans, lung cancer has been extensively studied for Fhit expression (reviewed in ref. 4). We demonstrated that most preneoplastic lesions, as well as more than 85% of squamous cell lung cancers have lost Fhit expression (32). In addition, loss of Fhit expression is more frequent in tumors of smokers (4). As Fhit is lost very early in lung carcinogenesis, FHIT gene delivery has been suggested as a therapeutic target, as well as a preventive treatment for these lesions (33).
Acknowledgments
This study has been supported in part by a program project grant and the core grant Translational Research in Cancer from the U.S. Public Health Service. K.R.D. was supported by a National Institutes of Health fellowship. This study would not have been possible without the generous support of Mr. George Strawbridge.
Abbreviations
- SCJ
squamocolumnar junction
- NMBA
N-nitrosomethylbenzylamine
- GFP
green fluorescent protein
- AAV
adenoassociated virus
- Ad
adenovirus
- FHL
focal hyperplastic lesions
- PCNA
proliferating cell nuclear antigen
References
- 1.Yunis J J, Soreng A L. Science. 1984;226:1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]
- 2.Huebner K, Garrison P N, Barnes L D, Croce C M. Annu Rev Genet. 1998;32:7–31. doi: 10.1146/annurev.genet.32.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Ohta M, Inoue H, Cotticelli M G, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 4.Huebner K, Sozzi G, Brenner C, Pierotti M A, Croce C M. Adv Oncol. 1999;15:3–10. [Google Scholar]
- 5.Siprashvili Z, Sozzi G, Barnes L D, McCue P, Robinson A K, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, et al. Proc Natl Acad Sci USA. 1997;94:13771–13776. doi: 10.1073/pnas.94.25.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii, H., Dumon, K. R., Vecchione, A., Trappaso, F., Mimori, K., Alder, H., Mori, M., Sozzi, G., Baffa, R., Huebner, K. & Croce, C. M. (2001) Cancer Res., in press. [PubMed]
- 7.Ji L, Fang B, Yen N, Fong K, Minna J D, Roth J A. Cancer Res. 1999;59:3333–3339. [PubMed] [Google Scholar]
- 8.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo M P, Gramegna M, Croce C M, Pierotti M A, Sozzi G. Proc Natl Acad Sci USA. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover T W, Hoge A W, Miller D E, Ascara-Wilke J E, Adam A N, Dagenais S L, Wilke C M, Dierick H A, Beer D G. Cancer Res. 1998;58:3409–3414. [PubMed] [Google Scholar]
- 10.Pekarsky Y, Druck T, Cotticelli M G, Ohta M, Shou J, Mendrola J, Montgomery J C, Buchberg A M, Siracusa L D, Manenti G, et al. Cancer Res. 1998;58:3401–3408. [PubMed] [Google Scholar]
- 11.Fong L Y, Fidanza V, Zanesi N, Lock L F, Siracusa L D, Mancini R, Siprashvili Z, Ottey M, Martin S E, Druck T, et al. Proc Natl Acad Sci USA. 2000;97:4742–4747. doi: 10.1073/pnas.080063497. . (First Published April 11, 2000; 10.1073/pnas.080063497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinson S F, Squire R A, Sporn M B. J Natl Cancer Inst. 1978;61:1471–1475. [PubMed] [Google Scholar]
- 13.Blot W J, Devesa S S, Kneller R W, Fraumeni J F., Jr J Am Med Assoc. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 14.Grimm D, Kern A, Rittner K, Kleinschmidt J A. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 15.During M J, Xu R, Young D, Kaplitt M G, Sherwin R S, Leone P. Nat Med. 1998;4:1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- 16.During M J, Symes C W, Lawlor P A, Lin J, Dunning J, Fitzsimons H L, Poulsen D, Leone P, Xu R, Dicker B L, et al. Science. 2000;287:1453–1460. doi: 10.1126/science.287.5457.1453. [DOI] [PubMed] [Google Scholar]
- 17.Labuc G E, Archer M C. Carcinogenesis. 1982;3:519–523. doi: 10.1093/carcin/3.5.519. [DOI] [PubMed] [Google Scholar]
- 18.Fong L Y, Magee P N. Cancer Lett. 1999;143:63–69. doi: 10.1016/s0304-3835(99)00191-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang C S. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 20.Lu S H, Montesano R, Zhang M S, Feng L, Luo F J, Chui S X, Umbenhauer D, Saffhill R, Rajewsky M F. J Cell Physiol. 1986;Suppl.4:51–58. doi: 10.1002/jcp.1041290411. [DOI] [PubMed] [Google Scholar]
- 21.Lu S H, Chui S X, Yang W X, Hu X N, Guo L P, Li F M. Int Agency Res Cancer Sci Publ. 1991;105:11–17. [PubMed] [Google Scholar]
- 22.Magee P N. Cancer Surv. 1989;8:207–239. [PubMed] [Google Scholar]
- 23.Celis J E, Madsen P, Nielsen S, Celis A. Leuk Res. 1986;10:237–249. doi: 10.1016/0145-2126(86)90021-4. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff G, Worgall S, van Rooijen N, Song W R, Harvey B G, Crystal R G. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muruve D A, Barnes M J, Stillman I E, Libermann T A. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 27.Khuri F R, Nemunaitis J, Ganly I, Arseneau J, Tannock I F, Romel L, Gore M, Ironside J, MacDougall R H, Heise C, et al. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 28.Wildner O, Blaese R M, Candotti F. Cancer Res. 1999;59:5233–5238. [PubMed] [Google Scholar]
- 29.Berns K I, Hauswirth W W. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- 30.Michael D, Beer D G, Wilke C W, Miller D E, Glover T W. Oncogene. 1997;15:1653–1659. doi: 10.1038/sj.onc.1201330. [DOI] [PubMed] [Google Scholar]
- 31.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce C M. Cancer Res. 2000;60:1177–1182. [PubMed] [Google Scholar]
- 32.Sozzi G, Musso K, Ratcliffe C, Goldstraw P, Pierotti M A, Pastorino U. Clin Cancer Res. 1999;5:2689–2692. [PubMed] [Google Scholar]
- 33.Croce C M, Sozzi G, Huebner K. J Clin Oncol. 1999;17:1618–1624. doi: 10.1200/JCO.1999.17.5.1618. [DOI] [PubMed] [Google Scholar]