Abstract
Class IA phosphatidylinositol 3-kinase (PI 3-kinase) is a key component of important intracellular signalling cascades. We have identified an adaptor protein, Rukl, which forms complexes with the PI 3-kinase holoenzyme in vitro and in vivo. This interaction involves the proline-rich region of Ruk and the SH3 domain of the p85α regulatory subunit of the class IA PI 3-kinase. In contrast to many other adaptor proteins that activate PI 3-kinase, interaction with Rukl substantially inhibits the lipid kinase activity of the enzyme. Overexpression of Rukl in cultured primary neurons induces apoptosis, an effect that could be reversed by co-expression of constitutively activated forms of the p110α catalytic subunit of PI 3-kinase or its downstream effector PKB/Akt. Our data provide evidence for the existence of a negative regulator of the PI 3-kinase signalling pathway that is essential for maintaining cellular homeostasis. Structural similarities between Ruk, CIN85 and CD2AP/CMS suggest that these proteins form a novel family of adaptor molecules that are involved in various intracellular signalling pathways.
Keywords: adaptor protein/neuronal apoptosis/phosphoinositide 3-kinase/signal transduction
Introduction
Signal transduction through inositol-containing lipids as second messengers is one of the most important signalling pathways in eukaryotic cells. 3′-OH phosphorylated derivatives of phosphatidylinositol are important phospholipid second messengers because they are directly involved in the regulation of multiple cell functions, including proliferation, differentiation, survival, migration, metabolic control, organization of the cytoskeleton and membrane trafficking. The production of these phospholipids is catalysed by phosphoinositide 3-kinases (PI 3-kinases), a subfamily of lipid kinases. In eukaryotic cells, there are three classes of PI 3-kinase that differ in their primary structure, regulation and substrate specificity (Domin and Waterfield, 1997; Vanhaesebroeck et al., 1997; Fruman et al., 1998). Phosphoinositides exert their action by recruiting effector proteins to intracellular membranes. This is achieved by the interaction of membrane-associated phosphoinositides with specific phospholipid-binding protein domains within the effector proteins. Two protein domains that differ in their specificities towards different phosphoinositides have been identified, namely the FYVE and pleckstrin homology (PH) domains.
The FYVE domain is specific for binding phosphatidylinositol trisphosphate (PtdIns3P) and is found in several proteins implicated in membrane trafficking (Stenmark et al., 1996; Patki et al., 1997, 1998; Burd and Emr, 1998; Gaullier et al., 1998; Fruman et al., 1999; Kutateladze et al., 1999; Misra and Hurley, 1999). PH domains are more widespread in proteins and demonstrate less sequence conservation than FYVE domains. Accord ingly, PH domains exhibit different affinities and binding specificities for inositol lipids (Salim et al., 1996; Rameh et al., 1997; Isakoff et al., 1998; Kavran et al., 1998; Fruman et al., 1999). From among >100 different eukaryotic proteins with PH domains, those that bind PtdIns(3,4)P2 and PtdIns(3,4,5)P3 preferentially have attracted special attention. This group includes serine/threonine protein kinases (PDK1 and PKB/Akt), non-receptor tyrosine kinases of the Tek family, phospholipase Cγ, guanine nucleotide exchange factors and GTPase-activating proteins. Phospholipid binding to the PH domain induces conformational changes and translocation of these normally cytosolic molecules to membranes where further activation takes place. The numerous downstream targets of these PH domain proteins contribute to the multiple effects of PtdIns(3,4)P2 and PtdIns(3,4,5)P3 on cell physiology (reviewed in Alessi and Cohen, 1998; Alessi and Downes, 1998; Downward, 1998a; Leevers et al., 1999; Rameh and Cantley, 1999; Vanhaesebroeck and Waterfield, 1999). The levels of these two phospholipids are very low in quiescent cells and can be increased by extracellular signals. This increase is achieved by activation of class I PI 3-kinases, a group of heterodimeric enzymes that are subdivided further into class IA and class IB.
The widely expressed class IA PI 3-kinases participate in signal transduction from both receptor and non-receptor tyrosine kinases. Delivery of inactive cytosolic class IA PI 3-kinases to the plasma membrane, where they gain access to their lipid substrates, requires the regulatory subunit that is encoded by one of three different genes: p85α, p85β or p55γ (reviewed in Wymann and Pirola, 1998; Vanhaesebroeck and Waterfield, 1999). The SH2 domain of the regulatory subunit binds to phosphorylated tyrosine residues within specific docking sites (YXXM) in the intracellular domain of receptor tyrosine kinases or in adaptor proteins. Although interaction of SH2 domains with phosphorylated targets initiates the activation of class IA PI 3-kinase, two other domains of the regulatory subunit have been implicated in modulating the lipid kinase activity of the holoenzyme. SH3 domain–proline-rich region interactions between the p85 regulatory subunit and certain adaptor proteins causes a conformational change in the p85–p110 holoenzyme (Gout et al., 1993; Liu et al., 1993; Prasad et al., 1993; Kapeller et al., 1994; Pleiman et al., 1994; Harrison-Findik et al., 1995; Wang et al., 1995; Mak et al., 1996; Soltoff and Cantley, 1996; Hunter et al., 1997). Although the molecular mechanism is not known, this conformational change increases the ability of the class IA PI 3-kinase to phosphorylate the 3′ position of the inositol ring.
While the mechanism of activation of the PtdIns(3,4)P2 and PtdIns(3,4,5)P3 signalling pathway by extracellular stimuli has become relatively well understood, much less is known about negative regulation of this pathway. The importance of negative regulation for normal cell physiology has been emphasized by the recent demonstration that the protein encoded by the tumour suppressor gene PTEN acts as a lipid phosphatase that dephosphorylates the 3′ position of PtdIns(3,4)P2 and PtdIns(3,4,5)P3, and as such acts as a functional antagonist of PI-3 kinases that produce these second messengers (Myers et al., 1997; Furnari et al., 1998; Haas-Kogan et al., 1998; Maehama and Dixon, 1998; Stambolic et al., 1998). The consequences of the loss of PTEN function are a constitutive activation of the PKB/Akt pathway (and probably other downstream pathways) that regulates cell growth and survival, which ultimately leads to development of neoplasia (reviewed in Cantley and Neel, 1999; Maehama and Dixon, 1999). However, by acting at the level of phospholipid products, PTEN negatively regulates all signalling pathways downstream of class I and at least some class II PI 3-kinases.
A few years ago, we isolated a clone (3E7) that encoded a novel SH3 domain protein (Akopian et al., 1996), later named Ruk (for regulator of ubiquitous kinase). Here we demonstrate that Ruk is the first known adaptor protein that is able to inhibit the lipid kinase activity of the class IA PI 3-kinase via specific binding to the p85α regulatory subunit. This inhibitory activity of Ruk could represent a novel mechanism for the fine regulation of the PI 3-kinase signalling pathway.
Results
Cloning of ruk cDNA and analysis of ruk mRNA expression
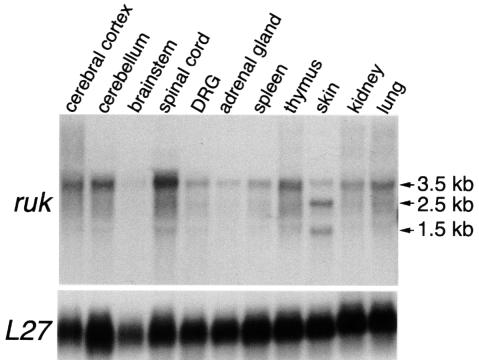
Using the insert of the original 3E7 clone (Akopian et al., 1996) as a probe for northern hybridization, a 3.5 kb transcript was detected in all tissues of newborn rats studied (Figure 1 and data not shown). In addition to the 3.5 kb (rukl) transcript, several other ruk transcripts were detected in some tissues; the most prominent of these were 2.5 (rukm) and 1.5 kb (ruks) transcripts in newborn and adult rat skin (Figure 1).
Fig. 1. Autoradiograms of a northern blot of total RNA isolated from newborn rat tissues hybridized with 32P-nick-translated ruk and L27 probes showing the relative levels of expression of ruk mRNAs in different tissues.
To isolate full-length clones that represent different ruk transcripts, newborn rat skin and cerebellum cDNA libraries were screened with a ruk probe. Sequence analysis of isolated clones demonstrated that rukl, rukm and ruks transcripts are generated by different promoter usage and alternative splicing in the coding or 5′-untranslated region (5′-UTR) regions (for details see DDBJ/EMBL/GenBank accession Nos AF255884 for ruks, AF255886 for rukm1, AF255887 for rukm3 and AF255888 for rukl).
Proteins coded by ruk mRNA
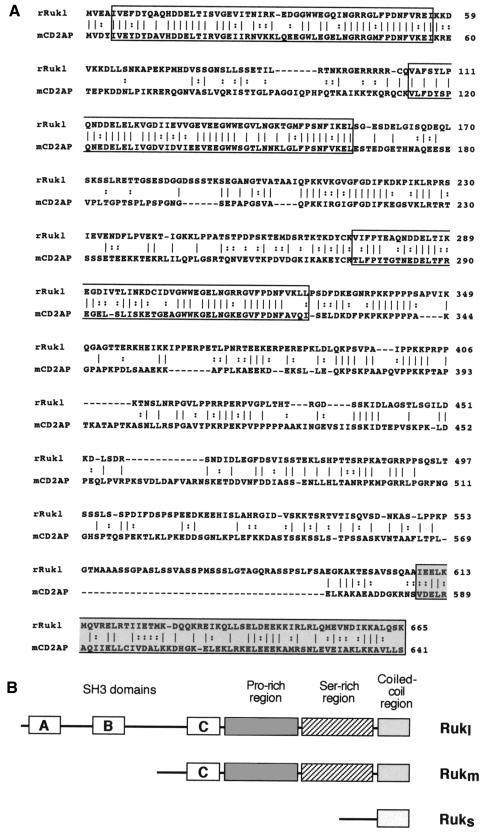
Analysis of ruk mRNA sequences showed that a set of proteins with various features is coded by the ruk gene. In all of the ruk mRNAs analysed, an upstream, in-frame termination codon was found and the first in-frame ATG codon was surrounded by a good Kozak consensus sequence (Kozak, 1989). The C-terminal region (shaded box in Figure 2) and serine-rich upstream region of the protein coded by rukl mRNA had no similarities to other proteins in data banks available at the time of cloning. Three typical SH3 domains (open boxes A, B and C in Figure 2) followed by a proline-rich region occupy most of the N-terminal half of the Rukl protein. This domain organization is very similar to that of the recently identified adaptor protein, mCD2AP/hCMS (Dustin et al., 1998; Kirsch et al., 1999). Direct alignment of mouse CD2A and rat Rukl amino acid sequences revealed 37% identity and 53% similarity between these two proteins (Figure 2A). Although the most similar regions are the SH3 domains, a putative coiled-coil C-terminal region is also well conserved. The major difference between CD2A and Rukl is the structure of a spacer between proline-rich and putative coiled-coil domains. This region of Ruk has twice as many serine residues as the corresponding region of CD2A. The proteins coded by rukm mRNAs possess only one SH3 domain (C, Figure 2B) but all downstream domains are the same as in the Rukl protein. In contrast, a short protein coded by ruks retains only the C-terminal coiled-coil region.
Fig. 2. (A) Alignment of the amino acid sequences of rat Rukl and mouse CD2AP proteins. The vertical bars (|) designate identical amino acids and the colons (:) designate similar amino acids. Gaps (–) were introduced for better alignment. SH3 domains A, B and C are boxed and the presumptive coiled-coil domain is shaded. (B) Schematic representation of the domain organization of three Ruk proteins.
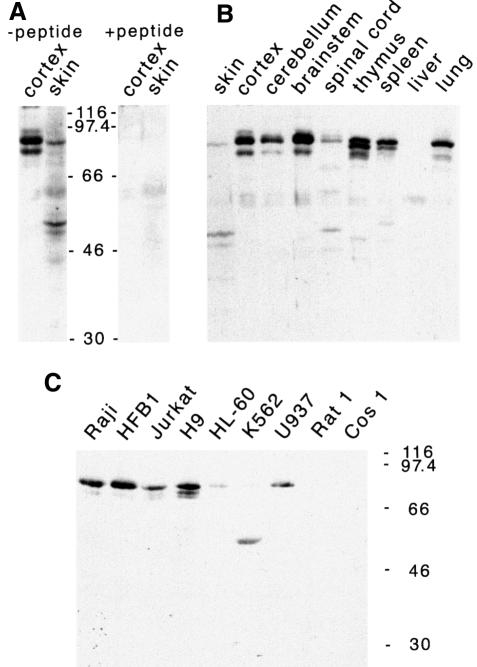
Antibodies were raised by immunizing rabbits with a C-terminal peptide that is common to all Ruk isoforms. Western blot analysis showed various Ruk isoforms in a variety of rat tissues (Figure 3A and B) at levels that correlated with the levels of the corresponding mRNAs shown in Figure 1. This antibody also detected Ruk proteins of similar sizes in human cell lines (Figure 3C). The mobility of the Rukl protein corresponded to a molecular mass of 85 kDa, which is much higher than that predicted from the amino acid sequence, suggesting the existence of post-translational modifications or a conformation that retards mobility. Pre-incubation of the antibody with the peptide used for immunization completely eliminated specific Ruk bands on western blotting (Figure 3A). Staining of cultured cells and cryosections of adult rat brain with anti-Ruk antibodies showed that Ruk is a cytoplasmic protein (data not shown).
Fig. 3. Detection of Ruk proteins in total protein extracted from adult rat tissues (A and B) and cell lines (C) by western blotting. (A) Shows elimination of specific bands after pre-incubation of the antibody with excess of the peptide used for immunization. The positions of molecular mass markers are shown.
Rukl binds to various SH3 domain proteins in vitro
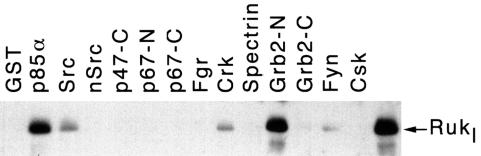
The structural organization of the Ruk protein suggests that it might interact with various molecules involved in signal transduction. To check this, we used a panel of SH3 domains expressed as glutathione S-transferase (GST) fusion proteins (Gout et al., 1993) to pull down recombinant Rukl protein produced in baculovirus. As shown in Figure 4, Rukl interacts in vitro with several SH3 domains, most strongly with the SH3 domain of the p85α regulatory subunit of PI 3-kinase and the N-terminal SH3 domain of Grb-2. Full-length Grb-2 and p85α GST fusion proteins were also able to interact with recombinant Rukl in vitro (data not shown). Because both Grb-2 and the p85α regulatory subunit of PI 3-kinase play important roles in signal transduction, we carried out further experiments to demonstrate interaction of these proteins with Rukl in vivo. Although the interaction between Rukl and p85α was clearly demonstrated in various experiments (see below), we were not able to detect complexes between Rukl and Grb2 in vivo (data not shown).
Fig. 4. Binding of Ruk to a panel of GST–SH3 domain fusion proteins. GST alone or GST–SH3 fusion proteins were coupled to glutathione–Sepharose beads and incubated with lysates of Sf9 cells, infected with baculovirus expressing Rukl. Bound proteins were analysed by western blotting with anti-Ruk antibodies. p47 and p67 are subunits of NADPH oxidase. The right lane contains recombinant Rukl, purified from Sf9 cells.
Rukl binds PI 3-kinase via SH3 domain of the p85α in Sf9 cells
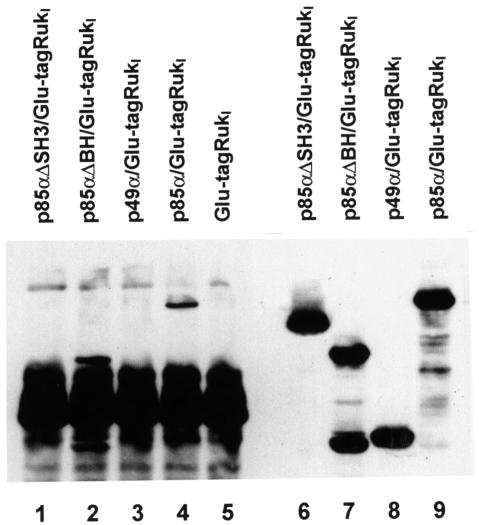
To study the interaction between Rukl and p85α in vivo, we co-infected Sf9 insect cells with baculoviruses expressing p85α and Glu-tagged Rukl. Anti-Glu-tag antibody was used for immunoprecipitation of complexes from Sf9 cell lysates and anti-p85α antibody for detection of the regulatory subunit in these immunoprecipitates. The results of this experiment (Figure 5, lane 4) clearly demonstrated that Rukl forms a stable complex with p85α when co-expressed in insect cells. In further experiments, deletion mutants of p85α were co-expressed with Rukl. Whereas deletion of the SH3 domain of p85α completely abolished interaction with Rukl, deletion of the BH domain (including proline-rich regions) did not affect this interaction (Figure 5). This confirms that the SH3 domain of p85α and the proline-rich region of Rukl are responsible for interaction of these proteins in vivo.
Fig. 5. Interaction of Rukl and p85α variants in Sf9 cells. Sf9 cells were infected with baculoviruses expressing Glu-tagged Rukl and either wild-type p85α or p85α deletion mutants as indicated. Rukl protein was immunoprecipitated with mouse monoclonal anti-Glu antibody. Western blotting with mouse monoclonal anti-p85α antibody was used to detect p85α in whole lysates of infected Sf9 cells (lanes 6–9) and immunoprecipitates (lanes 1–5).
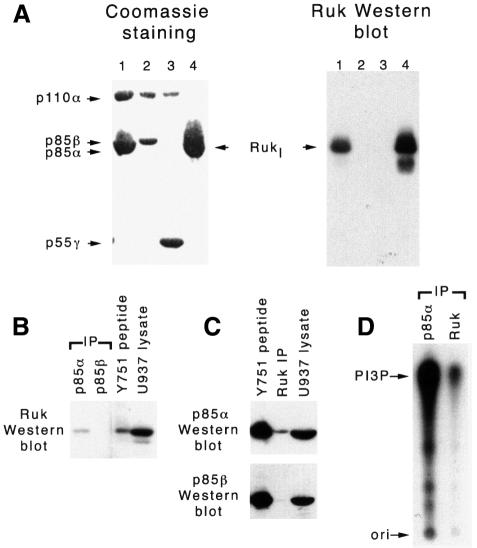
To demonstrate that Rukl forms a complex with the PI 3-kinase holoenzyme, we infected Sf9 cells with baculoviruses that express Rukl, the p110α catalytic subunit and one of the regulatory subunits (p85α, p85β or p55γ) of class IA PI 3-kinase. Complexes were pulled down from cell lysates by binding to a matrix-bound phosphotyrosine peptide of the platelet-drived growth factor-β (PDGF-β) receptor via the SH2 domains of the regulatory subunits as described in Materials and methods. SDS–polyacrylamide gel analysis of bound proteins and western blotting with anti-Ruk antibody revealed that although all three regulatory subunits form complexes with the p110α catalytic subunit in infected cells, only p85α recruits Rukl to the complex (Figure 6A).
Fig. 6. Interaction of Rukl with PI 3-kinase in vivo. (A) Specific association between Rukl and PI 3-kinase in Sf9 cells. Sf9 cells were infected with baculoviruses expressing Rukl alone (lane 4) or in combination with baculoviruses expressing p85α–p110α (lane 1), p85β–p110α (lane 2) and p55γ–p110α (lane 3). Cell lysates were incubated with Actigel-immobilized phosphotyrosine peptide (Y751) of the human PDGF-β receptor that is able to interact with SH2 domains of regulatory subunits of PI 3-kinase (lanes 1–3) or Actigel-immobilized proline-rich peptide that is able to interact with SH3 domains of Ruk (lane 4). Bound proteins were separated by SDS–PAGE and visualized by Coomassie staining or western blotting with anti-Ruk antibodies. (B–D) Association between Ruk and PI 3-kinase in U937 cells. U937 cell lysates were either incubated with Actigel-immobilized Y751 phosphopeptide or immunoprecipitated using monoclonal antibodies to p85α and β subunits of the PI3-kinase or polyclonal antibodies to Ruk. Proteins were analysed by western blotting with anti-Ruk (B) or anti-p85 (C) antibodies. (D) Results of the PI 3-kinase assays performed with anti-p85α or anti-Ruk immunoprecipitates from U937 cell lysates. The position of the phosphorylated product of the kinase reaction (PI3P) is shown by the arrow.
Rukl forms a complex with PI 3-kinase in mammalian cells
The human myelomonocytic leukaemia cell line U937 was used to study complexes of Rukl with PI 3-kinase in mammalian cells. The matrix-bound phosphotyrosine peptide and anti-p85α but not anti-p85β antibody co-precipitate Rukl from U937 cell lysates (Figure 6B). Consistently, the p85α but not the p85β regulatory subunit could be co-precipitated from U937 cell lysates using anti-Ruk antibody (Figure 6C), and lipid kinase activity could be detected in the immunoprecipitate (Figure 6D), suggesting the presence of the active catalytic subunit of PI 3-kinase.
Rukl inhibits lipid kinase activity of PI 3-kinase in vitro
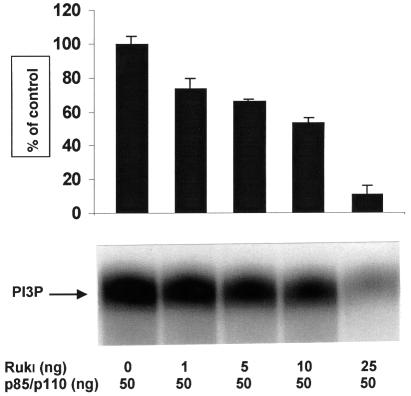
Rukl and p85α–p110α were affinity purified from Sf9 cells infected with the corresponding baculoviruses. A 50 ng aliquot of the PI 3-kinase holoenzyme was mixed with different amounts of recombinant Rukl and the lipid kinase assay was performed using phosphatidylinositol as substrate. The presence of Rukl substantially inhibited the activity of PI 3-kinase, with a maximum inhibition seen with an equimolar ratio of Rukl and PI 3-kinase in the reaction (Figure 7). Similar results were obtained when PtdIns(4,5)P2 was used as substrate in the lipid kinase assay (data not shown). Consistently, the lipid kinase activity in complexes pulled down from Sf9 cells co-expressing p85α–p110α–Rukl was lower than the activity in complexes from cells that expressed only p85α and p110α (data not shown).
Fig. 7. The effect of Rukl on PI 3-kinase activity in vitro. Rukl and p85α–p110α were affinity purified from Sf9 cells infected with the corresponding recombinant baculoviruses. Purified recombinant Rukl and p85α–p110α were mixed in the indicated ratios and PI 3-kinase assay was performed as described in Materials and methods. A phosphoimage of a representative thin-layer chromatogram is shown. The bar chart shows the combined results of three independent experiments.
Modulating Ruk expression affects neuronal survival
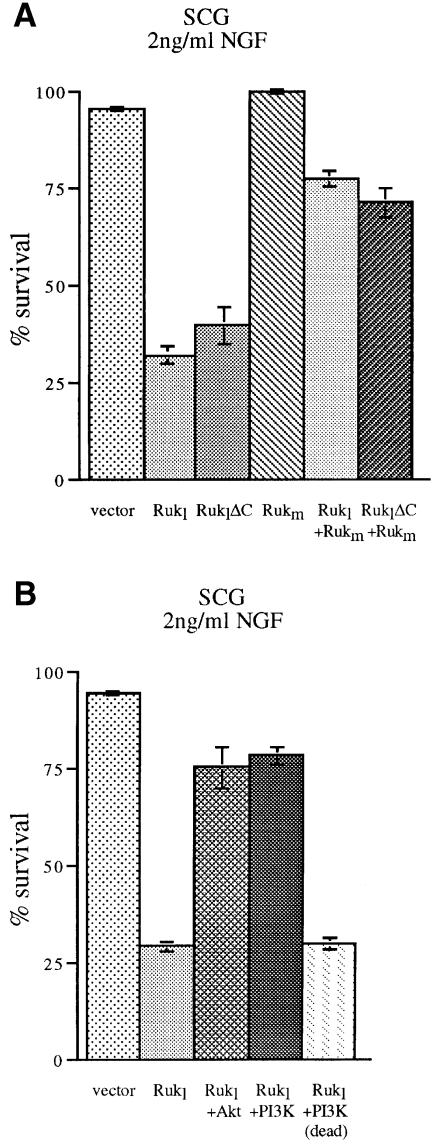
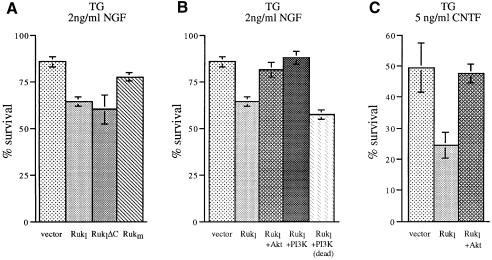
The ability of Rukl to inhibit the p85α-dependent activity of class IA PI 3-kinase suggests that overexpression of this protein in cells that utilize the PI 3-kinase signalling pathway should seriously compromise their physiology. Early post-natal sympathetic and sensory neurons survive in culture only in the presence of certain neurotrophic factors, and the PI 3-kinase pathway is necessary for efficient transduction of survival signals in these cells (Klesse and Parada, 1998). Thus, we used this well established experimental paradigm to investigate the potential effect of Ruk on cell survival. Ruk-expression plasmids were microinjected into the nuclei of cultured primary sensory neurons of the post-natal day 1 (P1) mouse trigeminal ganglion (TG) or sympathetic neurons of the P1 mouse superior cervical ganglion (SCG). At this stage of development, these neurons survive in medium containing nerve growth factor (NGF). TG neurons also survive in the presence of ciliary neurotrophic factor (CNTF) and rapidly die in the absence of these factors. Neurons were incubated in the presence of NGF or CNTF and injected with expression plasmids as described in our previous publications (Allsopp et al., 1993; Ninkina et al., 1996; Buj-Bello et al., 1997). The number of surviving neurons was counted 48 h later and expressed as a percentage of the number counted 2 h after injection (between 50 and 100 cells per expression plasmid per culture dish). A substantial reduction of survival was evident in neurons microinjected with a plasmid expressing the Rukl protein as compared with neurons injected with the empty expression vector plasmid (Figures 8 and 9). Staining the cultures 24 h after microinjection using the TUNEL technique to detect DNA fragmentation demonstrated that a proportion of Rukl-injected cells undergo apoptotic death (data not shown). In the presence of NGF, this effect was much more pronounced in SCG neurons (Figure 8) than in TG neurons (Figure 9). The survival-inhibiting effect of Rukl is dependent on the N-terminal part of the protein that contains the first two SH3 domains because a reduction in neuronal survival was observed with an expression plasmid that has a deletion of the C-terminal part of the Rukl protein, but was not observed with plasmids expressing Rukm, which does not contain the first two SH3 domains (Figures 8A and 9A).
Fig. 8. Ruk affects survival of sympathetic neurons in culture. P1 mouse SCG neurons were plated in medium containing NGF and injected with expression plasmids. The number of surviving neurons was counted 48 h later and is expressed as a percentage of the number counted 2 h after injection. For each condition, the means and SEM of at least three independent experiments are shown. (A) Overexpression of Rukl or RuklΔC results in reduced neuronal survival. Rukm does not reduce survival and in co-expression experiments abrogates the effect of Rukl or RuklΔC. (B) Reversal of the effect of Rukl overexpression by co-expression of constitutively activated forms of PKB/Akt kinase and the p110α catalytic subunit of PI 3-kinase but not functionally compromised (dead) p110α.
Fig. 9. Ruk affects survival of sensory neurons in culture. P1 mouse TG neurons were plated in medium containing NGF (A and B) or CNTF (C) and injected with expression plasmids. The number of surviving neurons was counted 48 h later and is expressed as a percentage of the number counted 2 h after injection. For each condition, the means and SEM of at least three independent experiments are shown. (A and C) Overexpression of Rukl or RuklΔC but not Rukm results in reduced neuronal survival. (B and C) Reversal of the effect of Rukl overexpression by co-expression of constitutively activated forms of PKB/Akt kinase and the p110α catalytic subunit of PI 3-kinase but not functionally compromised (dead) p110α.
To ascertain if Rukl kills neurons by inhibiting the PI 3-kinase signalling pathway, we carried out co-injection experiments. In cultures containing either NGF or CNTF, the survival-inhibitory effect of Rukl overexpression was prevented by co-injecting plasmids expressing the constitutively activated form of either PKB/Akt or the p110α catalytic subunit of PI 3-kinase (Figures 8B, 9B and 9C). However, overexpression of a non-functional form of the PI 3-kinase catalytic subunit did not significantly affect the survival-inhibitory effect of Rukl overexpression (Figures 8B and 9C).
We also studied the survival of SCG neurons co-expressing Rukm and either Rukl or RuklΔC. As shown in Figure 8A, more than half of the neurons could be rescued from Rukl-induced death by overexpression of the Rukm isoform. This result suggests that Rukm could function as a dominant-negative regulator of Rukl.
Discussion
Signalling pathways involving PI 3-kinase and its phosphoinositide products modulate numerous aspects of cell physiology and are subject to complex multilevel regulation. This is particularly important for class IA PI 3-kinases, which are expressed in the majority of mammalian tissues and become activated in response to many extracellular signals. The lipid kinase activity of all three known types of catalytic p110 subunit (α, β and δ) is stimulated by binding of one of the regulatory subunits, products of three different genes, p85α, p85β or p55γ (reviewed in Wymann and Pirola, 1998; Vanhaesebroeck and Waterfield, 1999). This interaction involves the inter-SH2 region of the regulatory subunit and the N-terminal region of the catalytic subunit, and results in a conformational change of the substrate-binding site (Klippel et al., 1993, 1994; Dhand et al., 1994; Holt et al., 1994). Interactions of other domains of the regulatory subunit with receptors and adaptor proteins could augment this conformational change further and increase the lipid kinase activity of the complex. In addition to these structural modifications, interactions between PI 3-kinase subunits and membrane-associated receptors or adaptor proteins have another important function in vivo, namely translocation of the enzyme to the membrane where the lipid substrates are localized (reviewed in Wymann and Pirola, 1998; Leevers et al., 1999; Rameh and Cantley, 1999). These interactions are mediated by the SH2 domains of the regulatory subunit and specific phosphotyrosine motifs in the intracellular domains of receptors and adaptor proteins (Zhou and Cantley, 1995). However, the multiplicity and variety of protein-binding domains in both subunits of PI 3-kinase imply that this enzyme could form various multiprotein complexes in the cytoplasm or on the surface of intracellular membranes. The composition of these complexes could have a substantial effect on the enzymatic activity and, therefore, on the cellular responses that are regulated via the PI 3-kinase pathway. Indeed, many proteins that increase PI3 lipid kinase activity by interaction with the SH3 or proline-rich domains of the regulatory subunit have been identified (Gout et al., 1993; Liu et al., 1993; Prasad et al., 1993; Kapeller et al., 1994; Pleiman et al., 1994; Harrison-Findik et al., 1995; Wang et al., 1995; Mak et al., 1996; Soltoff and Cantley, 1996; Hunter et al., 1997). However, proteins that down-regulate class IA PI3 lipid kinase activity by binding to the regulatory subunit have not been reported previously.
We described a novel protein, Rukl, which has a domain organization typical of adaptor proteins and acts as a negative regulator of class IA PI 3-kinase. A 10-fold decrease in in vitro lipid kinase activity was observed when equimolar amounts of Rukl were added to p85α–p110α complexes in a test reaction. Results of our in vitro and in vivo experiments suggest that the molecular basis of this inhibition is the formation of a complex between three proteins. In such complexes, Rukl is bound, via its proline-rich region, to the SH3 domain of p85α, although different effects of Rukl and its truncated isoform, Rukm, on cell survival hint at the importance of two N-terminal SH3 domains. It is possible that interaction of p85α with Rukl but not with Rukm induces conformational changes in the active site of the catalytic subunit that compromise its ability to phosphorylate lipid substrates.
The presence of Rukl–PI 3-kinase complexes in mammalian cells suggests that at least one of the possible biological functions of Rukl is the negative regulation of the PI 3-kinase signalling pathway. It is well established that the PI 3-kinase signalling pathway plays an important role in mediating the survival effects of several growth factors (Hemmings, 1997; Kaplan and Miller, 1997; Marte and Downward, 1997; Downward, 1998a,b). In certain types of cells, inhibition of this pathway by wortmannin, LY294002 or dominant-negative PI 3-kinase isoforms results in apoptotic cell death and, conversely, activation of PI 3-kinase or downstream effector PKB/Akt rescues cells from apoptosis (Khwaja and Downward, 1997; Khwaja et al., 1997; Philpott et al., 1997). We reasoned that if Rukl was a negative regulator of PI 3-kinase in vivo, its overexpression in cells should result in apoptotic death. To check this, we chose a well characterized system, primary cultures of sympathetic (SCG) and sensory (TG) neonatal mouse neurons that depend on NGF for survival. Previous work has shown that PI 3-kinase subunits are expressed in both neuronal populations (Bartlett et al., 1999) and that the PI3 signalling pathway is involved in transduction of the NGF survival signal (Carter and Downes, 1992; Soltoff et al., 1992; Bartlett et al., 1997; Klesse and Parada, 1998; Klesse et al., 1999). When Rukl was overexpressed in these neurons, a substantial proportion of them died by an apoptotic mechanism. This effect could be prevented by co-expression of constitutively activated forms of the p110α catalytic subunit of PI 3-kinase or its downstream effector PKB/Akt. Consistent with an earlier observation that the PI 3-kinase pathway is more important for survival of sympathetic than sensory neurons (Bartlett et al., 1997), we found that the pro-apoptotic effect of Rukl is much more pronounced in SCG than in TG cultures. We also studied the effect of Rukl overexpression on the survival of TG neurons in the presence of another neurotrophic factor, CNTF, which also provides trophic support for these neurons. CNTF, as well as other cytokines that use the gp130 receptor subunit for transduction of the signal through the plasma membrane, primarily activate the JAK–STAT intracellular signalling pathway. However, in various cell types, downstream activation of PI 3-kinase is required for effective prevention of apoptosis and other effects of these cytokines (Bonni et al., 1997; Oh et al., 1998; Al-Shami and Naccache, 1999). Accordingly, we observed a substantial decrease in the number of trigeminal neurons surviving after overexpression of Rukl in the presence of CNTF. This effect could also be blocked by co-expression of constitutively activated PKB/Akt. Taken together, our microinjection results support the hypothesis that Rukl is an intrinsic negative regulator of PI 3-kinase in vivo.
The pro-apoptotic effect of Rukl does not require the function of the C-terminal coiled-coil domain of the protein. This domain has substantial homology with the C-terminal domain of CD2AP/CMS, which is involved in dimerization of this adaptor protein (Kirsch et al., 1999). However, two N-terminal SH3 domains of Rukl that are not directly involved in the interaction with p85α are still necessary for induction of apoptosis because the Rukm isoform that lacks these domains does not have a pro-apoptotic effect. Overexpression of Rukm is not sufficient for prevention of neuronal apoptosis following neurotrophic factor deprivation; however, it rescues neurons from the apoptosis induced by Rukl or RuklΔC. We propose that Rukm competes with Rukl for binding to the SH3 domain of p85α and by this means acts as a dominant-negative regulator of Rukl.
So far, only the effects of Ruk proteins on cell survival have been studied. However, one might expect that Ruk would play a role in regulating other cell functions because the downstream effects of PI 3-kinase are multiple and diverse. Moreover, the presence of several protein–protein binding domains in the Ruk molecule together with our in vitro binding data imply that p85α is not the only interaction partner of Ruk in vivo. In a recent study (Take et al., 2000), published when this manuscript was in preparation, it was shown that a human protein named CIN85, which is possibly a human orthologue of Rukl, is able to bind c-Cbl, an SH2 domain adaptor protein that negatively regulates receptor tyrosine kinases by induction of their ubiquitination. This interaction involves the proline-rich region of c-Cbl and the SH3 domains of CIN85, and is enhanced by epidermal growth factor stimulation of 293 cells. Taken together, these data suggest that Rukl/CIN85 is an adaptor protein involved in multiple intracellular signalling cascades.
The domain organization of Rukl is identical to that of the recently identified adaptor protein CD2AP/CMS (Dustin et al., 1998; Kirsch et al., 1999). The similarity is not restricted to the general organization of the protein molecule; CD2AP/CMS is also the only known protein that has amino acid homology to Ruk outside the SH3 and proline-rich domains, namely in the C-terminal coiled-coil domain. CD2AP/CMS is involved in the orchestration of receptor patterning and rearrangements of the cytoskeleton, and is required for formation of a specialized junction between the T cell and the antigen-presenting cell (Dustin et al., 1998), and another specialized junction, the slit diaphragm, in kidney glomerular epithelial cells (Shih et al., 1999). It has also been shown that human CD2AP, known as CMS, interacts with the focal adhesion protein p130Cas and co-localizes with it and F-actin to membrane ruffles and leading edges of cells (Kirsch et al., 1999). In contrast to its interaction with the CD2 receptor, which requires the N-terminal SH3 domain of CD2AP, interaction with p130Cas requires the proline-rich region of CD2AP/CMS, which binds to the SH3 domain of p130Cas. Association of CD2AP/CMS with tyrosine kinases Fyn and Yes in vivo has also been demonstrated (Kirsch et al., 1999). These results reflect a complex pattern of protein–protein interactions that involve members of the novel family of adaptor proteins to which Ruk could be assigned. Although we do not have evidence of physical or functional interaction of Rukl protein with the cell cytoskeleton, it is possible that further studies will reveal such interactions in certain types of cells. Likewise, it is possible that CD2AP/CMS could have an effect on PI 3-kinase, as it binds in vitro to the SH3 domain of p85 regulatory subunit (Kirsch et al., 1999).
Only a single transcript coding for full-length CD2AP/CMS has been found in all human and mouse tissues studied so far. In contrast, the ruk gene generates a set of transcripts, some of which are expressed only in certain tissues. Sequence analysis of several such transcripts has shown that they are products of alternative splicing and different promoter usage. The proteins encoded by these ruk transcripts have a common C-terminal region but are truncated in their N-termini. Therefore, in addition to the full-length Rukl protein, which has a domain organization identical to that of CD2AP/CMS, Ruk isoforms with single SH3 and proline-rich domains, and isoforms without SH3 and proline-rich domains could be detected in certain tissues and cell lines. Our results clearly demonstrate that Ruk isoforms could have different functions in vivo, and it will be important to clarify these functions in further studies.
Materials and methods
Miscellaneous procedures
RNA extraction, isolation of RNA, preparation of hybridization probes, northern hybridization, library screening and plasmid sequencing were performed as described earlier (Buchman et al., 1992, 1994). An adult rat skin cDNA library in the λZAPII vector was constructed using a kit from Stratagene.
Anti-Ruk antibody and protein detection
Rabbits were immunized with the 17mer C-terminal peptide of rat Ruk (CRLQMEVNDIKKALQSK) conjugated to keyhole limpet haemocyanin (Calbiochem) activated by 3-maleimidobenzoic acid N-hydroxysuccinimide ester (Sigma). Monospecific antibody was purified from the antisera by affinity chromatography using the antigen bound to N-hydroxysuccinimide-activated columns (Supelco) and used at dilutions of 1:500 for western blot/ECL detection of Ruk in total cell protein samples. Protein extraction and western blotting/ECL detection were carried out as described earlier (Buchman et al., 1998). In some experiments, 10 ml of diluted antibody was pre-incubated with 15 µg of recombinant Ruk protein at room temperature for 2 h.
Tissue culture and microinjection techniques
Human leukaemia cell lines Raji, HFB1, Jurkat 6, H9, HL-60, K562 and U937 were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS). Rat1 and Cos1 fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS.
Purified P1 mouse TG or SCG neurons were grown on a polyornithine/laminin substratum in defined medium in the presence of 2 ng/ml NGF. For some experiments, TG neurons were grown in the presence of 5 ng/ml CNTF. At 48 h after plating, expression plasmids (pRc/CMV vector only, ruk cDNAs in pRc/CMV, Akt/PKB and PI 3-kinase cDNAs in pSG5) were injected into the nucleus of the neurons as described previously (Allsopp et al., 1993; Ninkina et al., 1996; Buj-Bello et al., 1997). Expression plasmids for constitutively activated PKB/Akt kinase, p110 PI 3-kinase and the functionally compromised (dead) form of PI 3-kinase were described earlier (Khwaja and Downward, 1997; Khwaja et al., 1997). The cultures were supplemented with 2 ng/ml fresh NGF or 5 ng/ml fresh CNTF and the starting number of neurons in each experimental condition was counted 2 h after injection. The number of surviving neurons was counted 48 h later and expressed as a percentage of the starting number.
Baculovirus expression and affinity purification of recombinant proteins
Full-length coding sequences of Rukl or Rukm, containing a Glu tag epitope at the N-terminus, were subcloned into pBlueBac4 transfer vector (Invitrogen). Propagation and transfection of Sf9 cells, isolation of recombinant viruses and infection of insect cells were performed according to the manufacturer’s recommendations. Recombinant Glu-tagged Ruk proteins were purified by affinity chromatography on a protein G–Sepharose/anti-Glu tag monoclonal antibody matrix. Elution of specifically associated proteins was carried out in the presence of 50 mg/ml Glu tag peptide. Recombinant baculoviruses that express PI 3-kinase subunits were described earlier (Gout et al., 1992; Harpur et al., 1999).
Protein–protein interaction studies
Lysates of baculovirus-infected Sf9 cells or human promonocytic U937 leukaemia cells were incubated with Actigel beads containing an immobilized 17 amino acid phosphotyrosine peptide whose sequence is based on that surrounding Tyr751 of the human PDGF-β receptor (Fry et al., 1992; Panayotou et al., 1992). After extensive washing with cell lysis buffer, bound proteins were eluted by boiling the beads in SDS–PAGE sample buffer. These proteins were separated on 8% gels and were visualized either by Coomassie staining or by western blotting using specific antibodies. Proteins were also immunoprecipitated from cell lysates using specific antibodies, and protein G–Sepharose was used to bring down the immune complexes.
PI 3-kinase assay
The assay of PI 3-kinase activity was performed essentially as described previously (Whitman et al., 1985). Lipid kinase assays contained 2 mM MgCl2, 1 mM ATP, 20 µCi of [γ-32P]ATP and 200 µg/ml phosphatidylinositol. Extracted phospholipids were separated by thin-layer chromatography in 65% 1-propanol, 0.7 M acetic acid, 50 mM phosphoric acid. The level of radioactivity in radiolabelled phospholipids was measured by phosphoimager (Bio-Rad). PI 3-kinase assay in immunoprecipitates was carried out as described previously (Kilgour et al., 1996).
Acknowledgments
Acknowledgements
We are grateful to J.Downward for PI 3-kinase and Akt/PKB expression plasmids, to G.Nuñez for the BclXl expression plasmid, and to E.Borthwick, P.Parker and P.Shepherd for critical reading of the manuscript. Our thanks to Liz Delaney for technical assistance and Alex Houston for automatic DNA sequencing. This work was supported by grants from The Wellcome Trust and the Russian Government.
References
- Akopian A.N., Baka,I.D., Chestkov,A.V., Bukhman,V.L. and Georgiev,G.P. (1996) Cloning of genes, differentially expressed in the cerebellum during postnatal rat development. Genetika, 32, 886–895. [PubMed] [Google Scholar]
- Alessi D.R. and Cohen,P. (1998) Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev., 8, 55–62. [DOI] [PubMed] [Google Scholar]
- Alessi D.R. and Downes,C.P. (1998) The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta, 1436, 151–164. [DOI] [PubMed] [Google Scholar]
- Allsopp T.E., Wyatt,S., Paterson,H.F. and Davies,A.M. (1993) The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell, 73, 295–307. [DOI] [PubMed] [Google Scholar]
- Al-Shami A. and Naccache,P.H. (1999) Granulocyte–macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J. Biol. Chem., 274, 5333–5338. [DOI] [PubMed] [Google Scholar]
- Bartlett S.E., Reynolds,A.J., Weible,M., Heydon,K. and Hendry,I.A. (1997) In sympathetic but not sensory neurones, phosphoinositide-3 kinase is important for NGF-dependent survival and the retrograde transport of 125I-βNGF. Brain Res., 761, 257–262. [DOI] [PubMed] [Google Scholar]
- Bartlett S.E., Reynolds,A.J., Tan,T., Heydon,K. and Hendry,I.A. (1999) Differential mRNA expression and subcellular locations of PI3-kinase isoforms in sympathetic and sensory neurons. J. Neurosci. Res., 56, 44–53. [DOI] [PubMed] [Google Scholar]
- Bonni A., Sun,Y., Nadal-Vicens,M., Bhatt,A., Frank,D.A., Rozovsky,I., Stahl,N., Yancopoulos,G.D. and Greenberg,M.E. (1997) Regulation of gliogenesis in the central nervous system by the JAK–STAT signaling pathway. Science, 278, 477–483. [DOI] [PubMed] [Google Scholar]
- Buchman V.L., Ninkina,N.N., Bogdanov,Y.D., Bortvin,A.L., Akopian,H.N., Kiselev,S.L., Krylova,O.Y., Anokhin,K.V. and Georgiev,G.P. (1992) Differential splicing creates a diversity of transcripts from a neurospecific developmentally regulated gene encoding a protein with new zinc-finger motifs. Nucleic Acids Res., 20, 5579–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman V.L., Sporn,M. and Davies,A.M. (1994) Role of transforming growth factor-β isoforms in regulating the expression of nerve growth factor and neurotrophin-3 mRNA levels in embryonic cutaneous cells at different stages of development. Development, 120, 1621–1629. [DOI] [PubMed] [Google Scholar]
- Buchman V.L., Hunter,H.J.A., Pinõn,L.G.P., Thompson,J., Privalova,E.M., Ninkina,N.N. and Davies,A.M. (1998) Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J. Neurosci., 18, 9335–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buj-Bello A., Adu,J., Pinon,L.G.P., Horton,A., Thompson,J., Rosenthal,A., Chinchetru,M., Buchman,V.L. and Davies,A.M. (1997) Neurturin responsiveness requires a GPI-linked receptor plus the Ret receptor tyrosine kinase. Nature, 387, 721–724. [DOI] [PubMed] [Google Scholar]
- Burd C.G. and Emr,S.D. (1998) Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell, 2, 157–162. [DOI] [PubMed] [Google Scholar]
- Cantley L.C. and Neel,B.G. (1999) New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. USA, 96, 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.N. and Downes,C.P. (1992) Phosphatidylinositol 3-kinase is activated by nerve growth factor and epidermal growth factor in PC12 cells. J. Biol. Chem., 267, 14563–14567. [PubMed] [Google Scholar]
- Dhand R. et al. (1994) PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J., 13, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J. and Waterfield,M.D. (1997) Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett., 410, 91–95. [DOI] [PubMed] [Google Scholar]
- Downward J. (1998a) Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol., 10, 262–267. [DOI] [PubMed] [Google Scholar]
- Downward J. (1998b) Ras signalling and apoptosis. Curr. Opin. Genet. Dev., 8, 49–54. [DOI] [PubMed] [Google Scholar]
- Dustin M.L. et al. (1998) A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell, 94, 667–677. [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Meyers,R.E. and Cantley,L.C. (1998) Phosphoinositide kinases. Annu. Rev. Biochem., 67, 481–507. [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Rameh,L.E. and Cantley,L.C. (1999) Phosphoinositide binding domains: embracing 3-phosphate. Cell, 97, 817–820. [DOI] [PubMed] [Google Scholar]
- Fry M.J., Panayotou,G., Dhand,R., Ruiz-Larrea,F., Gout,I., Nguyen,O., Courtneidge,S.A. and Waterfield,M.D. (1992) Purification and characterization of a phosphatidylinositol 3-kinase complex from bovine brain by using phosphopeptide affinity columns. Biochem. J., 288, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari F.B., Huang,H.J. and Cavenee,W.K. (1998) The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res., 58, 5002–5008. [PubMed] [Google Scholar]
- Gaullier J.M., Simonsen,A., D’Arrigo,A., Bremnes,B., Stenmark,H. and Aasland,R. (1998) FYVE fingers bind PtdIns3P. Nature, 394, 432–433. [DOI] [PubMed] [Google Scholar]
- Gout I., Dhand,R., Panayotou,G., Fry,M.J., Hiles,I., Otsu,M. and Waterfield,M.D. (1992) Expression and characterization of the p85 subunit of the phosphatidylinositol 3-kinase complex and a related p85β protein by using the baculovirus expression system. Biochem. J., 288, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout I. et al. (1993) The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell, 75, 25–36. [PubMed] [Google Scholar]
- Haas-Kogan D., Shalev,N., Wong,M., Mills,G., Yount,G. and Stokoe,D. (1998) Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol., 8, 1195–1198. [DOI] [PubMed] [Google Scholar]
- Harpur A.G., Layton,M.J., Das,P., Bottomley,M.J., Panayotou,G., Driscoll,P.C. and Waterfield,M.D. (1999) Intermolecular interactions of the p85α regulatory subunit of phosphatidylinositol 3-kinase. J. Biol. Chem., 274, 12323–12332. [DOI] [PubMed] [Google Scholar]
- Harrison-Findik D., Susa,M. and Varticovski,L. (1995) Association of phosphatidylinositol 3-kinase with SHC in chronic myelogeneous leukemia cells. Oncogene, 10, 1385–1391. [PubMed] [Google Scholar]
- Hemmings B.A. (1997) Akt signaling: linking membrane events to life and death decisions. Science, 275, 628–630. [DOI] [PubMed] [Google Scholar]
- Holt K.H., Olson,L., Moye-Rowley,W.S. and Pessin,J.E. (1994) Phosphatidylinositol 3-kinase activation is mediated by high-affinity interactions between distinct domains within the p110 and p85 subunits. Mol. Cell. Biol., 14, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S., Koch,B.L. and Anderson,S.M. (1997) Phosphorylation of cbl after stimulation of Nb2 cells with prolactin and its association with phosphatidylinositol 3-kinase. Mol. Endocrinol., 11, 1213–1222. [DOI] [PubMed] [Google Scholar]
- Isakoff S.J., Cardozo,T., Andreev,J., Li,Z., Ferguson,K.M., Abagyan,R., Lemmon,M.A., Aronheim,A. and Skolnik,E.Y. (1998) Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J., 17, 5374–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R., Prasad,K.V., Janssen,O., Hou,W., Schaffhausen,B.S., Rudd,C.E. and Cantley,L.C. (1994) Identification of two SH3-binding motifs in the regulatory subunit of phosphatidylinositol 3-kinase. J. Biol. Chem., 269, 1927–1933. [PubMed] [Google Scholar]
- Kaplan D.R. and Miller,F.D. (1997) Signal transduction by the neurotrophin receptors. Curr. Opin. Cell Biol., 9, 213–221. [DOI] [PubMed] [Google Scholar]
- Kavran J.M., Klein,D.E., Lee,A., Falasca,M., Isakoff,S.J., Skolnik,E.Y. and Lemmon,M.A. (1998) Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J. Biol. Chem., 273, 30497–30508. [DOI] [PubMed] [Google Scholar]
- Khwaja A. and Downward,J. (1997) Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J. Cell Biol., 139, 1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A., Rodriguez-Viciana,P., Wennstrom,S., Warne,P.H. and Downward,J. (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J., 16, 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgour E., Gout,I. and Anderson,N.G. (1996) Requirement for phosphoinositide 3-OH kinase in growth hormone signalling to the mitogen-activated protein kinase and p70s6k pathways. Biochem. J., 315, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch K.H., Georgescu,M.M., Ishimaru,S. and Hanafusa,H. (1999) CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl Acad. Sci. USA, 96, 6211–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesse L.J. and Parada,L.F. (1998) p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J. Neurosci., 18, 10420–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesse L.J., Meyers,K.A., Marshall,C.J. and Parada,L.F. (1999) Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene, 18, 2055–2068. [DOI] [PubMed] [Google Scholar]
- Klippel A., Escobedo,J.A., Hu,Q. and Williams,L.T. (1993) A region of the 85-kilodalton (kDa) subunit of phosphatidylinositol 3-kinase binds the 110-kDa catalytic subunit in vivo. Mol. Cell. Biol., 13, 5560–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A., Escobedo,J.A., Hirano,M. and Williams,L.T. (1994) The interaction of small domains between the subunits of phosphatidylinositol 3-kinase determines enzyme activity. Mol. Cell. Biol., 14, 2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. (1989) The scanning model for translation: an update. J. Cell Biol., 108, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze T.G., Ogburn,K.D., Watson,W.T., de Beer,T., Emr,S.D., Burd,C.G. and Overduin,M. (1999) Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol. Cell, 3, 805–811. [DOI] [PubMed] [Google Scholar]
- Leevers S.J., Vanhaesebroeck,B. and Waterfield,M.D. (1999) Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol., 11, 219–225. [DOI] [PubMed] [Google Scholar]
- Liu X., Marengere,L.E., Koch,C.A. and Pawson,T. (1993) The v-Src SH3 domain binds phosphatidylinositol 3′-kinase. Mol. Cell. Biol., 13, 5225–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T. and Dixon,J.E. (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem., 273, 13375–13378. [DOI] [PubMed] [Google Scholar]
- Maehama T. and Dixon,J.E. (1999) PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol., 9, 125–128. [DOI] [PubMed] [Google Scholar]
- Mak P., He,Z. and Kurosaki,T. (1996) Identification of amino acid residues required for a specific interaction between Src-tyrosine kinase and proline-rich region of phosphatidylinositol-3′ kinase. FEBS Lett., 397, 183–185. [DOI] [PubMed] [Google Scholar]
- Marte B.M. and Downward,J. (1997) PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci., 22, 355–358. [DOI] [PubMed] [Google Scholar]
- Misra S. and Hurley,J.H. (1999) Crystal structure of a phosphatidylinositol 3-phosphate-specific membrane-targeting motif, the FYVE domain of Vps27p. Cell, 97, 657–666. [DOI] [PubMed] [Google Scholar]
- Myers M.P., Stolarov,J.P., Eng,C., Li,J., Wang,S.I., Wigler,M.H., Parsons,R. and Tonks,N.K. (1997) P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl Acad. Sci. USA, 94, 9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkina N., Adu,J., Fisher,A., Pinon,L.G.P., Buchman,V.L. and Davies,A.M. (1996) Expression and function of TrkB variants in developing sensory neurons. EMBO J., 15, 6385–6393. [PMC free article] [PubMed] [Google Scholar]
- Oh H., Fujio,Y., Kunisada,K., Hirota,H., Matsui,H., Kishimoto,T. and Yamauchi-Takihara,K. (1998) Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J. Biol. Chem., 273, 9703–9710. [DOI] [PubMed] [Google Scholar]
- Panayotou G. et al. (1992) Interaction of the p85 subunit of PI 3-kinase and its N-terminal SH2 domain with a PDGF receptor phosphorylation site: structural features and analysis of conformational changes. EMBO J., 11, 4261–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V., Virbasius,J., Lane,W.S., Toh,B.H., Shpetner,H.S. and Corvera,S. (1997) Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA, 94, 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V., Lawe,D.C., Corvera,S., Virbasius,J.V. and Chawla,A. (1998) A functional PtdIns3P-binding motif. Nature, 394, 433–434. [DOI] [PubMed] [Google Scholar]
- Philpott K.L., McCarthy,M.J., Klippel,A. and Rubin,L.L. (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J. Cell Biol., 139, 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiman C.M., Hertz,W.M. and Cambier,J.C. (1994) Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science, 263, 1609–1612. [DOI] [PubMed] [Google Scholar]
- Prasad K.V., Janssen,O., Kapeller,R., Raab,M., Cantley,L.C. and Rudd,C.E. (1993) Src-homology 3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3-kinase in T cells. Proc. Natl Acad. Sci. USA, 90, 7366–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L.E. and Cantley,L.C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem., 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Rameh L.E. et al. (1997) A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J. Biol. Chem., 272, 22059–22066. [DOI] [PubMed] [Google Scholar]
- Salim K. et al. (1996) Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J., 15, 6241–6250. [PMC free article] [PubMed] [Google Scholar]
- Shih N.Y., Li,J., Karpitskii,V., Nguyen,A., Dustin,M.L., Kanagawa,O., Miner,J.H. and Shaw,A.S. (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science, 286, 312–315. [DOI] [PubMed] [Google Scholar]
- Soltoff S.P. and Cantley,L.C. (1996) p120cbl is a cytosolic adapter protein that associates with phosphoinositide 3-kinase in response to epidermal growth factor in PC12 and other cells. J. Biol. Chem., 271, 563–567. [DOI] [PubMed] [Google Scholar]
- Soltoff S.P., Rabin,S.L., Cantley,L.C. and Kaplan,D.R. (1992) Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. J. Biol. Chem., 267, 17472–17477. [PubMed] [Google Scholar]
- Stambolic V. et al. (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell, 95, 29–39. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Aasland,R., Toh,B.H. and D’Arrigo,A. (1996) Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem., 271, 24048–24054. [DOI] [PubMed] [Google Scholar]
- Take H., Watanabe,S., Takeda,K., Yu,Z.X., Iwata,N. and Kajigaya,S. (2000) Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem. Biophys. Res. Commun., 268, 321–328. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B. and Waterfield,M.D. (1999) Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res., 253, 239–254. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Panayotou,G. and Waterfield,M.D. (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci., 22, 267–272. [DOI] [PubMed] [Google Scholar]
- Wang J., Auger,K.R., Jarvis,L., Shi,Y. and Roberts,T.M. (1995) Direct association of Grb2 with the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem., 270, 12774–12780. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan,D.R., Schaffhausen,B., Cantley,L. and Roberts,T.M. (1985) Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature, 315, 239–242. [DOI] [PubMed] [Google Scholar]
- Wymann M.P. and Pirola,L. (1998) Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta, 1436, 127–150. [DOI] [PubMed] [Google Scholar]
- Zhou S. and Cantley,L.C. (1995) Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem. Sci., 20, 470–475. [DOI] [PubMed] [Google Scholar]