Abstract
Protein tyrosine phosphatase epsilon (PTPε) is strongly expressed in the nervous system; however, little is known about its physiological role. We report that mice lacking PTPε exhibit hypomyelination of sciatic nerve axons at an early post-natal age. This occurs together with increased activity of delayed- rectifier, voltage-gated potassium (Kv) channels and with hyperphosphorylation of Kv1.5 and Kv2.1 Kv channel α-subunits in sciatic nerve tissue and in primary Schwann cells. PTPε markedly reduces Kv1.5 or Kv2.1 current amplitudes in Xenopus oocytes. Kv2.1 associates with a substrate-trapping mutant of PTPε, and PTPε profoundly reduces Src- or Fyn-stimulated Kv2.1 currents and tyrosine phosphorylation in transfected HEK 293 cells. In all, PTPε antagonizes activation of Kv channels by tyrosine kinases in vivo, and affects Schwann cell function during a critical period of Schwann cell growth and myelination.
Keywords: myelination/potassium channel/Schwann cells/tyrosine kinase/tyrosine phosphatase
Introduction
Reversible phosphorylation of tyrosine residues in proteins plays a central role in regulation of cellular functions, and is a process controlled by the opposing actions of protein tyrosine kinases (PTKs) and tyrosine phosphatases (PTPases) (Hunter, 1995). Aberrant PTK activity has been linked repeatedly to a wide variety of human diseases, underscoring the pivotal role of accurate tyrosine phosphorylation in physiological processes. PTPases, which are molecularly, biochemically and physiologically distinct from PTKs, have not been studied as extensively as PTKs, although their intimate link to phosphorylation events indicates that PTPases are highly relevant in this respect.
PTPases are a structurally diverse family of transmembranal and cytoplasmic enzymes, of which >70 members have been identified in organisms ranging from viruses to man (Tonks and Neel, 1996). Transmembranal PTPases typically contain two catalytic domains each and are believed to bind extracellular molecules and to participate in signal transduction events (Schaapveld et al., 1997; Zondag and Moolenaar, 1997). Cytoplasmic PTPases generally contain a single catalytic domain flanked by protein domains, which either regulate the catalytic activity of the molecule or target it to particular regions within the cell (Mauro and Dixon, 1994; Denu and Dixon, 1998). Depending on its context, PTPase activity can enhance or decrease the intensity of transduced signals and is physiologically significant (Tonks and Neel, 1996; Neel and Tonks, 1997; Fischer, 1999). Not surprisingly, several key members of this family have been implicated in control of growth, differentiation and malignant transformation (Tonks and Neel, 1996; Parsons, 1998; den Hertog, 1999).
Protein tyrosine phosphatase epsilon (PTPε; gene symbol Ptpre) is somewhat unique among PTPases in that the single PTPε gene contains two distinct promoters, each of which gives rise to a unique protein product: a transmembranal, receptor-type protein (tm-PTPε) and a second protein, which is predominantly cytoplasmic (cyt-PTPε) (Krueger et al., 1990; Elson and Leder, 1995a,b; Nakamura et al., 1996; Tanuma et al., 1999). Although most of their sequences—including their catalytic domains—are identical, tm- and cyt-PTPε possess unique N-termini that determine their different subcellular localizations and probably distinct physiological roles (Elson and Leder, 1995b). Both forms of PTPε bind Grb2 (Toledano-Katchalski and Elson, 1999); tm-PTPε down-regulates insulin receptor signaling in transfected cells (Moller et al., 1995), has been linked with promotion of mammary tumorigenesis in vivo (Elson and Leder, 1995a; Elson, 1999) and may play a role in regulating osteoclast function (Schmidt et al., 1996).
It is reasonable to expect that additional physiological roles of PTPε will involve molecules whose activity is regulated by tyrosine phosphorylation. Prominent among these are K+ channels, as several studies have shown that these are substrates of protein kinase activities (Levitan, 1994, 1999; Siegelbaum, 1994; Jonas and Kaczmarek, 1996). Heterologous expression studies indicate that the activity of several members of the Kv1 family of delayed-rectifier K+ (Kv) channels is altered following phosphorylation by non-receptor and receptor tyrosine kinases (Huang et al., 1993; Timpe and Fantl, 1994; Holmes et al., 1996; Bowlby et al., 1997; Fadool et al., 1997; Tsai et al., 1997; Wang, 1999). In vivo, phosphorylation of K+ channels by PTKs can either activate or down-regulate channel activity. Along these lines, activated insulin receptor increases delayed-rectifier K+ currents in Aplysia bag cell neurons (Jonas et al., 1996), while in the Jurkat human T-cell line, p56lck-mediated phosphorylation of the Kv1.3 potassium channels down-regulates voltage-sensitive K+ currents (Szabo et al., 1996). Recently, we showed that the delayed-rectifier IK is markedly up-regulated following intracellular application of recombinant Fyn tyrosine kinase to mouse Schwann cells, and is down-regulated upon exposure to tyrosine kinase inhibitors (Sobko et al., 1998a; Peretz et al., 1999). In contrast, very little is known about the in vivo regulation of K+ channel activity by PTPases, although a recent study showed that in vitro expression of PTPα correlates with activation of Kv1.2 channels (Tsai et al., 1999).
The intimate connection between regulation of Kv channels and PTKs prompted us to examine whether PTPε is involved in this process in vivo. For this purpose we examined Kv channel function in gene-targeted mice that lack PTPε (Ptpre–/– mice). Lack of PTPε causes hypomyelination of sciatic nerve axons in newborn mice, together with increased Kv channel activity and hyperphosphorylation of the Kv1.5 and Kv2.1 α-subunits in cultured primary Schwann cells and sciatic nerve tissue. In vitro studies show that Kv2.1 interacts with the active site of PTPε, and that active PTPε can down-regulate Kv1.5 and Kv2.1 channel activity and phosphorylation. Our results indicate that a unique role of PTPε in vivo is to down-regulate Kv channel activity in post-natal mice, thereby antagonizing channel activation by tyrosine kinases such as Src and Fyn. PTPε is then part of the finely tuned molecular mechanism, which regulates Kv channel activity during a critical period of Schwann cell development and myelination of peripheral nerves.
Results
PTPε-deficient mice exhibit peripheral myelination abnormalities
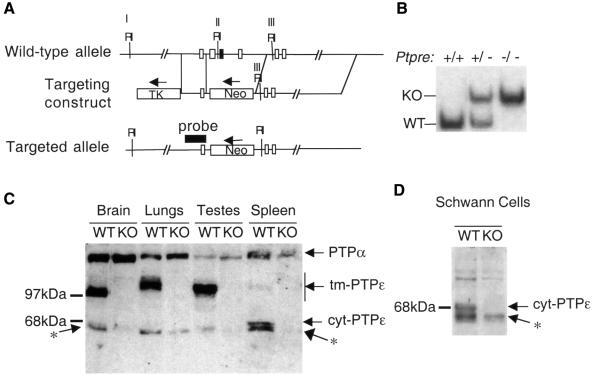
The Ptpre gene was targeted by replacing the genomic sequence corresponding to amino acid residues 262–411 of mature tm-PTPε with a selectable neomycin resistance gene (Figure 1A). This region is common to both tm- and cyt-PTPε (Elson and Leder, 1995b), and was chosen so as to disrupt both forms of the enzyme. Following electroporation and selection, clones of Ptpre+/– embryonic stem (ES) cells were used to generate chimeric mice and subsequently Ptpre+/– and Ptpre–/– mice (Figure 1B). tm-PTPε is known to be expressed mainly in brain, as well as in lungs and testes, while cyt-PTPε is expressed mainly in the hematopoietic system (Elson and Leder, 1995b; Mukouyama et al., 1997); protein blot analysis indicated that Ptpre–/– mice do not express tm- or cyt-PTPε proteins in these tissues (Figure 1C). As the antibody used was raised against a peptide located upstream of the targeting site (Elson and Leder, 1995a), it would have allowed the detection of possible truncated protein products; none were detected. The antibody cross-reacts with the closely related PTPα; no compensatory changes in the amounts of PTPα protein were detected in the organs examined (Figure 1C).
Fig. 1. Generation and characterization of Ptpre–/– mice. (A) Schematic representations of the region of the mouse Ptpre gene chosen for targeting (top), targeting construct (middle) and recombinant allele (bottom). Homologous recombination removed three exons (rectangles), including one (black rectangle) containing C277, the catalytic cysteine of the membrane-proximal catalytic domain. Flanking regions are 1.2 (5′) and 10 kb (3′) in length. RI-EcoRI sites in the targeted region. (B) DNA blot analysis of genomic DNA from Ptpre+/+, Ptpre+/– and Ptpre–/– mice. Genomic DNA was digested with EcoRI and probed with the genomic fragment indicated in (A). Fragments originating in the wild-type (WT; 10.0 kb) and targeted (KO; 11.8 kb) alleles, respectively, are indicated. (C) Protein blot documenting lack of expression of PTPε in Ptpre–/– mice. Protein extracts from the indicated organs of WT or KO mice were analyzed using an anti-PTPε antibody (Elson and Leder, 1995a). Antibody also cross-reacts with PTPα as indicated in the figure. Variations in size of tm-PTPε from brain, lungs and testes are due to tissue-specific glycosylation (Elson and Leder, 1995a). Note the presence in several lanes of PTPε-related bands migrating at ∼68 kDa, (asterisk), slightly faster than cyt-PTPε. (D) Protein blot analysis as in (C), documenting expression levels of cyt-PTPε in primary WT or KO Schwann cells derived from 3- to 5-day-old pups.
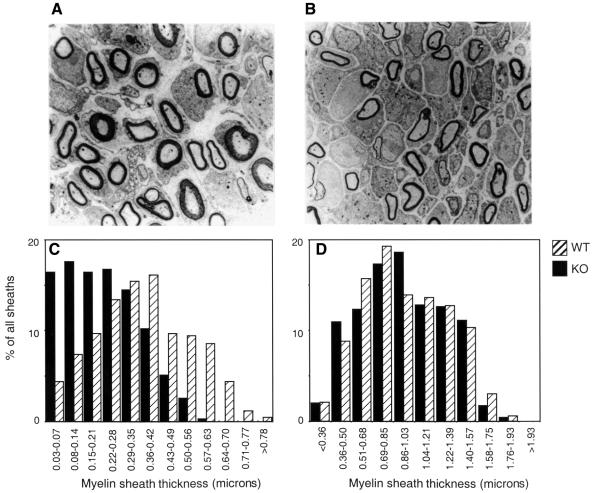
Ptpre–/– mice were born at the expected Mendelian ratios from matings of Ptpre+/– mice and were normal in appearance. However, detailed examination of early post-natal (3- to 5-day-old) Ptpre–/– mice revealed myelination defects in the peripheral nervous system. Specifically, myelin sheaths surrounding axons in sciatic nerve fibers of Ptpre–/– mice were significantly thinner than in WT (wild-type Ptpre+/+) mice (Figure 2A and C). Thinning of myelin sheaths was most evident in the nearly total disappearance of the very thickest sheaths (>0.6 µm) from Ptpre–/– sciatic nerves, together with a 2.6-fold increase in the fraction of thinly myelinated axons (<0.14 µm). Interestingly, myelination of sciatic nerve axons in adult (8 months) Ptpre–/– mice was normal (Figure 2D). These results suggest that PTPε performs a unique role in Schwann cells during early myelinogenesis, an important developmental period when Schwann cells cease dividing and differentiate. In adult mice, on the other hand, this function is either not required or can be performed by other PTPases.
Fig. 2. Reduced myelination of axons in sciatic nerves of 5-day-old Ptpre–/– mice. Cross-section of sciatic nerve of WT (A) or Ptpre–/– (B) mice. Magnification 3400×. (C) Distribution of widths of myelin sheaths of axons in sciatic nerves of 5-day-old WT (hatched bars) and KO (black bars) mice. Mean sheath thicknesses (in µm, ± SEM) were: Ptpre–/–: 0.23 ± 0.05, n = 456; wild type: 0.37 ± 0.06, n = 526 (p <0.0001 by the Mann–Whitney test). (D) As in (C), data from adult, 8-month-old male mice. Mean sheath thicknesses (in µm, ± SEM) were: Ptpre–/–: 0.92 ± 0.02, n = 448; wild type: 0.94 ± 0.02, n = 331.
Up-regulation of Kv channel activity and phosphorylation in PTPε-deficient Schwann cells
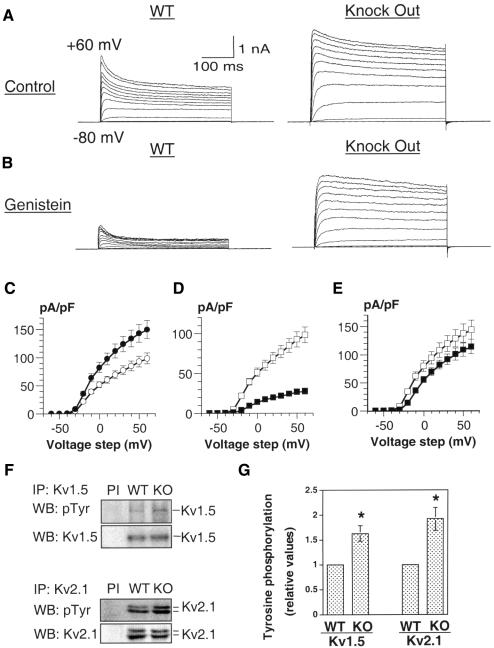
Recent evidence indicates that phosphorylation of Kv channels by Src family kinases could be important for Schwann cell development and peripheral myelinogenesis. In particular, the Fyn tyrosine kinase constitutively activates Kv channels in proliferating Schwann cells (Sobko et al., 1998a; Peretz et al., 1999). After birth, a developmental decrease in Fyn kinase and Kv channel activities may contribute to the exit of Schwann cells from the cell cycle and onset of myelination (Sobko et al., 1998a,b). As tyrosine phosphatases generically counter activities of tyrosine kinases, we sought to determine whether lack of PTPε could modulate Kv channels and Schwann cell function in vivo. WT Schwann cells express cyt-PTPε; the enzyme is missing from cells of Ptpre–/– mice (Figure 1D). The activity of Kv channels was monitored by recording K+ currents in Schwann cells from WT and Ptpre–/– mice using the whole-cell configuration of the patch–clamp technique. These measurements revealed that the maximal K+ current density of Ptpre–/– Schwann cells increased significantly by 52% when compared with WT cells [from 98.02 ± 9.86 pA/pF (WT) to 149.13 ± 16.11 pA/pF (Ptpre–/–) at +60 mV; Figure 3A and C]. No difference was found in the voltage-dependence characteristics of channel activation between Ptpre–/– and WT Schwann cells (data not shown), suggesting that PTPε could affect the number of functional channels or the unitary channel conductance. In parallel, two Kv α-subunits, Kv1.5 and Kv2.1, were significantly hyperphosphorylated in Ptpre–/– Schwann cells (Figure 3F and G) and in sciatic nerve tissue (data not shown). In Ptpre–/– Schwann cells, the Kv1.5 and Kv2.1 phospho tyrosine levels were up-regulated by 62.5 ± 15.4% (n = 4, p <0.01) and 91.3 ± 22.8% (n = 6, p <0.01), respectively, when compared with WT cells (Figure 3F and G). The data indicate that cyt-PTPε activity normally leads to dephosphorylation of Kv α-subunits and to down-regulation of Kv channel activity in vivo. The data also support the correlation observed between hyperphosphorylation of Kv channel α-subunits and increased channel activity (Sobko et al., 1998a; Peretz et al., 1999).
Fig. 3. Characteristics of voltage-gated K+ currents in Schwann cells from WT and Ptpre–/– mice and tyrosine phosphorylation of delayed-rectifier Kv channel α-subunits. (A and B) Whole-cell K+ currents recorded from WT and Ptpre–/– Schwann cells, before (A) and after (B) application of 100 µM genistein for 20 min. Cells were stepped from a holding potential of –80 mV to +60 mV in +10 mV increments for 400 ms pulse duration. (C) The K+ current density (pA/pF) of WT (n = 35, open circles) and Ptpre–/– (n = 40, solid circles) Schwann cells was plotted against voltage steps (mV). Ptpre–/– Schwann cells exhibit a significantly higher current density when compared with WT cells (p <0.01). (D and E) Current density–voltage relationships (n = 28) of WT (D) and Ptpre–/– (E) Schwann cells, before (open squares) and following (solid squares) exposure to 100 µM genistein for 20 min. (F) Representative experiment showing hyperphosphorylation of Kv1.5 and Kv2.1 in Schwann cells of Ptpre–/– mice as compared with WT cells. Total protein was immunoprecipitated with anti-Kv1.5 or anti-Kv2.1 antibodies followed by blotting and probing with anti-phosphotyrosine antibodies. Blots were then stripped and re-probed for Kv1.5 or Kv2.1; similar results were obtained for Kv2.1 in sciatic nerve tissue (not shown). (G) Quantification of tyrosine phosphorylation of Kv1.5 and Kv2.1 in WT and Ptpre–/– Schwann cells. Phosphorylation of Kv1.5 is increased by 62.5 ± 15.4% (n = 4), while that of Kv2.1 is increased by 91.3 ± 22.8% (n = 6); an asterisk indicates statistical significance (p <0.01) in both cases.
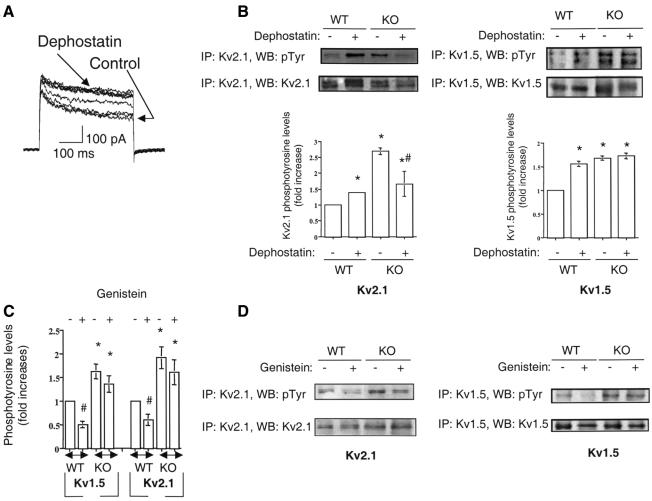
Up-regulation of Kv currents observed in Ptpre–/– Schwann cells agrees with results obtained in WT Schwann cells following broad-spectrum inhibition of tyrosine phosphatases or tyrosine kinases. Dephostatin, an inhibitor of tyrosine phosphatases, causes up-regulation of Kv currents and hyperphosphorylation of Kv1.5 and Kv2.1 channel α-subunits in WT Schwann cells (Figure 4A and B). Interestingly, dephostatin-mediated up-regulation of Kv1.5 and Kv2.1 tyrosine phosphorylation is lost in Ptpre–/– Schwann cells (Figure 4A and B). Dephostatin can eventually reduce the phosphotyrosine levels of Kv2.1 in cells from knockout animals, probably by inhibiting other tyrosine phosphatases, which modulate Kv2.1 channels in a manner different from that of cyt-PTPε (Figure 4B). In agreement with this result, the tyrosine kinase inhibitor genistein exerts the opposite effect and down-regulates Kv channel activity in WT Schwann cells (Sobko et al., 1998a; Peretz et al., 1999). Thus, in WT cells, genistein down-regulates tyrosine phosphorylation levels of Kv1.5 and Kv2.1 channel α-subunits by 50 ± 7% (n = 3, p <0.01) and 40 ± 12% (n =3, p <0.05), respectively (Figure 4C and D). Tyrosine phosphorylation of Kv channel α-subunits is then finely regulated by opposing activities of tyrosine kinases and tyrosine phosphatases (Jonas and Kaczmarek, 1996; Levitan, 1999). These experiments also link cyt-PTPε in particular to down-regulation of Kv currents, as the effects of genistein and dephostatin are markedly altered in Ptpre–/– Schwann cells. Genistein (100 µM) reduces Kv current density by 62% in WT Schwann cells, compared with only a 27% reduction in Ptpre–/– cells (Figure 3A, B, D and E). In line with this result, genistein does not alter significantly the Kv1.5 or Kv2.1 phosphotyrosine steady-state levels in Ptpre–/– Schwann cells (Figure 4C and D). Similar results were obtained with herbimycin A (data not shown). The inhibitory effect of genistein or herbimycin A on Kv channel activity is then partly countered by lack of cyt-PTPε activity in Ptpre–/– Schwann cells.
Fig. 4. Effects of dephostatin and genistein on tyrosine phosphorylation of Kv1.5 and Kv2.1 channel α-subunits. (A) Train of current traces recorded from the same WT Ptpre+/+ Schwann cell, before and after 50 µM dephostatin treatment (20 min). The cell was stepped every minute from a holding potential of –80 mV to +40 mV (400 ms). (B) Effects of dephostatin (50 µM, 20 min) on tyrosine phosphorylation of Kv1.5 and Kv2.1 α-subunits in WT Ptpre+/+ and KO Ptpre–/– Schwann cells. Upper panel, representative experiment showing that dephostatin produces an increase in tyrosine phosphorylation of Kv1.5 and Kv2.1 channel α-subunits in WT but not in KO Schwann cells. Lower panel, quantification of tyrosine phosphoryla tion of Kv1.5 and Kv2.1 in WT and KO Schwann cells before and after dephostatin treatment. Dephostatin increases Kv1.5 and Kv2.1 tyrosine phosphorylation by 56 ± 6% and 39 ± 2%, respectively (n = 3, p <0.05). An asterisk indicates a statistically higher value when compared with WT untreated Schwann cells (p <0.05). Dephostatin significantly reduces, by 38 ± 14%, the phosphotyrosine levels of Kv2.1 in KO Schwann cells (n = 3, #p <0.05). Total proteins were immunoprecipitated, blotted and probed as in Figure 3F. (C) Quantification of the effects of genistein (100 µM, 20 min) on tyrosine phosphorylation of Kv1.5 and Kv2.1 in WT and KO Schwann cells. Genistein reduces by 50 ± 7% and 40 ± 13% the tyrosine phosphorylation levels of Kv1.5 and Kv2.1, respectively (n = 3, #p <0.05). An asterisk indicates a statistically higher value when compared with WT untreated Schwann cells (p <0.05). (D) Representative experiment showing that genistein decreases significantly the tyrosine phosphorylation of Kv1.5 and Kv2.1 channel α-subunits in WT. The inhibitory effect of genistein is much weaker, if present at all, in KO Schwann cells. Total proteins were immunoprecipitated, blotted and probed as in Figure 3F.
cyt-PTPε down-regulates Kv channel activity and phosphorylation in vitro
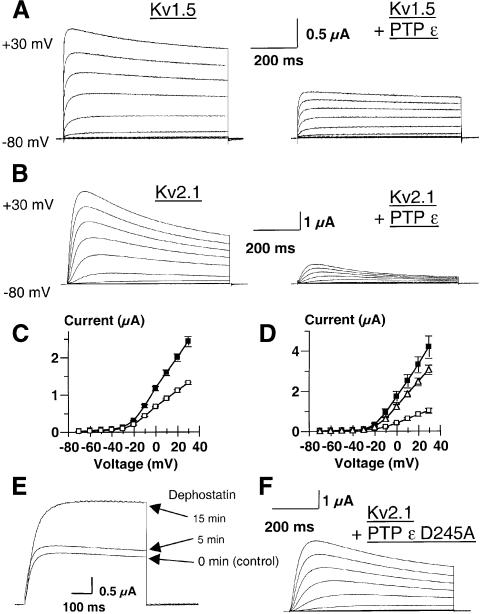
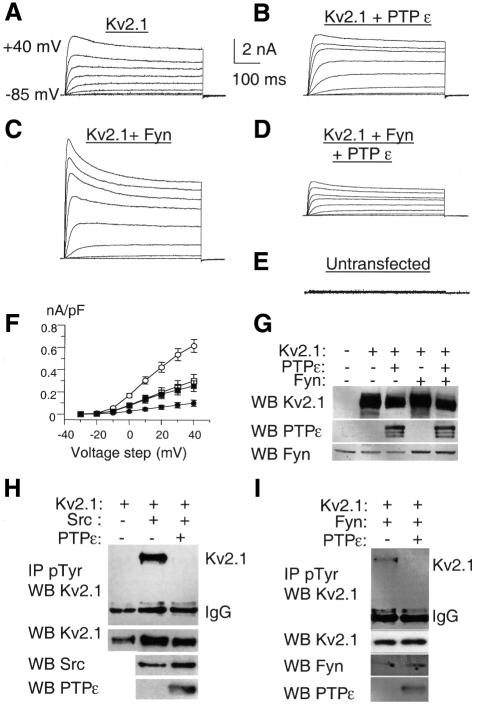
To investigate direct modulation of Kv channels by cyt-PTPε further, we co-expressed Kv1.5 or Kv2.1 with cyt-PTPε in Xenopus oocytes and HEK 293 cells (Figures 5 and 6). In Xenopus oocytes, co-expression of Kv1.5 or Kv2.1 with cyt-PTPε leads to a marked decrease in Kv current amplitudes, with 46 and 76% inhibition, respectively (Figure 5A–D). Similar to conclusions from our primary Schwann cell experiments, the inhibitory action of PTPε does not involve significant changes in the kinetics or in the voltage dependence of channel activation (Figure 5A and B). PTPε catalytic activity is important for this process, as a catalytically inactive, substrate-trapping mutant (Flint et al., 1997) of cyt-PTPε (D245A cyt-PTPε) inhibits only 27% of Kv current amplitude, compared with 76% inhibition by wild-type cyt-PTPε (Figure 5D and F). Residual inhibition by D245A cyt-PTPε may result from binding of the inactive phosphatase to Kv2.1 (see below) in a manner which, though not dephosphorylating Kv2.1, may nonetheless impede its activity. These data are in line with those obtained with Ptpre–/– mice and indicate that cyt-PTPε can down-regulate activity of Kv channels by reducing tyrosine phosphorylation of their α-subunits. Consistent with these results, the tyrosine phosphatase inhibitor dephostatin causes up-regulation of K+ currents produced by co-expression Kv2.1 and cyt-PTPε in Xenopus oocytes (Figure 5E). In HEK 293 cells, cyt-PTPε does not depress the basal Kv2.1 current activity (Figure 6A, B and F). This feature differs from Xenopus oocyte expression and is probably due to the lack of basal tyrosine phosphorylation of Kv2.1 channel α-subunits in HEK 293 cells, as revealed by our immunoprecipitation experiments (Figure 6H). However, when tyrosine phosphorylation of Kv2.1 α-subunits is stimulated by co-expressing activated Fyn kinase (Y531F), the Kv2.1 K+ current amplitude is up-regulated by >2-fold (617 ± 58 pA/pF, n = 12, p <0.01) when compared with control Kv2.1-expressing cells (298 ± 63 pA/pF, n = 15) (Figure 6A, C, F and I). Under these stimulatory conditions, co-expression of cyt-PTPε leads to a profound down-regulation of Kv2.1 current amplitude (101 ± 28 pA/pF, n = 14, p <0.001). cyt-PTPε totally suppresses the Fyn kinase stimulation of Kv2.1 current and tyrosine phosphorylation (Figure 6C, D, F and I). Similar results were obtained when Kv2.1 tyrosine phosphorylation was stimulated by activated Src kinase (Figure 6H). Correlative western blots show that co-expression of either activated Fyn kinase, activated Src kinase or cyt-PTPε does not alter significantly the expression levels of Kv2.1 channel α-subunits (Figure 6G–I; see also Figure 7A).
Fig. 5. Effects of cyt-PTPε and D245A cyt-PTPε substrate-trapping mutant on Kv2.1 and Kv1.5 K+ currents expressed in Xenopus oocytes. (A) Macroscopic K+ currents recorded from oocytes microinjected with Kv1.5 (left) or Kv1.5 and cyt-PTPε cRNAs (right) were elicited by depolarizing pulses (700 ms) from a –80 mV holding potential to +30 mV in 10 mV increments. (B) Macroscopic K+ currents recorded as in (A), from oocytes microinjected with Kv2.1 (left) or Kv2.1 and cyt-PTPε cRNAs (right). (C) Current–voltage relationships of Kv1.5 (n = 14, solid squares) and Kv1.5 plus cyt-PTPε-mediated K+ currents (n = 16, open squares). cyt-PTPε decreases Kv1.5 currents significantly (p <0.01). (D) Current–voltage relationships of Kv2.1 (n = 15, solid squares), Kv2.1 plus cyt-PTPε- (n = 16, open squares) and Kv2.1 plus D245A PTPε-mediated K+ currents (n = 14, open triangles). cyt-PTPε decreases Kv2.1 currents significantly (p <0.01). (E) Representative train of current traces recorded from oocytes microinjected with Kv2.1 and cyt-PTPε cRNAs, before and after 50 µM dephostatin treatment. The oocyte was stepped from a holding potential of –80 mV to +20 mV (600 ms). (F) Macroscopic K+ currents recorded as in (A), from oocytes microinjected with Kv2.1 and the D245A PTPε mutant.
Fig. 6. Effects of cyt-PTPε, activated Fyn and Src kinases on Kv2.1 tyrosine phosphorylation and K+ currents expressed in HEK 293 cells. (A–E) Representative whole-cell K+ currents from cells transfected with Kv2.1 (A), Kv2.1 + cyt-PTPε (B), Kv2.1 + activated Fyn kinase (C), Kv2.1 + activated Fyn kinase + cyt-PTPε (D) cDNAs, respectively, as well as from an untransfected cell (E). Currents were elicited by depolarizing pulses (500 ms) from a –85 mV holding potential to +40 mV in 20 mV increments. The scale bars are the same for A–E. (F) Current–voltage relationships of K+ currents produced by cells transfected with Kv2.1 (n = 15, open squares), Kv2.1 + cyt-PTPε (n = 5, solid squares), Kv2.1 + activated Fyn kinase (n = 12, open circles) and Kv2.1 + activated Fyn kinase + cyt-PTPε (n = 14, filled circles) cDNAs. (G) Documentation of expression of Kv2.1, cytPTPε and Fyn in HEK 293 cells used for electrophysiological studies presented in (A–E). Cells were transfected with plasmids for the indicated proteins, and protein blots prepared from their extracts were probed with the indicated antibodies. (H and I) Cyt-PTPε reduces, respectively, Src- (H) and Fyn-mediated (I) phosphorylation of Kv2.1 in transfected cells. HEK 293 cells were transiently transfected with Kv2.1, activated Fyn (Y531F), activated Src (Y527F), cyt-PTPε cDNAs, or combinations thereof. Tyrosine-phosphorylated proteins were immunoprecipitated and the amount of Kv2.1 in the precipitate was analyzed by protein blotting (top panel). The second, third and fourth panels document expression of Kv2.1, Fyn/Src and cyt-PTPε, respectively, in the transfected cells.
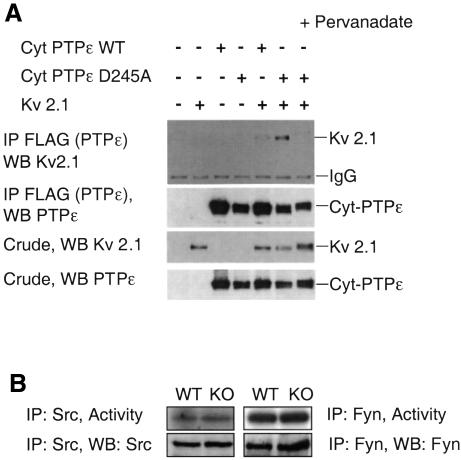
Fig. 7. (A) Kv2.1 preferentially associates with the D245A cyt-PTPε substrate-trapping mutant. HEK 293 cells were transiently transfected with wild-type cyt-PTPε, D245A cyt-PTPε, Kv2.1, or combinations thereof. Following lysis, PTPε was immunoprecipitated via its attached FLAG tag; associated Kv2.1 was detected by protein blot analysis of immunoprecipitates (top panel). The second, third and fourth panels document expression of cyt-PTPε and Kv2.1 and immunoprecipitation of cyt-PTPε in the various transfections. The presence of pervanadate (right-most lane) somewhat reduces the electrophoretic mobility of some of the proteins examined, most likely by more effectively reducing their dephosphorylation by phosphatases present in cell lysates during precipitation. (B) Expression and activities of Src and Fyn are unaltered in Ptpre–/– Schwann cells. Total protein from primary Schwann cells of 3- to 5-day-old mice were immunoprecipitated with anti-Src or anti-Fyn antibodies. A fraction of the precipitated kinases was allowed to phosphorylate acid-denatured enolase in vitro in the presence of [γ-32P]ATP, followed by SDS–PAGE analysis and exposure to film (top panels). Another fraction of the immunoprecipitated material was analyzed by protein blotting for expression levels of Src or Fyn (bottom panels). Tyrosine phosphorylation levels of both kinases were unaltered (not shown). The experiment shown is representative of three performed.
Kv2.1 binds the active site of cyt-PTPε
The above results suggest that cyt-PTPε binds and dephosphorylates α-subunits of Kv channels. In agreement with this model, several-fold more Kv2.1 co-precipitate with a D245A substrate-trapping mutant (Flint et al., 1997) of cyt-PTPε than with wild-type cyt-PTPε (Figure 7A). Significantly, enhanced binding to D245A cyt-PTPε does not occur in the presence of sodium pervanadate (Figure 7A), which irreversibly oxidizes the conserved cysteine residue located at the active site of tyrosine phosphatases (Huyer et al., 1997). Oxidation of this cysteine residue by pervanadate is known to disrupt active site-mediated binding of D-to-A type phosphatase mutants to their putative substrates (Flint et al., 1997; Huyer et al., 1997). These results indicate that Kv2.1 interacts mainly with the active site of cyt-PTPε, consistent with Kv2.1 being a substrate of cyt-PTPε. An alternative model based on findings in PTPα-deficient mice (Ponniah et al., 1999; Su et al., 1999) suggests that cyt-PTPε could indirectly control phosphorylation of Kv channel α-subunits by regulating activities of Src family tyrosine kinases. Fyn is the major Src-family tyrosine kinase active towards Kv channels in Schwann cells (Sobko et al., 1998a); yet, expression, activity and overall tyrosine phosphorylation of both Fyn and Src are unchanged in Ptpre–/– Schwann cells (Figure 7B and data not shown). Although this result does not rule out interactions between Fyn/Src and cyt-PTPε, it appears that these kinases are not the major mediators of cyt-PTPε activity towards Kv channels in the system studied here.
Discussion
Results presented here indicate that lack of cyt-PTPε expression results in reduced myelination of sciatic nerve axons in early post-natal Ptpre–/– mice. This finding parallels increased activity of voltage-gated potassium channels in primary Schwann cells derived from these mice, which in turn is mediated by hyperphosphorylation of Kv channel α-subunits. Ptpre–/– Schwann cells modulate phosphorylation of Kv1.5 and Kv2.1 differently than wild-type cells in response to broad-spectrum inhibition of tyrosine phosphatases or kinases, further attesting to the importance of this PTPase in early post-natal Schwann cells. The ability of cyt-PTPε to reduce phosphorylation and to down-regulate Kv1.5 and Kv2.1 can be reproduced in heterologous expression systems. Taken together, these findings indicate that cyt-PTPε dephosphorylates α-subunits of delayed-rectifier Kv channels, and that lack of cyt-PTPε most likely causes Schwann cell dysfunction in vivo in young mice. This study provides the first in vivo evidence of a functional link between a specific tyrosine phosphatase and regulation of Kv channel activity, and clearly defines its physiological consequences.
Although the above data suggest that cyt-PTPε plays a significant role in regulation of Kv phosphorylation in Schwann cells, the fact that myelination of sciatic nerve axons is normal in adult Ptpre–/– mice indicates that other PTPases participate in this process as well. The need for cyt-PTPε for proper Schwann cell function then likely decreases as mice age. A possible candidate for compensating lack of cyt-PTPε in Schwann cells is PTPσ, as young mice lacking PTPσ exhibit reduced myelination of sciatic nerve axons caused by a yet undetermined molecular mechanism (Wallace et al., 1999; M.Tremblay, personal communication). Nonetheless, the existence of myelination defects in post-natal Ptpre–/– mice demonstrates that cyt-PTPε performs a unique function in Schwann cells of such mice, and that other phosphatases cannot replace this enzyme at this developmental stage.
Recent data have shown that K+ channel activity can be either up-regulated or down-regulated by PTKs depending on the physiological context. Heterologous expression studies indicate that the K+ channel activity of several Kv1 family members is down-regulated by tyrosine kinases. In HEK 293 cells, phosphorylation of Kv1.3 by activated v-src or by epidermal growth factor treatment leads to a decrease in current amplitude (Holmes et al., 1996; Bowlby et al., 1997; Fadool et al., 1997). The current amplitudes of several other Kv channels, such as Kv1.2 and Kv1.5, are known to be strongly suppressed by tyrosine kinases (Timpe and Fantl, 1994; Holmes et al., 1997; Tsai et al., 1997). However, a recent in vitro study demonstrates that tyrosine phosphorylation can up-regulate the activity of the Kv1.1 channel (Wang et al., 1999). Differential regulation of K+ channel activity by tyrosine phosphorylation has also been observed in vivo. Activation of the insulin receptor inhibits Kv current amplitude in olfactory bulb neurons (Fadool and Levitan, 1998). On the other hand, positive modulation of transient K+ channels by PTKs was described in rat ventricular cardiac cells (Guo et al., 1995). Ca2+-activated K+ channels are up-regulated by PTK activation in mouse fibroblasts and in Chinese hamster ovary cells (Prevarskaya et al., 1995; Decker et al., 1998). More recently, ceramide was found to increase the delayed-rectifier K+ current via PTK activation in cultured cortical neurons, and this process parallels increased apoptosis of these cells (Yu et al., 1999). Similarly, our recent work showed that the Fyn tyrosine kinase increases delayed-rectifier K+ channel activity in mouse Schwann cells, and that exposure to tyrosine kinase inhibitors markedly down-regulates Kv current amplitude and inhibits cell proliferation in this system (Sobko et al., 1998a,b).
The intimate connections between activities of PTKs and PTPases argue that dephosphorylation of Kv channels may also affect channel activity in a manner dependent upon the circumstances of the reaction. Interestingly, in vitro expression of PTPα, a phosphatase closely related to the transmembranal, receptor-like form of PTPε, has been shown to correlate with activation of Kv1.2 (Tsai et al., 1999), the opposite of the effect that cyt-PTPε has on Kv1.5 and Kv2.1. This result may reflect basic differences in the manner by which PTPε and PTPα function, or in the effect dephosphorylation has on different Kv α-subunits. Alternatively, as our results are based on both in vivo (Schwann cells) and heterologous in vitro (Xenopus oocyte, HEK 293 cells) studies, this discrepancy may reflect differences in the molecular backgrounds of native versus heterologous cells and their influence on PTPα activity.
Our findings indicate that Kv channel phosphorylation in Schwann cells is regulated by opposing activities of tyrosine kinases and tyrosine phosphatases. One could then expect that inhibition of tyrosine kinases by genistein would offset Kv channel activation normally observed in Ptpre–/– Schwann cells. This effect is indeed observed upon treatment of Ptpre–/– Schwann cells with genistein; however, the magnitude of the effect is very mild. This may reflect residual kinase activity, which is not inhibited by genistein and which is now detectable in the absence of PTPε. Alternatively, blockade of tyrosine kinases by genistein may be less effective in the context of defective regulation of tyrosine dephosphorylation, which exists in Ptpre–/– Schwann cells.
The precise link between Kv channel activity and Schwan cell-mediated myelination is unclear at the present time. However, there is temporal correlation between decreases in activities of Src-related tyrosine kinases and of Kv channels, on the one hand, and exit of Schwann cells from the cell cycle and onset of myelination, on the other hand (Sobko et al., 1998a,b; MacFarlane and Sontheimer, 2000). Our data indicate that lack of PTPε results in increased tyrosine phosphorylation and activities of Kv channels, possibly delaying exit of PTPre–/– Schwann cells from the cell cycle and resulting in delayed myelination.
In all, results presented here indicate that, together with the Src family tyrosine kinases, cyt-PTPε is part of the finely tuned molecular mechanism that regulates Kv channel activity during Schwann cell development and myelination of peripheral nerves in vivo. Schwann cell dysfunction and myelination defects are known to be associated with severe human neurological diseases. Better understanding of how Schwann cell functions are regulated at the molecular level could increase the chances of eventually controlling such diseases.
Materials and methods
Gene targeting and genotyping
Two recombinant phage-containing fragments of mouse genomic DNA from the Ptpre locus were isolated from a strain 129 mouse genomic library (Stratagene) using a fragment of the mouse tm-PTPε cDNA (Elson and Leder, 1995a). The intron–exon structure of selected regions of these clones was determined and was found to be identical to that of PTPα exons 12–17 (Wong et al., 1993), with the PTPase signature motif spanning exons analogous to PTPα exons 13 and 14 (not shown). The targeting construct (Figure 1A) was based on the pPNT vector (Tybulewicz et al., 1991), with the Neor expression cassette of pPNT replacing a 2.9 kb PTPε genomic sequence containing exons 13–15. Following linearization and electroporation into TC1 ES cells (Deng et al., 1996), genomic DNA from ES cell clones that survived G418 and FIAU selection was analyzed by Southern blotting and PCR (not shown). Cells from two ES cell clones heterozygous for the targeted mutation were injected into C57BL/6 blastocysts, leading to chimeric mice and subsequently to germline transmission of the mutant allele. Mice were genotyped using DNA from tail biopsies by the Southern blot technique as shown in Figure 1B. All mice used in this report were of a mixed 129×C57BL/6 background.
Cell culture
Primary Schwann cells were prepared from 3- to 5-day-old WT (Ptpre+/+) or Ptpre–/– pups as described (Sobko et al., 1998a). The young age of the pups prevented their individual genotyping prior to cell preparation, hence age-matched WT and Ptpre–/– pups were obtained from separate matings of WT or of Ptpre–/– mice in which all pups were of the desired genotype. Sciatic nerves obtained from mice of a given litter were pooled prior to cell preparation. HEK 293 cells were grown and transfected by the calcium phosphate technique as described (Toledano-Katchalski et al., 1999). In some cases HEK 293 cells were transfected with vectors expressing rat Kv2.1 (gift of Drs J.Barhanin and M.Lazdunski), chicken Y527F Src and human Fyn Y531F (gifts of Dr S.Courtneidge), or mouse cyt-PTPε (Toledano-Katchalski et al., 1999).
Electrophysiology
Macroscopic whole-cell currents were recorded in Schwann cells or in transfected HEK 293 cells, as previously described (Sobko et al., 1998a). HEK 293 cells were co-transfected with pIRES-CD8 (kindly provided by Drs J.Barhanin and A.Patel, CNRS, Sophia Antipolis, France) as a marker for transfection. Transfected cells were visualized 40 h following transfection, using the anti-CD8 (Dynal) antibody-coated bead method (Jurman et al., 1994). Signals were amplified using an Axopatch 200B patch–clamp amplifier (Axon Instruments), sampled at 2 kHz and filtered below 0.8 kHz via a four-pole Bessel low pass filter. Data acquisition was carried out using pClamp 6.0.2 software (Axon Instruments). The patch pipettes with tip resistance of 4–8 MΩ were filled with (in mM): 164 KCl, 2 MgCl2, 1 CaCl2, 11 EGTA, 10 HEPES, 11 glucose at pH 7.4. The external solution contained (in mM): 140 NaCl, 5 KCl, 5 CaCl2, 2 MgCl2, 11 glucose and 10 HEPES at pH 7.4. Series resistances were within 8–16 MΩ and were compensated by 85–90%. The sustained component of the K+ currents was measured at the end of the depolarizing traces. All data were expressed as mean ± SEM. Statistically significant differences were assessed by Student’s t-test.
For heterologous expression in Xenopus laevis, stage V and VI oocytes were used to inject cRNA, as described previously (Abitbol et al., 1999). Capped complementary RNAs (cRNAs) were injected at 2.5 ng cRNA/oocyte. Standard two-electrode voltage–clamp measurements were performed 3–5 days following cRNA microinjection into oocytes as described (Abitbol et al., 1999). Current signals were filtered at 0.5 kHz and digitized at 2 kHz. Chord conductance (G) was calculated using the following equation: G = I/(V – Vrev) where I corresponds to the current amplitude and Vrev is the measured reversal potential, assumed to be –90 mV (–90 ± 2 mV; n = 7). G was estimated at various test voltages V and then, normalized to a maximal conductance value Gmax, calculated at +30 mV. Activation curves were fitted by a Boltzmann distribution: G/Gmax = 1/{1 + exp[(V50 – V)/s]}, where V50 is the voltage at which the current is half-activated and s is the slope factor.
Protein blot analysis
Selected organs and primary Schwann cells were homogenized in buffer A (50 mM Tris–HCl pH 7.5, 100 mM NaCl, 1% NP-40), supplemented with 0.5 mM sodium pervanadate and protease inhibitors (Sigma), and analyzed as described (Elson and Leder, 1995a). Antibodies used in this study included polyclonal anti-PTPε (Elson and Leder, 1995a), monoclonal (clone D4/11) or polyclonal anti-Kv2.1 (Upstate Biotechnology), polyclonal anti-Kv1.5 (Upstate Biotechnology and Alomone Labs), monoclonal anti-Fyn (Santa Cruz Biotechnology), monoclonal anti-v-Src (Calbiochem), anti-FLAG M2 affinity beads (Sigma) or anti-phosphotyrosine (clone PY20; Transduction Laboratories).
Immunoprecipitation and substrate-trapping experiments
The D245A mutation was introduced into the mouse cyt-PTPε cDNA (Elson and Leder, 1995b) by site-directed mutagenesis; the presence of the desired mutation and absence of other mutations were verified by DNA sequencing. PTPε cDNAs all contained a FLAG tag at their C-termini and were expressed from the pCDNA3 vector (Invitrogen). Wild-type cyt-PTPε, but not D245A cyt-PTPε, was catalytically active towards paranitrophenyl phosphate (not shown). For immunoprecipitations, cells were lysed in buffer A supplemented with 5 mM iodoacetic acid and protease inhibitors. Cellular proteins (0.5–1 mg) were reacted with the relevant antibodies for 6–8 h, followed by four extensive washes with RIPA buffer. When used, pervanadate (0.5 mM) replaced iodoacetate in the lysis buffer.
Electron microscopy
Three 5-day-old mice of either genotype were studied. Nerve fibers were fixed in 3% paraformaldehyde/2% glutaraldehyde and processed for electron microscope analysis as described (Shinder and Devor, 1994). Samples from different mice were always taken from the same location, 5 mm from the proximal end of the nerve. Vertical cross-sections 70–90 nm thick were cut with a Leica Ultracut UCT ultra-microtome, stained with uranyl acetate and lead citrate, and examined in a Philips 410 transmission electron microscope at 100Kv. Five to seven non-overlapping cross-section fields were examined from each mouse at 2800× magnification. The thicknesses of myelin sheaths of all axon profiles in a given field were measured, and data for all mice of the same genotype were pooled. Similar experiments were conducted on sciatic nerve tissue obtained from 8-month-old male mice (three mice from either genotype).
Acknowledgments
Acknowledgements
We thank P.Leder for his interest and support during this work; A.Harrington and C.Daugherty for help in preparation of Ptpre–/– mice; I.Spiegel for help in early experiments; J.Barhanin, M.Lazdunski and S.Courtneidge for their kind gifts of reagents; and H.Garty, Y.Groner and Y.Yarden for critical reading of this manuscript. This research was supported by the United-States–Israel Binational Science Foundation (BSF), The Israel Science Foundation, founded by The Israel Academy of Sciences and Humanities, the US Army Medical Research and Materiel Command (DAMD17-98-1-8266), the Kekst Family Foundation for Molecular Genetics, and the Abisch-Frenkel Foundation (to A.E.), and by EEC BIO-CT97-2207, the Ministry of Health and the Minerva Foundation (to B.A.). A.E. is an Alon Fellow and incumbent of the Adolfo and Evelyn Blum Career Development Chair in Cancer Research; B.A. is an incumbent of the Philip Harris and Gerald Ronson Career Development Chair at the Weizmann Institute.
References
- Abitbol I., Peretz,A., Lerche,C., Busch,A. and Attali,B. (1999) Allosteric interactions rescue the loss of IKS channel function induced by an LQT5 mutation and other IsK mutants. EMBO J., 18, 4137–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby M.R., Fadool,D.A., Holmes,T.C. and Levitan,I.B. (1997) Modulation of the Kv1.3 potassium channel by receptor tyrosine kinases. J. Gen. Physiol., 110, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Koschinski,A., Trouliaris,S., Tamura,T., Dreyer,F. and Repp,H. (1998) Activation of a Ca2+-dependent K+ current by the oncogenic receptor protein tyrosine kinase v-Fms in mouse fibroblasts. Naunyn Schmiedebergs Arch. Pharmacol., 357, 378–384. [DOI] [PubMed] [Google Scholar]
- Deng C., Wynshaw-Boris,A., Zhou,F., Kuo,A. and Leder,P. (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell, 84, 911–921. [DOI] [PubMed] [Google Scholar]
- den Hertog J. (1999) Protein-tyrosine phosphatases in development. Mech. Dev., 85, 3–14. [DOI] [PubMed] [Google Scholar]
- Denu J.M. and Dixon,J.E. (1998) Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr. Opin. Chem. Biol., 2, 633–641. [DOI] [PubMed] [Google Scholar]
- Elson A. (1999) Protein tyrosine phosphatase ε increases the risk of mammary hyperplasia and mammary tumors in transgenic mice. Oncogene, 18, 7535–7542. [DOI] [PubMed] [Google Scholar]
- Elson A. and Leder,P. (1995a) Protein-tyrosine phosphatase ε. An isoform specifically expressed in mouse mammary tumors initiated by v-Ha-ras or neu. J. Biol. Chem., 270, 26116–26122. [DOI] [PubMed] [Google Scholar]
- Elson A. and Leder,P. (1995b) Identification of a cytoplasmic, phorbol ester-inducible isoform of protein tyrosine phosphatase ε. Proc. Natl Acad. Sci. USA, 92, 12235–12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool D.A. and Levitan,I.B. (1998) Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J. Neurosci., 18, 6126–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool D.A., Holmes,T.C., Berman,K., Dagan,D. and Levitan,I.B. (1997) Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol., 78, 1563–1573. [DOI] [PubMed] [Google Scholar]
- Fischer E.H. (1999) Cell signaling by protein tyrosine phosphorylation. Adv. Enzyme Regul., 39, 359–369. [DOI] [PubMed] [Google Scholar]
- Flint A.J., Tiganis,T., Barford,D. and Tonks,N.K. (1997) Development of ‘substrate-trapping’ mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl Acad. Sci. USA, 94, 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Kamiya,K. and Toyama,J. (1995) bFGF promotes functional expression of transient outward currents in cultured neonatal rat ventricular cell. Pflugers Arch., 430, 1015–1017. [DOI] [PubMed] [Google Scholar]
- Holmes T.C., Fadool,D.A., Ren,R. and Levitan,I.B. (1996) Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science, 274, 2089–2091. [DOI] [PubMed] [Google Scholar]
- Huang X.Y., Morielli,A.D. and Peralta,E.G. (1993) Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell, 75, 1145–1156. [DOI] [PubMed] [Google Scholar]
- Hunter T. (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell, 80, 225–236. [DOI] [PubMed] [Google Scholar]
- Huyer G., Liu,S., Kelly,J., Moffat,J., Payette,P., Kennedy,B., Tsaprailis,G., Gresser,M.J. and Ramachandran,C. (1997) Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem., 272, 843–851. [DOI] [PubMed] [Google Scholar]
- Jonas E.A. and Kaczmarek,L.K. (1996) Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol., 6, 318–323. [DOI] [PubMed] [Google Scholar]
- Jonas E.A., Knox,R.J., Kaczmarek,L.K., Schwartz,J.H. and Solomon,D.H. (1996) Insulin receptor in Aplysia neurons: characterization, molecular cloning and modulation of ion currents. J. Neurosci., 16, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman M.E., Boland,L.M., Liu,Y. and Yellen,G. (1994) Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques, 17, 876–881. [PubMed] [Google Scholar]
- Krueger N.X., Streuli,M. and Saito,H. (1990) Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J., 9, 3241–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I.B. (1994) Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu. Rev. Physiol., 56, 193–212. [DOI] [PubMed] [Google Scholar]
- Levitan I.B. (1999) Modulation of ion channels by protein phosphorylation. How the brain works. Adv. Second Messenger Phosphoprotein Res., 33, 3–22. [DOI] [PubMed] [Google Scholar]
- MacFarlane S.N. and Sontheimer,H. (2000) Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia, 30, 39–48. [DOI] [PubMed] [Google Scholar]
- Mauro L.J. and Dixon,J.E. (1994) ‘Zip codes’ direct intracellular protein tyrosine phosphatases to the correct cellular ‘address’. Trends Biochem. Sci., 19, 151–155. [DOI] [PubMed] [Google Scholar]
- Moller N.P., Moller,K.B., Lammers,R., Kharitonenkov,A., Hoppe,E., Wiberg,F.C., Sures,I. and Ullrich,A. (1995) Selective down-regulation of the insulin receptor signal by protein-tyrosine phosphatases α and ε. J. Biol. Chem., 270, 23126–23131. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y., Kuroyanagi,H., Shirasawa,T., Tomoda,T., Saffen,D., Oishi,D. and Watanabe,T. (1997) Induction of protein tyrosine phosphatase ε transcripts during NGF-induced neuronal differentiation of PC12D cells and during the development of the cerebellum. Brain Res. Mol. Brain Res., 50, 230–236. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizuno,Y. and Kikuchi,K. (1996) Molecular cloning of a novel cytoplasmic protein tyrosine phosphatase PTP ε. Biochem. Biophys. Res. Commun., 218, 726–732. [DOI] [PubMed] [Google Scholar]
- Neel B.G. and Tonks,N.K. (1997) Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol., 9, 193–204. [DOI] [PubMed] [Google Scholar]
- Parsons R. (1998) Phosphatases and tumorigenesis. Curr. Opin. Oncol., 10, 88–91. [DOI] [PubMed] [Google Scholar]
- Peretz A., Sobko,A. and Attali,B. (1999) Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J. Physiol. (Lond.), 519, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S., Wang,D.Z., Lim,K.L. and Pallen,C.J. (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol., 9, 535–538. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N.B., Skryma,R.N., Vacher,P., Daniel,N., Djiane,J. and Dufy,B. (1995) Role of tyrosine phosphorylation in potassium channel activation. Functional association with prolactin receptor and JAK2 tyrosine kinase. J. Biol. Chem., 270, 24292–24299. [DOI] [PubMed] [Google Scholar]
- Schaapveld R., Wieringa,B. and Hendriks,W. (1997) Receptor-like protein tyrosine phosphatases: alike and yet so different. Mol. Biol. Rep., 24, 247–262. [DOI] [PubMed] [Google Scholar]
- Schmidt A. et al. (1996) Protein-tyrosine phosphatase activity regulates osteoclast formation and function: Inhibition by alendronate. Proc. Natl Acad. Sci. USA, 93, 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder V. and Devor,M. (1994) Structural basis of neuron-to-neuron cross-excitation in dorsal root ganglia. J. Neurocytol., 23, 515–531. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S.A. (1994) Ion channel control by tyrosine phosphorylation. Curr. Biol., 4, 242–245. [DOI] [PubMed] [Google Scholar]
- Sobko A., Peretz,A. and Attali,B. (1998a) Constitutive activation of delayed-rectifier potassium channels by a src family tyrosine kinase in Schwann cells. EMBO J., 17, 4723–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobko A., Peretz,A., Shirihai,O., Etkin,S., Cherepanova,V., Dagan,D. and Attali,B. (1998b) Heteromultimeric delayed-rectifier K+ channels in Schwann cells: a role in proliferation? J. Neurosci., 18, 10398–10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Muranjan,M. and Sap,J. (1999) Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol., 9, 505–511. [DOI] [PubMed] [Google Scholar]
- Szabo I., Gulbins,E., Apfel,H., Zhang,X., Barth,P., Busch,A.E., Schlottmann,K., Pongs,O. and Lang,F. (1996) Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J. Biol. Chem., 271, 20465–20469. [DOI] [PubMed] [Google Scholar]
- Tanuma N., Nakamura,K. and Kikuchi,K. (1999) Distinct promoters control transmembrane and cytosolic protein tyrosine phosphatase ε expression during macrophage differentiation. Eur. J. Biochem., 259, 46–54. [DOI] [PubMed] [Google Scholar]
- Timpe L.C. and Fantl,W.J. (1994) Modulation of a voltage-activated potassium channel by peptide growth factor receptors. J. Neurosci., 14, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano-Katchalski H. and Elson,A. (1999) The transmembranal and cytoplasmic forms of protein tyrosine phosphatase ε physically interact with the adaptor protein Grb2. Oncogene, 18, 5024–5031. [DOI] [PubMed] [Google Scholar]
- Tonks N.K. and Neel,B.G. (1996) From form to function: signaling by protein tyrosine phosphatases. Cell, 87, 365–368. [DOI] [PubMed] [Google Scholar]
- Tsai W., Morielli,A.D. and Peralta,E.G. (1997) The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J., 16, 4597–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W., Morielli,A.D., Cachero,T.G. and Peralta,E.G. (1999) Receptor protein tyrosine phosphatase α participates in the m1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity. EMBO J., 18, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V.L., Crawford,C.E., Jackson,P.K., Bronson,R.T. and Mulligan,R.C. (1991) Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell, 65, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Wallace M.J., Batt,J., Fladd,C.A., Henderson,J.T., Skarnes,W. and Rotin,D. (1999) Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPσ. Nature Genet., 21, 334–338. [DOI] [PubMed] [Google Scholar]
- Wang Q. (1999) Regulation of a human neuronal voltage-gated potassium channel (hKv1.1) by protein tyrosine phosphorylation and dephosphorylation. Ann. N Y Acad. Sci., 868, 447–449. [DOI] [PubMed] [Google Scholar]
- Wong E.C., Mullersman,J.E. and Thomas,M.L. (1993) Leukocyte common antigen-related phosphatase (LRP) gene structure: conservation of the genomic organization of transmembrane protein tyrosine phosphatases. Genomics, 17, 33–38. [DOI] [PubMed] [Google Scholar]
- Yu S.P., Yeh,C.H., Gottron,F., Wang,X., Grabb,M.C. and Choi,D.W. (1999) Role of the outward delayed rectifier K+ current in ceramide-induced caspase activation and apoptosis in cultured cortical neurons. J. Neurochem., 73, 933–941. [DOI] [PubMed] [Google Scholar]
- Zondag G.C. and Moolenaar,W.H. (1997) Receptor protein tyrosine phosphatases: involvement in cell–cell interaction and signaling. Biochimie, 79, 477–483. [DOI] [PubMed] [Google Scholar]