Abstract
Mast cells are key effectors in allergy and inflammation. Endowed with a large panel of surface receptors and a huge arsenal of bioactive mediators, they readily communicate with various cellular partners during innate and adaptive immune responses. Recent lines of evidence show that mast cells are also able to establish cognate interactions with helper T lymphocytes for antigen presentation and bidirectional cell-cell cooperation. In this short review we focus on the role of mast cells as unconventional antigen presenting cells for helper T lymphocytes. We discuss how looking at mast cell biology from this new angle can help to better understand their pleiotropic role in health and disease.
Key words: mast cell, antigen presentation, immunological synapse, helper T cell, T-cell antigen receptor
Introduction
Mast cells are a heterogeneous subset of blood-derived granulated cells located in most vascularized tissues in the proximity of vessels, nerves, glandular ducts and epithelial surfaces.1–4
In addition to their strategic location at the host-environment interface, mast cells exhibit functional requirements to fulfill a central role during various defense reactions against pathogens. On the one hand, mast cells express a wide variety of immune receptors on their surface (including Fc receptors, complement receptors and receptors for PAMPs, pathogen-associated molecular patterns) allowing them to efficiently detect pathogens and contribute to their clearance.2 On the other hand, following activation, mast cells swiftly release different soluble mediators that modulate the recruitment and activation of other leukocytes. Among the rapidly-released mast cell mediators are histamine, proteases, leukotrienes, prostaglandins and various cytokines/chemokines.1
The implication of mast cells in the hypersensitivity reaction triggered by cross-linking of IgE-bound FceRI has been thoroughly characterized.5,6 In addition, a growing body of evidence indicates that mast cells are involved in various additional steps of innate and adaptive immune responses.7–9
Interestingly, mast cells are the only cytokine-releasing cells known to store preformed TNFα in their cytoplasmic granules and to rapidly release this cytokine upon activation.10–16 They are therefore considered key effector cells in the initial steps of immune responses against pathogens.2,17,18
Mast cells also participate to various further steps of immunity and their role both in physiological responses against pathogens and in inflammatory/auto-immune conditions is now well established (for reviews on mast cell function at the interface between innate and adaptive immune response see).2,7–9,19
An intriguing and not yet fully clarified aspect of mast cell biology concerns their functional and/or physical interaction with different subsets of T lymphocytes. If functional cross-talks between mast cells and T lymphocytes have been well documented, the modalities of their interactions are less well understood.20
In this review, we will initially survey the literature describing the functional communication between helper T cells and mast cells during immune responses. We will then focus on results showing that mast cells can be primed to express functional MHC class II and costimulatory molecules and can serve as antigen presenting cells for CD4+ T lymphocytes. Finally, we will discuss recent evidences showing that functional immunological synapses can be formed at the T cell/mast cell interface.
Functional Cooperation Between Mast Cells and T Helper Cells
Several lines of evidence indicate that mast cells are involved in the recruitment of T lymphocytes to the sites of immune responses.
As mentioned above, by releasing stored histamine and TNFα, mast cells can rapidly activate endothelial cells, favoring the recruitment of various subsets of leukocytes, including T cells, to the site of infection.2,17,18 Mast cells also release various chemotactic factors. IL-16, a chemo-attractant for CD4+ T cells, is stored preformed in bone marrow-derived human mast cells and in the human mast cell line HMC-1 and is released upon mast cell degranulation.21 Furthermore, it has been shown, in a mouse model of hapten-induced contact hypersensitivity, that subcutaneous mast cells get activated and migrate to the draining lymph nodes where they secrete CCL4, responsible for T-cell recruitment.22,23 More in general, mast cells have been shown to release several chemotactic factors for which different T-cell subsets express functional receptors (CCL3, CCL4, CXCL9 and CXCL10 for Th1; CCL5 and CCL11 for Th2 and CCL2 and CCL20 for Th17).2
In addition to their capacity to recruit CD4+ T lymphocytes, mast cell-derived mediators are also known to modulate different steps of T helper (Th) cell response.
Mouse bone marrow derived mast cells (BMMC) triggered via FcεRI cross-linking costimulate proliferation and cytokine production in anti-CD3 stimulated CD4+ T lymphocytes.24 This effect is mediated by both release of soluble TNFα and by cell-cell contacts involving the interaction of OX40 on T cells with OX40L on BMMC.24,25 Similar results have been obtained using human cells. Human tonsil mast cells enhance proliferation of freshly purified CD4+ T cells stimulated with anti-CD3 antibodies via a mechanism requiring the OX40/OX40L interaction.26 Accordingly, our unpublished observations show that co-culture of the human mast cell line HMC-1 with freshly isolated human CD4+ T lymphocytes enhances their anti-CD3 induced proliferation (Espinosa E, unpublished observations).
Mast cells play also a crucial role in the polarization of Th cell responses. Under different stimulation conditions, mast cells release in vitro polarizing cytokines that promote the differentiation of various subsets of CD4+ T lymphocytes: IL-12 and IFNγ (for Th1), IL-4 (for Th2), IL-6 and TGFβ1 (for Th17) or IL-10 and TGFβ1.8,27,28
In vivo studies support this view by showing that mast cells can differently influence Th cell polarization depending on the stimulation conditions and on the mouse model employed. FcεRI activated mast cells favor Th2 lineage commitment in vivo after subcutaneous immunization with antigen and adjuvant.29 Conversely, mast cell-derived IL-4 reportedly promotes Th1 response in an experimental auto-immune encephalomyelitis mouse model.30 Finally, mast cell derived histamine also promotes Th1 responses.31
Together these reports challenge the classical view of mast cells as mere cellular effectors of type 2 adaptive immune responses, arguing in favor of a broader role for mast cells including their interaction with different T-cell subsets.
If it is well established that mast cells modulate T-cell responses, it is also known that helper T-cell-derived factors affect mast cell biology.
In mouse models, the Th cell-derived IL-3 has been shown to induce mouse mast cell development and survival.32,33 Moreover, Th cytokines (IL-3, IL-4, IL-5 and IL-6) enhance in vitro survival of cord blood-derived mast cells lines.34
Th cells have been reported to also influence mast cell activation. It has been shown that mouse T cells (activated either by PMA or by anti-CD3 antibodies) enhance FcεRI mediated degranulation in mast cells.35,36 This effect is contact dependent and is mediated at least in part by LFA1/ICAM-1 interaction.35 Similarly, human Jurkat T cells triggered by anti-CD3 antibodies stimulate HMC-1 cells to secrete β-hexosaminidase, TNFα and matrix metalloproteinase-9.37,38
Finally, morphological analysis of inflamed tissues has often provided data compatible with the existence of functional cross-talks between these two cell subsets. For instance, mast cells and T cells have been shown in close proximity in human tonsils and in a mouse model of contact hypersensitivity24,26 (reviewed in refs. 39–42).
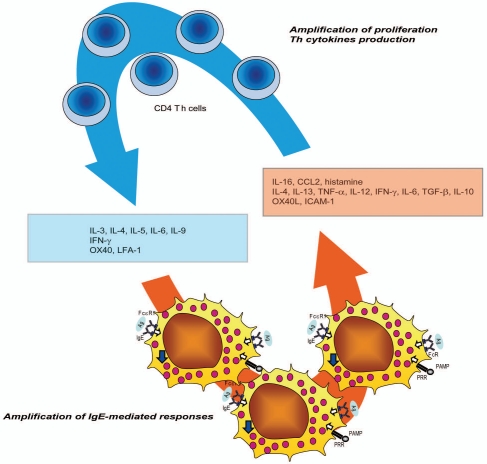
All in all, an increasing amount of literature documents that mast cell and T-cell functions are strongly interconnected and that these two cell populations cooperate by the means of soluble mediators and direct cellular interactions (Fig. 1).
Figure 1.
Mast cell/T cell cooperation. Upon activation via FcR or PRR, mast cell release several mediators that modulate Th cells responses (chemotaxis, panel of cytokines produced, and proliferation). In turn activated Th cells release mediators that influence mast cell responses.
Mast Cells Express Functional MHC Class II and Accessory Molecules
It is commonly accepted that murine resting mast cells do not express MHC class II molecules. This notion is based on an initial study performed using ex vivo peritoneal mast cells43 and on further studies performed using mast cells derived from bone marrow precursors such as BMMC.24,44 More recently, M. Daeron and collaborators described a new in vitro model of murine mast cells: peritoneal cell-derived mast cells (PCMC).45 PCMC have the advantage over other cellular models of exhibiting several phenotypic and functional properties of mature connective-tissue mast cells.45 In particular they express characteristic mast cell markers on their surface, such as FcεRI and CD117 and stain positive for toluidin blue. PCMC exhibit alcian blue low and safranin high staining and degranulate following stimulation with polycationic compounds, such as compound 48/80 and substance P, consistent with a connective tissue mast cell phenotype.1,45–47 Using PCMC, others and we have recently shown that, similarly to BMMC, also these mature mast cells are essentially negative for MHC class II expression under resting conditions.48,49
Various treatments have been described to induce MHC class II expression in both BMMC and PCMC. A critical cytokine involved in the induction of MHC class II molecules is IFNγ. Treatment with IFNγ for a time ranging between 24 and 96 hours has been shown to induce MHC class II molecules expression both in BMMC and in PCMC.49–52 In addition IFNγ treatment also induces MHC class II expression in other mast cell models such as freshly isolated rat peritoneal mast cells, human cord blood-derived mast cells and the human mast cell line HMC-1.53–57
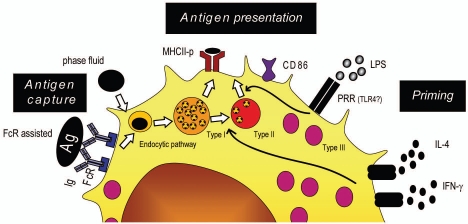
Additional stimuli synergize with IFNγ treatment to induce MHC class II expression on in vitro cultured mast cells. We have recently shown that IL-4, when given together with IFNγ, enhances MHC class II expression in PCMC.49 Similar results were obtained when LPS was used to induce MHC class II expression in BMMC and spleen-derived mast cells stimulated by IFNγ.48 Together, these observations indicate that IFNγ is necessary and sufficient to induce MHC class II expression on in vitro cultured mast cells, yet the presence of co-stimuli such as LPS or IL-4 optimizes the antigen presenting cell (APC) phenotype of these cells (Fig. 2).
Figure 2.
MHC class II restricted antigen presentation by mast cells. Different stimuli prime mast cells to express functional MHC class II molecules. Antigens are internalized via FcR and addressed to the endocytic pathway for presentation to Th.
MHC class II molecules expressed under these conditions are functional since they can be detected on mast cells surface using mAbs recognizing the mature form of I-Ab molecules.48,49
The observed synergy between IFNγ and IL-4 for class II upregulation in mast cells is somehow intriguing. While it is well established that IL-4 and IFNγ are major stimuli for MHC class II expression in the B-lymphoid and monocytic/macrophage lineages, respectively,58–61 it is not common that these two cytokines synergize on the same cellular subset. It is tempting to speculate that the capacity of mast cells to respond simultaneously to IFNγ and IL-4 might be instrumental to allow them to serve as tissue antigen presenting cells for both Th1 and Th2 lymphocytes.
It should be noted that although MHC class II expression can be detected on the surface of cultured mast cells, this phenomenon does not concern the entire population. Depending on the different stimuli applied (IFNγ alone or in association with LPS or IL-4) or on the cellular system employed (BMMC or PCMC) MHC class II expression is detected on cellular fractions ranging between 20 and 80%.20,48,49,52,62 Thus among the heterogeneous mast cell population only a fraction has the potential of acquiring an APC phenotype.
One limitation of studies performed using in vitro cultured mast cells is that these cells are deprived of the multiple tissue factors conditioning their responses in vivo. This limitation raises the question of whether the expression of functional class II molecules on mast cell surface might be an in vitro artifact. Two lines of evidence contribute to exclude this possibility. First, it has been shown that IFNγ treatment induces expression of mature MHC class II molecules on freshly isolated peritoneal mast cells.49 Second, a small fraction of mast cells expresses MHC class II molecules in tissues, namely 10–20% in normal human lung, ∼7% in normal human skin and ∼10% in pleural cavity. This fraction increases in tuberculin reactive skin and in autoimmune thyroiditis.54,63–65
The MHC class II biosynthetic pathway in mast cells is presently not fully characterized. Antigen processing and peptide loading into MHC class II molecules is a complex process that has been extensively described in professional APC such B cells or dendritic cells. In professional APC, class II MHC αβ dimers associated with invariant chain (Ii) are routed to early endosomal compartments. These molecular complexes follow the endocytic pathway to join late endosomes where Ii is degraded by endosomal proteases such as cathepsins. Only a short fragment of Ii (CLIP peptide) is left in the peptide-binding groove of MHC class II molecule. CLIP is then exchanged for peptides generated in the endosomes as a result of proteolytic degradation. Finally, MHC-peptide complexes are transported to the cell surface.66,67
This process is less investigated and still ill-understood in mast cells. Three types of lysosome-related organelles have been morphologically and biochemically identified in BMMC: type I granules (prelysosomal compartment) containing multivesicular bodies, type II granules (with features of both lysosomal compartment and secretory granules) containing electron-dense bodies and multivesicular bodies and type III granules containing electron-dense bodies.68 In contrast to professional APC, BMMC appear not to have a dedicated MHC class II compartment: MHC class II molecules are actually found accumulated in both type I and type II granules. Most of these MHC class II molecules are in an immature form and are still associated with Ii fragments. A low percentage (3%) of these MHC class II molecules is detected at the cell surface.68
MHC class II molecules accumulate in compartments similar to secretory granules also in the MHC-class II negative RBL rat mast cell line with transfected murine IAα, β and Ii chains.69 In this case, MHC class II molecules appear to be mature and loaded with antigenic peptides. Yet, the maturation of the IAαβIi complexes is very slow in RBL cells, as compared to B cells, since Ii is poorly hydrolyzed and remains associated with αβ dimers because of a lack of cathepsin S activity.69 Cathepsin S is an IFNγ-inducible protease that contributes to hydrolyze Ii in professional APC.70,71 It is tempting to speculate that in immature mast cells cathepsin S is expressed at low levels and that appropriate stimuli (such as IFNγ) enhance cathepsin S expression in mast cells allowing maturation of MHC-class II molecules. Further work is required to clarify this and related aspects of mast cell MHC class II biosynthetic pathway.
In addition to MHC class II molecule expression, mast cells also exhibit an inducible expression of selected accessory molecules that contribute to their APC phenotype. Similarly to MHC class II molecules, the upregulation of accessory molecules varies depending on the mast cell model employed and the stimulus applied. Following is a non-exhaustive list of accessory molecules described to be inducible in mast cell models.
Mast cells exhibit a basal expression of ICAM-1, yet ICAM-1 expression is upregulated by stimuli such as PMA, IL-4, IL-13, IFNγ, TNFα.20 Expression of the costimulatory molecule CD137-L is induced in BMMC by FcεRI cross-linking or by treatment with stem cell factor or LPS.72 Human in vitro cultured tonsil mast cells express CD54, OX40L and CD137-L and upregulate their expression following FceRI cross-linking.26 Finally, IFNγ/IL-4 treatment induces CD86 expression but not CD80 expression on mouse PCMC.49
In conclusion studies performed both in vitro and in vivo during the last 10–15 years have put forth the notion that mast cells can acquire an APC phenotype permitting Ag-presentation to class II restricted CD4+ T cells. This phenotype is variable, possibly due to the intrinsic heterogeneity of the mast cell populations and to their capacity to respond to a large panel of mediators.
Mast Cells Can Serve as Antigen Presenting Cells for CD4+ T Lymphocytes
The possibility that mast cells could present antigenic determinants to class II restricted T cells has been for a long time controversial. Initial studies showed that BMMC can present antigenic determinants to T-cell lines or hybridomas resulting in T-cell proliferation and IL-2 production.52,69,73,74 Furthermore, IgE-mediated capture of the antigen was shown to enhance its presentation by BMMC.75 One limitation of these initial studies was that in the experimental conditions employed it was not possible to formally exclude the possibility that bystander professional APC (possibly present as contaminant in T cells or mast cell preparation) could present antigenic determinants to T cells thus contributing to, or even determining, T-cell responses.
Kambayashi et al. recently addressed this point by carefully eliminating possible professional APC contaminants from cellular preparations before investigating antigen presentation by BMMC.44 They report that, in the absence of professional APC, BMMC cannot elicit activation of T cells, since although they can uptake antigens, they do not express on their surface functional class II molecules (both in resting conditions and after priming with IL-4, GM-CSF or IFNγ44). The study also reports that once BMMC capture ovalbumin via an IgE assisted mechanism, they undergo apoptosis and are phagocyted by DC that in turn present class II bound antigenic determinants.44
More recently, the same authors re-proposed the possibility that mast cells could present antigen to CD4+ T cells by showing that IFNγ plus LPS primed spleen-derived mast cells indeed express functional MCH class II molecules and can thus elicit CD4+ T responses also in the absence of bystander professional APC.48 In line with these observations are our findings obtained using PCMC primed with IFNγ plus IL-4.49 IFNγ/IL-4 primed PCMC express class II and costimulatory molecules and are able to capture, process and present OVA antigen to CD4+ effector OT-II T cells, but not to their naive counterparts.49 We also showed that not only in vitro cultivated PCMC but also freshly isolated peritoneal mast cells are able to activate effector CD4+ T cells to proliferation and cytokine production.49 The fact that mast cells selectively activate effector T cells suggests that mast cells, although primed to acquire APC phenotype are functionally defective when compared to profession APC and therefore cannot activate resting T cells. The fact that mast cells reside in tissues in the proximity of vessels and epithelial surfaces make it likely that they encounter effector Th cells and serve as tissular APC for these cells that have been previously activated in secondary lymphoid organs.
It is interesting to note that the capacity to present antigen in the context of MHC class II molecules also allows mast cells to interact with regulatory T cells. Kambayashi et al. showed that mast cell preferentially stimulate in vitro Treg as compared to conventional CD4+ T lymphocytes.48 Accordingly it has been recently shown that Foxp3+ Treg and mast cells are in close proximity in lymph nodes.36
Mast cells may therefore play a versatile role as antigen presenting cells in tissues. Being able to interact with both Th subsets and regulatory T cells they might, in some conditions amplify the effector phase of the immune response and in some other conditions contribute to peripheral tolerance.
Mast cells are not the only “nonconventional” APC that can present antigenic ligands to MHC class II restricted CD4+ T cells. It has been recently shown that basophils can capture, process and present class II restricted antigens and are implicated in the initiation of Th2 responses via IL-4 secretion in vivo.76–78 It is likely that, similarly to mast cells, basophils can also serve as occasional APC in tissues where, by forming cognate interaction with effector T cells, they might contribute to modulate adaptive immune responses and inflammation.
All in all recent data highlight the antigen-presenting role of mast cells for CD4+ T cells. It is predictable that a better definition of the molecular mechanisms and of the functional outcome of these interactions will be a central issue in cellular immunology during the next years.
The Th Cell/Mast Immunological Synapse
Immunological synapses (IS) are specialized signaling areas formed at the contact site between T lymphocytes and cognate antigen presenting cells characterized by the three-dimensional clustering and segregation of surface molecules and intracellular signaling components. Depending on the activation state of T lymphocytes, on the nature of the APC and on the strength and quality of antigenic stimulation, the IS exhibit various three-dimensional structures and mediate different biological functions.79,80 At the IS, complex inter-cellular information is exchanged and is traduced into versatile biological responses. Thus, investigating the structure and dynamics of IS is indeed important to decipher cell-cell communication.79,80
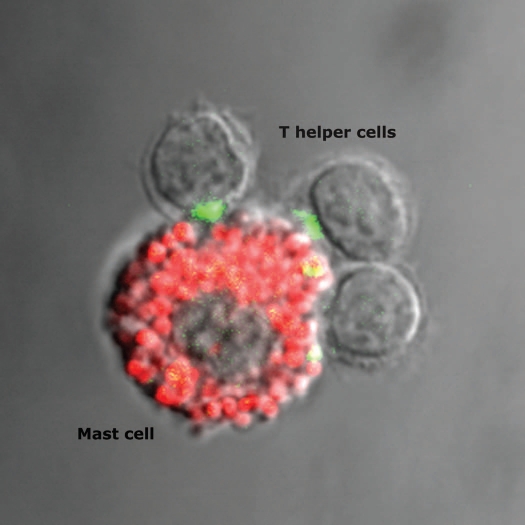
We recently investigated whether a productive IS is assembled at the Th cell/mast cell contact site. Co-culturing previously activated OT-II T cells with pulsed PCMC (either with native ovalbumin or the immunodominant I-Ab binding ovalbumin peptide) allowed the morphological assessment of IS formation.49 Using avidin-sulforhodamine 101 staining that specifically labels mast cell granules81,82 we could unambiguously demonstrate that OT-II T cells interact with Ag-loaded mast cells by forming an IS at the cell interface (Fig. 3 and ref. 49). Our study shows that a significant fraction of Th cell/PCMC conjugates, exhibit enrichment of filamentous actin and of phosphotyrosine staining (an early marker of signaling at the IS83). Additional early markers of productive TCR engagement, such as TCR/CD3 complexes and p56lck are also recruited to the T- cell/PCMC contact site.49
Figure 3.
Polarization of CD4+ T cells secretory machinery towards the antigen presenting mast cells. CD4+ OT-II T cells interacting with a peptide-pulsed peritoneal cell-derived mast cell (stained with avidin-sulforhodamine 101, red) are shown. Cells are stained for IFNγ (green). The IFNγ-filled Golgi apparatus (green) of two T cells is polarized towards the antigen presenting mast cell.
Furthermore, T cells in conjugation with cognate PCMC show sustained PKCθ recruitment at the IS and polarize their secretory machinery towards the antigen-presenting mast (as detected by tubulin and IFNγ staining). This last observation supports the notion that synapses formed between PCMC and Th cells are indeed productive. It is well established that Th cell activation results from the combination of sustained signaling (required for T-cell cytokine production and proliferation) and the rapid T-cell polarization response (required for dedicated help delivery).83,84 The observations that PKCθ is durably recruited for a sustained time to the IS and that the T-cell Golgi apparatus is filled with IFNγ and polarized towards the antigen-presenting mast cells shows that mast cells correctly trigger Th cell responses.
Similarly to in vitro differentiated PCMC, freshly isolated peritoneal mast cells can establish productive IS with OT-II T cells.49 This result shows that the capacity to form IS is an intrinsic property of bona fide mast cells. Interestingly, also in the case of cognate interactions between basophils and CD4+ T cells morphological evidence of IS formation has been recently provided.78
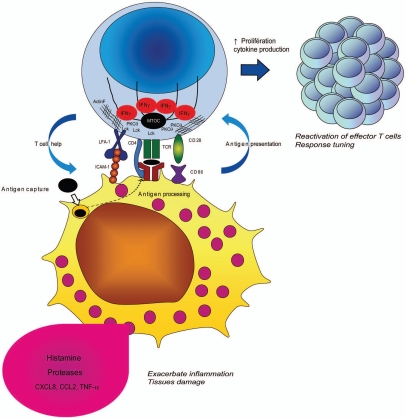
The description of immunological synapses formed at the Th cell/mast cell contact site highlights the central role of mast cells in adaptive immunity. It is tempting to speculate that the formation of IS between mast cells and effector CD4+ T lymphocytes might optimize activation of both cell subsets during tissular immune responses (Fig. 4).
Figure 4.
Cell-cell cooperation at the helper T cell/mast cell immunological synapse. Upon conjugation with antigen presenting mast cells, T cells polarize their secretory machinery and form an immunological synapse. The cognate interaction allows functional cooperation between the two cells resulting in amplification of the immune response and/or inflammation.
For Th cells, antigen-presenting mast cells might serve as platforms for re-stimulation and fine-tuning, strategically scattered in tissues. By encountering antigen-presenting mast cells in tissues, effector T cells may keep an activation state instrumental for the efficiency of their effector function. In addition, presentation by these versatile APC (able to influence T-cell differentiation by releasing a variety of mediators) might favor the plasticity of Th cell responses in tissues. For mast cells, the formation of IS with Th cells might create an area of intimate cellular contact favorable to the dedicated secretion of cytokines by Th cells and to the engagement of stimulatory receptors with their ligands. In other words, the physical interaction of mast cells with Th cells may allow them to be more efficiently activated by Th cell-derived factors and, in turn, enhance their biological responses. It is tempting to speculate that Th cell/mast cell cognate interactions might cooperate with additional signals received by mast cells via Fc receptors and/or PRR allowing cross-talks between innate and adaptive immune stimuli. While these cognate interactions can be instrumental to boost immune responses in tissues, they might become detrimental in some inflammatory conditions.
In conclusion, an increasing number of reports on functional cross-talk and cognate interactions between mast cell and Th lymphocytes contributed to put forth the idea that mast cells are central actors in adaptive immune responses. Moreover, the participation of mast cells to adaptive immune response is not limited to their interaction with Th cell. Mast cells have been indeed shown to interact with and present antigen also to different lymphocyte subsets including Treg and CD8+ T lymphocytes.48,85
The antigen-presenting role of mast cell might be further recognized in the future and is presently an active field of investigation.
Abbreviations
- APC
antigen presenting cell
- BMMC
bone marrow-derived mast cells
- HMC-1
human mast cell line-1
- IS
immunological synapse
- LPS
lipopolysaccharid
- MHC
major histocompatibility complex
- MTOC
microtubule-organizing center
- PMA
phorbol myristate acetate
- PCMC
peritoneal cell-derived mast cells
- PRR
pattern recognition receptor
- TCR
T-cell receptor
- TLR
toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/selfnonself/article/11795
References
- 1.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 4.Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010;3:111–128. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–2614. [PubMed] [Google Scholar]
- 7.Heib V, Becker M, Taube C, Stassen M. Advances in the understanding of mast cell function. Br J Haematol. 2008;142:683–694. doi: 10.1111/j.1365-2141.2008.07244.x. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 9.Stelekati E, Orinska Z, Bulfone-Paus S. Mast cells in allergy: innate instructors of adaptive responses. Immunobiology. 2007;212:505–519. doi: 10.1016/j.imbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 10.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 11.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNFalpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 12.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNFalpha)/cachectin by mouse mast cells stimulated via the FcepsilonRI. A mechanism for the sustained action of mast cell-derived TNFalpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNFalpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 14.Kaartinen M, Penttila A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNFalpha. Circulation. 1996;94:2787–2792. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 15.Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- 16.Kunder CA, St. John AL, Li G, Leong KW, Berwin B, Staats HF, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol. 2007;178:2549–2555. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- 20.Henz BM, Maurer M, Lippert U, Worm M, Babina M. Mast cells as initiators of immunity and host defense. Exp Dermatol. 2001;10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x. [DOI] [PubMed] [Google Scholar]
- 21.Rumsaeng V, Cruikshank WW, Foster B, Prussin C, Kirshenbaum AS, Davis TA, et al. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J Immunol. 1997;159:2904–2910. [PubMed] [Google Scholar]
- 22.Tedla N, Wang HW, McNeil HP, Di Girolamo N, Hampartzoumian T, Wakefield D, et al. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1alpha and MIP-1beta. J Immunol. 1998;161:5663–5672. [PubMed] [Google Scholar]
- 23.Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 26.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 27.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18:751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 29.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177:3577–3581. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 30.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 32.Razin E, Ihle JN, Seldin D, Mencia-Huerta JM, Katz HR, LeBlanc PA, et al. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132:1479–1486. [PubMed] [Google Scholar]
- 33.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 34.Yanagida M, Fukamachi H, Ohgami K, Kuwaki T, Ishii H, Uzumaki H, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5 and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- 35.Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD. Induction and enhancement of Fc(epsilon)RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J Immunol. 1998;160:4026–4033. [PubMed] [Google Scholar]
- 36.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharyya SP, Drucker I, Reshef T, Kirshenbaum AS, Metcalfe DD, Mekori YA. Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J Leukoc Biol. 1998;63:337–341. doi: 10.1002/jlb.63.3.337. [DOI] [PubMed] [Google Scholar]
- 38.Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNFalpha. J Immunol. 2001;167:4008–4016. doi: 10.4049/jimmunol.167.7.4008. [DOI] [PubMed] [Google Scholar]
- 39.Smith TJ, Weis JH. Mucosal T cells and mast cells share common adhesion receptors. Immunol Today. 1996;17:60–63. doi: 10.1016/0167-5699(96)80580-9. [DOI] [PubMed] [Google Scholar]
- 40.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 41.Friedman MM, Kaliner M. In situ degranulation of human nasal mucosal mast cells: ultrastructural features and cell-cell associations. J Allergy Clin Immunol. 1985;76:70–82. doi: 10.1016/0091-6749(85)90807-3. [DOI] [PubMed] [Google Scholar]
- 42.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 43.Daeron M, Voisin GA. Mast cell membrane antigens and Fc receptors in anaphylaxis I. Products of the major histocompatibility complex involved in alloantibody-induced mast cell activation. Immunology. 1979;38:447–458. [PMC free article] [PubMed] [Google Scholar]
- 44.Kambayashi T, Baranski JD, Baker RG, Zou T, Allenspach EJ, Shoag JE, et al. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–1496. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, et al. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465–6475. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 46.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, et al. Induction of mast cell proliferation, maturation and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enerback L. Mast cells in rat gastrointestinal mucosa 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- 48.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 50.Wong GH, Clark-Lewis I, McKimm-Breschkin JL, Schrader JW. Interferon-gamma-like molecule induces Ia antigens on cultured mast cell progenitors. Proc Natl Acad Sci USA. 1982;79:6989–6993. doi: 10.1073/pnas.79.22.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch N, Wong GH, Schrader JW. Ia antigens and associated invariant chain are induced simultaneously in lines of T-dependent mast cells by recombinant interferon-gamma. J Immunol. 1984;132:1361–1369. [PubMed] [Google Scholar]
- 52.Frandji P, Oskeritzian C, Cacaraci F, Lapeyre J, Peronet R, David B, et al. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J Immunol. 1993;151:6318–6328. [PubMed] [Google Scholar]
- 53.Warbrick EV, Taylor AM, Botchkarev VA, Coleman JW. Rat connective tissue-type mast cells express MHC class II: upregulation by IFNgamma. Cell Immunol. 1995;163:222–228. doi: 10.1006/cimm.1995.1120. [DOI] [PubMed] [Google Scholar]
- 54.Banovac K, Neylan D, Leone J, Ghandur-Mnaymneh L, Rabinovitch A. Are the mast cells antigen presenting cells? Immunol Invest. 1989;18:901–906. doi: 10.3109/08820138909050768. [DOI] [PubMed] [Google Scholar]
- 55.Poncet P, Arock M, David B. MHC class II-dependent activation of CD4+ T cell hybridomas by human mast cells through superantigen presentation. J Leukoc Biol. 1999;66:105–112. doi: 10.1002/jlb.66.1.105. [DOI] [PubMed] [Google Scholar]
- 56.Grabbe J, Karau L, Welker P, Ziegler A, Henz BM. Induction of MHC class II antigen expression on human HMC-1 mast cells. J Dermatol Sci. 1997;16:67–73. doi: 10.1016/s0923-1811(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 57.Love KS, Lakshmanan RR, Butterfield JH, Fox CC. IFNgamma-stimulated enhancement of MHC class II antigen expression by the human mast cell line HMC-1. Cell Immunol. 1996;170:85–90. doi: 10.1006/cimm.1996.0137. [DOI] [PubMed] [Google Scholar]
- 58.Littman BH, Dastvan FF, Carlson PL, Sanders KM. Regulation of monocyte/macrophage C2 production and HLA-DR expression by IL-4 (BSF-1) and IFNgamma. J Immunol. 1989;142:520–525. [PubMed] [Google Scholar]
- 59.Pai RK, Askew D, Boom WH, Harding CV. Regulation of class II MHC expression in APCs: roles of types I, III and IV class II transactivator. J Immunol. 2002;169:1326–1333. doi: 10.4049/jimmunol.169.3.1326. [DOI] [PubMed] [Google Scholar]
- 60.Lombard-Platet S, Meyer V, Ceredig R. Both IFNgamma and IL-4 induce MHC class II expression at the surface of mouse pro-B cells. Dev Immunol. 1997;5:115–120. doi: 10.1155/1997/76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuart PM, Zlotnik A, Woodward JG. Induction of class I and class II MHC antigen expression on murine bone marrow-derived macrophages by IL-4 (B cell stimulatory factor 1) J Immunol. 1988;140:1542–1547. [PubMed] [Google Scholar]
- 62.Frandji P, Tkaczyk C, Oskeritzian C, David B, Desaymard C, Mecheri S. Exogenous and endogenous antigens are differentially presented by mast cells to CD4+ T lymphocytes. Eur J Immunol. 1996;26:2517–2528. doi: 10.1002/eji.1830261036. [DOI] [PubMed] [Google Scholar]
- 63.Reshef A, MacGlashan DW. Immunogold probe for the light-microscopic phenotyping of human mast cells and basophils. J Immunol Methods. 1987;99:213–219. doi: 10.1016/0022-1759(87)90130-x. [DOI] [PubMed] [Google Scholar]
- 64.Suzumura Y, Ohasi M. Immunoelectron microscopic localization of HLA-DR antigen on mast cells and vessels in normal and tuberculin-reactive skin. Am J Dermatopathol. 1991;13:568–574. doi: 10.1097/00000372-199113060-00007. [DOI] [PubMed] [Google Scholar]
- 65.Banovac K, Ghandur-Mnaymneh L, Leone J, Neylan D, Rabinovitch A. Intrathyroidal mast cells express major histocompatibility complex class-II antigens. Int Arch Allergy Appl Immunol. 1989;90:43–46. doi: 10.1159/000234998. [DOI] [PubMed] [Google Scholar]
- 66.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 67.Pierre P, Mellman I. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 1998;93:1135–1145. doi: 10.1016/s0092-8674(00)81458-0. [DOI] [PubMed] [Google Scholar]
- 68.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincent-Schneider H, Thery C, Mazzeo D, Tenza D, Raposo G, Bonnerot C. Secretory granules of mast cells accumulate mature and immature MHC class II molecules. J Cell Sci. 2001;114:323–334. doi: 10.1242/jcs.114.2.323. [DOI] [PubMed] [Google Scholar]
- 70.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 71.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, et al. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 72.Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamoto M, Kinoshita T, et al. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood. 2005;106:4241–4248. doi: 10.1182/blood-2005-04-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fox CC, Jewell SD, Whitacre CC. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994;158:253–264. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]
- 74.Frandji P, Tkaczyk C, Oskeritzian C, Lapeyre J, Peronet R, David B, et al. Presentation of soluble antigens by mast cells: upregulation by interleukin-4 and granulocyte/macrophage colony-stimulating factor and down-regulation by interferon-gamma. Cell Immunol. 1995;163:37–46. doi: 10.1006/cimm.1995.1096. [DOI] [PubMed] [Google Scholar]
- 75.Tkaczyk C, Viguier M, Boutin Y, Frandji P, David B, Hebert J, et al. Specific antigen targeting to surface IgE and IgG on mouse bone marrow-derived mast cells enhances efficiency of antigen presentation. Immunology. 1998;94:318–324. doi: 10.1046/j.1365-2567.1998.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 77.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trautmann A, Valitutti S. The diversity of immunological synapses. Curr Opin Immunol. 2003;15:249–254. doi: 10.1016/s0952-7915(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 80.Valitutti S, Dupre L. Plasticity of immunological synapses. Curr Top Microbiol Immunol. 340:209–228. doi: 10.1007/978-3-642-03858-7_11. [DOI] [PubMed] [Google Scholar]
- 81.Tharp MD, Seelig LL, Jr, Tigelaar RE, Bergstresser PR. Conjugated avidin binds to mast cell granules. J Histochem Cytochem. 1985;33:27–32. doi: 10.1177/33.1.2578142. [DOI] [PubMed] [Google Scholar]
- 82.Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol. 1984;83:214–218. doi: 10.1111/1523-1747.ep12263584. [DOI] [PubMed] [Google Scholar]
- 83.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 84.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]