Abstract
The standard therapeutic approaches for acute myeloid leukemia (AML) continue to be based on anthracyclines and cytarabine. However, the prognosis for AML remains poor, especially for patients with high-risk disease. During the past decade, promising novel agents that target DNA replication and repair, as well as cell cycling and apoptosis, have been developed and are being actively investigated in AML. Among these agents is flavopiridol, which interferes with key steps of the cell cycle and effectively promotes cell death, and voreloxin, an intercalating agent that also targets topoisomerase II. Also under clinical study in AML are oligonucleotide antisense constructs, which suppress the translation of proteins essential for leukemic blast survival and proliferation, and agents that target antiapoptotic cascades. In summary, it is hoped that novel therapies such as these will augment and/or supplant our current cytarabine- and anthracycline-based approaches, overcome active drug-resistance pathways, and eventually improve outcomes for patients with AML.
Introduction
Acute myeloid leukemia (AML) is characterized by an arrest in differentiation and an uncontrolled proliferation of myeloid precursors in the bone marrow. This process leads to hematopoietic insufficiency and occasionally significant leukocytosis, with subsequent severe and life-threatening sequelae. The treatment of AML has remained a daunting challenge for oncologists. Although roughly 70% of adults under age 60 with so-called de novo AML achieve a complete remission (CR) with traditional anthracycline- and cytarabine-based induction regimens, the overall long-term survival rate with therapy continues to be unsatisfactory at approximately 30% to 40% [1,2]. The prognosis is even grimmer for older patients, those with AML derived from myelodysplastic syndromes (MDS) or myeloproliferative disorders, and those with secondary AML related to environmental exposures or prior chemotherapy. In these patients, CR is achieved in less than 40% of cases and long-term survival in less than 10% [1,3]. Therefore, novel approaches and adjuncts to therapy are desperately needed and are being actively investigated for adult patients with AML.
In recent years, investigators have focused increasingly on molecularly targeted agents that affect cell signaling and cycling, new therapeutics that interfere with DNA repair and replication, epigenetic approaches directed at methylation and acetylation, and antibody-based immunotherapies, among others [4]. Many of these nascent approaches are in very early phases of development and study. Others have already shown promise in preclinical and clinical investigation. Ultimately, the aim will be to broaden the narrow, and often transient, therapeutic potential of traditional induction regimens in AML by the rational incorporation of mechanistically novel agents.
A burgeoning area of drug development and investigation in the treatment of AML involves therapies that interfere with cell survival, cycling, and proliferation. The cell cycle is a tightly regulated process mediated by a variety of proteins, including cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors. Other proteins, such as p53, bcl-2, p16, and Rb have important roles in determining the balance between cell survival and cell death [5]. Cell cycle–active agents, such as cytarabine and anthracyclines, have been the mainstay of antileukemic therapy for many years. An exciting area of research and drug development is now focused on a new generation of agents that target selected regulators of cycling and survival by a variety of novel mechanisms. This review presents and discusses some of these promising and novel approaches, alone and in combination with traditional antileukemic regimens.
Flavopiridol
Flavopiridol is a semisynthetic flavone derived from the stem bark of Amoora rohituka and Dysoxylum binectariferum, plants used in India as herbal medicines [5]. It is the first extensively studied CDK inhibitor and has been investigated in a variety of malignancies, including AML. It displays strong activity against several CDKs, including CDK1, CDK2, CDK4, CDK6, and CDK7; arrests the cell cycle at the G2/M phase; and delays the G1 to S phase progression [6]. Flavopiridol further inactivates the CDK9/cyclin T complex, also known as pTEF-b, resulting in inhibition of RNA polymerase II and suppression of RNA and polypeptide synthesis. This transcriptional inhibition then leads to a decrease in levels of proteins essential for cell cycling and survival, such as cyclin D1, VEGF, MCL-1, and STAT-3 (Table 1) [7–9]. Flavopiridol is also active to a lesser degree on tyrosine kinases, such as the epidermal growth factor receptor (EGFR), protein kinase C, and Erk [6].
Table 1.
Mechanistic targets of flavopiridol
| Action | Impact on cell survival and proliferation |
|---|---|
| Inhibition of serine–threonine CDKs through non–cell cycle–dependent and cycle-dependent mechanisms | Cell cycle arrest at the G1–S and G2–M checkpoints |
| Binding and inactivation of the CDK9/cyclin T1 complex (pTEF-b) | Inhibition of the RNA polymerase II complex and resultant blockade of transcriptional elongation |
| Binding to DNA and disruption of transcription | Disruption of DNA binding to key transcription factors, such as STAT-3, leading to a decrease in the expression of target proteins such as Mcl-1 |
| Decrease in cyclin D1 activity | Abrogation of cell cycle progression |
| Decrease in VEGF activity | Inhibition of angiogenesis and cell growth |
In preclinical studies, flavopiridol was found to be active in hematopoietic cell lines [10,11]. In AML, its novel mechanism of action and ability to target both cycling and noncycling cells in vitro have rendered flavopiridol an intriguing candidate for combination with traditional cytotoxic therapies. When administered concomitantly with S-phase–dependent agents, such as cytarabine and topotecan, it produces antagonistic effects through its propensity to induce cell cycle arrest [12]. However, it was noted that when flavopiridol administration and withdrawal preceded cytarabine and topotecan, dormant surviving cells were allowed to reenter the cell cycle and were thus further sensitized to the latter agents [9,12].
This observation prompted a phase 1 trial of flavopiridol followed by cytarabine and mitoxantrone (FLAM) in adults with refractory or relapsed AML and acute lymphoblastic leukemia (ALL). Flavopiridol was administered as an initial cytoreductive agent for 3 days, after which the remaining leukemic cells could be recruited into the cell cycle and thus be kinetically “ripe” for targeting by the 72-hour continuous administration of cytarabine beginning on day 6 and mitoxantrone on day 9. The overall response rate was 31% in AML and 12.5% in ALL. Pharmacokinetics demonstrated that a linear two-compartment model, with first-order elimination, provided the best fit of the observed concentration versus time data. Flavopiridol downregulated multiple cell cycle or survival target proteins in marrow blasts. These data suggested that flavopiridol is directly cytotoxic to leukemic cells and when followed by cytarabine and mitoxantrone, exerts biologic and clinical effects in patients with relapsed and refractory acute leukemias [13].
A phase 2 clinical trial of FLAM in relapsed, refractory, or newly diagnosed secondary AML used the maximum tolerated dose of flavopiridol at 50 mg/m2 per day for 3 days, as a 1-hour bolus, in 62 adult patients with relapsed, refractory, and secondary AML. Again, the direct cytotoxic activity of flavopiridol was demonstrated, with 44% of patients experiencing a ≥ 50% decrease in peripheral blasts by day 2 and 26% experiencing a ≥ 80% decrease in blasts by day 3. Self-limited tumor lysis occurred in 53% of patients. CRs were achieved in 12 of 15 patients (75%) with newly diagnosed secondary AML, 18 of 24 (75%) with a first relapse after a short CR, and 2 of 13 (15%) with primary refractory disease, but 0 of 10 patients with multiply refractory AML. Disease-free survival for all patients with a CR was 40% at 2 years, with newly diagnosed patients having a 2-year disease-free survival of 50%. This trial substantiated and augmented the initial phase 1 findings, namely that flavopiridol has anti-AML activity, directly and in combination with traditional antileukemic therapy. Moreover, the timed sequential FLAM regimen induces durable CRs in a significant proportion of adults with newly diagnosed secondary AML and those in first relapse after a short CR [14••].
Expansion of these findings and further exploration of alternative flavopiridol-based regimens are under way. One approach, a “hybrid” bolus infusion schedule of flavopiridol, has been investigated in chronic lymphocytic leukemia, with promising results. In this approach, a pharmacologically modeled “hybrid” schedule of flavopiridol is administered, with a 30-minute bolus of roughly half the total dose, followed by a 4-hour infusion of the remaining half, in an attempt to overcome the observed effects of avid binding of flavopiridol by human plasma proteins [15,16•]. This alternative hybrid dosing (hFLAM) is being studied in a dose escalation phase 1 trial in patients with primary refractory and relapsed AML. In concert with this trial, in vivo pharmacodynamic studies demonstrate flavopiridol-induced suppression of multiple target genes, including VEGF, E2F1, STAT-3, cyclin D1, and RNA polymerase II. Interestingly, bcl-2 mRNA levels increased after flavopiridol administration in most cases, representing a possible antiapoptotic compensatory mechanism [17]. The next step in the development of flavopiridol in AML therapy is to compare the hybrid infusion with bolus administration in patients with newly diagnosed, poor-risk AML.
Voreloxin
Voreloxin, formerly called SNS-595, is a novel naphthyridine analogue that effectively intercalates into DNA and inhibits topoisomerase II. It leads to replication-dependent DNA damage, irreversible cell cycle arrest in the G2 phase, and ultimately apoptosis. Unlike anthracyclines, which also inhibit topoisomerase II, voreloxin is not a substrate for P-glycoprotein and has not demonstrated a potential for cardiac toxicity, thereby making it attractive for use in settings of anthracycline resistance and/or dose limitation [18,19].
In a phase 1b trial of voreloxin in patients with refractory AML, weekly and twice-weekly regimens of voreloxin were investigated. In those receiving weekly dosing, 12 of 30 patients (40%) experienced antileukemic activity and 3 had a CR. Twice-weekly scheduling demonstrated less antileukemic activity, with 2 of 14 patients (14%) experiencing responses, but the regimen’s tolerability and potential for synergy support its use in future combination trials with cytarabine. Antileukemic activity correlated with a time above threshold drug concentration of 1 µM. For both schedules, the dose-limiting toxicity was oral mucositis [20••]. In a subsequent phase 2 study in patients aged ≥ 60 years with newly diagnosed AML, 67% of those evaluated had a bone marrow blast percentage of less than 5% after induction, with three patients in CR and three others in count recovery. Of note, two deaths were reported within 60 days of initiation of voreloxin therapy [21].
In addition, an ongoing phase 1b/2 trial is studying the combination of voreloxin and cytarabine in refractory or relapsed AML [22]. The twice-weekly approach has been used in this combination trial. Of the 38 patients enrolled thus far, 9 (24%) have achieved a CR. Correlative laboratory studies have demonstrated extreme drug resistance to cytarabine in five of seven pretreatment bone marrow blast populations tested. In contrast, only one bone marrow aspirate sample displayed extreme drug resistance to voreloxin, suggesting that the latter agent was the greater contributor to the achievement of CR in these patients [22]. These results intimate activity for voreloxin as single-agent therapy and in combination with traditional induction therapies; the final conclusions of ongoing studies are eagerly anticipated
Inhibitors of the PI3K/mTOR Signal Transduction Pathway
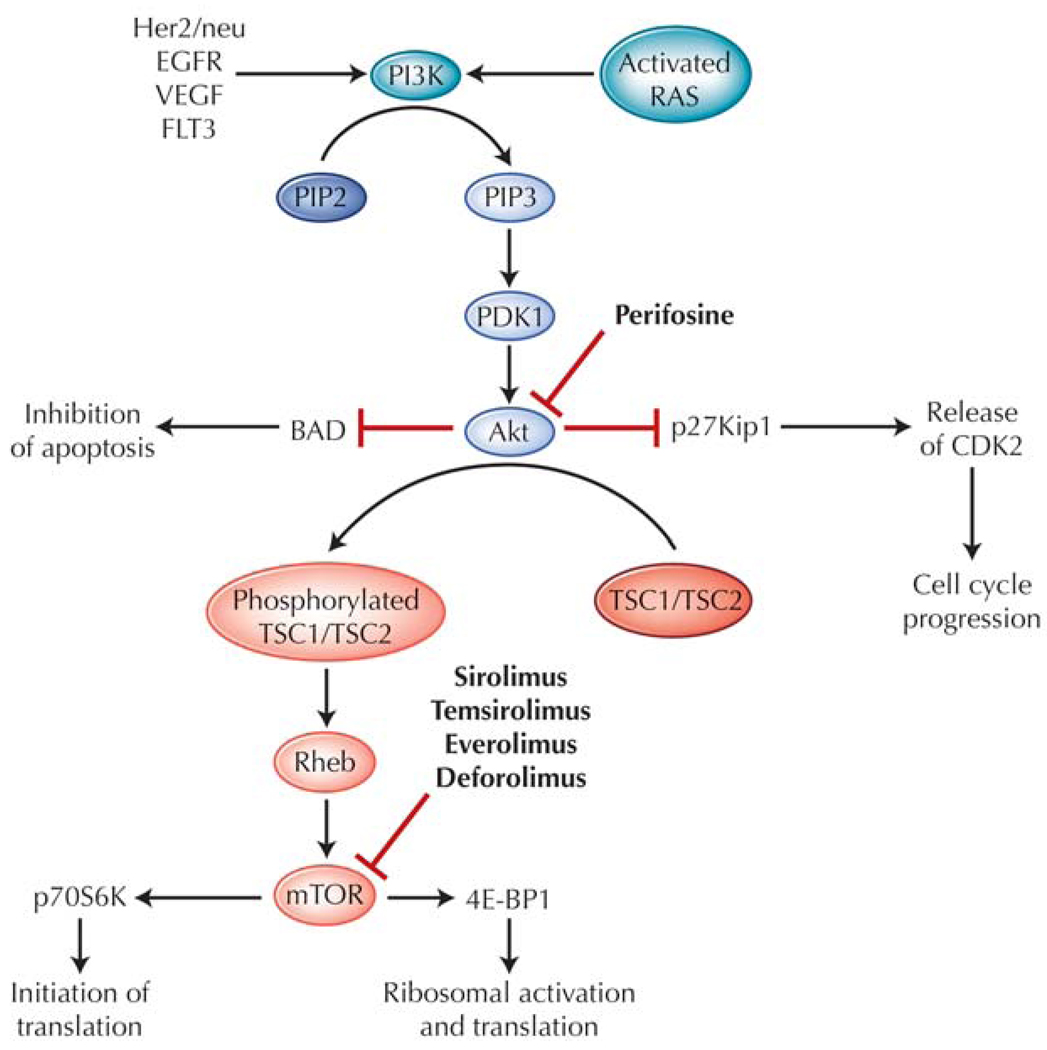
The phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signal transduction pathway is a vital intracellular cascade that helps regulate translation, ribosomal biogenesis, cell cycling, and proliferation, as well as apoptosis. The pathway comprises a sequence of kinases starting with PI3K, a lipid kinase at the surface membrane. Although this pathway has been reviewed extensively elsewhere [23•], a succinct summary of the cascade provides a background for this discussion (Fig. 1). PI3K is activated when bound by a variety of receptor tyrosine kinases, such as FLT3, EGFR, and HER2/neu. The catalytic subunit of PI3K also may be activated by downstream targets of Ras or may be constitutively activated by mutations in malignancies. PI3K converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the inner surface of the membrane. Enzymes such as phosphoinositide-dependent kinase (PDK1) and Akt are then recruited to the inner membrane surface by PIP3, and Akt is subsequently activated by PDK1 as a result of this interaction. Akt appears to be crucial in the process of regulating cell survival, cell cycling, and proliferation and activates a variety of downstream enzymes to stimulate proliferation and inhibit proapoptotic signals [24]. For example, Akt targets and suppresses p27Kip1, a direct inhibitor of CDK2, which is then free and able to promote transcription and resultant cell proliferation [25], and inhibits the proapoptotic bcl-2 antagonist of cell death (BAD), leading to its sequestration and eventual prevention of cell death [26]. Another target enzyme is tuberous sclerosis protein 2 (TSC2), which when phosphorylated, releases the protein Rheb to interact with and activate the mTOR kinase. mTOR, an essential mediator in cell cycling, allows progression from G1 phase to S only if sufficient energy and nutrient levels are available for cell division and proliferation [24]. The multiple targets of mTOR include p70S6K, an activator of ribosomal machinery and protein synthesis, and 4E-BP1, which enhances translation of RNA. These processes together lead to increased protein synthesis of enzymes essential for regulating cycling and survival, such as cyclin D1, c-Myc, and p27 [24].
Figure 1.
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) cascade in acute myeloid leukemia, and relevant targeted therapies. BAD—bcl-2 antagonist of cell death; CDK2—cyclin-dependent kinase 2; EGFR—epidermal growth factor receptor; PDK1—phosphoinositide-dependent kinase 1; PIP2—phosphatidylinositol-4,5-bisphosphate; PIP3—phosphatidylinositol-3,4,5-trisphos phate; TSC1, TSC2—tuberous sclerosis proteins 1 and 2; VEGF—vascular endothelial growth factor.
Alterations in the PI3K/Akt/mTOR pathway have been noted in a variety of neoplasms and include individual mutations of PI3K and Akt. Such mutations lead to increased constitutive signaling, increased survival and proliferation of malignant cells, and resistance to chemotherapy [27]. In AML, constitutive activation of the PI3K/Akt signaling cascade is relatively common, with 50% to 70% of patients with AML exhibiting activation of Akt [26] and those with phosphorylated Akt having worse overall survival [28]. In addition to activating mutations of Akt, constitutive phosphorylation of Akt may result from increased activity of upstream factors. Indeed, up to a quarter of AML cases exhibit N-Ras or K-Ras point mutations, leading to continuous activation of Ras and subsequent stimulation of the PI3K/Akt cascade, and a significant percentage of patients have activating mutations of c-Kit, which also activate the cascade [26]. Moreover, patients with activating FLT3 internal tandem duplication mutations have a resultant downstream constitutive activation of the PI3K/Akt cascade, leading to prolonged cell survival and proliferation [29]. Given these observations, targeting members of the PI3K/Akt cascade as a modality in cancer therapeutics has garnered some interest.
Therapeutic agents that target Akt are being actively studied in acute leukemia. Perifosine, an inhibitor of Akt, has been evaluated in AML cell lines and demonstrated to promote dephosphorylation of Akt and subsequent apoptosis. It also was noted to sensitize blast cells to etoposide, a topoisomerase inhibitor, decreasing the survival of cells exposed to the combination of these agents [30]. Based on encouraging preclinical data, clinical trials in AML have been launched. A phase 2 study of perifosine in refractory or relapsed acute or chronic leukemia recently completed enrollment (clinicaltrials.gov, NCT00391560). Another trial combining UCN-01, a Chk1 inhibitor, and perifosine in patients with refractory acute leukemia, chronic myelogenous leukemia, and refractory MDS, is currently enrolling patients (clinicaltrials.gov, NCT00301938).
Direct inhibition of mTOR protein also was evaluated recently for therapeutic potential in AML. Rapamycin (sirolimus), an antibiotic derived from the bacterial species Streptococcus hygroscopicus, was initially approved and extensively used as an immunosuppressant [31]. However, it was subsequently found to effectively inhibit mTOR when complexed with FK506 binding protein 12 (FKBP12) [23•] and thus has been used as an agent to target the PI3K/Akt/mTOR pathway in malignancies. Other agents have since been developed, including temsirolimus, an ester derivative of rapamycin; everolimus; and deforolimus (AP23573) [32].
In the area of AML, investigators have demonstrated that rapamycin effectively suppresses leukemic cell lines and arrests the cell cycle at the G1 phase, which correlates with an upregulation of the CDK inhibitor p27Kip1. Moreover, p70S6K and 4E-BP1, downstream targets of mTOR, were noted to be constitutively phosphorylated in multiple AML patient samples, and this phosphorylation was suppressed with administration of rapamycin. Also included was a pilot clinical study of nine patients with refractory or relapsed AML, in whom daily rapamycin produced four partial responses with a median duration of 38 days [33]. However, another small study of rapamycin in MDS-derived secondary AML in patients over the age of 65 demonstrated no clinical responses [34].
A phase 1/2 study of temsirolimus, an oral rapamycin derivative, was performed in patients with hematologic malignancies, including nine patients with AML and five with MDS. Two patients with MDS achieved minor hematologic responses, and the phosphorylation of downstream targets of mTOR were effectively suppressed [35]. Yet another rapamycin analogue, deforolimus, was assessed in a phase 2 clinical trial in patients with relapsed and refractory hematologic malignancies. Of the 55 patients who received the drug, 23 had AML and 2 had MDS. These patients experienced only minor clinical responses, with normalization of neutrophil count in one patient with MDS-AML and stabilization of disease in three others. However, suppression of mTOR targets was again demonstrated with decreased phosphorylation of 4E-BP1 in most of the evaluated patients [36]. Therefore, although clinical responses have been uncommon, the effective inhibition of mTOR by rapamycin and its analogues and their promising synergy with other agents have prompted further investigation. In fact, multiple clinical trials are under way or are being planned to evaluate mTOR inhibitors in combination with traditional AML therapies for patients with poor-risk AML (clinicaltrials.gov, NCT00235560, NCT00780104, NCT00634244).
Antisense Therapies
Antisense oligonucleotides are short sequences of single-stranded deoxyribonucleotides designed to complement and bind specific coding regions on mRNA; after binding, they form DNA–mRNA complexes that are then degraded by a ribonuclease. This process thus prevents gene expression and eventual translation of the targeted proteins and increasingly is being used as a novel approach to developing antineoplastic agents [37]. Among the more extensively studied antisense therapies are those that target the antiapoptotic proteins bcl-2 and XIAP (X-linked inhibitor of apoptosis).
Bcl-2, a mitochondrial protein that impedes programmed cell death, often is upregulated in AML. Studies have suggested that bcl-2 expression portends a poor prognosis, with lower CR rates and poorer survival, possibly as a result of the contribution of bcl-2 to chemotherapy resistance [38,39]. Oblimersen (Genasense; Genta, Berkeley Heights, NJ), a phosphorothioate 18-base antisense oligonucleotide to bcl-2, was found in preclinical studies to effectively suppress bcl-2 mRNA expression [37]. The clinical efficacy of oblimersen, as a single agent and in combination with traditional antileukemic therapy, is being evaluated in serially designed phase 1 through 3 clinical trials in adults with AML.
Phase 1 studies of oblimersen in AML tested the hypothesis that bcl-2 suppression could reduce resistance to traditional chemotherapeutic agents in patients with refractory or relapsed disease. In one trial, 17 patients with AML and 3 patients with ALL were administered escalating doses of oblimersen with FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor) salvage chemotherapy. Of these, 6 patients (30%), including 5 of 17 (29%) with AML, had a CR, and intracellular bcl-2 mRNA and protein levels were quantitatively suppressed in 9 of 12 evaluated patients. Side effects included gastrointestinal toxicities and fever, though none were dose limiting [40]. Another phase 1 trial assessed the concurrent use of oblimersen with cytarabine/anthracycline induction and high-dose cytarabine consolidation regimens in patients aged ≥ 60 years with newly diagnosed AML. Of 29 patients enrolled in the study, 14 (48%) achieved a CR, with a proportion of these patients experiencing prolonged remissions (> 13 months). The data again suggested the safety of combining this agent with traditional AML regimens, with no observed dose-limiting toxicities [41]. A randomized multicenter phase 3 trial followed comparing daunorubicin/cytarabine-based induction and consolidation with or without oblimersen in patients older than 60 years with newly diagnosed AML. Unfortunately, the addition of oblimersen did not improve outcomes for these patients, with statistically similar CR rates, as well as similar relapse-free and overall survival, between the two arms [42••].
Another antisense construct being studied in AML targets XIAP. This important protein binds and inhibits caspases 3, 7, and 9, which are essential downstream mediators of the apoptotic cascade. Like bcl-2, XIAP has been found to be overexpressed in AML, is postulated to be involved in leukemic cell survival and resistance to cytotoxic therapy, and when highly expressed, may be linked to poor clinical outcomes [43]. AEG35156, also known as GEM640, is a 19-base phosphorothioate antisense agent targeting XIAP and was selected from multiple similar oligonucleotides because of its efficacy in suppressing XIAP mRNA and protein levels. This agent has been investigated in preclinical and some clinical studies and has shown promise [44].
A phase 1/2 trial of the antisense construct in combination with idarubicin/cytarabine reinduction therapy was completed recently in refractory/relapsed AML patients. In the phase 1 portion of the study, 1 of 24 individuals achieved a CR, whereas in the phase 2 portion, 41% of the 27 treated patients responded, with seven CRs. Importantly, this regimen was not efficacious in patients with multi-refractory AML, and the noted remissions were in those with primary refractory or first-relapse disease. Blasts from the peripheral blood also were collected from 22 patients. XIAP mRNA levels were quantified by reverse transcriptase polymerase chain reaction assay, and their suppression was detected after AEG35156 administration, with greater reductions with higher doses of drug [45•,46]. In summary, this combination appeared to effectively suppress XIAP levels in treated patients, with clinical results showing sufficient promise to warrant further investigation in advanced AML.
An antisense target that recently entered the clinical arena is the DNA synthesis and repair enzyme ribonucleotide reductase (RNR). GTI-2040, a 20-mer antisense construct complementary to the R2 component of RNR. RNR is necessary for the conversion of ribonucleotides to deoxyribonucleotides, is often upregulated in malignant cells, increasing the pool of deoxynucleotides [47]. Inhibiting RNR and the resultant decrease in available deoxynucleotide triphosphate pools could theoretically enhance the cytotoxicity of all nucleoside analogues, including cytarabine, and other agents that damage DNA and require deoxynucleotide triphosphates for repair [47,48]. With this in mind, a phase 1 clinical trial in 23 patients with refractory or relapsed AML studied the combination of high-dose cytarabine and GTI-2040. Eight patients (35%) achieved a CR, with the degree of reduction in bone marrow levels of R2 correlating with response [48]. Given the relative safety of the combination and the promising results, a phase 2 randomized multicenter trial of this combination is currently enrolling patients (clinicaltrials.gov, NCT00565058).
Conclusions and Future Directions
AML continues to be a lethal disease and a challenge for treating oncologists. The survival rate has not changed significantly in years, and new strategies and therapies are desperately needed. During the past decade, investigators have evaluated multiple approaches to target the survival, cycling, and proliferation of AML blasts. Newly developed agents have been shown to effectively interrupt DNA replication and repair, cause arrest of the cell cycle, and promote apoptosis. Among these, flavopiridol and voreloxin already have shown significant promise in clinical trials of relapsed and refractory AML and are undergoing expanded clinical investigation. Agents that interrupt intracellular cascades, including Akt and mTOR inhibitors, have been associated with more modest clinical results, again with more expansive trials under way. Novel therapeutics, such as antisense oligonucleotides, are also being developed and studied, with unclear potential.
Lastly, new and ambitious approaches to clinical trial design are also needed to improve AML therapies in the near future. These include the testing of novel agents in combination with traditional, established therapeutics in earlier phases of clinical investigation, as well as more frequent randomization of larger phase 2 trials [49]. Finally, novel therapies not only should be tested in the setting of induction and consolidation, but also should be considered for treating minimal residual disease as maintenance regimens [50]. It is hoped that the ongoing progress in expanding novel therapies and approaches soon will yield useful adjuncts to AML therapy and significantly improve AML’s poor prognosis.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Karp JE, Smith MA. The molecular pathogenesis of treatment-induced (secondary) leukemias: foundations for treatment and prevention. Semin Oncol. 1997;24:103–113. [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Bao T, Smith BD, Karp JE. New agents in the treatment of acute myeloid leukemia: a snapshot of signal transduction modulation. Clin Adv Hematol Oncol. 2005;3:287–296. 302. [PubMed] [Google Scholar]
- 4.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 5.Klasa RJ, List AF, Cheson BD. Rational approaches to design of therapeutics targeting molecular markers. Hematology Am Soc Hematol Educ Program. 2001:443–462. doi: 10.1182/asheducation-2001.1.443. [DOI] [PubMed] [Google Scholar]
- 6.Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–170. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 7.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 8.Melillo G, Sausville EA, Cloud K, et al. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999;59:5433–5437. [PubMed] [Google Scholar]
- 9.Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9:307–315. [PubMed] [Google Scholar]
- 10.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 11.Decker RH, Dai Y, Grant S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 2001;8:715–724. doi: 10.1038/sj.cdd.4400868. [DOI] [PubMed] [Google Scholar]
- 12.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997;57:3375–3380. [PubMed] [Google Scholar]
- 13.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res. 2005;11:8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 14. Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–4473. doi: 10.1158/1078-0432.CCR-07-0381. This phase 2 trial of FLAM showed promising results in poor-risk, relapsed, and secondary AML.
- 15.Blum W, Klisovic RB, Johnson A, et al. Final results of a dose escalation study of flavopiridol in acute leukemias using a novel treatment schedule [abstract] Blood (ASH Annual Meeting Abstracts, part 1) 2007;110:890. [Google Scholar]
- 16. Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. This article describes an early trial of the hybrid bolus regimen of FLAM in patients with chronic lymphocytic leukemia.
- 17.Resar L, Hillion J, Alino K, et al. Flavopiridol downregulates genes involved in cell cycle regulation and tumor progression in adults with refractory or poor-risk acute leukemia. Blood. 2008;112:351. [Google Scholar]
- 18.Richardson DS, Johnson SA. Anthracyclines in haematology: preclinical studies, toxicity and delivery systems. Blood Rev. 1997;11:201–223. doi: 10.1016/s0268-960x(97)90020-5. [DOI] [PubMed] [Google Scholar]
- 19.Hoch U, Lynch J, Sato Y, et al. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64:53–65. doi: 10.1007/s00280-008-0850-3. [DOI] [PubMed] [Google Scholar]
- 20. Lancet JE, Kantarjian H, Ravandi F, et al. SNS-595 demonstrates clinical responses in a phase I study in acute leukemia [abstract] Blood. 2007;110:442. Preliminary results suggest significant clinical responses to voreloxin in patients with poor-risk AML.
- 21.Maris M, Cripe LD, Stuart RK, et al. Phase 2 study of voreloxin (formerly known as SNS-595) as single agent therapy for elderly patients with newly diagnosed acute myeloid leukemia (AML): preliminary safety and clinical responses (The Reveal-1 Study) [abstract] Blood. 2008;112:1951. [Google Scholar]
- 22.Lancet J, Karp JE, Cripe LD, et al. Voreloxin in combination with cytarabine demonstrates preliminary clinical responses in a phase 1b/2 study in relapsed/refractory acute myeloid leukemia [abstract] Blood. 2008;112:1955. [Google Scholar]
- 23. Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. This is an excellent review of the PI3K/Akt/mTOR pathway and its relevance to targeted therapies in hematologic malignancies.
- 24.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Cappellini A, Tabellini G, Zweyer M, et al. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia. 2003;17:2157–2167. doi: 10.1038/sj.leu.2403111. [DOI] [PubMed] [Google Scholar]
- 26.Martelli AM, Nyakern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 28.Min YH, Eom JI, Cheong JW, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17:995–997. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 29.Brandts CH, Sargin B, Rode M, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 30.Papa V, Tazzari PL, Chiarini F, et al. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–160. doi: 10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- 31.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 32.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 33.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–2534. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 34.Callera F, Lopes CO, Rosa ES, Mulin CC. Lack of antileukemic activity of rapamycin in elderly patients with acute myeloid leukemia evolving from a myelodysplastic syndrome. Leuk Res. 2008;32:1633–1634. doi: 10.1016/j.leukres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 36.Rizzieri DA, Feldman E, Dipersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 37.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 38.Campos L, Rouault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. [PubMed] [Google Scholar]
- 39.Karakas T, Maurer U, Weidmann E, et al. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann Oncol. 1998;9:159–165. doi: 10.1023/a:1008255511404. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci G, Byrd JC, Dai G, et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101:425–432. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- 41.Marcucci G, Stock W, Dai G, et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol. 2005;23:3404–3411. doi: 10.1200/JCO.2005.09.118. [DOI] [PubMed] [Google Scholar]
- 42. Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old [abstract] J Clin Oncol (ASCO Annual Meeting Proceedings Part 1) 2007;25:7012. A randomized phase 3 trial of oblimersen combined with traditional cytotoxic chemotherapy did not demonstrate improvement in outcomes.
- 43.Lacasse EC, Kandimalla ER, Winocour P, et al. Application of XIAP antisense to cancer and other proliferative disorders: development of AEG35156/ GEM640. Ann N Y Acad Sci. 2005;1058:215–234. doi: 10.1196/annals.1359.032. [DOI] [PubMed] [Google Scholar]
- 44.Tamm I. AEG-35156, an antisense oligonucleotide against X-linked inhibitor of apoptosis for the potential treatment of cancer. Curr Opin Investig Drugs. 2008;9:638–646. [PubMed] [Google Scholar]
- 45. Schimmer AD, Estey EH, Borthakur G, et al. Phase ½ trial of the XIAP antisense oligonucleotide (AEG35156) in combination with idarubicin and cytarabine in patients with relapsed/refractory AML. Blood. 2008;112:283. Preliminary results of a phase 1/2 study of XIAP antisense combined with traditional cytotoxic therapy demonstrate a decrease in XIAP transcripts and some clinical responses in patients with poor-risk AML.
- 46.Carter BZ, Mak DH, Morris S, et al. Pharmacodynamic study of phase 1/2 trial of the XIAP antisense oligonucleotide (AEG35156) in combination with chemotherapy in patients with relapsed/refractory AML. Blood. 2008;112:678. [Google Scholar]
- 47.Fan H, Villegas C, Wright JA. Ribonucleotide reductase R2 component is a novel malignancy determinant that cooperates with activated oncogenes to determine transformation and malignant potential. Proc Natl Acad Sci U S A. 1996;93:14036–14040. doi: 10.1073/pnas.93.24.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klisovic RB, Blum W, Wei X, et al. Phase I study of GTI-2040, an antisense to ribonucleotide reductase, in combination with high-dose cytarabine in patients with acute myeloid leukemia. Clin Cancer Res. 2008;14:3889–3895. doi: 10.1158/1078-0432.CCR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estey EH, Thall PF. New designs for phase 2 clinical trials. Blood. 2003;102:442–448. doi: 10.1182/blood-2002-09-2937. [DOI] [PubMed] [Google Scholar]
- 50.Karp JE, Smith BD, Gojo I, et al. Phase II trial of tipifarnib as maintenance therapy in first complete remission in adults with acute myelogenous leukemia and poor-risk features. Clin Cancer Res. 2008;14:3077–3082. doi: 10.1158/1078-0432.CCR-07-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]