Abstract
A concentration-function approach to vitamin C (ascorbate) has yielded new physiology and pharmacology discoveries. To determine the range of vitamin C concentrations possible in humans, pharmacokinetics studies were conducted. They showed that when vitamin C is ingested by mouth, plasma and tissue concentrations are tightly controlled by at least 3 mechanisms in healthy humans: absorption, tissue accumulation, and renal reabsorption. A 4th mechanism, rate of utilization, may be important in disease. With ingested amounts found in foods, vitamin C plasma concentrations do not exceed 100 μmol/L. Even with supplementation approaching maximally tolerated doses, ascorbate plasma concentrations are always <250 μmol/L and frequently <150 μmol/L. By contrast, when ascorbate is i.v. injected, tight control is bypassed until excess ascorbate is eliminated by glomerular filtration and renal excretion. With i.v. infusion, pharmacologic ascorbate concentrations of 25–30 mmol/L are safely achieved. Pharmacologic ascorbate can act as a pro-drug for hydrogen peroxide (H2O2) formation, which can lead to extracellular fluid at concentrations as high as 200 μmol/L. Pharmacologic ascorbate can elicit cytotoxicity toward cancer cells and slow the growth of tumors in experimental murine models. The effects of pharmacologic ascorbate should be further studied in diseases, such as cancer and infections, which may respond to generation of reactive oxygen species via H2O2.
Introduction and background
For many years, the RDA for all vitamins were based on preventing deficiency, with a margin of safety (1, 2). For vitamin C, 2 other criteria were added. The vitamin C RDA was set as that amount that conferred protection against scurvy for 1 mo if intake stopped and that approximated the threshold for accelerated renal excretion (2, 3). Vitamin C was thought to always be excreted in urine, but excretion was thought to sharply increase when plasma concentrations became high enough (2, 4, 5).
Rather than deficiency, a more desirable strategy, with the potential to optimize nutrient intake, is a concentration-function approach. Its roots are in experiments from 70 y ago in which kinetics relationships were described between nutrient concentration and bacterial growth (6). Both Linus Pauling and Roger Williams (7, 8) (who discovered pantothenic acid and named folic acid) expanded the approach by recognizing that enzyme mutations could shift Km and/or Vmax for nutrients, concepts explored more recently and in greater detail by Ames (9). The next logical step of a concentration-function approach is to determine kinetics relationships for a vitamin with respect to dominant wild-type (i.e. not mutated) enzyme action in situ, meaning literally “in position,” in cells and tissues (10). For human recommendations, this should be followed by determination of vitamin kinetics in vivo (11). Any vitamin can be selected to test this approach, although a water-soluble vitamin would have simpler distribution properties, and choice of vitamin C was arbitrary. A concentration-function approach, in a simpler fashion, can be considered an x-y axes approach, where x is concentration (the abscissa, the independent variable) and y is function (the ordinate, the dependent variable) (12, 13).
Kinetics in situ were validated for the first time for vitamin C, and probably for any vitamin, using intact adrenal medullary secretory vesicles, also termed chromaffin granules, which are localized within adrenal medullary cells. Kinetics of vitamin C for dopamine β-monooxygenase (also called dopamine β-hydroxylase) and for transmembrane electron transfer were described, and the mechanism of ascorbate action was different and more complex than its action with the isolated enzyme (13–17). In the presence of its substrate dopamine, the isolated enzyme dopamine β-monooxygenase directly accepts electrons from ascorbate in solution. In situ, dopamine β-monooxygenase is localized within chromaffin granules. Ascorbate is found both in the cytosol of adrenal medullary cells and within chromaffin granules, but chromaffin granules are impermeant to ascorbate itself. When dopamine is present, dopamine β-monooxygenase within chromaffin granules accepts single electrons from intragranular ascorbate, generating ascorbate radical. Intragranular ascorbate radical is reduced back to intragranular ascorbate via transmembrane electron transfer from cytosolic ascorbate, mediated by the chromaffin granule transmembrane protein cytochrome b561. In situ, dopamine β-monooxygenase and transmembrane electron transfer have distinct kinetics properties with respect to ascorbate (15, 16). Together, these data provided key conceptual evidence and showed that kinetics in situ were necessary rather than those for isolated enzymes or reactions.
Current knowledge
Clinical pharmacokinetics for vitamin C: tight control and underlying mechanisms
In situ kinetics for organelles, cells, and even organ systems were suggestive but clearly not adequate for nutrient recommendations in humans. What was needed next was knowledge of x-axis concentrations in vivo, in humans, that then drive y-axis function. x-Axis concentrations in vivo are determined by pharmacokinetics, which describe relationships between doses and concentrations achieved. For this reason, clinical vitamin C pharmacokinetics were characterized in healthy young men and women, using a depletion-repletion design (18–21). Participants were hospitalized to carefully control vitamin C intake and received an inpatient diet containing <5 mg/d of vitamin C. When their plasma concentrations fell to <8 μmol/L, they were administered vitamin C twice daily in the fasted state. The study was designed to approximate vitamin C doses from foods or supplements. Doses ranged from 30 to 2500 mg/d and were not increased until participants achieved steady state for each dose. Steady state was rigorously defined as at least 5 separate measurements of plasma concentrations over at least 1 wk with <10% SD. At each steady state, the following was performed: absolute bioavailability studies, isolation of circulating cells for vitamin C content, and collection of urine to determine vitamin C and metabolite excretion. Although dosing more frequently than twice daily would have been ideal, it was impractical to administer more doses to fasted participants and retain them in the study, because the average hospitalization time was ∼6 mo.
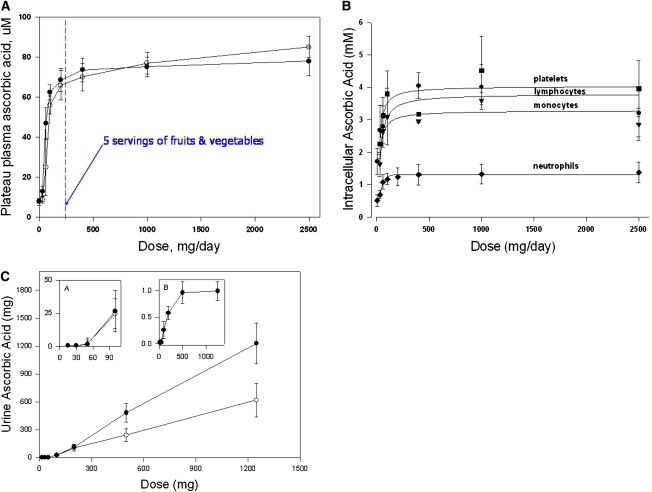
The clinical pharmacokinetics studies showed that vitamin C concentrations in plasma and tissues were tightly controlled (Fig. 1A-C) (18, 21). At doses < 100 mg/d, there was a steep sigmoidal relationship between dose and concentrations. At doses > 100 mg/d, plasma concentrations reached a plateau between 70 and 80 μmol/L. At doses > 400 mg/d, further increases in plasma concentrations were minimal. At least 3 mechanisms were responsible for tight control: absorption (bioavailability), tissue transport, and renal reabsorption and excretion. Not explored in these studies is a potential 4th mechanism, utilization, which is discussed below. These clinical pharmacokinetics data are used to derive RDA for vitamin C in many countries (21, 22).
Figure 1.
(A) Plasma vitamin C concentrations as a function of dose. The relationship between oral doses of vitamin C and the mean fasting steady-state plasma vitamin C concentration in 7 healthy men and 15 healthy women are shown. The daily doses of vitamin C were: 30, 60, 100, 200, 400, 1000, and 2500 mg. The dose concentration curve is sigmoidal with its steep portion between 30 and 100 mg of vitamin C daily. Plasma vitamin C concentrations likely to be attained by the consumption of 5 servings of fruits and vegetables per day (containing ∼200 mg of vitamin C) are also shown. It is possible that vitamin C bioavailability from fruits and vegetable is less than that from oral doses of pure vitamin C in solution. Adapted from (18) and (21). (B) Intracellular vitamin C concentrations in circulating cells as a function of dose in healthy women. Cells were isolated when steady state was achieved for each dose. Reproduced from (21) with permission of The National Academy of Sciences, Washington DC. (C) Urinary vitamin C excretion as a function of single vitamin C doses at steady state in 7 healthy men. Vitamin C excretion over 24 h was determined after administration of single doses given either orally or i.v. (Inset A) Vitamin C excretion for single oral or intravenous doses of 15–100 mg. x-axis indicates dose, y-axis indicates amount (mg) excreted in urine. (Inset B) Fractional excretion (the fraction of the dose excreted) after i.v. administration of single doses of vitamin C. x-axis indicates dose, y-axis indicates fractional excretion (vitamin C excreted in urine in milligrams divided by the vitamin C dose in milligrams). Reproduced from (18) with permission of The National Academy of Sciences, Washington DC.
Ascorbate transport and its transporters, SLC23A1 (SVCT1) and SLC23A2 (SVCT2), play a key role in tight control (23–25). The widely distributed tissue transporter SLC23A2 is responsible for ascorbate accumulation against its concentration gradient in many tissues. Knockout mice for this transporter have generalized severe ascorbate deficiency and die at birth (24), indicating that ascorbate transport, as ascorbate itself, is necessary for tissue accumulation. Some investigators have advanced the idea that the dominant mechanism of ascorbate accumulation occurs by transport of oxidized ascorbate, dehydroascorbic acid, followed by its intracellular reduction (26, 27). If this were correct, then SLC23A2 knockout mice should not have had severe tissue deficiency, as was found. It remains possible that dehydroascorbic acid transport has a role in specialized cells, such as those that oxidize ascorbate by generating oxidants (i.e. neutrophils) or those that lack ascorbate transporters entirely (red blood cells). Nevertheless, these data provide clear evidence that ascorbate transport as such is the primary mechanism of ascorbate accumulation and that dehydroascorbic acid and ascorbate cannot be considered equivalent.
The epithelial ascorbate transporter SLC23A1 plays a central role in tight control by mediating ascorbate renal reabsorption. Knockout SLC23A1 mice completely lose their ability to reabsorb vitamin C in kidney and lose tight control (25). However, these mice continue to absorb intestinal ascorbate. It is not known whether SLC23A2 is upregulated to compensate or if another uncharacterized intestinal ascorbate transporter exists or is induced.
Data from SLC23A1 knockout mice imply that there is a renal threshold for ascorbate reabsorption (25). Clinical data concerning a threshold of ascorbate reabsorption are contradictory. Earlier data sets indicated that there was always ascorbate loss in urine regardless of plasma concentration, but that loss accelerated beyond a certain plasma value (4, 5). Comprehensive pharmacokinetics studies indicate that there is a distinct renal threshold. It is likely that the discrepancy is due to assay artifact in the former studies, which was eliminated with more precise assays (28). The exact renal threshold remains to be defined. Correlation with this threshold, plasma concentration, and ascorbate intake could influence vitamin intake recommendations.
An unexpected finding with clinical implications is that SLC23A1 knockout mice have a functional outcome in relation to ascorbate concentrations that is not scurvy. Pregnant SLC23A1 knockout mice lose approximately one-half of their pups despite ascorbate concentrations that are several-fold above those associated with scurvy. Raising plasma ascorbate concentrations from ∼30 to 40 μmol/L prevents fetal loss in these mice (25).
When ascorbate and pregnancy findings are extended to humans, findings appear contradictory, although an explanation is obvious when tight control is considered. In 1 British study, vitamin C plus vitamin E supplements decreased preeclampsia and associated complications (29). In this study, vitamin C concentrations at entry and after supplementation were measured. Initial concentrations were ∼26 μmol/L and increased to 45 μmol/L with supplementation. This study had relatively few patients with complications and used both ascorbate and tocopherol. In 2 larger and more recent studies using ascorbate alone, no effects were found on preeclampsia and complications (30, 31). However, in both of these studies, participants at study entry were ingesting at least 100 mg/d of ascorbate. Unlike the prior British study, no ascorbate concentrations were measured, and it is likely that participants were close to saturation, beyond the steep portion of the dose concentration curve (32) (Fig. 1A). Because of tight control, ascorbate supplements in these participants were expected to make minimal if any difference to plasma and tissue concentrations and would not be expected to change any outcomes. This is precisely what was observed. A key lesson from these types of studies was pointed out >10 y ago and deserves reemphasis (33). To detect potential differences from ascorbate supplementation, concentrations at study entry must be measured. If concentrations are low and are subsequently raised by ascorbate supplements, then outcome differences can be assessed. However, if ascorbate concentrations at study entry are to the right of the steep portion of the ascorbate tight control curve (Fig. 1), then supplements will make minimal difference in attained concentrations, and outcome assessments are likely to show no differences and should not be undertaken. The issue of initial and concluding nutrient concentrations in nutrient supplementation outcomes studies is of central importance. Vitamin C supplementation, without consideration of initial vitamin C concentrations, is likely to produce a negative result without resolving the question of whether vitamin C supplementation is useful for the condition studied (33–36).
Another unexpected finding in SLC23A1 mice with translational implications is that they upregulated ascorbate biosynthesis in response to low ascorbate concentrations from their renal leak (25). Ascorbate concentrations could feedback directly on the biosynthetic pathway. Alternatively, there are supporting data for pituitary mediation of ascorbate feedback sensing (37). Humans and other primates do not make ascorbate. Via feedback sensing, it remains possible that the sensing pathways remain intact in humans, with ascorbate precursors displaying inverse concentrations in relation to ascorbate concentrations. Similar sensing mechanisms explain many hormone systems in humans and guide clinical hormone replacement therapies. It will be extremely worthwhile to determine whether ascorbate “sensing” occurs in humans, because this could provide a completely new and quantitative means to assess individual ascorbate needs (25).
In theory, dose concentration relationships can be affected by changes in utilization rates. Healthy participants had different utilization rates, although this did not affect steady-state concentrations for different doses (10, 18, 21, 38, 39). It is possible that ascorbate utilization increases in disease by acceleration of ascorbate-dependent enzymatic reaction rates, by decrease in reduction of ascorbate radical dehydroascorbic acid, or by accelerated nonenzymatic degradation due to disease-associated oxidants. Ascorbate concentrations are lower in smokers, probably because of smoking-associated oxidants (40). Ascorbate concentrations are lower in patients with pancreatitis, sepsis, crucial illness, acute myocardial infarction, and diabetes (41–45). Why concentrations are lower is often difficult to determine. Patients may have had low ascorbate concentrations due to poor intake from the underlying disease. Low concentrations can also be due to transient redistribution, inappropriate renal loss, or assay loss or artifact without proper controls (46). Despite these difficulties, it is worth knowing whether ascorbate utilization increases in disease, because this could guide replacement and possibly affect outcomes. For ideal characterization of ascorbate utilization, it will be necessary to study metabolism of an ascorbate stable heavy isotope in healthy participants to obtain a range of utilization rates at different steady-state concentrations.
Tight control and the bioavailability surprise
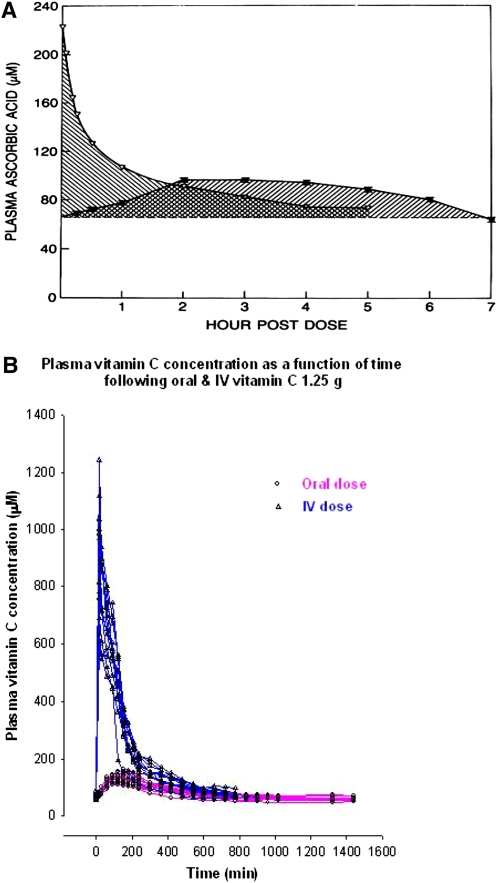
As part of determining mechanisms of tight control, absolute bioavailability was evaluated for different vitamin C doses. Bioavailability was determined by comparing area under curve for oral compared with i.v. administration when participants were at steady state (Fig. 2A,B; Table 1). As the doses increased, bioavailability declined, indicating that intestinal absorption contributed to tight control.
Figure 2.
(A) Determination of ascorbic acid bioavailability in a single participant using area under curve (AUC) methodology. Bioavailability was determined by calculating the ratio of the AUC of vitamin C plasma concentrations, following oral and i.v. administration of the same dose of vitamin C on successive days. AUC was calculated by the linear trapezoidal method. In the example shown, a healthy male was given 200-mg doses of vitamin C after he had attained steady state for that dose. (B) Plasma vitamin C concentrations in healthy volunteers as a function of time. Twelve participants (3 men, 9 women) were administered vitamin C 1.25 g in the fasting state after they had attained steady state for that dose. Plasma vitamin C concentrations following i.v. or oral administration are shown. Following i.v. administration, blood samples were collected at 0, 2.5, 5, 10, 15, and 30 min, and at 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, and 10 h. Following oral administration, blood samples were collected at 0, 15, and 30 min, and at 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9,10, 11, 12, 13, 14, 15, 16, 19, 22, and 24 h. Adapted from (58) with permission of the Annals of Internal Medicine.
Table 1.
Bioavailability of vitamin C in 7 healthy men at steady state for each dose1
| Dose, mg | Median bioavailability, % |
| 15 | 89 |
| 30 | 87.3 |
| 50 | 58 |
| 100 | 80 |
| 200 | 72 |
| 500 | 63 |
| 1250 | 46 |
Vitamin C bioavailability was calculated using a multi-compartment mathematical model as described (20).
Bioavailability data showed that at doses > 200 mg/d, i.v. administration bypassed tight control. This was most easily seen at the highest dose tested for bioavailability: 1.25 g (Fig. 2A). Administration of this dose i.v. to participants at steady state for the dose produced plasma concentrations approaching 1 mmol/L, a concentration not possible with oral dosing. These data indicated that i.v. administration of pharmacologic ascorbate doses, i.e. those > 500 mg, was similar to drug administration.
That i.v. dosing might matter had unexpected roots and even more unexpected consequences. In 1959 the Canadian physician William McCormick hypothesized that vitamin C could be useful in cancer treatment (47). The hypothesis was simple. Cancer metastasized by invading collagen, and collagen could be strengthened by vitamin C. Ewan Cameron (48), a Scottish surgeon, expanded the hypothesis by invoking the role of hyaluronidase in cancer metastases. Hyaluronidase was proposed to destroy collagen to facilitate metastases, and ascorbate would inhibit hyaluronidase. Cameron (49, 50) began administering up to 10 g of ascorbate to patients with advanced cancers, with meticulous documentation of several cases. Encouraged by these findings, Cameron contacted Linus Pauling, the 2-time Nobel Laureate who was advocating ascorbate for treatment of common colds. Cameron and Pauling (51, 52) published 2 high-profile case series that suggested gram doses of ascorbate could improve survival in cancer. Pauling (53) publicly challenged the medical establishment with resisting consideration of his findings. Charles Moertel et al. at the Mayo Clinic rose to Pauling’s challenge. To replicate the Cameron/Pauling findings, Mayo Clinic investigators conducted 2 double-blinded, placebo-controlled trials using the same ascorbate dose of 10 g/d (54, 55). The first trial showed no difference in survival, but Pauling objected, because the patients had received prior cancer treatment. The second trial was conducted on patients with metastatic colon cancer who had not received chemotherapy. Again, there was no difference in survival between treated and untreated patients, and ascorbate was deemed ineffective as cancer therapy (55, 56). Although much smoke, fire, and bad feelings were generated, the story seemed to have dead ended (53) until the bioavailability findings emerged. Ascorbate clearly was different if administered i.v. vs. orally. The Mayo Clinic investigators administered only oral ascorbate, but Cameron administered both i.v. and oral ascorbate. This difference was not recognized previously by any investigators, because no one provided plasma measurements so that the pharmacology explanation remained obscured.
Given its potential promise, a call was issued for oncologists to investigate ascorbate anew (57). But there was no response, perhaps due to skepticism and even bitterness engendered by the earlier battles among Pauling, Moertel, oncologists, and complementary and alternative medicine (integrative medicine) practitioners (53).
Pharmacologic ascorbate
As a prerequisite for reexamining ascorbate in cancer, it was necessary to confirm whether the bioavailability findings were correct. All bioavailability data from all participants were pooled for doses of 15–1250 mg and bioavailability was modeled for doses of 2–100 g. Analyses of all data showed that i.v. pharmacologic doses of ascorbate could produce plasma concentrations 70-fold higher than those possible with maximally tolerated oral doses (58). Plasma concentrations of 10 mmol/L (10,000 μmol/L) were predicted to be attainable for >3 h with infusion rates of 0.5–1 g/min. For i.v. administration, plasma concentrations are expected to vary depending on the dose, rate of infusion, and frequency of infusion. This was subsequently confirmed in animals and humans (59–61).
In contrast to data from i.v. (parenteral) administration, maximal peak plasma concentrations from oral dosing were predicted, and subsequently found by others, to be <0.25 mmol/L (58, 62, 63). Although peak oral concentrations perhaps could also vary due to dose frequency, variations are minimal compared with concentrations attainable with i.v. administration. The upper limit vitamin C dose in the Dietary Reference Intakes guidelines is 2 g and maximally tolerated oral doses are in the range of 3–4 g (22). Oral administration is limited by osmotic diarrhea and saturation of intestinal absorption and by currently available means could never produce peak plasma concentrations approaching 1 mmol/L (58). Additional risks of gram doses are hemolysis in patients with glucose 6 phosphate deficiency and paroxysmal nocturnal hemoglobinuria; oxalate renal stones in patients with hyperoxaluria; and worsening of iron overload in patients with hemochromatosis or who receive chronic RBC transfusions (33, 39).
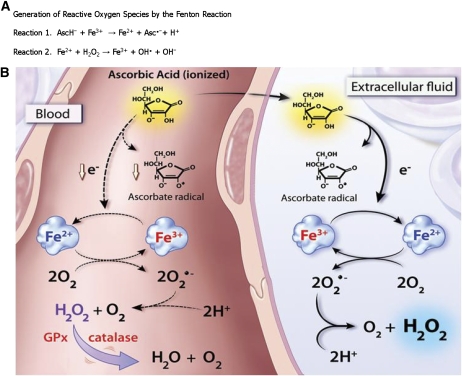
Given the new knowledge of the clinical pharmacology profile of parenteral ascorbate, the next steps were to determine whether and how pharmacologic ascorbate could selectively kill cancer cells. To mimic clinical pharmacology, cancer and normal cells were incubated for 1 h in cell culture media containing pharmacologic ascorbate concentrations ranging from 0 to 20 mmol/L. Of the normal cells tested, none were affected by 20 mmol/L ascorbate. In contrast, more than three-quarters of the 43 cancer lines tested showed sensitivity of ≤10 mmol/L, defined as the effective ascorbate concentrations for killing 50% of a cancer cell type (EC50) (60, 64). Addition of the enzyme catalase to the cell culture media, which catalyzes decomposition of hydrogen peroxide (H2O2) to oxygen and water, ameliorated the cytotoxicity of pharmacologic ascorbate. Based on these and other in vitro experiments, the killing of cancer cells was proposed to be mediated by H2O2 formation, which in the presence of reduced transition metal catalysts is classically thought to produce the highly reactive hydroxyl radical (OH·) species. This is commonly referred to as Fenton chemistry, where ferrous iron (Fe2+) is oxidized by H2O2 to yield ferric iron (Fe3+) and OH· (Fig. 3A).
Figure 3.
(A) In reaction 1, ascorbate (AscH−) reacts with ferric iron (Fe3+) to produce ferrous iron (Fe2+) and ascorbate radical (Asc·−). In reaction 2, the classic Fenton reaction generates the hydroxyl radical (OH·) species from H2O2. (B) Proposed mechanism of formation of ascorbate radical and H2O2 in extracellular fluid compared with blood. After oral and parenteral administration, ascorbic acid achieves equivalent pharmacological concentrations in blood (left side of the diagram) and extracellular fluid (right side). In extracellular fluid, a molecule of ascorbic acid loses 1 electron and forms ascorbate radical. This electron subsequently reduces a protein-centered metal, shown as the reduction of Fe3+ to Fe2+. This complex donates an electron to molecular oxygen, forming superoxide anion (O2·−) with ensuing dismutation to H2O2. In blood (left side), these reactions are damped or inhibited (dashed lines). The appearance of ascorbate radical is inhibited by RBC membrane-bound reducing proteins and/or by large plasma proteins that do not distribute to the extracellular space. RBC enzymes glutathione peroxidase and catalase destroy H2O2 so that none is detectable in blood. The identities of the metal-centered proteins are unknown. Reproduced from (59) with permission of The National Academy of Sciences, Washington DC.
For >100 y, the exact chemical and biochemical nature of these reaction intermediates and the complicated cycles they participate in has been a subject of study and debate. Transition metals such as iron (and copper) are easily reduced by ascorbate. These reduced metal centers can donate their electron to molecular oxygen to produce the species known as superoxide (O2·−). Likewise, the reverse reaction can occur where O2·− reduces the oxidized metal. This reverse reaction may be in competition with superoxide dismutases that catalytically remove O2·-−, thereby producing H2O2. In the biological milieu, pharmacologic ascorbate may therefore set in motion a preponderance of reactions that favor formation of H2O2, the key cytotoxic effector species. H2O2 was proposed to achieve effective steady-state concentrations of ≥25–50 μmol/L to elicit cell death (60, 64). We observed that these concentrations could be achieved in extracellular fluid but not whole blood (Fig. 3B). RBC contain large quantities of catalase and peroxidases, which efficiently quell Fenton chemistry to protect hemoglobin from oxidative damage.
Actions of pharmacologic ascorbate as a pro-drug for H2O2 formation were explored in vivo (59, 60). The goal was to learn whether proposed H2O2 formation mediated by pharmacologic ascorbate occurs in intact experimental animal systems. Physiologic and pharmacologic ascorbate in blood fully equilibrated with extracellular fluid. H2O2 selectively formed in extracellular fluid but was not detected in blood (Fig. 3B) due to the red cell’s rapid catabolism of H2O2 to below detectable amounts (59, 64). Ascorbate concentrations > 2–4 mmol/L were required to attain H2O2 concentrations ≥ 5 μmol/L in extracellular fluid. H2O2 formed only when ascorbate radical concentrations were >100 nmol/L. Appearance of ascorbate radical in conjunction with H2O2 formation was consistent with the hypothesis that a threshold ascorbate concentration was necessary, above which sufficient effector species would mediate cancer cell death.
Whether there were consequences to formation of H2O2 in vivo was further examined in mice bearing experimentally implanted cancer cells (60). Mice were treated with either vehicle (hypertonic saline) or pharmacologic ascorbate. Compared with controls, pharmacologic ascorbate decreased growth of established tumors by ∼50%, which included models for ovarian, glioblastoma (brain), and pancreatic cancer. In situ instrumentation showed that parenteral administration of pharmacologic ascorbate resulted in steady-state formation of H2O2 within the interstitial fluid of both normal tissues and tumors, where concentrations approaching 200 μmol/L were detected. In the glioblastoma model, metastases that occurred in controls were not observed in the treated animals.
In these and subsequent animal studies, ascorbate was administered by i.p. injection. Doses of 4 mg/g i.p. in rodents are approximately equivalent to 1.5 mg/g (1.5 g/kg) by i.v. infusion in humans and produce peak plasma concentrations of 25–30 mmol/L (60, 61). Peak plasma concentrations approaching 30 mmol/L are produced by i.v. infusion of pharmacologic ascorbate in humans, at a dose of ∼1.5 g/kg administered at rates of 0.5–1.0 g/min (60, 61). With these parameters, plasma concentrations > 10 mmol/L are maintained in humans for at least 3 h (61).
At least 2 data sets indicate that pharmacologic ascorbate, with proper screening, is safe for humans (61, 65). In a data set from a phase I clinical trial, reported adverse events were minimal (61). Patients had advanced, metastatic, or recurrent disease refractory to standard treatment. After completion of infusions, 2 patients had stable disease and only 6 patients received the highest dose of 1.5 g/kg. Patients received i.v. ascorbate 3 times/wk at a maximal infusion rate of 1 g/min for 90 min. Screening included the exclusion of glucose 6 phosphate dehydrogenase deficiency; history of kidney stones (renal calculi); concurrent therapy with vitamin K antagonists; dyspnea at rest; severe anemia; and renal insufficiency. Another data set provided evidence of pharmacologic ascorbate safety (65). Complementary and alternative medicine (integrative medicine) practitioners were surveyed at annual meetings in 2 different years to learn whether they used i.v. ascorbate and, if so, whether their patients experienced adverse events. Findings were corroborated by surveying manufacturers of i.v. ascorbate for units sold yearly, searching PUBMED, and searching FDA databases. Based on survey results and industry sales, ∼10,000 patients in the United States each year were treated with i.v. ascorbate, at 20 treatments per patient, at dosing averages of 0.5 g/kg. With properly screened patients, i.v. ascorbate was remarkably safe, based on survey results from practitioners and PUBMED and FDA data (65).
The effects of pharmacologic ascorbate on tumor growth in animals have now been confirmed in many laboratories, using the following models: hepatoma, pancreatic cancer, colon cancer, sarcoma, leukemia, prostate cancer, and mesothelioma (62, 66–71). In these studies, ascorbate doses were the same or <4 mg/g i.p. These findings point to the feasibility of pharmacologic ascorbate in cancer treatment.
Ascorbate had been intermittently reported for many years to have varying effects on tumor cells (72). Essential information, absent from these reports, were frameworks for concentration and mechanism of action. It was unclear whether application of micromolar or millimolar concentrations of ascorbate for hours or days had any translational application. Clinical pharmacokinetics data were essential to recognize translation potential (18, 57, 58). Although the mechanism had been postulated by many to be related to H2O2, the concentration threshold context of pharmacologic ascorbate was lacking. Key to advancing knowledge were the discoveries that H2O2 formed only with pharmacologic but not physiologic ascorbate concentrations and that micromolar steady-state concentrations of H2O2 produced in the tumor interstitium were effective in decreasing tumor growth (59, 60, 64).
Pharmacologic ascorbate: outstanding questions and issues for future research
For pharmacologic ascorbate to promote cancer cell death, H2O2 must be formed (60, 64). There are many downstream targets of H2O2 and they almost certainly involve the actions of reactive oxygen species (ROS)3 (73). ROS can have direct effects on a wide variety of biologically important molecules, including lipids, DNA, RNA, and proteins. The summation of damage and repair elicited by H2O2 differs according to cell and tissue type (74), which is integral in an individual cancer cell’s sensitivity and ultimate ability to either survive or succumb to pharmacologic ascorbate. The inherent promiscuity of H2O2 and ROS effects dictate that no single Achilles heel exists among cancer cell types for cytotoxic response to pharmacologic ascorbate. The concentration of H2O2 induced by pharmacologic ascorbate is far higher, as much as 2 orders of magnitude, than those concentrations that regulate normal cellular processes (74). Whether transient changes in H2O2 concentration have long-term effects are unknown, although available clinical data do not indicate pharmacologic ascorbate produces long-term adverse effects or complications.
The identity of a specific metal catalyst necessary for the action of pharmacologic ascorbate is currently unknown. Our analysis showed protein catalysts of between 10 and 30 kDa exist in human plasma and the extracellular fluid of tissues and tumors (64). Metals in proteins can suffice as a catalyst in extracellular fluid or on plasma membrane proteins facing extracellularly. Free iron and copper are very unlikely to be mediators, because these cations are tightly bound in vivo. It is likely that there are multiple candidate metalloprotein catalysts that would have a high Km for ascorbate and/or molecular oxygen to become permissive toward this process only when ascorbate was present in sufficient pharmacologic concentrations.
It is apparent both from in vitro and clinical studies that adverse effects of pharmacologic ascorbate are few. It is possible that as clinical studies increase and/or as dose frequency increases, more adverse effects will emerge. Nevertheless, the absence of toxicities is striking compared with many chemotherapeutic agents. Why are normal cells and tissues unaffected? Normal cells have redundant mechanisms for H2O2 disposal and/or repair of H2O2 damage. In contrast, susceptible cancer cells may have a series of mutations that signal cell death in the context of H2O2 formed by pharmacologic ascorbate; the specific pathways affected likely vary between cancer cells.
Given the absence of gross toxicities of pharmacologic ascorbate, it is reasonable to wonder why tight control exists at all and whether chronic pharmacologic ascorbate concentrations have adverse effects. One potential explanation as a metabolic untoward consequence of constant pharmacologic ascorbate concentrations is chronic hyperoxalemia, which could occur from constant catabolism of excess ascorbate. Although oxalate urine excretion increased acutely following administration of pharmacologic ascorbate concentrations, concentrations were not dramatically elevated (75). Perhaps constant elevations in H2O2 concentrations have untoward effects on cell repair and growth, but available data do not support these concerns.
Clinical investigation of pharmacologic ascorbate should be considered as an addition to existing cancer treatments. Its mechanism of action as a pro-drug for H2O2 generation is distinct from most currently used agents. For this reason, there is potential for synergy, or at least an additive effect, in combination with other drugs. This strategy is similar to that used for treatment of many cancers, tuberculosis, serious bacterial infections, hepatitis, and HIV. Emerging data indicate that there are additive effects of ascorbate with other neoplastic agents (76). One exception is for bortezomib. Cell and animal data suggest the possibility of antagonism with physiologic ascorbate concentrations (77), although clinical data do not support this possibility (78). The doses of bortezomib used in animals were ∼4 times higher than those used clinically, and the ascorbate concentrations that produced antagonism were in the physiologic and not the pharmacologic range. Other investigators described that the ascorbate metabolite dehydroascorbic acid antagonizes chemotherapeutic agents (27). Dehydroascorbic acid concentrations used in these experiments were at least 100-fold higher than those found in vivo. Dehydroascorbic acid was termed as and was used interchangeably with vitamin C, which is incorrect and misleading (79, 80). Such misnomers can muddy the underlying science, and as a consequence of the Internet, may result in confusion among patients and caregivers. Clinical studies have begun that will test the effects of pharmacologic ascorbate in combination with other agents in patients with pancreatic cancer (see clinicaltrials.gov).
Clinical advancement of pharmacologic ascorbate
It would be ideal if there were a means to predict cancer responsiveness to pharmacologic ascorbate. In theory, characterization of downstream effectors would help to realize this goal. However, given the promiscuity of ROS derived from H2O2, there may not be a common downstream effector. Searching for such effectors is complicated by the variety of different tumor models and their differences in vitro and in vivo. For antineoplastic agents with toxic side effects, identification of effectors may be necessary to guide treatment. In contrast to many commonly used antineoplastic treatments, ascorbate does not have apparent harm and has potential for benefit.
Our opinions are that characterization of downstream effectors, although ideal for predicting response, is not a requirement for pharmacologic ascorbate to move to clinical investigation. Objective clinical testing of ascorbate can proceed even as new mechanism data emerge. Acetaminophen and aspirin were both used for nearly a century before their mechanisms of action were unraveled (81, 82). Apparent benefit outweighed apparent harm and the absence of information about the mechanism of drug effects. Pharmacologic ascorbate can be viewed similarly. Already available are data about mechanisms in vitro and in vivo and pharmacology and safety in vivo. In light of new knowledge, what is lacking is evidence of efficacy in people and this is what merits proper and rigorous testing. Patients currently do not have choices of many treatments that provide benefit with minimal toxicity, and pharmacologic ascorbate potentially offers this possibility (57, 83).
The design of clinical trials for pharmacologic ascorbate should consider type of cancer, available treatment modalities, and expected median survival. Tumor types that may be suitable for testing pharmacologic ascorbate in combination with available modalities are those where there is rapid progression, there are few treatment options, and where physical functional limitations at presentation are relatively few. Current dosing schemes in trials were selected based on apparent safety and long-term use (60, 61, 65, 84). A frequency of 3 times/wk most likely was selected simply for patient and practitioner convenience in an outpatient setting. There is a clear need for studies that define toxicity, especially with increased dosing frequency (i.e. daily), as well as with increased dosing amount.
Finally, pharmacologic ascorbate as a treatment can be applied to conditions where H2O2 and/or ROS could be beneficial. Obvious candidates are infectious agents, including viruses, bacteria, and other human pathogens. Particularly attractive candidates are infectious agents for which few or no treatments currently exist. As in cancer treatment, pharmacologic ascorbate also has the potential to be added to existing therapies for synergy. This may be especially useful for bacteria that have developed multiple antibiotic resistances and for which only limited treatments are available. H2O2 is the means by which neutrophils generate ROS, and it is possible that pharmacologic ascorbate may be effective against some bacteria especially in combination with antibiotics. Although it is intuitive that H2O2 could be toxic to viruses, surprisingly little data are available (60). Unlike cancer, for which chronic treatment may be necessary, administration of pharmacologic ascorbate might only be short term or in an acute setting, until infection is cleared. Use of pharmacologic ascorbate is ripe for investigation.
Conclusions
A concentration-function approach to vitamin C yields new insights into its physiology and pharmacology. Vitamin C concentrations are tightly controlled with oral ingestion by at least 4 mechanisms. Disruption of one mechanism, renal reabsorption, reveals a new potential role of ascorbate in perinatal health and unanticipated feedback regulation of ascorbate biosynthesis. For proper clinical translation, dose concentration relationships must be accounted for in clinical studies. Tight control of ascorbate concentrations is bypassed with i.v. administration until renal excretion restores homeostasis. With i.v. administration, ascorbate is turned from vitamin to drug, as pharmacologic concentrations are produced that are as much as 100-fold higher than those possible with maximal oral dosing. Pharmacologic ascorbate, by acting as a pro-drug for H2O2 in the extracellular fluid, has potential in treatment of cancer, infectious diseases, and perhaps other conditions in which H2O2 may have efficacy. Ascorbate administered intravenously has already been tested in a phase I clinical trial, is in wide use by complementary and alternative medicine (integrative medicine) practitioners, and appears to have minimal side effects in patients who are properly screened.
Acknowledgments
M.L., S.J.P., and M.G.E. performed literature research, wrote the manuscript, and read and approved the final manuscript.
Footnotes
Supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Author disclosures: M. Levine, S. J. Padayatty, and M. G. Espey, no conflicts of interest.
Abbreviations used: AscH−, ascorbate; Asc·-, ascorbate radical; ROS, reactive oxygen species.
Literature Cited
- 1.Harper AE. The recommended dietary allowances for ascorbic acid. Ann N Y Acad Sci. 1975;258:491–7 [DOI] [PubMed] [Google Scholar]
- 2.Food and Nutrition Board (U.S.R.C.) Recommended dietary allowances. Washington, DC: National Academy Press; 1989 [Google Scholar]
- 3.Food and Nutrition Board Vitamin C (ascorbic acid). In: Committee on Dietary Allowances, editor. Recommended dietary allowances Washington, DC: National Academy of Sciences; 1980. p. 72–81 [Google Scholar]
- 4.Friedman GJ, Sherry S, Ralli E. The mechanism of excretion of vitamin C by the human kidney at low and normal plasma levels of ascorbic acid. J Clin Invest. 1940;19:685–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallner A, Hartmann D, Hornig D. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr. 1979;32:530–9 [DOI] [PubMed] [Google Scholar]
- 6.Beadle GW, Tatum EL. Genetic control of biochemical reactions in neurospora. Proc Natl Acad Sci USA. 1941;27:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauling L. Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease. Science. 1968;160:265–71 [DOI] [PubMed] [Google Scholar]
- 8.Williams RJ, Deason G. Individuality in vitamin C needs. Proc Natl Acad Sci USA. 1967;57:1638–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002;75:616–58 [DOI] [PubMed] [Google Scholar]
- 10.Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892–902 [DOI] [PubMed] [Google Scholar]
- 11.Levine M, Rumsey SC, Wang Y, Park J, Kwon O, Amano N. In Situ Kinetics: an approach to recommended intake of vitamin C. Methods Enzymol. 1997;281:425–37 [DOI] [PubMed] [Google Scholar]
- 12.Levine M, Eck P. Vitamin C: working on the x-axis. Am J Clin Nutr. 2009;90:1121–3 [DOI] [PubMed] [Google Scholar]
- 13.Levine M, Dhariwal KR, Washko PW, Butler JD, Welch RW, Wang YH, Bergsten P. Ascorbic acid and in situ kinetics: a new approach to vitamin requirements. Am J Clin Nutr. 1991;54:S1157–62 [DOI] [PubMed] [Google Scholar]
- 14.Kaufman S, Friedman S. DOPAMINE-BETA-HYDROXYLASE. Pharmacol Rev. 1965;17:71–100 [PubMed] [Google Scholar]
- 15.Dhariwal KR, Washko P, Hartzell WO, Levine M. Ascorbic acid within chromaffin granules. In situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989;264:15404–9 [PubMed] [Google Scholar]
- 16.Dhariwal KR, Shirvan M, Levine M. Ascorbic acid regeneration in chromaffin granules. In situ kinetics. J Biol Chem. 1991;266:5384–7 [PubMed] [Google Scholar]
- 17.Fleming PJ, Kent UM. Cytochrome b561, ascorbic acid, and transmembrane electron transfer. Am J Clin Nutr. 1991;54:S1173–8 [DOI] [PubMed] [Google Scholar]
- 18.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a Recommended Dietary Allowance. Proc Natl Acad Sci USA. 1996;93:3704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King J, Wang Y, Welch RW, Dhariwal KR, Conry-Cantilena C, Levine M. Use of a new vitamin C-deficient diet in a depletion/repletion clinical trial. Am J Clin Nutr. 1997;65:1434–40 [DOI] [PubMed] [Google Scholar]
- 20.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14:1133–9 [DOI] [PubMed] [Google Scholar]
- 21.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds Vitamin C. Dietary Reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press; 2000. p. 95–185, 1–20, 434–435, 442–443 [Google Scholar]
- 23.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–5 [DOI] [PubMed] [Google Scholar]
- 24.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–7 [DOI] [PubMed] [Google Scholar]
- 25.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagun KC, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19:1657–67 [DOI] [PubMed] [Google Scholar]
- 27.Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O'Connor OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68:8031–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine M, Wang Y, Rumsey SC. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999;299:65–76 [DOI] [PubMed] [Google Scholar]
- 29.Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–6 [DOI] [PubMed] [Google Scholar]
- 30.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–806 [DOI] [PubMed] [Google Scholar]
- 31.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padayatty SJ, Levine M. Vitamins C and E and the prevention of preeclampsia. N Engl J Med. 2006;355:1065. [DOI] [PubMed] [Google Scholar]
- 33.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23 [DOI] [PubMed] [Google Scholar]
- 34.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padayatty SJ, Levine M. Antioxidant supplements and cardiovascular disease in men. JAMA. 2009;301:1336–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010;103:1251–9 [DOI] [PubMed] [Google Scholar]
- 37.Burns JJ. Evans C, Trousof N. Stimulatory effect of barbital on urinary excretion of L-ascorbic acid and nonconjugated D-glucuronic acid. J Biol Chem. 1957;227:785–94 [PubMed] [Google Scholar]
- 38.Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC, Canham JE. Metabolism of 14C- and 3H-labeled L-ascorbic acid in human scurvy. Am J Clin Nutr. 1971;24:444–54 [DOI] [PubMed] [Google Scholar]
- 39.Padayatty S, Espey MG, Levine M. Vitamin C. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, Encyclopedia of dietary supplements. New York: Informa Healthcare; . 2010. p. 821–31 [Google Scholar]
- 40.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–37 [DOI] [PubMed] [Google Scholar]
- 41.Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis KB, Wilkinson A, Winterbourn CC, Windsor JA. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999;86:1296–301 [DOI] [PubMed] [Google Scholar]
- 42.Riemersma RA, Carruthers KF, Elton RA, Fox KA. Vitamin C and the risk of acute myocardial infarction. Am J Clin Nutr. 2000;71:1181–6 [DOI] [PubMed] [Google Scholar]
- 43.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, Franks W, Lawson TC, Sauberlich HE. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. 2003;109:144–8 [DOI] [PubMed] [Google Scholar]
- 44.Price KD, Price CS, Reynolds RD. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis. 2001;158:1–12 [DOI] [PubMed] [Google Scholar]
- 45.Estivariz CF, Griffith DP, Luo M, Szeszycki EE, Bazargan N, Dave N, Daignault NM, Bergman GF, McNally T, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2008;32:389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padayatty SJ, Levine M. Vitamin C and myocardial infarction: the heart of the matter. Am J Clin Nutr. 2000;71:1027–8 [DOI] [PubMed] [Google Scholar]
- 47.McCormick WJ. Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr. 1959;76:166–71 [PubMed] [Google Scholar]
- 48.Cameron E, Rotman D. Ascorbic acid, cell proliferation, and cancer. Lancet. 1972;1:542. [DOI] [PubMed] [Google Scholar]
- 49.Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9:285–315 [DOI] [PubMed] [Google Scholar]
- 50.Cameron E, Campbell A, Jack T. The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: double complete regression induced by high-dose ascorbic acid therapy. Chem Biol Interact. 1975;11:387–93 [DOI] [PubMed] [Google Scholar]
- 51.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73:3685–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75:4538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins H, Pinch T. Alternative medicine: the case of vitamin C and cancer. In: Collins H, Pinch T, editors. Dr. Golem: how to think about medicine Chicago: University of Chicago Press; . 2005. p. 84–111 [Google Scholar]
- 54.Creagan ET, Moertel CG, O'Fallon JR, Schutt AJ, O'Connell MJ, Rubin J, Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301:687–90 [DOI] [PubMed] [Google Scholar]
- 55.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137–41 [DOI] [PubMed] [Google Scholar]
- 56.Wittes RE. Vitamin C and cancer. N Engl J Med. 1985;312:178–9 [DOI] [PubMed] [Google Scholar]
- 57.Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19:423–5 [DOI] [PubMed] [Google Scholar]
- 58.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–7 [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Kirshna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH., Jr Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–74 [DOI] [PubMed] [Google Scholar]
- 62.Park CH, Kimler BF, Yi SY, Park SH, Kim K, Jung CW, Kim SH, Lee ER, Rha M, et al. Depletion of L-ascorbic acid alternating with its supplementation in the treatment of patients with acute myeloid leukemia or myelodysplastic syndromes. Eur J Haematol. 2009;83:108–18 [DOI] [PubMed] [Google Scholar]
- 63.Micallef J, Attarian S, Dubourg O, Gonnaud PM, Hogrel JY, Stojkovic T, Bernard R, Jouve E, Pitel S, et al. Effect of ascorbic acid in patients with Charcot-Marie-Tooth disease type 1A: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2009;8:1103–10 [DOI] [PubMed] [Google Scholar]
- 64.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE. 2010;5:e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47:32–40 [DOI] [PubMed] [Google Scholar]
- 67.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Knudsen CM, Cullen JJ. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16:509–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belin S, Kaya F, Duisit G, Giacometti S, Ciccolini J, Fontes M. Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS ONE. 2009;4:e4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeom CH, Lee G, Park JH, Yu J, Park S, Yi SY, Lee HR, Hong YS, Lee S. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med. 2009;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollard HB, Levine MA, Eidelman O, Pollard M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo. 2010;24:249–55 [PMC free article] [PubMed] [Google Scholar]
- 71.Takemura Y, Satoh M, Satoh K, Hamada H, Sekido Y, Kubota S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem Biophys Res Commun. 2010;394:249–53 [DOI] [PubMed] [Google Scholar]
- 72.Verrax J, Calderon PB. The controversial place of vitamin C in cancer treatment. Biochem Pharmacol. 2008;76:1644–52 [DOI] [PubMed] [Google Scholar]
- 73.Sies H. de GH. Role of reactive oxygen species in cell toxicity. Toxicol Lett. 1992;64–65:547–51 [DOI] [PubMed] [Google Scholar]
- 74.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–70 [DOI] [PubMed] [Google Scholar]
- 75.Robitaille L, Mamer OA, Miller WH, Levine M, Assouline S, Melnychuk D, Rousseau C, Hoffer LJ. Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism. 2009;58:263–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fromberg A, Gutsch D, Schulze D, Vollbracht C, Weiss G, Czubayko F, Aigner A. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemother Pharmacol. Epub; . 2010 Aug 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, Santo L, Cirstea D, Raje N, et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23:1679–86 [DOI] [PubMed] [Google Scholar]
- 78.Berenson JR, Yellin O, Woytowitz D, Flam MS, Cartmell A, Patel R, Duvivier H, Nassir Y, Eades B, et al. Bortezomib, ascorbic acid and melphalan (BAM) therapy for patients with newly diagnosed multiple myeloma: an effective and well-tolerated frontline regimen. Eur J Haematol. 2009;82:433–9 [DOI] [PubMed] [Google Scholar]
- 79.Levine M, Katz A, Padayatty SJ. Vitamin C. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, Modern nutrition in health and disease. Philadelphia: Lippincott Williams and Wilkins; . 2006. p. 507–24 [Google Scholar]
- 80.Espey MG, Chen Q, Levine M. Comment re: Vitamin C antagonizes the cytotoxic effects of chemotherapy. Cancer Res. 2009;69:8830–1 [DOI] [PubMed] [Google Scholar]
- 81.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci USA. 2002;99:7130–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levine M, Espey MG, Chen Q. Losing and finding a way at C: new promise for pharmacologic ascorbate in cancer treatment. Free Radic Biol Med. 2009;47:27–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003;22:118–23 [DOI] [PubMed] [Google Scholar]