Abstract
The cyclooxygenase (COX) product, prostacyclin (PGI2), inhibits platelet activation and vascular smooth-muscle cell migration and proliferation. Biochemically selective inhibition of COX-2 reduces PGI2 biosynthesis substantially in humans. Because deletion of the PGI2 receptor accelerates atherogenesis in the fat-fed low density lipoprotein receptor knockout mouse, we wished to determine whether selective inhibition of COX-2 would accelerate atherogenesis in this model. To address this hypothesis, we used dosing with nimesulide, which inhibited COX-2 ex vivo, depressed urinary 2,3 dinor 6-keto PGF1α by approximately 60% but had no effect on thromboxane formation by platelets, which only express COX-1. By contrast, the isoform nonspecific inhibitor, indomethacin, suppressed platelet function and thromboxane formation ex vivo and in vivo, coincident with effects on PGI2 biosynthesis indistinguishable from nimesulide. Indomethacin reduced the extent of atherosclerosis by 55 ± 4%, whereas nimesulide failed to increase the rate of atherogenesis. Despite their divergent effects on atherogenesis, both drugs depressed two indices of systemic inflammation, soluble intracellular adhesion molecule-1, and monocyte chemoattractant protein-1 to a similar but incomplete degree. Neither drug altered serum lipids and the marked increase in vascular expression of COX-2 during atherogenesis. Accelerated progression of atherosclerosis is unlikely during chronic intake of specific COX-2 inhibitors. Furthermore, evidence that COX-1-derived prostanoids contribute to atherogenesis suggests that controlled evaluation of the effects of nonsteroidal anti-inflammatory drugs and/or aspirin on plaque progression in humans is timely.
Platelet-dependent vascular occlusion complicating fracture of an atherosclerotic plaque is thought to be the pathophysiological basis of both acute myocardial infarction and the majority of cases of stroke (1, 2). The efficacy of platelet-inhibitory drugs—aspirin (3–5), clopidogrel (6), and dipyridamole (7)—in the secondary prevention of coronary and cerebrovascular disease has been established in randomized controlled clinical trials. Much less is known about the potential contribution of platelet activation to development and progression of the underlying atherosclerotic disease. Although platelets release mitogenic growth factors and bioactive lipids when activated (8, 9), their relative absence in the descriptive morphology of atherosclerotic lesions in humans has led to a reduction in emphasis on their role in pathogenesis (10). Therefore, such studies do not address the possibility that platelets activated in the circulation of patients with atherosclerosis (11) might release products that modulate lesion progression, and controlled clinical trials of antiplatelet agents on plaque progression have not been performed. Similarly, mouse models of atherosclerosis seem quite resistant to spontaneous thrombosis (12), and it is unknown whether their development of vascular lesions alone results in platelet activation in vivo, as is the case in human atherosclerosis (11, 13).
The efficacy of low-dose aspirin in platelet-dependent vascular occlusion (3–5) is consistent with the role of platelet-derived thromboxane (Tx) A2 as an amplifying signal for activation by more potent primary platelet agonists such as thrombin (14). Platelet Tx is formed via prostaglandin endoperoxide intermediates (15) by cyclooxygenase (COX)-1 (16). An alternative source of Tx formation by macrophages and smooth-muscle cells in the vessel wall is COX- 2 (17, 18). Although this isoform is the most readily induced by inflammatory stimuli (19, 20), both COXs are induced ex vivo in humans administered bacterial lipopolysaccharide (21) and are colocalized in human atherosclerotic plaque (22). Recently, it has been reported that a Tx receptor (TP) antagonist retards atherogenesis in ApoE-deficient mice and that this reduction is accompanied by a decrease in elevated levels of soluble intercellular adhesion molecule (sICAM)-1, a marker of inflammation (23).
These observations prompt interest in the relative contributions of the COX isoforms to atherosclerotic lesion development. Classical nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin are essentially isoform-nonspecific at doses administered in humans (24), whereas inhibitors more selective for COX-2 have emerged into clinical practice. Recently, we found that the major COX product of macrovascular endothelial cells, prostacyclin (PGI2), is formed predominantly, albeit not exclusively, by COX-2 in healthy humans (25, 26), perhaps because of its induction in the vasculature by physiological rates of shear (27). Deletion of the PGI2 receptor results in an increased sensitivity to thrombotic stimuli (28), and the likely effects of its suppression by COX-2 inhibitors without concomitant inhibition of platelet function has prompted much speculation. The present studies were designed to address two questions. First, we wished to determine whether suppression of PGI2 by a pharmacologically selective COX-2 inhibitor would accelerate atherogenesis. Second, we wished to determine whether coincident inhibition of both COX isozymes with concomitant suppression of Tx and PGI biosynthesis would retard atherogenesis. To address these hypotheses, we defined biochemically the specificity of our pharmacological probes and characterized the biosynthesis of these eicosanoids as atherogenesis developed in the fat-fed, low density lipoprotein (LDL) receptor (R) knockout (KO) mouse.
Materials and Methods
Animals.
LDLR-KO mice (back-crossed 10 times to C57BL/6 mice) were obtained from The Jackson Laboratories at 6 weeks of age. All procedures and care of animals were approved by the Institutional Animal Care and Usage Committee of the University of Pennsylvania. After 2 weeks of acclimatization, they were fed a Western-type diet (normal chow supplemented with 0.15% cholesterol and 20% butter fat) for the entire study. At this time, animals were divided into three groups (n = 14 each) and randomized to receive indomethacin (6 mg/liter), an isoform-nonspecific inhibitor (29), nimesulide (40 mg/liter), a selective COX-2 inhibitor (30), or placebo. Preliminary experiments demonstrated that the selected dose of nimesulide inhibited COX-2 activity ex vivo without concomitant inhibition of platelet TxA2. Urine was collected in metabolic cages at 8, 16, and 26 weeks of age. Blood samples were obtained as previously described from animals fasted overnight by retro-orbital bleeding (31).
Assays for COX Activity.
For serum TxB2, nonanticoagulated whole blood was allowed to clot at 37°C for 1 h as described (32). Serum was separated by centrifugation at 1,000 × g for 15 min and stored at −80°C until analysis.
Inhibition of COX-2 activity ex vivo was assessed as described (33). Briefly, aliquots of heparinized blood were incubated with 10 μg/ml lipopolysaccharide (Sigma) at 37°C for 24 h. The contribution of platelet COX-1 was suppressed by the addition of 100 μM aspirin. Because aspirin is inactivated rapidly by hydrolysis, induced COX-2 activity is unaffected, and the prostaglandin E2 (PGE2) formed in this assay depends on COX-2. Plasma was separated by centrifugation at 1,000 × g for 15 min and stored at −80°C until analysis of PGE2.
Biochemical Analysis.
Serum TxB2, plasma PGE2, urinary 2,3-dinor TxB2, and 2,3-dinor-6-keto PGF1α were measured by stable-dilution isotope GC/MS assays as described (34, 35). Briefly, a known amount of each tetradeuterated internal standard was added to the samples. After solid-phase extraction, the samples were derivatized, purified by TLC, and analyzed on GC/MS. Plasma cholesterol and triglyceride levels were determined enzymatically by using Sigma reagents. Levels of sICAM-1 and monocyte chemoattractant protein-1 (MCP-1) were measured by ELISA kits [Endogen (Cambridge, MA) and R & D Systems, respectively].
Platelet-Aggregation Studies.
Platelet aggregation was studied as described (36). Briefly, anticoagulated blood was centrifuged immediately at 100 × g for 10 min at room temperature, and platelet-rich plasma was collected. The remaining fraction was centrifuged at 2,000 × g to obtain platelet-poor plasma. Platelet aggregation was determined by light absorbance using a platelet aggregometer with constant magnetic stirring. Arachidonic acid (100 μM) was used as an agent to induce an irreversible aggregation.
Ribonuclease Protection Assay.
Expression of COX-1 and COX-2 in the murine vasculature was assessed by using a ribonuclease-protection assay. Briefly, a 300- to 400-bp fragment of interest cDNA was PCR amplified, a T7 promoter was added to the 3′ ending by PCR (Lig'n scribe kit, Ambion, Austin, TX), and a radiolabeled RNA probe was synthesized by in vitro-transcription system (MAXIScript T7 kit, Ambion). The probe was hybridized to 10–20 μg total RNA for approximately 16 h at 42°C (RPA III kit, Ambion), and the samples were digested with RNase A/T, precipitated, and loaded onto 7% denaturing polyacrylamide gel. The gel was dried, and signals were detected by autoradiography and quantitated on a PhosphorImager (Molecular Dynamics). The following mouse-probe primers were used: (i) for COX-1, forward primer 5′-GTTCCGAGCCCAGTTCCAA-3′ and reverse primer 5′-CATCTCCTTCTCTCCTGTG-3′; and (ii) for COX-2, forward primer 5′-CCAGCACTTCACCCATCAG-3′ and reverse primer 5′-CTCATCACCCCACTCAGGA-3′. Both pairs of primers are hosted on C-terminal sequences, which diverge between the two isoforms (37).
Preparation of Mouse Aortas and Quantitation of Atherosclerosis.
After the final blood collection, mice were killed, and the aortic tree was perfused for 10 min with ice-cold PBS containing 20 μmol/liter butylated hydroxytoluene (BHT) and 2 mmol/liter EDTA ( pH 7.4) by inserting a cannula into the left ventricle and allowing free efflux from an incision in the vena cava. After removal of the surrounding adventitial tissue, the aorta was opened longitudinally from the aortic root to the iliac bifurcation, fixed in formal-sucrose (4% paraformaldehyde/5% sucrose/20 μmol/liter BHT/2 mmol/liter EDTA, pH 7.4), and then stained with Sudan IV. The extent of atherosclerosis was determined by using the “en face” method (31). Quantitation was performed by capturing images of aortas with a Dage-MTI 3CCD three-chips color video camera connected to a Leica MZ12 dissection microscope as described (31).
Statistics.
Results were expressed as mean ± SEM. Total plasma cholesterol, triglycerides, PGE2, serum TxB2, urinary 2,3-dinor TxB2, the major urinary metabolite of PGI2 (2,3-dinor-6-keto PGF1α), and the extent of aortic atherosclerosis were analyzed by ANOVA and subsequently by Student's unpaired two-tailed t test as indicated.
Results
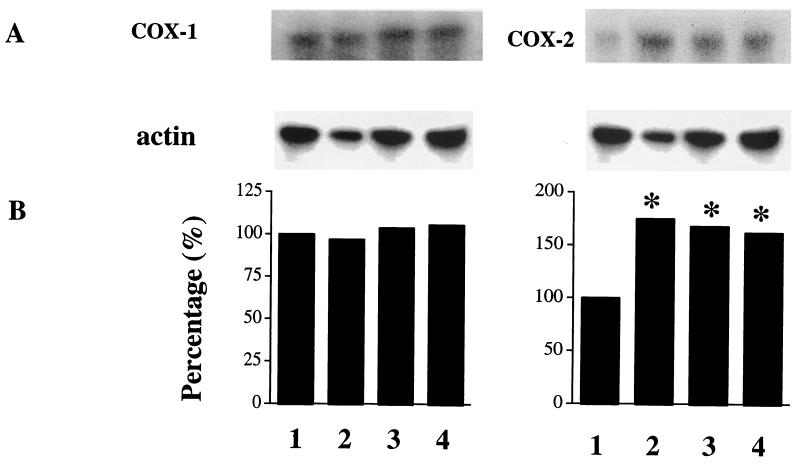
Mice were housed as described (31). Starting at 8 weeks of age, they were fed a Western-type diet for the entire study. Body weight, total plasma cholesterol, and triglycerides levels were not different between animals when randomized to the treatment groups at the beginning of the study. LDLR-KO mice on placebo had achieved a significant increase in plasma cholesterol, triglycerides levels, and body weight by the end of the study, i.e., at 26 weeks of age (Table 1). Excretion of 2,3-dinor TxB2, the major murine Tx metabolite in mice (34, 36), was increased also. This increment was evident already after 8 weeks on high-fat diet (5 ± 1.5 vs. 48 ± 7 ng/mg creatinine; P < 0.001). These levels increased further during the weeks of follow-up and were up to 10-fold higher than the initial values by the end of the study at 26 weeks of age (Fig. 1). A similar pattern was observed for urinary 2,3-dinor-6-keto PGF1α, the PGI2 metabolite (ref. 35; Fig. 2). The mRNAs of both COX isoforms were detectable in the aortas of LDLR-KO mice at baseline. However, COX-2 mRNA was increased markedly in the presence of atherosclerosis, whereas COX-1 mRNA was not increased significantly (Fig. 3).
Table 1.
Body weight, total plasma cholesterol, triglycerides and the platelet aggregation response to arachidonic acid in LDLR-KO mice at 8 weeks of age (A) and after 18 weeks on a Western-type diet (B)
| Placebo | Indomethacin | Nimesulide | ||
|---|---|---|---|---|
| Body weight, g | A | 20 ± 1.5 | 21 ± 2.0 | 22 ± 2.0 |
| B | 37.5 ± 3* | 36.8 ± 2.5* | 38.5 ± 2.1* | |
| Cholesterol, mg/dL | A | 190 ± 20 | 200 ± 16 | 205 ± 16 |
| B | 1750 ± 40* | 1700 ± 55* | 1850 ± 50* | |
| Triglycerides, mg/dL | A | 85 ± 12 | 95 ± 10 | 95 ± 10 |
| B | 800 ± 45* | 830 ± 55* | 850 ± 55* | |
| Platelet aggregation, LT % | B | 85 ± 10 | 10 ± 2** | 75 ± 17 |
Mice were randomized to receive placebo, indomethacin, or nimesulide (n = 14 animals for each group). Results are expressed as mean ± SEM. LT, light transmission. *, P < 0.001; **, P < 0.0001 vs. base or placebo.
Figure 1.
Increasing Tx biosynthesis during atherogenesis in LDLR-KO mice. Excretion of the major urinary metabolite of Tx, 2,3-dinor TxB2, in LDLR-KO mice on a Western-type diet receiving placebo (open bars), indomethacin (hatched bars), or nimesulide (closed bars) at 8 weeks of age and after 18 weeks of treatment (26 weeks of age). (*, P < 0.001.)
Figure 2.
Increasing PGI2 biosynthesis during atherogenesis in LDLR-KO mice. Excretion of 2,3-dinor-6-keto PGF1α in LDLR-KO mice on a Western-type diet receiving placebo (open bars), indomethacin (hatched bars), or nimesulide (closed bars) at 8 weeks of age and after 18 weeks of treatment (26 weeks of age). (*, P < 0.001.)
Figure 3.
Murine atherosclerotic aortas express high levels of COX-2 mRNA. Ribonuclease-protection assay analysis of COX-1 and COX-2 mRNA expression in aortas from LDLR-KO mice at baseline (8 weeks of age; lane 1), and LDLR-KO mice on a Western-type diet receiving placebo (lane 2), indomethacin (lane 3), or nimesulide (lane 4) for 18 weeks of treatment. Blots shown are representative of four separate experiments.
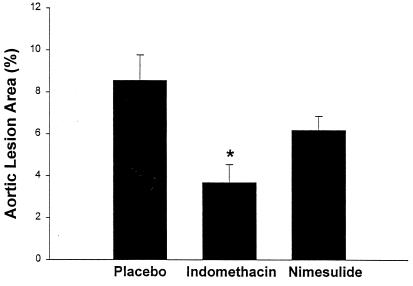
A group of 14 LDLR-KO mice (seven females and seven males) were randomized to receive indomethacin (6 mg/liter). Assuming that each mouse drinks 3–4 ml of water per day, the estimated daily intake of the drug was calculated around 10–20 ng. Mice on indomethacin for only 8 weeks already showed a reduction of urinary 2,3-dinor TxB2 compared with the animals receiving vehicle (7.5 ± 1.5 vs. 48 ± 3 ng/mg creatinine, P < 0.001). By the end of the study, indomethacin had suppressed urinary 2,3-dinor TxB2 by approximately 90–95% (Fig. 1). Serum TxB2 was depressed from 195 ± 25 ng/ml on placebo to 12 ± 1.1 ng/ml (P < 0.001) on indomethacin. Platelet aggregation induced by arachidonic acid was inhibited markedly also (Table 1). There was a corresponding reduction of 2,3-dinor-6-keto PGF1α excretion by approximately 60% (Fig. 2). By contrast, indomethacin had no effect on body weight or plasma lipids (Table 1). Consistent with the inflammatory basis of atherosclerosis (10) and observations in the apoE-KO model (23), circulating levels of the inflammatory molecules sICAM-1 and MCP-1 increased in LDLR-KO mice as atherosclerosis developed over time (8 ± 0.7 vs. 11 ± 0.5 and 130 ± 15 vs. 184 ± 40 ng/ml, respectively; P < 0.001 for both). These numbers fell significantly to 9.4 ± 0.4 and 145 ± 21 ng/ml, respectively, on indomethacin (P = 0.02 for both). By contrast, indomethacin treatment did not modify the expression of either COX-1 or COX-2 mRNA in atherosclerotic murine aortas (Fig. 3). Mice were killed at the end of the study, and their aortas analyzed for the extent of atherosclerosis. Although extensive atherosclerotic lesions were observed throughout the aorta in untreated mice (Fig. 4), indomethacin significantly reduced the lesion area by 55 ± 4% (P < 0.001 vs. placebo and P = 0.03 vs. nimesulide; Figs. 4 and 5).
Figure 4.

Photomicrographs of representative Sudan IV-stained aortas of LDLR-KO mice on a Western-type diet receiving placebo, indomethacin, or nimesulide for 18 weeks.
Figure 5.
Percentage of total aortic-lesion areas in LDLR-KO mice (26 weeks of age) on Western-type diet receiving placebo, indomethacin, or nimesulide for 18 weeks (*, P < 0.001 vs. placebo).
Finally, another group of LDLR-KO mice were randomized to receive nimesulide (40 g/liter) in their drinking water (n = 14, seven females and seven males). Again assuming that each mouse drinks 3–4 ml of water per day, the estimated daily intake of this drug was calculated around 0.10–0.15 mg. This treatment had no effect on body weight, plasma cholesterol, and triglycerides (Table 1). Consistent with this regimen selectively inhibiting COX-2, nimesulide failed to depress serum TxB2 (195 ± 2.5 vs. 190 ± 2.6 ng/ml) or to inhibit arachidonic acid-induced platelet aggregation (Table 1). By contrast, lipopolysaccharide-induced whole blood PGE2 generation was suppressed markedly from 12.8 ± 1.8 to 2.0 ± 0.7 ng/ml (P < 0.001). Urinary 2,3-dinor TxB2 was unaltered also by nimesulide (Fig. 1), whereas excretion of 2,3-dinor-6-keto PGF1α was suppressed markedly (Fig. 2). As with indomethacin, circulating levels of sICAM-1 and MCP-1 were reduced from 11 ± 0.5 to 9.1 ± 0.3 (P = 0.02) and from 184 ± 40 to 140 ± 18 ng/ml, respectively (P < 0.01) on nimesulide. However, despite this effect, nimesulide did not modify significantly the expression of either COX isoform in the atherosclerotic aorta (Fig. 3) or the extent of atherosclerosis (Figs. 4 and 5).
Discussion
The present study was designed to address two hypotheses. First, we wished to determine the effects of biochemically defined selective inhibition of COX-2 on atherogenesis. Second, we wished to assess the effects of combined inhibition of both COX isozymes on the process. The importance of the first objective relates to our observation that structurally distinct COX-2 inhibitors depress PGI2 biosynthesis by approximately 60% in healthy individuals (25, 26). PGI2 inhibits vascular smooth-muscle cell proliferation, platelet activation, and leukocyte–endothelial cell interactions in vitro (38–40), and overexpression of PGI2 synthase reduces blood pressure in pulmonary hypertension and the response to carotid injury in rodents (41, 42). Recently, we reported that deletion of the PGI2 receptor (IP) accelerates atherogenesis in LDLR-KO mice.† However, this effect is much less evident in mice retaining one copy of the IP, which may resemble more closely the effect of an inhibitor. Given that patients with juvenile arthritis in Western countries are likely to consume COX-2 inhibitors for extended periods while they consume an atherogenic diet, we felt it was important to address this aspect of drug action. On the other hand, platelet COX-1 is a prominent source of TxA2, a platelet agonist and vasoconstrictor that stimulates vascular smooth-muscle cell adhesion, migration, and proliferation (43–45). Remarkably little information is available on the clinical effects of isoform-nonspecific inhibitors such as indomethacin or aspirin on atherosclerotic plaque progression in humans. However, a TP antagonist has been reported recently to retard atherogenesis in apoE-KO mouse (23).
We felt it was important to define the biochemical specificity of our pharmacological probes. Despite the nomenclature, “specific” inhibitors of COX-2 become isoform-nonspecific as a function of drug concentration (26, 46). Thus, although such compounds have been evaluated in rodent models of cancer (47) and cardiovascular disease,‡ the actual biochemical specificity of the drug regimens used has not been defined previously. We selected nimesulide (48) as a probe for COX-2 and used a regimen that markedly inhibited COX-2 but not COX-1 ex vivo. Consistent with these properties, nimesulide failed to inhibit platelet aggregation ex vivo. Urinary Tx biosynthesis, as reflected by metabolite excretion in urine, increased in the LDLR-KO mice as they developed atherosclerosis. Thus, despite the resistance of atherosclerotic mice to plaque fissure and thrombosis, they develop biochemical evidence of increased platelet activation, as is the case in atherosclerotic patients (11, 13). Nimesulide had no effect on endogenous Tx biosynthesis, again consistent with observations with selective doses of COX-2 inhibition in humans (48).
PGI2 biosynthesis also increased in the mice as they developed atherosclerosis. Again, this is consistent with observations in human atherosclerosis (11, 13) and probably reflects a homeostatic response to accelerated platelet–vessel-wall interactions. The biochemically selective dose of nimesulide suppressed excretion of the PGI metabolite by approximately 60%, suggesting that COX-2 was a prominent source of the increase in PGI2 biosynthesis. This finding is consistent with the marked up-regulation of COX-2 but not COX-1 in the aortas of the atherosclerotic mice as disease progressed. Although there is evidence that NSAIDs may regulate expression of COX-2 in vitro by acting as ligands for peroxisomal proliferator-activating receptors (49), there was no evidence that treatment with either nimesulide or indomethacin modulated expression of COX-2 during murine atherogenesis. Similarly, neither drug affected the lipid profile of the mice during atherogenesis.
The effects of indomethacin were consistent with inhibition of both COX isoforms, as is the case for clinically used doses of both traditional NSAIDs and aspirin (24, 46). Thus, serum TxB2 was suppressed markedly, coincident with platelet inhibition ex vivo. Similarly, urinary Tx metabolite was suppressed markedly by indomethacin, whereas depression of 2,3-dinor-6-keto PGF1α excretion was similar to that observed on nimesulide.
The two active treatments diverged significantly with respect to their effects on atherogenesis. There was no evidence that nimesulide accelerated atherogenesis, despite substantial suppression of PGI2 biosynthesis without coincident inhibition of platelet function. Conversely, selective inhibition of COX-2 did not retard atherogenesis significantly. Despite their divergent effects on atherogenesis, both nimesulide and indomethacin depressed two systemic markers of inflammation, sICAM and MCP-1, to a similar but incomplete degree. Atherosclerosis is an inflammatory condition, and COX-2 inhibitors, aspirin, and traditional NSAIDs are effective in the treatment of arthritis. Studies in humans administered lipopolysaccharide suggest that COX-1 may contribute approximately 15% to PG I2 biosynthesis in this model of inflammation (21). Given the relatively crude subjective and objective criteria combined to assess drug efficacy (50, 51), one would not expect to discriminate such a contribution in clinical comparisons of COX-2 inhibitors and NSAIDs in arthritis. The failure of nimesulide to retard atherogenesis in these studies may reflect incomplete suppression of the inflammatory response, the balance between pro- and anti-inflammatory effects of PGI2 and other PG products, the features of the model and the timing of the evaluation.
Our results suggest that products of COX-1 are of likely importance in atherogenesis. Although a role for platelets in atherogenesis, which solely express COX-1, has been dismissed largely on observational grounds (10), this approach does not exclude the importance of soluble products of platelet activation such as TxA2. Indeed, Cayette et al. demonstrated that a TP antagonist retards atherogenesis in the apoE-KO mouse (23). Aspirin failed to influence atherogenesis in this model, prompting the authors to speculate on a role for TP ligands other than those produced by COX. However, the aspirin regimen used in this case did not depress Tx to the degree necessary to inhibit Tx-dependent platelet activation (52, 53). By contrast, Tx formation was depressed into the functionally relevant range in the present study, although suppression of additional, incidental TP ligands formed by COX, including PG endoperoxides and the isoprostane, iPF2α-III (54, 55), also may have been relevant. Surprisingly, there is no information from prospective controlled clinical trials of either aspirin or traditional NSAIDs on plaque progression. A small subset of individuals selected in a nonrandom manner from a controlled comparison of low- and high-dose aspirin in patients with peripheral vascular disease (56) suggested that the former was more effective than placebo in retarding carotid-plaque progression as assessed by ultrasound. Clearly, these results and those of Cayette et al. (23) suggest that a controlled evaluation of aspirin, TP antagonists, or NSAIDs on plaque progression by using modern imaging methods such as electron-beam computerized tomography (57) is timely.
In summary, the development of atherosclerosis is associated in LDLR-KO mice, as in humans, with evidence of accelerated platelet–vascular interactions. Although increased vascular expression of COX-2 contributes to augmented PGI2 biosynthesis, substantial but incomplete suppression of this eicosanoid with a COX-2 inhibitor failed to modulate the extent of atherosclerosis that had developed after 16 weeks on a high-fat diet. By contrast, combined inhibition of both COX isoforms retarded atherogenesis coincident with suppression of platelet-derived Tx into a functionally important range. These observations support the controlled evaluation of aspirin and TP antagonists in plaque progression in humans and suggest that acceleration of plaque progression during chronic consumption of COX-2 inhibitors is unlikely.
Acknowledgments
This work was supported by grants from the American Heart Association (0030211N) and the National Institutes of Health (HL-62250 and HL-61364). G.A.F. is the Robinette Foundation Professor of Cardiovascular Medicine.
Abbreviations
- Tx
thromboxane
- COX
cyclooxygenase
- TP
Tx receptor
- sICAM
soluble intercellular adhesion molecule
- NSAIDs
nonsteroidal anti-inflammatory drugs
- 2,3-dinor-6-keto PGF1α
the major urinary metabolite of PGI2
- PG
prostaglandin
- PGI2
prostacyclin
- LDL
low density lipoprotein
- R
receptor
- KO
knockout
- PGE2
prostaglandin E2
- MCP-1
monocyte chemoattractant protein-1
Footnotes
Egan, K. M., Austin, S. C., Smyth, E. M. & FitzGerald, G. A. (2000) Circulation 102, 234 (abstr.).
Burleigh, M. E., Patel, M. B., Babaev, V. R., Oates, J. A., Morrow, J. D., Fazio, S. & Linton, M. F. (2000) Circulation 102, 195 (abstr.).
References
- 1.Falk E. Circulation. 1985;71:699–708. doi: 10.1161/01.cir.71.4.699. [DOI] [PubMed] [Google Scholar]
- 2.Davies M J. Cardiovasc Clin. 1987;18:151–159. [PubMed] [Google Scholar]
- 3.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 4.Chen Z M, Sandercock P, Pan H C, Counsell C, Collins R, Liu L S, Xie J X, Warlow C, Peto R. Stroke (Dallas) 2000;31:1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 5.Collins R, Baigent C, Sandercock P, Peto R. BMJ. 1994;309:1215–1217. doi: 10.1136/bmj.309.6963.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 7.ESPS-2 Working Group. J Neurol. 1992;239:299–301. doi: 10.1007/BF00867583. [DOI] [PubMed] [Google Scholar]
- 8.Zucker T P, Bonish D, Much S, Weber A A, Bretschneider E, Gluss E, Schror K. Adv Exp Med Biol. 1997;433:387–390. doi: 10.1007/978-1-4899-1810-9_84. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo P, Golino P, Ragni M, Battaglia C, Pacifico F, Formisano S, Buono C, Condorelli M, Chiariello M. Cardiovasc Res. 1999;43:210–218. doi: 10.1016/s0008-6363(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Reilly I A, Doran J B, Smith B, FitzGerald G A. Circulation. 1986;73:1300–1309. doi: 10.1161/01.cir.73.6.1300. [DOI] [PubMed] [Google Scholar]
- 12.Ni H, Denis C V, Subbarao S, Degen J L, Sato T N, Hynes R O, Wagner D D. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FitzGerald G A, Smith B, Pedersen A K, Brash A R. N Engl J Med. 1984;310:1065–1068. doi: 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald G A. Am J Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- 15.Needleman P, Moncada S, Bunting S, Vane J R, Hamberg M, Samuelsson B. Nature (London) 1976;261:558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 16.Funk C D, Funk L B, Kennedy M E, Pong A S, FitzGerald G A. FASEB J. 1991;5:2304–2312. [PubMed] [Google Scholar]
- 17.Rimarachin J A, Jacobson J A, Szabo P, Maclouf J, Creminon C, Weksler B B. Arterioscler Thromb. 1994;14:1021–1031. doi: 10.1161/01.atv.14.7.1021. [DOI] [PubMed] [Google Scholar]
- 18.Lee S H, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 19.Xie W L, Chipman J G, Robertson D L, Erikson R L, Simmons D L. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib A, Creminon C, Frobert Y, Grassi J, Pradelles P, Maclouf J. J Biol Chem. 1993;268:23448–23454. [PubMed] [Google Scholar]
- 21.McAdam B F, Mardini I, Habib A, Burke A, Lawson J A, Kapoor S, FitzGerald G A. J Clin Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schonbeck U, Sukova G K, Graber P, Coulter S, Libby P. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayatte A J, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren T J, Cohen R A. Arterioscler Thromb Vasc Biol. 2000;20:1724–1728. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catella-Lawson F, McAdam B F, Morrison B W, Kapoor S, Kujubu D, Antes L, Lasseter K C, Quan H, Gertz B J, FitzGerald G A. J Pharmacol Exp Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- 26.McAdam B F, Catella-Lawson F, Mardini I, Kapoor S, Lawson J A, FitzGerald G A. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimbrone M A, Jr, Topper J N, Nagel T, Anderson K R, Garcia-Cardena G. Ann NY Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 28.Murata T, Ushikubi F, Matsouka T, Hirata M, Yamasaki A, Sugimoto Y, İchikawa A, Aze Y, Tanaka T, Yoshida N, et al. Nature (London) 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 29.Cryer B, Feldman M. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 30.Shah A A, Murray F E, Fitzgerald D J. Rheumatology. 1999;38:19–23. doi: 10.1093/rheumatology/38.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- 31.Praticò D, Tangirala R K, Rader D J, Rokach J, FitzGerald G A. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 32.Alessandrini P, Avogaro P, Bittolo Bon G, Patrignani P, Patrono C. Thromb Res. 1985;37:1–8. doi: 10.1016/0049-3848(85)90027-1. [DOI] [PubMed] [Google Scholar]
- 33.Patrignani P, Panara M R, Greco A, Fusco A, Natoli C, Iacobelli S, Cipollone F, Gancia A, Creminon C, Maclouf J, et al. J Pharmacol Exp Ther. 1994;271:1705–1712. [PubMed] [Google Scholar]
- 34.Lawson J A, Brash A R, Doran J, FitzGerald G A. Anal Biochem. 1985;150:463–470. doi: 10.1016/0003-2697(85)90536-6. [DOI] [PubMed] [Google Scholar]
- 35.FitzGerald G A, Brash A R, Falardeau P, Oates J A. J Clin Invest. 1981;68:1272–1275. doi: 10.1172/JCI110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocca B, Loeb A L, Strauss J F, Vezza R, Habib A, Li H, FitzGerald G A. Nat Med. 2000;6:219–221. doi: 10.1038/72334. [DOI] [PubMed] [Google Scholar]
- 37.Creminon C, Habib A, Maclouf J, Pradelles P, Grassi J, Frobert Y. Biochim Biophys Acta. 1995;1254:341–348. doi: 10.1016/0005-2760(94)00197-7. [DOI] [PubMed] [Google Scholar]
- 38.Moncada S, Gryglewski R, Bunting S, Vane J R. Nature (London) 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 39.Tschopp T B, Baumgartner H R. Agents Actions Suppl. 1979;4:156–162. [PubMed] [Google Scholar]
- 40.Kudryashov S A, Tertov V V, Orekhov A N, Geiling N G, Smirnov V N. Biomed Biochim Acta. 1984;43:S284–S286. [PubMed] [Google Scholar]
- 41.Geraci M W, Gao B, Shepherd D C, Moore M D, Westcott J Y, Fagan K A, Alger L A, Tuder R M, Voelkel N F. J Clin Invest. 1999;103:1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara S, Morishita R, Tone Y, Yokayama C, Inoue H, Kaneda Y, Ogihara T, Tanabe T. Biochem Biophys Res Commun. 1995;216:862–867. doi: 10.1006/bbrc.1995.2701. [DOI] [PubMed] [Google Scholar]
- 43.Cowan D H. Br J Haematol. 1981;49:425–434. doi: 10.1111/j.1365-2141.1981.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 44.Ogletree M L. Fed Proc. 1987;46:133–138. [PubMed] [Google Scholar]
- 45.Thomas D W, Manno R B, Mannon P J, Latour A, Oliver J A, Hoffman M, Smithies O, Koller B H, Coffman T M. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FitzGerald G A, Patrono C. In: Therapeutic Roles of Selective COX-2 Inhibitors. Vane J R, Botting R M, editors. London: William Harvey Press; 2001. (in press). [Google Scholar]
- 47.Nakatsugi S, Fukutake M, Takahashi M, Fukuda K, Isoi T, Taniguchi Y, Sugimura T, Wakabayashi K. Jpn J Cancer Res. 1997;88:1117–1120. doi: 10.1111/j.1349-7006.1997.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullen L, Kelly L, Connor S O, Fitzgerald D J. J Pharmacol Exp Ther. 1998;287:578–582. [PubMed] [Google Scholar]
- 49.Lehmann J M, Lenhard J M, Oliver B B, Ringold G M, Kliewer S A. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 50.Emery P, Zeidler H, Kvien T K, Guslandi M, Naudin R, Stead H, Verburg K M, Isakson P C, Hubbard R C, Geis G S. Lancet. 1999;354:2106–2111. doi: 10.1016/S0140-6736(99)02332-6. [DOI] [PubMed] [Google Scholar]
- 51.Simon L S, Weaver A L, Graham D Y, Kivitz A J, Lipsky P E, Hubbard R C, Isakson P C, Verburg K M, Yu S S, Zhao W W, et al. J Am Med Assoc. 1999;282:1921–1928. doi: 10.1001/jama.282.20.1921. [DOI] [PubMed] [Google Scholar]
- 52.Reilly I A, FitzGerald G A. Blood. 1987;69:180–186. [PubMed] [Google Scholar]
- 53.Praticò D, Cheng Y, FitzGerald G A. Arterioscler Thromb Vasc Biol. 2000;20:1695–1698. doi: 10.1161/01.atv.20.7.1695. [DOI] [PubMed] [Google Scholar]
- 54.Fitzpatrick F A, Gorman R R. Prostaglandins. 1977;14:881–889. doi: 10.1016/0090-6980(77)90304-5. [DOI] [PubMed] [Google Scholar]
- 55.Audoly L P, Rocca B, Fabre J E, Koller B H, Thomas D, Loeb A L, Coffman T M, FitzGerald G A. Circulation. 2000;101:2833–2840. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- 56.Ranke C, Hecker H, Creutzig A, Alexander K. Circulation. 1993;87:1873–1879. doi: 10.1161/01.cir.87.6.1873. [DOI] [PubMed] [Google Scholar]
- 57.Schermund A, Mohlenkamp S, Baumgart D, Kriener P, Pump H, Gronemeyer D, Siebel R, Erbel R. Am J Cardiol. 2000;86:127–132. doi: 10.1016/s0002-9149(00)00847-x. [DOI] [PubMed] [Google Scholar]