Abstract
Chronic marijuana (MRJ) use is associated with altered cognition and mood state, altered brain metabolites, functional and structural brain changes. The objective of this study was to apply proton magnetic resonance spectroscopic imaging (MRSI) to compare proton metabolite levels in 15 young men with MRJ-dependence and 11 healthy non-using (NU) young men. Spectra were acquired at 4.0 Tesla using 2D J-resolved MRSI to resolve coupled resonances in J-space and to quantify the entire J-coupled spectral surface of metabolites from voxels containing basal ganglia and thalamus, temporal and parietal lobe, and occipital white and gray matter. This method permitted investigation of high-quality spectra for regression analyses to examine metabolites relative to tissue type. Distribution of myo-inositol (mI)/creatine (Cr) was altered in the MRJ group whereas the NU group exhibited higher mI/Cr in WM than GM, this pattern was not observed in MRJ subjects. Significant relationships observed between global mI/Cr and distribution in WM, and self-reported impulsivity and mood symptoms were also unique between MRJ and NU groups. These preliminary findings suggest that mI, and distribution of this glial metabolite in WM, is altered by MRJ use and is associated with behavioral and affective features reported by young MRJ-dependent men.
Keywords: Marijuana, 1H MRS, Myo-Inositol, Impulsivity, Mood
1. Introduction
Cannabis, or marijuana (MRJ), is the most widely used illicit drug in the US, with 3.9 million people using MRJ almost daily in 2008 (SAMHSA, 2009). The highest rate of MRJ use occurs in 18–25 year olds (16.5%), followed by 16–17 year olds (12.7%), 14–15 year olds (5.7%), and then those 26 years and older (4.2%). Not only is MRJ most commonly the first illicit substance used, with 2.2 million people becoming new MRJ users in 2008, equating to roughly 6000 initiates per day, but 61.8% of the recent initiates were 18 or younger. Further, MRJ has the highest rate for illicit drug dependence, accounting for 60.1% of all illicit drug abuse, and was the second highest substance for which treatment was received (for alcohol, 2.7 million persons and for MRJ, 947,000 persons reporting receiving treatment in 2008). Importantly, sex differences in individuals 12 years of age and older have been reported both for prevalence of past month MRJ use, with males reporting greater use (7.9%) than females (4.4%), and higher rates of dependence (males 11.5% versus females 6.4%).
Neuropsychological studies have shown deficits in attention, mental flexibility, learning and memory in MRJ-users (Block and Ghoneim, 1993; Bolla et al., 2002; Fletcher et al., 1996; Pope et al., 2001; Pope and Yurgelun-Todd, 1996; Solowij et al., 2002), some of which persist even after drug use has ceased (Pope et al., 2001; Pope and Yurgelun-Todd, 1996). In addition to cognitive alterations, greater levels of impulsivity and depressive symptoms have been associated with MRJ use (Degenhardt et al., 2003; Ramaekers et al., 2006). Although the neurobiological effects of MRJ have not been fully characterized, neuroimaging studies have shown that MRJ-users exhibit abnormal brain activation during performance of functional imaging tasks, including verbal list learning (Block et al., 2002; Bolla et al., 2005; Chang and Chronicle, 2007; Chang et al., 2006b), decision-making (Iowa Gambling task) (Bolla et al., 2005), visual-attention (Chang et al., 2006b), and response inhibition (Gruber and Yurgelun-Todd, 2005) (for review, see Chang and Chronicle, 2007). Altered activation may be related to reductions in grey matter (GM) volume in regions such as the medial temporal lobe (MTL) (Matochik et al., 2005; Yucel et al., 2008) (see also Block et al., 2000; Tzilos et al., 2005), which has a high density of cannabinoid receptors (CB1) (Iversen, 2003; Sullivan, 2000). Cerebral white matter (WM) is also vulnerable to the effects of MRJ use, as diffusion tensor imaging (DTI) studies in adolescent and adult MRJ-users demonstrate lower WM integrity relative to non-using comparison subjects, albeit inconsistently (Arnone et al., 2006; Arnone et al., 2008; Ashtari et al., 2009; Delisi et al., 2006; Gruber and Yurgelun-Todd, 2005). Thus, there is compelling evidence that MRJ exposure is associated with structural and functional alterations that may contribute to the disturbances in memory performance, executive functioning and affect modulation observed in MRJ-users (Gruber et al., 2009; Lubman et al., 2007).
Proton magnetic resonance spectroscopy (1H MRS) can be used to examine cellular health and integrity by measuring in vivo levels of several brain metabolites. N-acetyl-aspartate (NAA), the most prominent metabolite in brain, is found primarily in neurons, and is generally regarded as a marker indicating neuronal integrity (Birken and Oldendorf, 1989; Demougeot et al., 2004; Inglese et al., 2008; Moffett et al., 2007; Pouwels and Frahm, 1997; Sullivan et al., 2001). Creatine (Cr), with phosphocreatine (PCr), plays a major role in cerebral energy metabolism, and acts as an energy buffer by maintaining constant brain energy levels and by distributing energy (Kemp, 2000; Miller, 1991). Choline (Cho) reflects a number of choline-containing compounds that are involved in cellular membrane synthesis and degradation (Miller, 1991; Pouwels and Frahm, 1998). Myo-Inositol (mI) serves as an organic osmolyte by maintaining cell volume, and as a phosphorylated derivative for the synthesis of secondary messengers (Berridge and Irvine, 1989; Brand et al., 1993; Downes and Macphee, 1990; Kim et al., 2005; Wolfson et al., 2000). Additional metabolites quantifiable in the proton spectrum, albeit near the lower limit of detection, include glutamate (Glu), glutamine (Gln) and gamma amino butyric acid (GABA) (Behar and Rothman, 2001; Petroff et al., 2000; Shulman et al., 2004). Gln is a precursor for Glu, which is a major excitatory neurotransmitter found in all brain cell types, with the highest concentrations generally observed in neurons. Gamma amino butyric acid is the major inhibitory neurotransmitter in the mammalian brain. In addition to excitatory and inhibitory neurotransmission, together with Gln, Glu and GABA play important roles in glucose metabolism, neuronal energetics and ammonia detoxification (Behar and Rothman, 2001; Patel et al., 2005). However, these peaks are obscured by peaks of higher concentration metabolites in brain spectra, such as Cr, and thus can be difficult to resolve. Recently, use of high field MR scanners and specialized editing techniques have enhanced MR visibility and quantification of these peaks (Jensen et al., 2009; Keltner et al., 1997; Mescher et al., 1998; Weber et al., 1997).
To date, only two published reports have examined proton metabolites in MRJ-users. Chang and colleagues (Chang et al., 2006a), in an investigation of human immunodeficiency virus (HIV) and marijuana use, acquired single voxel MRS data at 4 Tesla (T) from multiple brain regions and found significantly lower mI, Cho and Glu in the basal ganglia and elevated Cr in the thalamus of HIV-negative chronic MRJ-users (36 ± 2 years) relative to HIV-negative non-using controls (42 ± 2 years), although metabolite differences were negligible after controlling for age effects. In a study by Hermann and colleagues (Hermann et al., 2007), magnetic resonance spectroscopic imaging (MRSI) was employed at 1.5 T to examine several regions of interest in young MRJ-users (22 ± 2 years) and controls (23 ± 2). Significantly lower ratios of NAA to total Cr (Cr + PCr, or tCr) were observed in the dorsal lateral prefrontal cortex of the MRJ group relative to non-users. A significant positive correlation was observed between NAA/tCr levels in the putamen/globus pallidum and cannabidiol (CBD), a constituent of the cannabis plant that, unlike tetrahydrocannabinol (THC), is nonpsychotropic.
While the main advantage of MRSI is to examine spectra across multiple regions post spectral acquisition, the objective of this preliminary study was to use MRSI combined with tissue segmentation to evaluate relative levels of proton metabolites in GM and WM from a maximal sample of high-quality voxels (Auer et al., 2001; Doyle et al., 1995; Hetherington et al., 1996; Jensen et al., 2005; Lim and Spielman, 1997; Pan et al., 1998; Pfefferbaum et al., 1999; Schuff et al., 2001). This approach permits a thorough and rigorous statistical regression analysis of metabolite levels relative to tissue type from large brain areas, including regions known to contain high concentrations of the CB1 receptor, such as the basal ganglia, the hippocampus and the MTL (Glass et al., 1997; Moldrich and Wenger, 2000). Given the higher prevalence of both use and dependence relative to older and younger cohorts, as well as relative to women, young adult MRJ-using men who met criteria for MRJ dependence were examined in this study. Measures of impulsivity and mood were also examined relative to the distribution of metabolite levels in GM and WM, as previous structural and functional changes in MRJ-users suggest neurobiological correlates for these behavioral traits in MRJ users.
2. Methods
2.1. Subjects
Fifteen men diagnosed with MRJ-dependence (MRJ; age=21.2±3.4 years, education=13.9±2.2 years) and eleven healthy non-using men (NU; age=25.0±4.8 yrs., education=16.1±2.7 years) served as participants. Clinical assessments were performed in all subjects using the Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-I/NP), which is widely used to reliably determine Axis I disorders in clinical populations (First et al., 2002). NU subjects were recruited from the local community, were free of Axis I psychiatric diagnoses based on the SCID interviews, and reported no psychoactive substance use other than minimal alcohol use. MRJ subjects met DSM-IV criteria for MRJ-dependence and were active MRJ-users, with an average age of first MRJ use=15.7±2.2 years and duration of MRJ use=5.5±2.6 years. MRJ subjects reported using MRJ 5.6±1.7 times per week, consuming 12.7±9.2 alcoholic beverages/week and, in only one MRJ subject, smoking 1 pack of cigarettes/day. Exclusion criteria for all subjects included DSM-IV Axis I diagnoses (other than marijuana dependence in the MRJ group), current psychoactive substance use (other than marijuana use in the MRJ group), history of organic mental disorder, head trauma, loss of consciousness, seizure disorder or central nervous system disease, or contraindications to scanning. All subjects underwent a clinical MRI scan, which was read and interpreted by a clinical neuroradiologist. No clinical brain abnormalities were present in any of the MRJ or NU subjects included in the MRSI investigation.
2.2. Procedure
The Institutional Review Board of McLean Hospital approved all aspects of the clinical research protocol. After complete description of the study, written informed consent was obtained from all subjects. Subjects were monetarily compensated for their participation.
Subjects provided a urine sample, under the direct observation of the study coordinator, which was tested for tetrahydrocannabinol (THC), amphetamines/methamphetamines, barbiturates, benzodiazepines, cocaine, opiates, phencyclidine, propoxyphene and tricyclic antidepressants (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, San Diego, CA). Results of the drug panel confirmed recent MRJ use in MRJ subjects and no drug use in NU subjects. An aliquot of the urine sample from MRJ subjects was sent for a standard laboratory urinalysis, which included gas chromatography-mass spectroscopy to quantify nor-9-carboxy-delta 9-tetrahydrocannabinol levels (Quest Diagnostics, Cambridge, MA). THC was normalized to urinary creatinine (Cre) for each subject, in order to correct for individual differences in urine concentration. Average THC/Cre levels observed in MRJ subjects were 4.1±4.5 ng/ml.
2.2.1. Clinical measures
All participants completed the Barratt Impulsiveness Scale (BIS-11, Patton et al., 1995), a self-report measure of impulsivity that yields a total score for trait impulsivity, and subscale impulsivity scores: cognitive (rapid shifts in attention/impatience with complexity), motor (impetuous action), and non-planning (lack of future orientation). Participants also completed the Profile of Mood States (POMS; McNair et al., 1971), a self-report inventory of six mood states (tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, confusion-bewilderment); the Positive Affect Negative Affect Schedule (PANAS; Watson et al., 1988), yielding scores for positive and negative affect; and the Beck Depression Inventory (BDI-II; Beck et al., 1996), which assesses symptoms of depression over the previous week.
2.2.2. Spatial and spectral localization
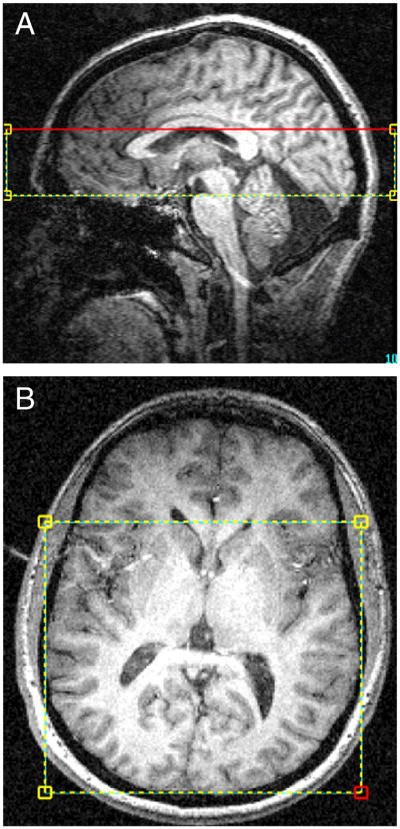
In vivo proton MRS and structural MRI scans were acquired using a full-body 4.0 Tesla Varian Unity/Inova MRI/MRS scanner (Varian, Inc., Palo Alto, CA, USA) and a volumetric TEM design (Bioengineering, Minneapolis, MN, USA) RF head coil operating at 170.3 MHz for 1H imaging. A rapid two-dimensional (2D) gradient-recalled echo imaging sequence (12s) was used to acquire sagittal, coronal, and axial images for rapid determination of head position within the coil. The global magnetic field was manually shimmed using first and second-order shim coils (x, y, z, z2, xy, yz, xz, x2-y2), resulting in unfiltered water linewidths ≤ 25Hz. High-contrast, T1-weighted sagittal and axial images were then acquired, using the following parameters: TR/TE= 6.2/11.4ms, field of view (FOV) (in-plane)=24x24cm, slice thickness=2.5mm (sagittal 16 slices, axial 32 slices), matrix size=256x256, to guide placement of a 4cm thick CSI-PRESS box inferior to the body of the corpus callosum over the mid-sagittal image (Figure 1a), and readjusted in the axial plane to align the top of the box with the top of the caudate (Figure 1b). This placement permitted sampling voxels from basal ganglia and thalamus, temporal and parietal lobe, and occipital white and gray matter.
Figure 1.
A,B. A,B) Sagittal and axial T1-weighted images depicting placement of the PRESS box over the volume of interest.
2.2.3. Spectral acquisition
A modified 2D J-resolved magnetic resonance spectroscopic imaging (J-MRSI) sequence and standard point-resolved spectroscopy (PRESS) selective chemical shift imaging (CSI) scheme was use to incrementally acquire spectra at multiple phase-encode steps, with increasing echo-times to sample the J-coupling of proton metabolites. Sixteen individual TE-stepped spectra were collected for each of the 96 circular, sparsely sampled k-space points, with echo-times ranging from 30–330 milliseconds (msec), in 20 msec increments. Acquisition parameters were: TR=1.25 sec, sampling matrix=14x14 (circular-sparse), spectral bandwidth=2kHz, complex time-points=1024, FOV=18x18 cm, NEX=1, nominal voxel volume=6.6 cc (10.2 cc effective voxel size), total spectral acquisition time=32 min. The advantages of TE stepping in the present study, as opposed to spectral acquisition at a single stable TE, were two-fold. First, TE-stepping permits the collection of metabolite transverse (T2) relaxation times and subsequent determination of T2-decay constants for several key metabolites, including NAA, Cho and Cr. T2 is sensitive to changes in the cellular microenvironment and can influence metabolite MRS signal decay characteristics, cell volume, molecular structure and/or metabolite-macromolecular interactions (Frahm et al., 1989; Posse et al., 1995; Traber et al., 2004). Thus, NAA, Cho and Cr can be T2-corrected, which is particularly important for Cr when Cr is used as the denominator for calculating metabolite ratios. Second, TE-stepped data, when Fourier-Transformed in the TE-dimension, yield J-resolved datasets for every voxel. It has been shown that the J-resolved technique allows for quantification of the entire J-coupled spectral surface, and accordingly, improved measurement precision of strongly-coupled resonance species, e.g., mI, Glu, and Gln, as compared to single-echo techniques (Jensen et al, 2009).
2.2.4. Spectral data processing
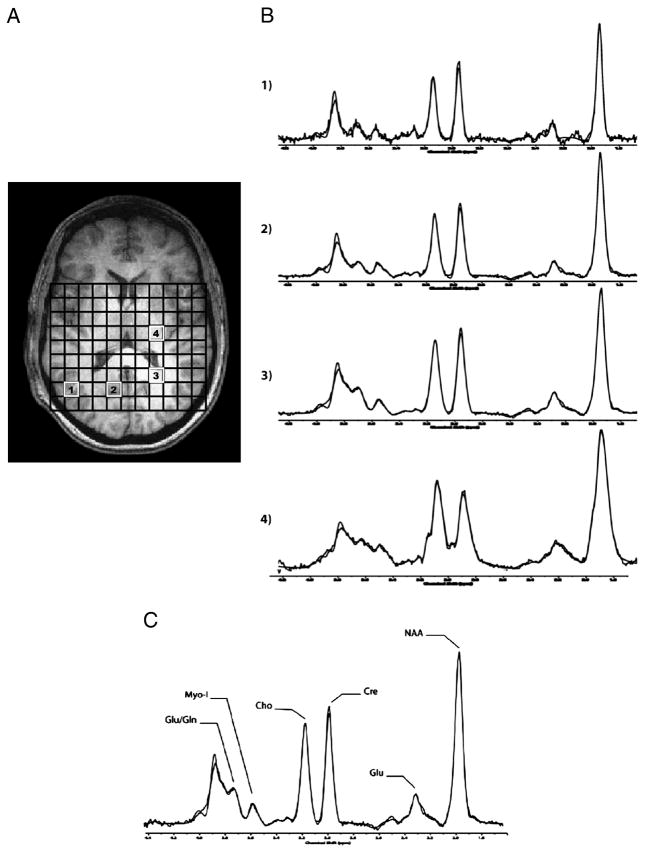
The 96 sparsely sampled k-space points per echo-time were first read into a zero-padded 16x16 matrix for all 16 echo-times. The TE-series (16 k-space J-MRSI data sets) were zero-filled out to 64 k-space and digitally-apodized prior to Fourier transforming in the TE dimension. Each J-resolved, 2D MRSI k-space set was then digitally filtered in kx and ky with a Hanning k-space filter to produce J- and spatially-resolved spectra. For each subject, the 2D J-MRSI grid was shifted in the x and y dimensions to position an 11x9 rectangular matrix of voxels inside the volume of interest (Figure 2a). Automated software developed on-site was used to omit voxels from the entire J-resolved CSI dataset that contained excessive lipid signal from sub-cranial fat layer. Remaining spectra were then automatically fitted using an optimized, two-dimensional LCModel algorithm produced from GAMMA-simulated basis sets (Jensen et al., 2009), which quantifies all 32 J-resolved spectral extractions within a J-bandwidth of 25Hz and provides two-dimensional integrals for Cho, mI, Glu, Gln, NAA, NAA + N-acetyl-aspartyl-glutamate (NAAG), and Cr. To ensure only high-quality spectra were included in the analysis, the J=0.0 Hz spectrum and LCModel fit for each voxel was inspected by an experienced spectroscopist (JEJ). The number of voxels that produced high quality spectra did not differ between groups, MRJ group=44.2±5.6 voxels, NU group=46.3±6.5 voxels, P=.82. Spectral data from 2 MRJ subjects and 1 NU subject were not useable due to poor spectral quality, likely resulting from subject movement. Sample spectra (J=0.0Hz) from parieto-occipital GM voxels (Figure 2B-1, B-2, B-3) and from striatal WM (Figure 2B-4), and a sample spectrum (J=0.0Hz) with LCModel fit and labeled metabolites are presented in Figure 2C.
Figure 2.
A-C. A) Axial T1-weighted images depicting MRSI voxel grid, with corresponding J=0.0Hz sample spectra from voxels in the parieto-occipital GM matter (B-1, B-2, B-3) and from striatal WM (B-4). C) J=0.0Hz spectrum shown with LCModel fit and labeled metabolites. Abbreviations: Glu, Glutamate; Gln, Glutamine; Myo-I, myo-Inositol; Cho, Choline; Cre, Creatine; NAA, N-acetyl-aspartate.
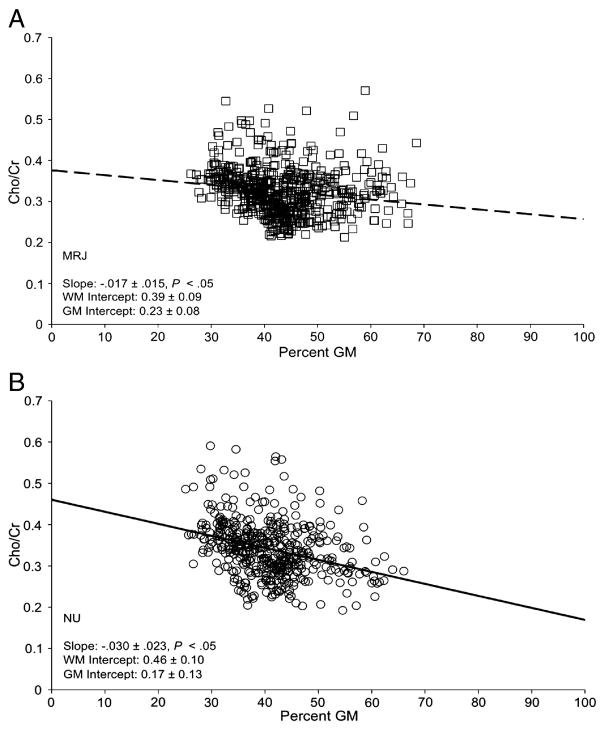
Figure 4.
A,B. Group regression plots of Cho/Cr levels as a function of %GM for all subjects in each group for all voxels with high quality spectra. Individual data/regression lines in Figure 4A represent the MRJ group, open squares a with dashed line, and in Figure 4B, the NU group, open circles with a solid line. Slopes of the linear regression were calculated per subject (data not shown), and group averages demonstrated that slopes were significantly different from zero for both the MRJ group (slope=−.016±.006, P=.002) and the NU group (slope=−.031±.006, P=.003).
2.2.5. Metabolite Quantification
Metabolite ratios were calculated as raw metabolite integrals relative to the Cr integral. Although there are disadvantages of using metabolite ratios, particularly relative to Cr and compared to absolute quantifications (Cheong et al., 2006; Knight-Scott et al., 2003; Kreis et al., 1993), a series of steps were taken in the present study to ensure that Cr was an unbiased denominator, i.e., Cr did not differ as a function of group. First, using the TE-stepped dataset, each of the 16 TE-stepped spectra were fitted with the corresponding LCModel template in order to derive T2 values for Cr, Cho, NAA, and NAA + NAAG, using an automated, least-squares algorithm. NAA, Cr and Cho have small, coupled components in the proton spectrum, but also dominating singlet resonances that LCModel can fit with a high degree of precision over the longer echo-times. In contrast, the highly coupled mI, Glu and Gln are much more difficult to fit properly at longer echo times, given the high degree of overlap with other resonances, thus producing an undulating and non-exponential single decay curve due to J-coupling evolution with echo time (Hurd et al., 2004). Thus, T2 values (msec) were derived for Cr, Cho and NAA on a voxel-by-voxel basis for each subject, and demonstrated no significant differences in Cr T2, Cho T2, NAA T2, or NAA + NAAG T2 between MRJ and NU groups. Accordingly, Cr, Cho, NAA and NAA + NAAG were corrected for each metabolite’s respective T2 value prior to normalization to Cr by multiplying each raw metabolite integral by 1/exp(-TE/T2) (TE=30ms and T2=derived T2 decay-constant for each respective metabolite, msec). Although this correction could not be applied to mI, Glu or Gln as indicated above, contributions of T2 to NAA, NAA + NAAG and Cho, and importantly, Cr, were likely minimized.
Second, the ratio of T2-corrected Cr to the partially T2-corrected total integrated proton signal (which did not differ by group, P=.49) was derived using all fitted metabolites including T2-corrected Cr, Cho, NAA and NAAG values, and non T2-corrected mI, Glu and Gln. Ratios of T2-corrected Cr/partially T2-corrected total signal did not differ between groups (P=.69). Accordingly, all proton metabolites reported were expressed as raw integral values (T2-corrected when applicable, for Cho, NAA, and NAA + NAAG) divided by T2-corrected Cr.
2.2.6. Tissue segmentation and linear regressions with metabolite levels
T1-weighted image sets were segmented into four individual tissue compartments using the automated commercial software package FSL 4.1 (FMRIB Software Library, Analysis Group, FMRIB, Oxford, UK), which included GM, WM, cerebral-spinal fluid (CSF) and subcortical deep GM (i.e., thalamus, putamen, caudate and globus pallidus, SDGM). Binary-segmented tissue image sets were convolved with the calculated two-dimensional point-spread function, including effects of digital k-space filtering, to provide partial tissue estimates for each voxel. Percent relative cortical GM tissue content was estimated per voxel by dividing the raw GM fraction by the total tissue sum [raw grey fraction/(raw grey fraction + raw white fraction + raw subcortical fraction)] x 100%. Subsequently, linear regressions were calculated between tissue-corrected values for GM content and tissue-corrected metabolite/Cr ratios for each voxel. For each subject, linear regressions were used to determine estimates for “pure” WM and “pure” GM metabolite/Cr ratios, based on the y-intercepts for 0% GM and 100% GM respectively, as well as the slope of the fitted regression line (Auer et al., 2001; Doyle et al., 1995; Hetherington et al., 1996; Jensen et al., 2005; Lim and Spielman, 1997; Pan et al., 1998; Pfefferbaum et al., 1999; Schuff et al., 2001). Given that linear regressions can be influenced by even a single extreme value, a multistep algorithm was used for voxel filtering to reduce inclusion of potential outliers in the analyses. In addition to inspection of spectra from all voxels for quality of fit, with obvious misfits and poor-quality spectra identified and discarded by an experienced MRS physicist (JEJ), each voxel cluster of metabolites was screened statistically by applying a filter in which metabolite values falling 3±SD from the mean were omitted.
2.3. Statistical analysis
One-way analyses of covariance (ANCOVAs), with age and education as covariates, were used to compare MRJ and NU subjects on clinical measures, T2 and metabolite values, tissue contributions, and linear regressions of metabolites relative to GM contributions. One-sample t-tests, conducted separately for each group, determined if average slopes of the linear regression of metabolites relative to percent GM were significantly different from zero. Relationships between clinical variables and metabolites were examined using Spearman’s rho correlation coefficients, a non-parametric statistic that is not sensitive to outliers. Given the exploratory nature of this preliminary investigation, significance levels for regressions, group comparisons on extrapolated y-intercepts for WM metabolite concentrations and correlations, were not corrected for multiple comparisons. SPSS 18.0 (SPSS, Chicago, IL), with a=.05, was used for all statistical analyses.
3. Results
3.1. Clinical measures
MRJ subjects exhibited significantly higher total impulsivity scores than NU subjects on the BIS-11 (P=.01), as well as higher scores on the BIS-11 cognitive (P=.03), and motor (P=.04) impulsivity subscales. No other significant differences on clinical measures were observed between groups. Data for clinical measures are presented in Table 1.
Table 1.
Clinical measures
| MRJ (n=**) | NU (n=10) | F | P | ||
|---|---|---|---|---|---|
| BIS | Cognitive | 19.8 ±3.6 | 14.9±3.7 | 5.63 | .03* |
| Motor | 26.3±4.9 | 20.8±5.3 | 4.71 | .04* | |
| Non-Planning | 27.5±4.5 | 23.1±5.1 | 2.90 | .10 | |
| Total Score | 73.5±10.1 | 58.8±9.8 | 7.75 | .01* | |
| POMS | Vigor | 20.3±4.9 | 21.0±5.2 | 0.06 | ns |
| Anger | 5.2±4.3 | 3.5±4.3 | 0.58 | ns | |
| Confusion | 8.6±4.6 | 6.7±3.4 | 0.84 | ns | |
| Tension | 8.7±7.1 | 6.9±4.7 | 0.79 | ns | |
| Fatigue | 5.6±5.0 | 5.5±6.9 | 0.03 | ns | |
| Depression | 5.6±10.8 | 3.7±5.3 | 0.37 | ns | |
| Total Mood Disturbance | 13.5±29.8 | 5.3±26.3 | 0.31 | ns | |
| PANAS | Positive Affect Score | 33.1±7.8 | 38.3±5.0 | 2.43 | .14 |
| Negative Affect Score | 15.7±8.4 | 13.1±3.0 | 1.52 | ns | |
| BDI | Total Score | 5.0±9.5 | 1.0±1.8 | 2.38 | .14 |
Data represent average scores±standard deviation (SD).
Indicates statistical significance, P < .05.
Sample sizes for the MRJ group: BIS n=15, POMS n=13, PANAS n=12, and BDI n=13. Abbreviations: BIS, Barratt Impulsivity Scale; POMS, Profile of Mood States; PANAS, Positive and Negative Affect Scale; BDI, Beck Depression Inventory
3.2. Tissue segmentation
No significant group differences were observed for the relative GM, WM, CSF or SDGM measured across all voxels within the MRSI volume of interest. Percent tissue volumes were as follow: GM, MRJ=39.1±0.8%, NU=37.5±0.9%, P=.22; WM, MRJ=50.2±1.0%, NU=51.0±1.2%, P=.65; CSF, MRJ=8.7±0.7%, NU=9.8±0.8%, P=.33; and SDGM, MRJ=2.1±0.4%, NU=1.8±0.5%, P=.66.
3.3. Proton metabolite ratios
Global mI/Cr ratios were significantly lower in MRJ subjects relative to NU comparison subjects, F1,23=11.85, P=.003. No other significant group differences in global metabolite ratios, averaged across ~45 voxels for each group, were observed (Table 2).
Table 2.
Proton metabolite ratios
| MRJ (n=13) | NU (n=10) | F1,23 | P | |
|---|---|---|---|---|
| Cho/Cr | 0.29±0.03 | 0.30±0.02 | 0.15 | ns |
| mI/Cr | 0.80±0.12 | 0.96±0.12 | 11.85 | .003* |
| NAA/Cr | 1.25±0.20 | 1.22±0.12 | 0.29 | ns |
| NAA + NAAG/Cr | 1.55±0.21 | 1.49±0.13 | 0.34 | ns |
| Glu/Cr | 0.88±0.16 | 0.88±0.09 | 0.38 | ns |
| Gln/Cr | 0.26±0.12 | 0.20±0.09 | 2.43 | .14 |
| Gln/Glu | 0.30±0.11 | 0.23±0.10 | 1.72 | ns |
Data represent average metabolite ratios (/T2-corrected Cr)±SD. Cho, NAA, and NAA + NAAG metabolite numerators were T2-corrected.
Indicates statistical significance, P < . 05. Abbreviations: Cho, Choline; mI, myo-Inositol; NAA, N-acetyl-aspartate; NAA + NAAG, N-acetyl-aspartate + N-acetyl-aspartyl-glutamate; Glu, glutamate; Gln, glutamine.
3.4. Linear regressions: Metabolite ratios vs. percent GM
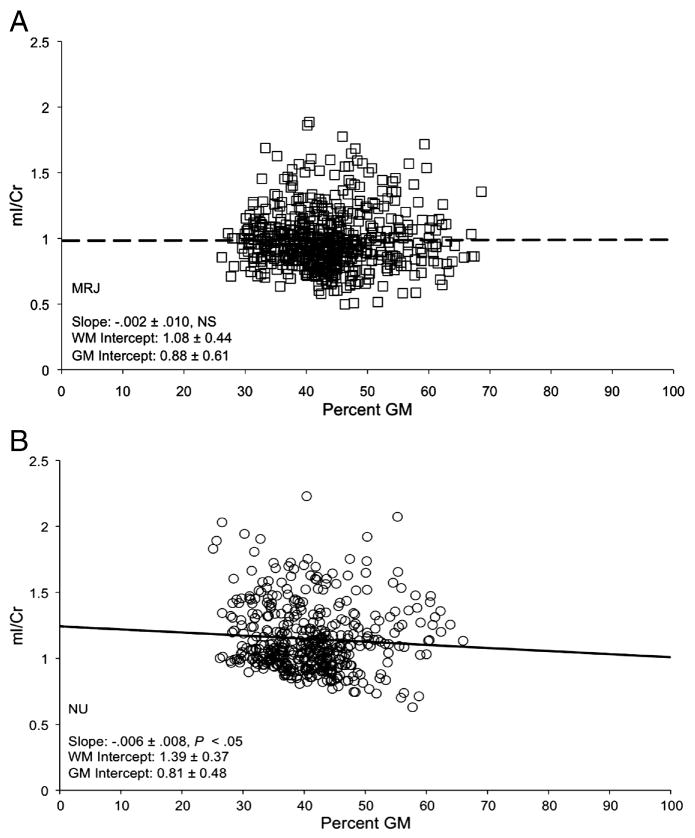
Significant group differences in the slope of the linear regression were observed for mI/Cr as a function of percent GM (Table 3, P=.04), with only the NU group exhibiting a negative slope that differed significantly from zero (P=.05). These data indicate that mI/Cr decreases as GM content increases in NU subjects (Figure 3b), but not in MRJ-users (Figure 3a). The MRJ group exhibited significantly lower estimated mI/Cr in pure WM (0% GM, y-intercept) than the NU group (Table 3, P=.005) (see also, Figure 2a). Similar to the profile observed for mI, MRJ subjects exhibited a less negative slope of Cho as a function of GM content than NU subjects (P=.11), although negative slopes for both groups differed significantly from zero (MRJ: P=.002; NU: P=.003) (Figure 4a, b). No other significant linear regressions were observed (Table 3).
Table 3.
Slopes and y-intercepts: Linear regression of metabolites vs. percent GM
| MRJ (n=13) | NU (n=10) | F1,23 | P | ||
|---|---|---|---|---|---|
| Slope | Cho/Cr | −.017±.015** | −.030±.023** | 2.82 | .11 |
| mI/Cr | −.002±.010 | −.006±.008** | 4.76 | .04* | |
| NAA/Cr | .002±.017 | .003±.013 | 0.01 | ns | |
| NAA + NAAG/Cr | −.002±.021 | −.004±.016 | 0.09 | ns | |
| Glu/Cr | −.001±.013 | −.003±.009 | .01 | ns | |
| Gln/Cr | −.002±.017 | .003±.006 | 1.97 | .18 | |
| y-intercept | Cho/Cr WM | 0.39±0.09 | 0.46±0.10 | 2.43 | .14 |
| Cho/Cr GM | 0.23±0.08 | 0.17±0.13 | 2.66 | .12 | |
| mI/Cr WM | 1.08±0.44 | 1.39±0.37 | 10.36 | .005* | |
| mI/Cr GM | 0.88±0.61 | 0.81±0.48 | 1.61 | ns |
Data represent group averages (± SD) of the slope of the linear regression of metabolites normalized to Cr and %GM, and estimated y-intercept values for metabolite ratios at 0% GM (WM) and at 100% GM.
Indicates statistically significant group difference and
indicates slope significantly different from zero, P < .05. Abbreviations: Cho, Choline; mI, myo-Inositol; NAA, N-acetyl-aspartate; NAA + NAAG, N-acetyl-aspartate + N-acetyl-aspartyl-glutamate; Glu, glutamate; Gln, glutamine; WM, white matter; GM, grey matter
Figure 3.
A,B. Group regression plots of mI/Cr levels as a function of %GM for all subjects in each group for all voxels with high quality spectra. Individual data/regression lines in Figure 3A represent the MRJ group, open squares a with dashed line, and in Figure 3B, the NU group, open circles with a solid line. Slopes of the linear regression were calculated per subject (data not shown), and group averages demonstrated that slopes were not significantly different from zero in the MRJ group (slope=.001±.002, P=. 49), but were significantly different in the NU group (slope= −.009±.003, P=.05).
3.5. Correlations: MRJ use and clinical measures
A greater frequency of reported MRJ use per week was positively associated with higher urinary THC/Cre levels (r=.55, P=.02) and showed a trend for a negative association with age of onset of MRJ use (r=−.41, P=.06). A greater frequency of MRJ use per week was significantly associated with lower BIS motor impulsivity (r=−.60, P=. 009) and trend for lower BDI total score (r=−.45, P=.06), whereas higher urinary THC/Cr was significantly correlated with lower BIS motor impulsivity (r=−.46, P=.04) and higher non-planning impulsivity (r=.58, P=.01), lower POMS tension (r=−.64, P=.009) and less PANAS negative affect (r=−.66, P=.01) in MRJ subjects.
3.6. Correlations: Metabolites, MRJ use and clinical measures
Global mI/Cr was not related to current frequency of MRJ use, age of onset or duration of MRJ use or urinary THC/Cr levels. However, greater frequency of use and earlier age on onset were significantly associated with a more negative mI/Cr slope (higher in WM than GM) (r=−.53, P=.03, and r=.62, P=.01, respectively) and higher estimated WM intercepts (r=.57, P=.02, and r=.66, P=.007, respectively).
Significant correlations observed between impulsivity (BIS-11), mood measures (POMS, PANAS, BDI) and global mI/Cr, slope of mI/Cr as a function of tissue type and WM intercept, differed between MRJ and NU groups. Higher global mI/Cr in NU subjects predicted less BIS cognitive impulsivity, r=−.65, P=.03; motor impulsivity, r=−.71, P=.02; and total BIS score, r=−.76, P=.01. Higher estimated WM intercept also predicted less BIS cognitive impulsivity in the NU group, r=−.60, P=.04. In contrast, lower global mI/Cr was significantly predictive of less non-planning impulsivity in MRJ subjects (r=.51, P=. 04), who also demonstrated that a higher slope (lower mI in WM) and a lower estimated WM intercept were correlated with less cognitive impulsivity (r=−.47, P=.05, r=.59, P=. 02, respectively).
As for mood measures, in NU subjects, lower global mI/Cr significantly predicted higher POMS tension (r=−.57, P=.05), depression (r=−.66, P=.03), anger (r=−.60, P=.05), confusion (r=−.70, P=.02), fatigue (r=−.63, P=.03) and total mood disturbance (r=−.78, P=.007), whereas lower global mI/Cr in the MRJ group only predicated higher POMS tension (r=−.62, P=.02). Also observed in the NU group was a significant relationship between lower global mI/Cr and higher PANAS negative affect (r=−.92, P=.001), while MRJ-users demonstrated that a higher mI/Cr slope (lower mI in WM) and a lower WM intercept predicted higher depression scores on the BDI (r=.71, P=.007, r=−.66, P=.01, respectively).
4. Discussion
Results of this preliminary study demonstrate lower global mI/Cr in young MRJ-dependent men compared to NU subjects across several brain regions known to contain high concentrations of CB1 receptors, including basal ganglia, thalamus and hippocampus. Importantly, tissue regression analyses showed that distribution of mI/Cr in WM was also lower in MRJ subjects, measured by a significantly smaller slope (that was not significantly different from zero) and a lower estimated intercept of mI/Cr in WM tissue, compared to NU subjects. Trends for a smaller slope and lower estimated WM intercept for Cho/Cr were also observed in MRJ subjects relative to the NU group in the present study. These data are consistent with previously reported alterations in mI and Cho from a similar region in MRJ-users (Chang et al., 2006a). This mI-specific finding was observed in the present study despite a lack of group differences in the number of voxels with high spectral quality or in overall tissue percentages across all voxels.
The primary localization and tissue dependence of mI is consistent with reports of susceptibility of regions, such as the MTL, to chronic MRJ use (Delisi et al., 2006; Matochik et al., 2005; Yucel et al., 2008). Reduced mI in the current cohort of young men with a relatively short duration of MRJ use (5.5 years) may reflect an early neurochemical response to toxicity prior to the manifestation of changes in neuronal integrity (Wu and Zhuo, 2008). Thus, reduced mI may be suggestive of glial function suppression, as activation of CB1 receptors expressed by microglia has been shown to control their immune-related functions (Stella, 2009). Lower mI may also result from increased efforts to maintain a constant cellular volume, since previous work has demonstrated decreased hippocampal cellular volume following chronic THC exposure (Scallet, 1995). Cellular volume is maintained through transmembrane influx of Inositol, which is mediated by volume sensitive organic osmolyte channels. Compensatory efforts to maintain cell volume would require increased consumption of Inositol, and subsequently, reduce the availability of the precursor, mI (Wolfson et al., 2000). Although purely speculative, at the receptor level, reductions in mI could be secondary to prolonged CB1 receptor activation. CB1 receptors are a family of G-protein coupled presynaptic receptors that have been shown to influence GABAergic inhibitory signaling (Hajos and Freund, 2002; Hoffman and Lupica, 2000; Katona et al., 2000). Reduced GABA release associated with CB1 receptor activation involves multiple intracellular signal transduction pathways, which is relevant given that mI is a precursor for Inositol phospholipids, secondary messengers diacylglycerol (DAG) and Inositol 1,4,5-triphosphate (IP3) (Downes and Macphee, 1990). Examination of secondary messenger systems in chronic MRJ-users is not possible with MRS, and thus is beyond the scope of this work, but nonetheless, it seems plausible that changes in mI could be associated with MRJ-related alterations in neurotransmission. Elevations in mI have been reported in cocaine (Chang et al., 1999b; Chang et al., 1997), alcohol (Gazdzinski et al., 2008; Meyerhoff et al., 2004; Schweinsburg et al., 2000) and ecstasy (Chang et al., 1999a) users with longer histories of abuse than in the MRJ-users examined in the present study. It is important to consider that mI reductions to overcome acute toxicity may be observed early during use history. In contrast, later elevations in mI, suggestive of glial hypertrophy associated with increased duration of drug use, may occur in the presence of changes in neuronal health, indicated by decreased NAA levels.
The present findings also demonstrate that lower mI/Cr may be a neurochemical correlate of MRJ dependence and some of its associated behavioral and affective features. Lower global mI/Cr and WM distribution of mI/Cr significantly predicted a higher level of total impulsivity in NU, but less non-planning impulsivity in MRJ-users, e.g., I am self-controlled, I plan for my future. These unique relationships were observed despite evidence that MRJ-users in the present study were significantly more impulsive than NU subjects, which is consistent with previous reports (Degenhardt et al., 2003; McDonald et al., 2003; Medina et al., 2007; Ramaekers et al., 2006). With regard to mood state, lower global mI/Cr in the NU group predicted a worse mood state across several domains of the POMS (tension, depression, anger, fatigue and total mood disturbance) and a lower negative affect score on the PANAS, but lower global mI/Cr was only related to higher POMS tension in MRJ-users. While global mI/Cr across the entire CSI voxel grid may not be a regionally specific indicator of MRJ-related influences on this important glial metabolite, tissue regression analyses demonstrated a significant influence of MRJ use on distribution of mI/Cr in WM. Reduced distribution of mI/Cr in WM, reflected by a smaller negative slope of the linear regression of mI/Cr as a function of GM content and a lower estimated mI/Cr WM intercept, significantly predicted higher depression scores on the BDI in MRJ-users. Taken together, while lower mI was related to greater impulsiveness in general in young men and may not discriminate between users and non-users, lower mI was predictive of a worse mood state in young MRJ-dependent men, suggesting that this latter relationship may have greater physiological relevance as a neurochemical correlate in addiction.
Frequency of reported MRJ use and urinary THC/Cre levels were associated with lower self-reports of motor impulsivity, greater non-planning impulsivity, lower negative affect, lower tension scores and a trend for a lower depression scores on the BDI. Thus, both subjective and objective indicators of MRJ use were correlated with less impulsivity and a less negative mood. These results are consistent with predictions of tension-reduction (Buckner and Schmidt, 2008; Galen and Henderson, 1999; Levenson et al., 1980), motivational (Buckner et al., 2007; Cooper et al., 2000; Simons et al., 2005), and stress-dampening (Hyman and Sinha, 2009; Sher and Levenson, 1982) models of substance use, suggesting that initiation or continuation of use may help mitigate negative emotions, which in turn increases the risk of substance-related problems. Though only a non-significant trend, there was evidence for a correlation between an earlier age of onset of MRJ use and lower positive affect on the PANAS, suggesting that a consequence of early onset use may be reduced positive affectivity, which may confer increased risk for depression (Chorpita, 2002). Alternatively, low positive affect may lead some individuals to start using MRJ at a younger age. It is also noteworthy that while global mI/Cr was unrelated to frequency, age of onset and duration of MRJ use, or urinary THC/Cre levels, a higher distribution of mI/Cr in WM (slope and y-intercept) was related to a greater amount of MRJ use and an earlier age of onset, suggesting that exposure to marijuana could be neuroprotective (Maccarrone and Finazzi-Agro, 2003; Pope et al., 2009). It cannot be ruled out, however, that mI alterations and relationships with clinical variables either predated the onset of MRJ use, and/or served as predisposing factors that contributed not only to initiation, but also to continuation of MRJ use.
There are strengths and weaknesses of the study that deserve attention when considering the findings. This investigation included a modest sample size limited to men who were well characterized as young chronic MRJ-users, clinically diagnosed with MRJ dependence. Importantly, MRJ-users did not meet criteria for any other substance use disorders, and reported no other illicit drug use, which was confirmed by self-report and by urine drug toxicology screen. Only one MRJ-user reported also using tobacco, which minimizes the possibility that the observed findings were related to effects of tobacco use on brain metabolites (Epperson et al., 2005; Gallinat et al., 2007; Gallinat and Schubert, 2007). Moreover, when we removed this single MRJ tobacco-user from the analyses, significantly lower global mI/Cr and significant correlations observed in MRJ-users were maintained. Measures of impulsivity and mood state, as well as drug use, were collected via self-report, which has its own inherent limitations (Magura, 2010; Williamson, 2007). However, urinary THC/Cre levels provided significant objective evidence confirming MRJ use.
Notable strengths included collection of multiple spectra at high field (4.0 Tesla) using 2D J-Resolved MRSI, along with the use of GAMMA-simulated basis sets for LCModel fitting, to provide an exact, theoretically-correct template for each spectral extraction. Further, the PRESS-CSI scheme minimized the number of spectra with poor quality due to subcutaneous lipids, while permitting collection of 45 high quality spectra that allowed for tissue regression analyses. The use of a broadband water-suppression pulse to maximize global water-suppression throughout the PRESS-box resulted in a small creatine misfit at 3.8ppm, although this would have affected all spectra for all subjects, minimizing the likelihood of influencing our results. While there is debate regarding the use of Cr as a denominator for determining metabolite ratios (Doelken et al., 2009; Safriel et al., 2005), notably, neither Cr T2 nor Cr/total signal (both T2 corrected) differed between groups, suggesting that Cr served as a relatively unbiased denominator for determining metabolite ratios in this study. Although T2 relaxation times and correction factors were calculated for Cho, NAA and NAA+NAAG, T2 correction was not feasible for mI. It is plausible that mI T2 could have affected the results, but this seems unlikely given that no other T2 differences were observed. A final caveat is that proton metabolite values in the present study were likely T1-weighted, given the relatively short TR of 1.25s. Although it is unlikely that the observed metabolite findings are due solely to T1 differences between groups, any tendency for pathologically-based T1 changes between groups could affect metabolite levels. Ideally, a longer TR would be necessary to obtain fully-relaxed spectra, however given the CSI/multiple TE-sampling acquisition scheme, the resulting acquisition time would have been unacceptably long for subjects to endure.
In conclusion, young MRJ-dependent men demonstrated significantly lower global mI/Cr and less distribution of this glial metabolite in WM relative to non-using counterparts. While this exploratory investigation did not address regionally specific MRJ-related alterations in mI/Cr, regression analyses of tissue distribution of mI/Cr, across several regions known to contain high concentrations of CB1 receptors, revealed novel findings, in that WM mI/Cr distribution is particularly vulnerable in young MRJ-dependent men with a relatively short duration of MRJ use. Importantly, the mI/Cr indices were significant neurobiological correlates of impulsivity and mood in MRJ-users, showing correlation patterns that were unique from those observed in same-aged subjects who do not use MRJ. Previous work has identified metabolite differences between drug dependent populations and non-using comparison subjects, particularly with regard to the influence of monitored abstinence on recovery of metabolite levels and associated improvements in cognitive and clinical functioning. Thus, the design of this study, which probed the neurochemical correlates of active use rather than abstinence, should be applied in the future to longitudinally investigate proton metabolites from the period of active use through extended drug abstinence, as well as to also include cohorts of women with and without MRJ-dependence.
Acknowledgments
This work was supported by NIAAA K01 grant AA014651 (MMS) and NIDA R01 grants DA020269 (DYT) and DA012483 (DYT). The authors wish to thank Dr. Staci Gruber for assistance in the clinical screening of study subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, Abou-Saleh MT, Barrick TR. Diffusion tensor imaging of the corpus callosum in addiction. Neuropsychobiology. 2006;54:107–13. doi: 10.1159/000096992. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–74. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophrenia Research. 2001;52:87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. Journal of Nutrition. 2001;131:2498S–504S. doi: 10.1093/jn/131.9.2498S. discussion 2523S–4S. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and Biobehavioral Reviews. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Block RI, Ghoneim MM. Effects of chronic marijuana use on human cognition. Psychopharmacology. 1993;110:219–28. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–6. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology, Biochemistry and Behavior. 2002;72:237–50. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience. 1993;15:289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, Schmidt NB. Marijuana use motives and social anxiety among marijuana-using young adults. Addictive Behaviors. 2007;32:2238–52. doi: 10.1016/j.addbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB. Marijuana effect expectancies: relations to social anxiety and marijuana use problems. Addictive Behaviors. 2008;33:1477–83. doi: 10.1016/j.addbeh.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neuroscientist. 2007;13:422–32. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006a;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. Journal of Magnetic Resonance Imaging. 1999a;10:521–6. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. American Journal of Psychiatry. 1999b;156:716–22. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chang L, Mehringer CM, Ernst T, Melchor R, Myers H, Forney D, Satz P. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biological Psychiatry. 1997;42:1105–14. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006b;129:1096–112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Cady EB, Penrice J, Wyatt JS, Cox IJ, Robertson NJ. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR American Journal of Neuroradiology. 2006;27:1546–54. [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF. The tripartite model and dimensions of anxiety and depression: an examination of structure in a large school sample. Journal of Abnormal Child Psychology. 2002;30:177–90. doi: 10.1023/a:1014709417132. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: the role of personality and affect regulatory processes. Journal of Personality. 2000;68:1059–88. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. Journal of Neurochemistry. 2004;90:776–83. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- Doelken MT, Mennecke A, Stadlbauer A, Kloska S, Struffert T, Engelhorn T, Thuerauf N, Doerfler A, Stefan H, Hammen T. Multi-voxel magnetic resonance spectroscopy of cerebral metabolites in healthy adults at 3 Tesla. Academic Radiology. 2009;16:1493–501. doi: 10.1016/j.acra.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Downes CP, Macphee CH. myo-inositol metabolites as cellular signals. European Journal of Biochemistry. 1990;193:1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Bedell BJ, Narayana PA. Relative concentrations of proton MR visible neurochemicals in gray and white matter in human brain. Magnetic Resonance in Medicine. 1995;33:755–9. doi: 10.1002/mrm.1910330603. [DOI] [PubMed] [Google Scholar]
- Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biological Psychiatry. 2005;57:44–8. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York Psychiatric Institute; New York: 2002. [Google Scholar]
- Fletcher J, Page J, Francis D, Copeland K, Naus M, Davis C, Morris R, Krauskopf D, Satz P. Cognitive correlates of long-term cannabis use in Costa Rican men. Archives of General Psychiatry. 1996;53:1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magnetic Resonance in Medicine. 1989;11:47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]
- Galen LW, Henderson MJ. Validation of cocaine and marijuana effect expectancies in a treatment setting. Addictive Behaviors. 1999;24:719–24. doi: 10.1016/s0306-4603(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, Seifert F, Schubert F. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. Journal of Clinical Psychopharmacology. 2007;27:80–4. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–7. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Research. 2008;162:133–45. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug and Alcohol Dependence. 2009;105:139–53. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research. Cognitive Brain Research. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chemistry and Physics of Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biological Psychiatry. 2007;61:1281–9. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magnetic Resonance in Medicine. 1996;36:21–9. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. Journal of Neuroscience. 2000;20:2470–9. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magnetic Resonance in Medicine. 2004;51:435–40. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Sinha R. Stress-related factors in cannabis use and misuse: implications for prevention and treatment. Journal of Substance Abuse Treatment. 2009;36:400–13. doi: 10.1016/j.jsat.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Rusinek H, George IC, Babb JS, Grossman RI, Gonen O. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage. 2008;41:270–6. doi: 10.1016/j.neuroimage.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–70. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Frederick Bde B, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR in Biomedicine. 2005;18:570–6. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, Renshaw PF. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR in Biomedicine. 2009;22:762–9. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Keltner JR, Wald LL, Frederick BD, Renshaw PF. In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magnetic Resonance in Medicine. 1997;37:366–71. doi: 10.1002/mrm.1910370312. [DOI] [PubMed] [Google Scholar]
- Kemp GJ. Non-invasive methods for studying brain energy metabolism: what they show and what it means. Developmental Neuroscience. 2000;22:418–28. doi: 10.1159/000017471. [DOI] [PubMed] [Google Scholar]
- Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders--focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. 2005;20:309–26. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- Knight-Scott J, Haley AP, Rossmiller SR, Farace E, Mai VM, Christopher JM, Manning CA, Simnad VI, Siragy HM. Molality as a unit of measure for expressing 1H MRS brain metabolite concentrations in vivo. Magnetic Resonance Imaging. 2003;21:787–97. doi: 10.1016/s0730-725x(03)00179-6. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 1993;30:424–37. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: pharmacological effects, expectancy, and tension reduction. Journal of Abnormal Psychology. 1980;89:528–38. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- Lim KO, Spielman DM. Estimating NAA in cortical gray matter with applications for measuring changes due to aging. Magnetic Resonance in Medicine. 1997;37:372–7. doi: 10.1002/mrm.1910370313. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Hides L, Jorm AF. Beliefs of young people and their parents about the harmfulness of alcohol, cannabis and tobacco for mental disorders. Medical Journal of Australia. 2007;187:266–9. doi: 10.5694/j.1326-5377.2007.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death and Differentiation. 2003;10:946–55. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- Magura S. Validating self-reports of illegal drug use to evaluate National Drug Control Policy: a reanalysis and critique. Evaluation and Program Planning. 2010;33:234–7. doi: 10.1016/j.evalprogplan.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11:266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism, Clinical and Experimental Research. 2004;28:650–61. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L- aspartate, creatine and choline. NMR in Biomedicine. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–42. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Pan JW, Twieg DB, Hetherington HP. Quantitative spectroscopic imaging of the human brain. Magnetic Resonance in Medicine. 1998;40:363–9. doi: 10.1002/mrm.1910400305. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5588–93. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Mattson RH, Rothman DL. Proton MRS: GABA and glutamate. Advances in Neurology. 2000;83:261–71. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magnetic Resonance in Medicine. 1999;41:276–84. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pope C, Mechoulam R, Parsons L. Endocannabinoid signaling in neurotoxicity and neuroprotection. Neurotoxicology. 2010;31:562–71. doi: 10.1016/j.neuro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HJ, Gruber A, Hudson J, Huestis M, Yurgelun-Todd D. Neuropsychological Performance in Long-term Cannabis Users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HJ, Yurgelun-Todd D. The Residual Cognitive Effects of Heavy Marijuana Use in College Students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- Posse S, Cuenod CA, Risinger R, Le Bihan D, Balaban RS. Anomalous transverse relaxation in 1H spectroscopy in human brain at 4 Tesla. Magnetic Resonance in Medicine. 1995;33:246–52. doi: 10.1002/mrm.1910330215. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR in Biomedicine. 1997;10:73–8. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magnetic Resonance in Medicine. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Safriel Y, Pol-Rodriguez M, Novotny EJ, Rothman DL, Fulbright RK. Reference values for long echo time MR spectroscopy in healthy adults. American Journal of Neuroradiology. 2005;26:1439–45. [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2008 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2009. [Google Scholar]
- Scallet AC. Quantitative histological evaluation of neuroprotective compounds. Annals of the New York Academy of Sciences. 1995;765:47–58. doi: 10.1111/j.1749-6632.1995.tb16559.x. discussion 59–61. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magnetic Resonance in Medicine. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I. Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: a preliminary MR spectroscopy study. Alcoholism, Clinical and Experimental Research. 2000;24:699–705. [PubMed] [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. Journal of Abnormal Psychology. 1982;91:350–67. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends in Neurosciences. 2004;27:489–95. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gaher RM, Correia CJ, Hansen CL, Christopher MS. An affective-motivational model of marijuana and alcohol problems among college students. Psychol Addict Behav. 2005;19:326–34. doi: 10.1037/0893-164X.19.3.326. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman R, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive Functioning of Long-term Heavy Cannabis Users Seeking Treatment. JAMA. 2002;287:1123–31. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56(Suppl 1):244–53. doi: 10.1016/j.neuropharm.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Spielman DM, Hurd RE, Pfefferbaum A. N-acetylaspartate--a marker of neuronal integrity. Annals of Neurology. 2001;50:823. doi: 10.1002/ana.1279. author reply 824–5. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learning and Memory. 2000;7:132–9. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. Journal of Magnetic Resonance Imaging. 2004;19:537–45. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JBR, Simpson NS, Young AD, Pope HG, Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. The American Journal on Addictions. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weber OM, Trabesinger AH, Duc CO, Meier D, Boesiger P. Detection of hidden metabolites by localized proton magnetic resonance spectroscopy in vivo. Technology and Health Care. 1997;5:471–91. [PubMed] [Google Scholar]
- Williamson A. Using self-report measures in neurobehavioural toxicology: can they be trusted? Neurotoxicology. 2007;28:227–34. doi: 10.1016/j.neuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wolfson M, Bersudsky Y, Hertz E, Berkin V, Zinger E, Hertz L. A model of inositol compartmentation in astrocytes based upon efflux kinetics and slow inositol depletion after uptake inhibition. Neurochemical Research. 2000;25:977–82. doi: 10.1023/a:1007556509371. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. Journal of Neurophysiology. 2008;99:2026–32. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]