Abstract
The primary objective of this study was to utilize MR molecular imaging to compare the 3-dimensional spatial distribution of Robo4 and αVβ3-integrin as biosignatures of angiogenesis, in a rapidly growing, syngeneic tumor. B16-F10 melanoma-bearing mice were imaged with magnetic resonance (MR; 3.0 T) 11 d postimplantation before and after intravenous administration of either Robo4- or αVβ3-targeted paramagnetic nanoparticles. The percentage of MR signal-enhanced voxels throughout the tumor volume was low and increased in animals receiving αVβ3- and Robo4-targeted nanoparticles. Neovascular signal enhancement was predominantly associated with the tumor periphery (i.e., outer 50% of volume). Microscopic examination of tumors coexposed to the Robo4- and αVβ3-targeted nanoparticles corroborated the MR angiogenesis mapping results and further revealed that Robo4 expression generally colocalized with αVβ3-integrin. Robo4- and αVβ3-targeted nanoparticles were compared to irrelevant or nontargeted control groups in all modalities. These results suggest that αVβ3-integrin and Robo4 are useful biomarkers for noninvasive MR molecular imaging in syngeneic mouse tumors, but αVβ3-integrin expression was more detectable by MR at 3.0 T than Robo4. Noninvasive, neovascular assessments of the MR signal of Robo4, particularly combined with αVβ3-integrin expression, may help define tumor character prior to and following cancer therapy.—Boles, K. S., Schmieder, A. H., Koch, A. W., Carano, R. A. D., Wu, Y., Caruthers, S. D., Tong, R. K., Stawicki, S., Hu, G., Scott, M. J., Zhang, H., Reynolds, B. A., Wickline, S. A., and Lanza, G. M. MR angiogenesis imaging with Robo4- vs. αVβ3-targeted nanoparticles in a B16/F10 mouse melanoma model.
Keywords: magnetic resonance imaging, integrin, neovasculature, roundabout, tumor
The roundabouts are transmembrane receptors expressed in developing tissues, such as the central nervous system (Robo1, Robo2, Robo3) and neovascular endothelium (Robo4) (1). Robo4, also referred to as “magic roundabout,” is an endothelial specific guidance receptor expressed at sites of active angiogenesis (2). In particular, Robo4 is elevated in tumor neovascular endothelium and down-regulated in mature vasculature (2, 3), which suggests that Robo4 may be a useful neovessel marker for noninvasive detection and characterization of nascent cancers undergoing active angiogenesis.
The essential associations of angiogenesis, VEGF, and tumor development are well-established concepts that have spurred efforts in molecular imaging to noninvasively detect and characterize tumor neovascularity. αVβ3-Integrin is a differentially up-regulated adhesion molecule in proliferating vs. quiescent endothelial cells and is considered a prominent neovascular biomarker for noninvasive molecular imaging of nascent cancers (4–6). However, unlike Robo4, the αVβ3-integrin, a heterodimeric transmembrane glycoprotein, is found abundantly on nonendothelial cell types, including macrophages (7), platelets (8), lymphocytes (8), smooth muscle cells (9), and tumor cells (10, 11). We have developed nanomedicine tools for imaging angiogenesis in tumors using αVβ3-targeted perfluorocarbon nanoparticles (12–16), which preclude significant interaction with integrins expressed by nonendothelial cells by utilizing particle size (∼250 nm) to constrain the homed perfluorocarbon nanoparticles within the vasculature (13, 16, 17).
We have recently reported the use of αVβ3-paramagnetic nanoparticles to create 3-dimensional (3-D) angiogenesis maps in the Vx2 rabbit and MDA 435 mouse tumor models (15, 16). The expression of αVβ3-integrin by neovascular endothelial cells in the Vx2 tumor was abundant, although asymmetrically and heterogeneously distributed along the tumor periphery (15); in contrast, the expression of αVβ3-integrin was much less prevalent by MRI in the MDA 435 xenograft mouse model (16). Similar sparse angiogenesis imaging patterns were found in the MDA 435 mice when α5β1-integrin alone or in combination with αVβ3-integrin were targeted with the paramagnetic nanoparticles (16).
The paucity of αVβ3-integrin neovessels previously detected by MR in the MDA 435 tumor model may be related to slow tumor growth in an immunodeficient athymic mouse model. Possibly, the prevalence of αVβ3 and related integrin neoendothelial expression levels were <100 pM/voxel, which is the lower level of MR detection achievable with acceptable conspicuity (i.e., a contrast-to-noise ratio≥5) (18). The objectives of the present study were to compare noninvasive MR molecular imaging utilizing Robo4 and αVβ3-integrin as biosignatures of angiogenesis, to determine the relative spatial distribution of Robo4- and αVβ3-nanoparticle labeled neovessels within the tumor, and to assess the prevalence of angiogenesis using these neovessel biomarkers in an immunocompetent mouse model implanted with a rapidly growing, syngeneic tumor.
MATERIALS AND METHODS
Nanoparticle formulation
Biotinylated Robo4 antibody was coupled to avidin-modified paramagnetic perfluorocarbon nanoparticles and compared with αVβ3-peptidomimetic targeted perfluorocarbon nanoparticles. Briefly, the paramagnetic emulsions comprised 20% (v/v) perfluorooctylbromide (PFOB), 2.0% (w/v) of a surfactant comixture, and 1.7% (w/v) glycerin in distilled, deionized water. The surfactant comixture of peptidomimetic nanoparticles included 68.8 mol% lecithin (Avanti Polar Lipids, Alabaster, AL, USA), 0.1 mol% of the αVβ3- peptidomimetic antagonist conjugated to PEG2000-phosphatidylethanolamine (Kereos, Inc., St. Louis, MO, USA), 0.1 mol% rhodamine-coupled phosphatidylethanolamine (Avanti Polar Lipids, Alabaster, AL, USA), or 0.1 mol% AlexaFluor 488 (Invitrogen, Carlsbad, CA, USA) coupled to phosphatidylethanolamine (Avanti Polar Lipids), 1.9 mol% phosphatidylethanolamine (Avanti Polar Lipids) and 30 mol% gadolinium diethylene-triamine-pentaacetic acid-bis-oleate (IQSynthesis, St. Louis, MO, USA). Nontargeted nanoparticles excluded all homing ligands from the surfactant above, which was replaced with equimolar phosphatidylethanolamine. Antibody (i.e., Robo4 and irrelevant IgG) targeted perfluorocarbon nanoparticles included 1.0 mol% carboxy-PEG2000-phosphatidylethanolamine substituted for the peptidomimetic antagonist and chemically coupled after emulsification to avidin, as detailed below. The surfactant components for each formulation were combined with the perfluorooctylbromide (Exfluor Research Corporation, Round Rock, TX USA), water, and glycerin; the pH was adjusted to 7.5; and the mixtures were emulsified (Microfluidics, Newton, MA, USA) at 20,000 psi for 4 min.
Avidin (35 μl, 0.15 mM) was incubated with perfluorocarbon nanoparticles incorporating carboxy-PEG2000-phosphatidylethanolamine in the surfactant and 140 μl (30.7 mM) 1-[3-(dimethylamino)propyl]-3-ethylcarbodimide for 42 min at ambient temperature. Avidin was conjugated with nanoparticles with 97% efficiency based on HPLC yielding 21 avidin/nanoparticle, nominally. Robo4 or an irrelevant antibody was monobiotinylated with biotin-NHS (Thermo Scientific, Rockford, IL, USA) and coupled to the particles equimolar to the avidin concentration. Nominal particle sizes were 167 and 236 nm (polydispersity≤0.18) for the peptidomimetic and avidin-functionalized nanoparticles, respectively (Brookhaven Instrument Corp., Holtsville, NY, USA).
Homing ligands
Robo4 and irrelevant antibody
The anti-Robo4-1 antibody was a human (IgG1) phage-derived, affinity-matured, antibody cross-reactive for human and murine Robo4 with an affinity of 1.0–1.2 nM, which was raised against the two N-terminal IgG-like domains of Robo4 (unpublished results). The irrelevant antibody was a human IgG fraction derived from peripheral blood (Southern Biotech, Birmingham, AL, USA).
αVβ3-integrin peptidomimetic antagonist
The αVβ3-integrin antagonist is a quinalone nonpeptide developed by Lantheus Medical Imaging (U.S. patent 6,511, 648 and related patents), which was initially reported and characterized as the 111In-DOTA conjugate RP478 and cyan 5.5 homologue TA145 (19). The specificity of the αVβ3-ligand mirrors that of the anti-αVβ3 LM609 antibody (Chemicon International, Billerica, MA, USA), as assessed by staining and flow cytometry, and it has a 15-fold preference for the Mn2+ activated receptor (21 nM). The IC50 for αVβ5, α5β1, and GP IIb/IIIa was determined to be >10 μM (Lantheus Medical Imaging, Billerica, MA, USA, unpublished data).
In vitro experiments
pSurfGNB construction and Robo4 cloning
A surface display vector was constructed to overexpress the extracellular domain of Robo4 on a mammalian cell surface. The base vector, pSurfGNB, was composed of a murine Ig κ-chain V-J2-C signal peptide sequence followed by a multiple cloning site (MCS) comprising NotI and asymmetrical SfiI sites followed by a Myc epitope tag, a PDGFR transmembrane domain, and a EGFP/neomycin resistance cassette. The entire expression cassette was assembled by overlap extension PCR (primer sequences available on request) from components of pDisplay (Invitrogen, Carlsbad, CA, USA) and pQBI-pgk (Quantum Biotechnology, Montréal, QC, Canada) and cloned into the pEF6V5HisTOPO vector (Invitrogen) linearized with KpnI and NotI. The extracellular domain of Robo4 from the start codon to near the transmembrane domain (K466) was amplified with primers containing KpnI and SfiI sites from a cDNA clone(Human MGC Verified FL cDNA 40068748, Thermo Fisher Scientific, Huntsville, AL, USA). The pSurfGNB vector and Robo4 insert were similarly restriction digested and ligated. All constructs were sequenced (Protein and Nucleic Acid Chemistry Laboratory, Washington University, St. Louis, MO, USA). All restriction enzymes, competent cells, and PCR reagents (Phusion enzyme) were from New England Biolabs (Ipswich, MA, USA). All oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA, USA).
Robo4 HEK-293T cell transfection
HEK-293T cells [CRL-11268 from American Type Culture Collection (ATCC), Manassas, VA, USA] were cultured in DMEM supplemented with 10% FBS and transfected with a human Robo4 expression plasmid or control plasmid using a 3:1 ratio of Fugene6 to DNA (F. Hoffmann-La Roche, Nutley, NJ, USA). HEK-293T cells transfected with Robo4 or control constructs were surface labeled with either biotinylated anti-Robo4 monoclonal antibody (mAb) or biotinylated hIgG (4 μg/105 cells), detected by streptavidin-allophycocyanin (APC; Southern Biotech), and analyzed with FACS (CyAn ADP flow cytometer, Dako, Carpinteria, CA, USA). Similarly, HEK-293T cells transfected with Robo4 or control constructs were surface labeled with Robo4- or irrelevant-targeted avidinized paramagnetic nanoparticles for 1 h at 37°C. Unbound nanoparticles were removed with 2% FBS in PBS. Avidinized nanoparticles bound to the cell surfaces were detected with biotinylated anti-avidin mAb (Sigma-Aldrich, St. Louis, MO, USA), detected by APC, and analyzed with the CyAn ADP flow cytometer.
In vivo experiments
Experimental design
C57BL/6 black mice (n=24; Charles River, Wilmington, MA, USA) were implanted subcutaneously with 5 × 105 B16/F10 mouse melanoma cells (CRL6475; ATCC) in 50 μl of DMEM in the right flank. On d 11, mice were anesthetized with 3% isoflurane in 100% oxygen (2.0 L/min), a 27-gauge tail-vein catheter was placed, and the mice were positioned in the MR scanner (3 T, Philips Achieva; Philips Healthcare, Andover, MA, USA), where body temperature was monitored and regulated with a heater (Small Animal Instruments, Stony Brook, NY, USA).
Tumor-implanted mice were randomly assigned into 4 experimental groups (n=6/group) that received either αVβ3-targeted, Robo4-targeted, irrelevant-targeted, or nontargeted paramagnetic nanoparticles (2 ml/kg, 0.2 nM). Dynamic, T1-weighted 3-D GRE images (TR=46 ms, TE=3.9 ms, 4 signal averages, flip angle 40°, 320×204 acquisition matrix, 0.39-×0.39-×0.5-mm resolution, with SPIR fat suppression) were acquired simultaneously for 3 mice/sequence before and 30, 60, 90, and 120 min posttreatment by placing them in an 11- × 14-cm SENSE flex M coil with two elliptical elements (Philips Healthcare). Immediately after imaging, animals were euthanized, and tumors were resected, weighed, and frozen in optimum cutting temperature (OCT) compound. The procedure was repeated until all 24 mice were scanned. All procedures were conducted in accordance with a protocol approved by the Animal Studies Committee of Washington University School of Medicine.
MR image analysis
A signal reference standard (Gd-DTPA-doped tube) was placed in a fixed position with respect to the coil and tumor during scans to eliminate errors in the signal calibration over time due to the spatial variation in coil sensitivity (12, 14). The voxel intensities from the normalized MR images were analyzed using a threshold algorithm implemented with custom Matlab programs (The MathWorks, Natick, MA, USA), as described previously to map the spatial distribution of antiangiogenic contrast rich voxels (15, 16).
For each animal, a region of interest (ROI) was manually placed around the tumor edge on each baseline slice, and the sd of the average tumor signal at baseline was calculated. Subsequent, serial images were spatially coregistered using a cross-correlation routine, and the tumor ROI mask was copied to each time point. At 120 min postinjection, pixels with an MR signal intensity increase ≥3 sd above the baseline tumor signal were considered significant. The tumor ROI was automatically partitioned into rim and core regions via an automated algorithm implemented in Matlab. The core region was defined by eroding the tumor periphery to 50% of the original volume. The average percentage signal enhancement and the percentage of tumor volume enhancement were calculated for each tumor region.
High-resolution 3-D tumor reconstructions were created in Matlab to map the spatial distribution of enhancing neovasculature in each experimental group. The overall 3-D structure of the tumor was displayed as a mesh surface plot using isosurface rendering and a smoothing filter. A surface plot of the enhancing voxels was reconstructed similarly, and overlaid onto the tumor volume.
Optical microscopy
In a separate cohort of mice (n=3), Robo4-targeted rhodamine paramagnetic nanoparticles (1 ml/kg) and αVβ3-targeted AlexaFluor-488 paramagnetic nanoparticles (1 ml/kg) were coadministered via tail-vein injection. After 2 h, animals were sacrificed, and tumors were resected, weighed, and quickly frozen in OCT compound. Frozen sections of the cotreated tumors were obtained (8 μm) and studied with an Olympus BX61 microscope and an F-View II camera using a Cy3.3 (Robo4) or FITC (αVβ3) filter (Olympus, Tokyo, Japan). The cell nuclei were visualized with DAPI. Immunohistochemistry for endothelial PECAM-1 was performed on adjacent frozen sections (clone MEC13.3; BD Biosciences, San Jose, CA, USA) developed with ABC method and VIP substrate kits (Vector Laboratories, Burlingame, CA, USA) and nuclear counterstained with Methyl Green nuclear. Light images were captured with a ColorView II camera (Olympus).
Statistical analysis
MR in vivo signal intensity and tumor data were analyzed using the general linear model procedure in SAS (SAS Institute, Cary, NC, USA). The Tukey-Kramer option was applied for multiple comparisons, which controls the maximum experiment wise error rate (P<0.05). Data are presented as means ± se.
RESULTS
Evaluation of ROBO-4 paramagnetic nanoparticles in vitro
Fluorescent assessments of in vitro targeting
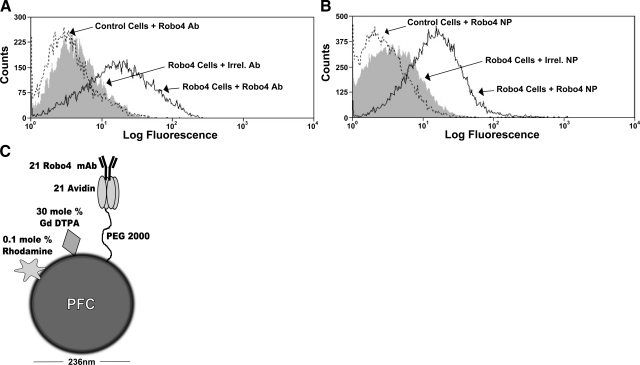
The effectiveness of Robo4-nanoparticle targeting was demonstrated using HEK293T cells transiently transfected with a human Robo4-EGFP construct or an EGFP control vector. Surface expression of Robo4 on the HEK293T cells was confirmed by flow cytometry using a biotinylated Robo4 mAb detected by streptavidin–APC (data not shown). The binding of Robo4 antibody to transfected Robo4-EGFP cells was detected on 52% of the GFP positive cells in contradistinction to 5% nonspecific binding for the irrelevant control antibody. Nonspecific binding of Robo4 antibody to the control transfected cells was 3% (Fig. 1A). The binding of Robo4-targeted nanoparticles to transfected Robo4-EGFP cells was detected on 49% of the GFP positive cells, while only 5% nonspecific binding for the irrelevant-targeted nanoparticles were measured. Minimal (2%) nonspecific binding was found for the Robo4-targeted nanoparticles to the control transfected cells (Fig. 1B).
Figure 1.
A, B) Binding of anti-Robo4 monoclonal antibody to hRobo4/EGFP-expressing cells in solution (A) and when on the surface of nanoparticles (B) by flow cytometry. Transiently transfected HEK-293T cells labeled with biotinylated monoclonal anti-Robo4 or control IgG were detected with streptavidin-APC. Similarly, Robo4- or irrelevant-targeted PFC nanoparticles were incubated with cells for 1 h and were detected with a biotinylated anti-avidin antibody and streptavidin-APC. Solid line, Robo4 targeting of Robo4-expressing cells; solid line; dashed line, Robo4 targeting of control cells; shaded histogram, irrelevant antibody targeting of Robo4-expressing cells. C) Pictorial representation of the Robo4 nanoparticle. Attached moieties are shown at a greater size for illustrative purposes.
MR confirmation of in vitro targeting
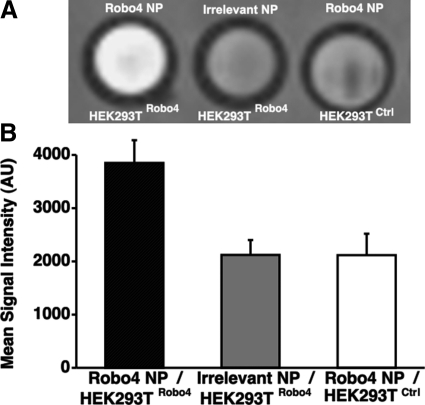
The Robo4 transfected HEK293T cells were targeted with Robo4 paramagnetic nanoparticles, washed, and imaged with a T1-weighted MRI scan (Fig. 2A), revealing strong contrast enhanced signal. Low MR signal intensity was observed when irrelevantly targeted nanoparticles were exposed to Robo4-expressing cells or when Robo4 nanoparticles were incubated with control cells. The mean signal intensity of the Robo4-targeted particles bound to the Robo4 transfected cells was ∼1-fold higher than the signal observed in the controls (Fig. 2B). These data corroborate the specific targeting results obtained with fluorescent imaging and illustrate the effectiveness of the Robo4-paramagnetic nanoparticles to generate T1w contrast in vitro.
Figure 2.
Representative slice of MR image of HEK-293T-cell pellets after incubation with Robo4- or irrelevant-targeted paramagnetic nanoparticles. HEK-293T cells were transiently transfected with a Robo4 or a control plasmid. A) T1-weighted contrast enhancement is clearly seen in the Robo4-targeted sample with Robo4-transfected cells. B) Mean signal intensity for each of the samples. Error bars = sem.
Evaluation of ROBO-4 paramagnetic nanoparticles in a mouse melanoma model
MR imaging
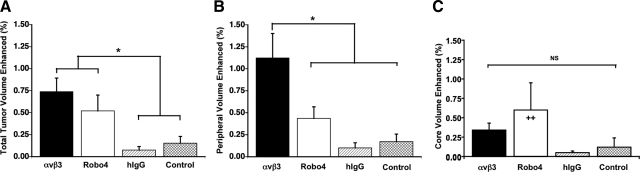
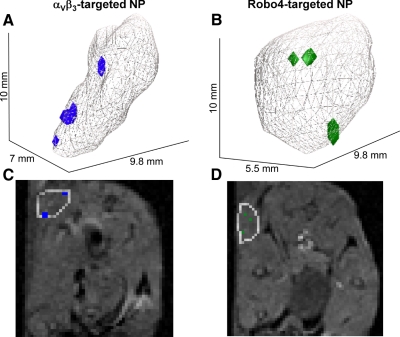
C57BL/6 black mice were implanted subcutaneously with syngeneic B16/F10 melanoma cells and imaged with MR 11 d later. Baseline images revealed that tumor growth over the interval was variable but not different (P>0.05) between experimental groups (188±37 mm3, across all animals). The percentage of MR signal-enhanced voxels in the overall tumor was increased in animals receiving αVβ3- and Robo4-targeted nanoparticles when compared to the irrelevant or nontargeted control groups (Fig. 3A). No difference in overall tumor neovessel contrast enhancement was appreciated between the αVβ3- and Robo4-nanoparticle-treated mice. Voxel signal enhancement was predominantly associated with the tumor periphery (i.e., outer 50%), where neovascular signal enhancement from the vascularly restricted particles was greater for the αVβ3- than Robo4-targeted tumors (P<0.05), the latter of which did not differ from the irrelevant or nontargeted control animal tumors (Fig. 3B). The mean MR contrast enhancement in the tumor cores did not differ from zero in the αVβ3-, irrelevant-, and nontargeted groups and was similarly low, but >0 (P<0.006) in the Robo4 animals (Fig. 3C). Three-dimensional angiogenesis maps from mice administered αVβ3 or Robo4 paramagnetic nanoparticles illustrated an overall sparse, peripheral distribution of contrast-enhanced voxels, as appreciated in the 3-D-rendered angiogenic maps and individual 2-D slices (Fig. 4).
Figure 3.
Percentage of tumor surface and core volumes with significant contrast enhancement. Number of enhanced voxels in the total tumor (A), peripheral tumor (i.e., outer 50% tumor volume; B), and core tumor (i.e., central 50% tumor volume; C) was calculated for αVβ3-targeted, Robo4-targeted, irrelevant-targeted, and nontargeted groups. *P < 0.05. ++P < 0.01 vs. 0.
Figure 4.
A, B) Representative 3-D reconstructions of MR signal enhancement revealing tumor neovascular morphology following in vitro administration of αvβ3-targeted (A) or Robo4-targeted paramagnetic nanoparticles (B). Tumor volumes are outlined in gray; voxels meeting enhancement thresholds at 2 h after injection of contrast agent are shown in blue (αVβ3) or green (Robo4). C, D) MR slices corresponding to each 3-D reconstruction; nascent tumors are outlined, and enhanced voxels are colored as above.
Microscopic imaging of αVβ3- and Robo4-targeted fluorescent nanoparticles
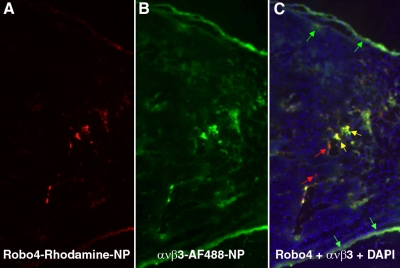
In a separate cohort, C57BL/6 black mice implanted with B16 cells were injected intravenously with a mixture of αVβ3-targeted AlexaFluor 488 and Robo4-targeted rhodamine paramagnetic nanoparticles. After 2 h of circulation, the tumors were excised, and cryosections were examined microscopically to assess the relative spatial localization of the homed particles. Consistent with the noninvasive MR mapping, fluorescence from αVβ3-targeted AlexaFluor 488 nanoparticles was more prominent peripherally than Robo4-targeted rhodamine nanoparticle, although both biosignatures were noted (Fig. 5). Observation at high magnification revealed the majority of unique integrin signal arose from vessels in connective tissue tracks (data not shown). Within the tumor, the vascular-constrained αVβ3-targeted AlexaFluor 488 and Robo4-targeted rhodamine particles were generally colocalized. Neither αVβ3-targeted AlexaFluor 488 nor Robo4-targeted rhodamine particles were observed within the tumor parenchyma, reconfirming that these intravascular agents (∼200 nm) were vascularly constrained.
Figure 5.
Histological assessment of the spatial distribution of Robo4- vs. αVβ3-targeted fluorescent nanoparticles. Fluorescent tumor images from mice injected intravenously with a comixture (1:1) of perfluorocarbon nanoparticles targeted to Robo4 (red) (A), or αVβ3 (green) (B), or combined and counterstained with DAPI (blue) (C). Colocalization is demonstrated by yellow color. Arrows indicate examples of colocalized (yellow), Robo4-bound (red) and αVβ3-bound particles (green).
DISCUSSION
In this study, MR imaging (3 T) of the Robo4 and integrin neovascular biosignatures was compared in a syngeneic B16/F10 melanoma tumor model (d 11). Three-dimensional MR angiogenesis maps revealed sparse, peripheral, and heterogeneous distribution of tumor neovessels, similar to earlier studies with the slower growing, MDA 435 xenograft mouse model. Both Robo4- and αVβ3-integrin-targeted nanoparticles provided contrast for detecting angiogenesis in the melanoma tumors. Angiogenesis mapping revealed that an abundance of the MR signal enhancement was derived from the tumor periphery with less contrast enhancement noted in the central 50% of the lesion. Fluorescence microscopy of tumors from mice administered a mixture of αVβ3-targeted AlexaFluor 488 and Robo4-targeted rhodamine nanoparticles showed that αVβ3-targeted nanoparticle intravascular binding was more prevalent than Robo4 particles, in general, and particularly in the tumor periphery. Within the tumor, Robo4 and αVβ3-integrin-targeted nanoparticles essentially colocalized. Moreover, neither targeted species of particles were observed within the tumor parenchyma beyond the vasculature, consistent with other studies involving PFC nanoparticles. Since αVβ3-integrin and Robo4 are both biosignatures of angiogenesis, these results suggest that the two markers provide additive understanding for tumor neovascular development, which may ultimately relate to tumor progression or anticancer therapeutic responses.
The association of αVβ3-integrin with angiogenesis in aggressive highly malignant tumors has been extensively studied and reviewed (20, 21), but the discovery of Robo4 and its role in neovascular development have been recent. Robo4 was discovered as an endothelial specific marker (2, 22) associated in mutant mice with embryonic vascular sprouting defects (23). Activated Robo4 is integral in maintenance of vascular integrity by inhibiting endothelial migration, tube formation, and permeability response to VEGF (1, 24) by inhibiting the activation of the Src kinases (25, 26).
In a recent paper, Jones et al. (24) generated Robo4AP/AP mice in which the immunoglobulin repeats required for interaction with Slit were substituted with human placental alkaline phosphatase repeats, negating Slit2-Robo4 interactions. The Robo4AP/AP mice were viable and developed normal vascular patterning. Jones et al. claim that the function of Robo4 is to stabilize the vasculature is supported by their observation of enhanced hypervascularization in Robo4AP/AP mice treated with VEGF. Furthermore, in the neovasculature of the neonatal retina, Robo4 expression was associated with more mature and stabilized stalk cells rather than the proliferating tip cells of the sprout. Molecular imaging and microscopic results observed in the present study are consistent with these findings.
A balance of proangiogenic signals, such as VEGF-VEGFR2 interaction, and the antiangiogenic influences regulate the proliferation of angiogenesis, notably Delta-like 4 (DLL4)-Notch receptor binding (27–30). Sprouting requires flipping of endothelial apical-basal polarity, initiation of invasive cellular migration, degradation of surrounding extracellular matrix, and modulation of cell-cell contacts (30). In this process, the expression of adhesion molecules, including αVβ3-integrin, are markedly up-regulated and expressed on the lumenal aspect of these proliferating cells, where they are available for binding to the αVβ3-targeted perfluorocarbon nanoparticles. Consistent with previous studies (12, 14–16, 31–33), we observed that the proliferation of endothelial cells expressing αsβ3-integrin was prevalent in the periphery, particularly in connective tissues encapsulating the tumor. Likely, the majority of these neovessel sprouts become apoptotic rather than coalesce into a continuous lumen, which may account for the disproportionate αVβ3-integrin to Robo4 signal appreciated in the periphery vs. the tumor interior.
The majority of Robo4-positive vessels was found in the tumor parenchyma in close association with αVβ3-integrin particles. Given the previous findings, one interpretation could be that these neovessels within the tumor expressing both signals represent sprouts fusing to form stable endothelial to endothelial cell interactions characterized by the lumenal expression of Robo4 (24). Since Robo4 nanoparticles were generally not observed independent of αVβ3-integrin signal, one could speculate that the transition of neovessels into maturing microvessels coincides with the loss of both epitopes on the lumen surface.
The relative MR detectability of Robo4 and αVβ3-integrin also relates to the relative endothelial lumenal expression density of each epitope; of the two, Robo4 may be lower than the integrin such that only one is detected, although both are present. Robo4 lumenal expression density relative to the integrin may be low where neovessel proliferation is initiating along the tumor growth front but become enriched within maturing neovessels in the tumor, where colocalization of Robo4- and αVβ3-integrin-targeted nanoparticles was prominent.
We have reported the use of 3-D MR imaging to study neovascular integrin expression in the syngeneic Vx2 rabbit tumor model, as well as the xenograft MDA 435 breast cancer mouse model (15, 16). In contradistinction to the marked MR-detectable angiogenic proliferation observed in the syngeneic Vx2 rabbit model, the neovascularity of the xenograft tumor was very sparse. Moreover, while treatment with a targeted antiangiogenic compound, i.e., fumagillin, significantly reduced angiogenesis and decreased Vx2 tumor development in the rabbit, the same treatment regimen diminished MR detectable angiogenesis to nearly zero without impacting tumor growth in the MDA 435 mouse model. At the time, the less prolific MR angiogenesis patterns observed and lack of tumor growth response to fumagillin treatment were considered to reflect the use of a slow developing human tumor in an immunodeficient mouse model, ostensibly due to diminished inflammatory and neovascular responses. However, in the present study, an aggressive syngeneic melanoma tumor (B16/F10) implanted into an immunocompetent mouse also resulted in low levels of neovessel proliferation, despite rapid tumor growth. The low neovascular patterns observed in these mice studies were similar to additional observations in an earlier xenograft study utilizing human melanoma in athymic mice and the sparse angiogenesis observed in cholesterol fed ApoE−/− mice, particularly when compared to aortic neovasculature in larger species (15, 34, 35).
The development of noninvasive angiogenic maps using MRI requires the segmentation of contrast-enhanced pixels from the nonenhanced tumor signal, which are subject to variability dependent on the discrimination signal threshold and algorithm employed. In addition, proton MR imaging is highly influenced by local environment effects, and a more quantitative augmentative approach to angiogenesis mapping can be envisioned that utilizes 19F spectroscopy to determine the total or regional amount of bound perfluorocarbon contrast (36–38). Ultimately, a qualitative microanatomical representation of tumor neovascular distribution based on 1H could be paired with a quantitative angiogenic index based on 19F, which would better support patient segmentation into individualized medical strategies. Moreover, analogous to the 2-color (red and green) fluorescent microscopy employed to assess the spatial distribution of the αVβ3-integrin to Robo4-targeted nanoparticles in tissue sections, nanoparticles with different species of perfluorocarbon could be noninvasively differentiated with 19F MR imaging and spectroscopy. Each nanoparticle subtype could be homed to a distinct biomarker, e.g., αVβ3-integrin or Robo4, providing quantitative differentiation of multiple biomarkers at once (36–42). The development of clinical 1H and 19F imaging is rapidly progressing, and today, 1H and 19F images can be acquired and displayed simultaneously, minimizing scanning time, ensuring proper registration, and providing unprecedented quantification of MR imaging results (43–48).
In the current study, we developed and used a novel avidinized fluorescent paramagnetic (bimodal) perfluorocarbon nanoparticle, to which monobiotinylated ligands were surface coupled without aggregation. This platform particle, which differed from the covalently coupled αVβ3-nanoparticles, provided a convenient and effective means for evaluating the role of vascular biosignatures, such as Robo4, where an antibody or similar ligand is available. The binding of the Robo4 nanoparticles in vitro to Robo4-transfected HEK293T cells was excellent with no nonspecific binding to nontransformed HEK293T cells. Both the αVβ3-integrin and Robo4 nanoparticles were dosed in mild excess to ensure saturation of the target markers without background blood signal at the time of MR imaging. Ultimately, the spatial relationship of the two species of nanoparticles observed by fluorescence microscopy agreed with the MR signal results, suggesting that the modest formulation differences did not limit the results of this study.
In this study, the differential perspective provided by two lumenal neovascular markers of angiogenesis, αVβ3-integrin, and Robo4, was evaluated with MR molecular imaging and corroborated with fluorescence microscopy. The neovascular expression of αVβ3-integrin was more prevalent than Robo4 when assessed by MR and microscopically. αVβ3-integrin was more prevalent than Robo4 in the tumor periphery, but the two were nearly always colocalized within the central tumor parenchyma. MR molecular imaging of αVβ3-integrin and Robo4 neovascular expression offered individual biochemical characterization of nascent tumor angiogenesis, which may be useful for guiding and following personalized strategic medical decisions in cancer.
Acknowledgments
The authors thank Kereos, Inc. (St. Louis, MO, USA), for the gift of the peptidomimetic integrin antagonist; Todd Williams and Xiaoxia Yang for MR and molecular biology technical support, respectively; and John S. Allen and Cordelia Caradine for assistance in the management of the animal studies.
Funding was provided by the U.S. National Institutes of Health to S.A.W. and G.M.L. (U54-CA119342 and R01-HL094470). Additional funding was provided by the Barnes-Jewish Hospital Foundation to K.S.B. (01152-0308-01).
REFERENCES
- 1. Legg J. A., Herbert J. M. J., Clissold P., Bicknell R. (2008). Slits and roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 11, 13– 21 [DOI] [PubMed] [Google Scholar]
- 2. Huminiecki L., Gorn M., Suchting S., Poulsom R., Bicknell R. (2002). Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 79, 547– 552 [DOI] [PubMed] [Google Scholar]
- 3. Seth P., Lin Y., Hanai J. I., Shivalingappa V., Duyao M. P., Sukhatme V. P. (2005). Magic roundabout, a tumor endothelial marker: Expression and signaling. Biochem. Biophys. Res. Commun. 332, 533– 541 [DOI] [PubMed] [Google Scholar]
- 4. Cheresh D. A. (1991). Integrins in thrombosis, wound healing and cancer. Biochem. Soc. Trans. 19, 835– 838 [DOI] [PubMed] [Google Scholar]
- 5. Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., Cheresh D. A. (1995). Definition of two angiogenic pathways by distinct alpha v integrins. Science. 270, 1500– 1502 [DOI] [PubMed] [Google Scholar]
- 6. Friedlander M., Theesfeld C. L., Sugita M., Fruttiger M., Thomas M. A., Chang S., Cheresh D. A. (1996). Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. U. S. A. 93, 9764– 9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Nichilo M., Burns G. (1993). Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate alpha v integrin expression on cultured human macrophages. Proc. Natl. Acad. Sci. U. S. A. 90, 2517– 2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helluin O., Chan C., Vilaire G., Mousa S., DeGrado W. F., Bennett J. S. (2000). The activation state of alphavbeta 3 regulates platelet and lymphocyte adhesion to intact and thrombin-cleaved osteopontin. J. Biol. Chem. 275, 18337– 18343 [DOI] [PubMed] [Google Scholar]
- 9. Itoh H., Nelson P. R., Mureebe L., Horowitz A., Kent K. C. (1997). The role of integrins in saphenous vein vascular smooth muscle cell migration. J. Vasc. Surg. 25, 1061– 1069 [DOI] [PubMed] [Google Scholar]
- 10. Carreiras F., Denoux Y., Staedel C., Lehmann M., Sichel F., Gauduchon P. (1996). Expression and localization of alpha v integrins and their ligand vitronectin in normal ovarian epithelium and in ovarian carcinoma. Gynecol. Oncol. 62, 260– 267 [DOI] [PubMed] [Google Scholar]
- 11. Kageshita T., Hamby C. V., Hirai S., Kimura T., Ono T., Ferrone S. (2000). Differential clinical significance of alpha (v) Beta(3) expression in primary lesions of acral lentiginous melanoma and of other melanoma histotypes. Int J Cancer. 89, 153– 159 [DOI] [PubMed] [Google Scholar]
- 12. Winter P. M., Caruthers S. D., Kassner A., Harris T. D., Chinen L. K., Allen J. S., Lacy E. K., Zhang H., Robertson J. D., Wickline S. A., Lanza G. M. (2003). Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 63, 5838– 5843 [PubMed] [Google Scholar]
- 13. Hu G., Lijowski M., Zhang H., Partlow K. C., Caruthers S. D., Kiefer G., Gulyas G., Athey P., Scott M. J., Wickline S. A., Lanza G. M. (2007). Imaging of Vx-2 rabbit tumors with alpha(nu)beta3-integrin-targeted 111In nanoparticles. Int. J. Cancer. 120, 1951– 1957 [DOI] [PubMed] [Google Scholar]
- 14. Schmieder A. H., Winter P. M., Caruthers S. D., Harris T. D., Williams T. A., Allen J. S., Lacy E. K., Zhang H., Scott M. J., Hu G., Robertson J. D., Wickline S. A., Lanza G. M. (2005). Molecular MR imaging of melanoma angiogenesis with alpha (v) beta (3)-targeted paramagnetic nanoparticles. Magn. Reson. Med. 53, 621– 627 [DOI] [PubMed] [Google Scholar]
- 15. Winter P. M., Schmieder A. H., Caruthers S. D., Keene J. L., Zhang H., Wickline S. A., Lanza G. M. (2008). Minute dosages of alpha(nu)beta3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 22, 2758– 2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmieder A. H., Caruthers S. D., Zhang H., Williams T. A., Robertson J. D., Wickline S. A., Lanza G. M. (2008). Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 22, 4179– 4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weissleder R., Bogdanov A., Jr, Tung C. H., Weinmann H. J. (2001). Size optimization of synthetic graft copolymers for in vivo angiogenesis imaging. Bioconjug. Chem. 12, 213– 219 [DOI] [PubMed] [Google Scholar]
- 18. Morawski A. M., Winter P. M., Crowder K. C., Caruthers S. D., Fuhrhop R. W., Scott M. J., Robertson J. D., Abendschein D. R., Lanza G. M., Wickline S. A. (2004). Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn. Reson. Med. 51, 480– 486 [DOI] [PubMed] [Google Scholar]
- 19. Meoli D. F., Sadeghi M. M., Krassilnikova S., Bourke B. N., Giordano F. J., Dione D. P., Su H., Edwards D. S., Liu S., Harris T. D., Madri J. A., Zaret B. L., Sinusas A. J. (2004). Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J. Clin. Invest. 113, 1684– 1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hood J. D., Cheresh D. A. (2002). Role of integrins in cell invasion and migration. Nat. Rev. Cancer. 2, 91– 100 [DOI] [PubMed] [Google Scholar]
- 21. Folkman J. (2002). Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29 (16), 15– 18 [DOI] [PubMed] [Google Scholar]
- 22. Huminiecki L., Bicknell R. (2000). In silico cloning of novel endothelial-specific genes. Genome Res. 10, 1796– 1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park K. W., Morrison C. M., Sorensen L. K., Jones C. A., Rao Y., Chien C. B., Wu J. Y., Urness L. D., Li D. Y. (2003). Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev. Biol. 261, 251– 267 [DOI] [PubMed] [Google Scholar]
- 24. Jones C. A., London N. R., Chen H., Park K. W., Sauvaget D., Stockton R. A., Wythe J. D., Suh W., Larrieu-Lahargue F., Mukouyama Y. S., Lindblom P., Seth P., Frias A., Nishiya N., Ginsberg M. H., Gerhardt H., Zhang K., Li D. Y. (2008). Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 14, 448– 453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Itoh A., Miyabayashi T., Ohno M., Sakano S. (1998). Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for Slit in the formation and maintenance of the nervous system. Brain Res. Mol. Brain Res. 62, 175– 186 [DOI] [PubMed] [Google Scholar]
- 26. Li H. S., Chen J. H., Wu W., Fagaly T., Zhou L., Yuan W., Dupuis S., Jiang Z. H., Nash W., Gick C., Ornitz D. M., Wu J. Y., Rao Y. (1999). Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 96, 807– 818 [DOI] [PubMed] [Google Scholar]
- 27. Ridgway J., Zhang G., Wu Y., Stawicki S., Liang W. C., Chanthery Y., Kowalski J., Watts R. J., Callahan C., Kasman I., Singh M., Chien M., Tan C., Hongo J. A., de Sauvage F., Plowman G., Yan M. (2006). Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 444, 1083– 1087 [DOI] [PubMed] [Google Scholar]
- 28. Noguera-Troise I., Daly C., Papadopoulos N. J., Coetzee S., Boland P., Gale N. W., Lin H. C., Yancopoulos G. D., Thurston G. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 444, 1032– 1037 [DOI] [PubMed] [Google Scholar]
- 29. Hellstrom M., Phng L. K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A. K., Karlsson L., Gaiano N., Yoon K., Rossant J., Iruela-Arispe M. L., Kalen M., Gerhardt H., Betsholtz C. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 445, 776– 780 [DOI] [PubMed] [Google Scholar]
- 30. Adams R., Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell. Biol. 8, 464– 478 [DOI] [PubMed] [Google Scholar]
- 31. Ellegala D. B., Leong-Poi H., Carpenter J. E., Klibanov A. L., Kaul S., Shaffrey M. E., Sklenar J., Lindner J. R. (2003). Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha (v) beta3. Circulation. 108, 336– 341 [DOI] [PubMed] [Google Scholar]
- 32. Mulder W. J., Strijkers G. J., Habets J. W., Bleeker E. J., van der Schaft D. W., Storm G., Koning G. A., Griffioen A. W., Nicolay K. (2005). MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 19, 2008– 2010 [DOI] [PubMed] [Google Scholar]
- 33. Lijowski M., Caruthers S., Hu G., Zhang H., Scott M. J., Williams T., Erpelding T., Schmieder A. H., Kiefer G., Gulyas G., Athey P. S., Gaffney P. J., Wickline S. A., Lanza G. M. (2008). High sensitivity: high-resolution SPECT-CT/MR molecular imaging of angiogenesis in the Vx2 mModel. Invest. Radiol. 43, 100– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winter P. M., Morawski A. M., Caruthers S. D., Fuhrhop R. W., Zhang H., Williams T. A., Allen J. S., Lacy E. K., Robertson J. D., Lanza G. M., Wickline S. A. (2003). Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 108, 2270– 2274 [DOI] [PubMed] [Google Scholar]
- 35. Winter P., Neubauer A., Caruthers S., Harris T., Robertson J., Williams T., Schmieder A., Hu G., Allen J., Lacy E., Wickline S., Lanza G. (2006). Endothelial alpha(nu)beta(3)-Integrin targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26, 2103– 2109 [DOI] [PubMed] [Google Scholar]
- 36. Yu X., Song S. K., Chen J., Scott M. J., Fuhrhop R. J., Hall C. S., Gaffney P. J., Wickline S. A., Lanza G. M. (2000). High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn. Reson. Med. 44, 867– 872 [DOI] [PubMed] [Google Scholar]
- 37. Neubauer A. M., Caruthers S. D., Hockett F. D., Cyrus T., Robertson J. D., Allen J. S., Williams T. D., Fuhrhop R. W., Lanza G. M., Wickline S. A. (2007). Fluorine cardiovascular magnetic resonance angiography in vivo at 1.5 T with perfluorocarbon nanoparticle contrast agents. J. Cardiovasc. Magn. Reson. 9, 565– 573 [DOI] [PubMed] [Google Scholar]
- 38. Caruthers S. D., Neubauer A. M., Hockett F. D., Lamerichs R., Winter P. M., Scott M. J., Gaffney P. J., Wickline S. A., Lanza G. M. (2006). In vitro demonstration using (19)F magnetic resonance to augment molecular imaging with paramagnetic perfluorocarbon nanoparticles at 1.5 tesla. Invest. Radiol. 41, 305– 312 [DOI] [PubMed] [Google Scholar]
- 39. Bottomley P. A. (1989). Human in vivo NMR spectroscopy in diagnostic medicine: clinical tool or research probe?. Radiology. 170, 1– 15 [DOI] [PubMed] [Google Scholar]
- 40. Lanza G. M., Yu X., Winter P. M., Abendschein D. R., Karukstis K. K., Scott M. J., Chinen L. K., Fuhrhop R. W., Scherrer D. E., Wickline S. A. (2002). Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: implications for rational therapy of restenosis. Circulation. 106, 2842– 2847 [DOI] [PubMed] [Google Scholar]
- 41. Ahrens E. T., Flores R., Xu H., Morel P. A. (2005). In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 23, 983– 987 [DOI] [PubMed] [Google Scholar]
- 42. Partlow K. C., Chen J., Brant J. A., Neubauer A. M., Meyerrose T. E., Creer M. H., Nolta J. A., Caruthers S. D., Lanza G. M., Wickline S. A. (2007). 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 21, 1647– 1654 [DOI] [PubMed] [Google Scholar]
- 43. Keupp J., Caruthers S., Rahmer J., Williams T., Wickline S., Lanza G. (2009). Fluorine-19 MR molecular imaging of angiogenesis on Vx-2 tumors in rabbits using ανβ3-targeted nanoparticles. Proc. Int. Soc. Magn. Reson. Med. 17, 223 [Google Scholar]
- 44. Keupp J., Mazurkewitz P. (2007). Simultaneous 19F/1H Imaging for quantification: calibration and sensitivity assessment. Proc. Int. Soc. Magn. Reson. Med. 15, 1334 [Google Scholar]
- 45. Keupp J., Mazurkewitz P., Frasslin, Schaeffter T. (2006). Simultaneous 19F and 1H imaging on a clinical 3T MR scanner. Proc. Int. Soc. Magn. Reson. Med. 15, 102 [Google Scholar]
- 46. Rahmer J., Keupp J., Caruthers S. (2008). Self-navigated motion compensation in simultaneous 19F/1H 3D radial imaging using golden means profile interleaving. Proc. Int. Soc. Magn. Reson. Med. 16, 1471 [Google Scholar]
- 47. Rahmer J., Keupp J., Caruthers S., Lips O., Williams T., Wickline S., Lanza G. (2009). Dual resolution simultaneous 19F/1H in vivo imaging of targeted nanoparticles. Proc. Int. Soc. Magn. Reson. Med. 17, 611 [Google Scholar]
- 48. Rahmer J., Keupp J., Caruthers S., Lips O., Williams T., Wickline S., Lanza G. (2009). 19F/1H Simultaneous 3D radial imaging of atherosclerotic rabbits using self-navigated respiratory motion compensation. Proc. Int. Soc. Magn. Reson. Med. 17, 4611 [Google Scholar]