Abstract
Trypanosomes were observed in a peripheral blood smear from a 45-day-old Thai infant displaying fever, anaemia, cough and anorexia. Human trypanosomiasis is not endemic to Thailand, so parasite identification was undertaken to determine likely sources of the infection. Several morphological parameters of the trypanosomes were similar to those of Trypanosoma evansi and statistically different from those of Trypanosoma lewisi-like parasites from a naturally infected indigenous rat. However, duplicate PCR assays with primers flanking trypanosome rRNA internal transcribed spacer 1 (ITS1) resulted in amplicons of ~623 bp that corresponded to the expected size for T. lewisi-like parasites. The ITS1 sequence from the infant’s blood was 98 and 49% identical to T. lewisi and T. evansi sequences, respectively. Based on molecular results, it was concluded that the infant was infected with a T. lewisi-like (Herpetosoma) species.
Introduction
Trypanosomes are flagellated protozoan parasites, some of which can cause distinct zoonoses. The genus Trypanosoma is divided into two major groups that infect vertebrates, the salivaria and the stercorcaria (Hoare, 1964). Although both Trypanosoma groups are enzootic to Thailand, human trypanosomiasis has not been reported to date in this country, and reports of human infections with the Trypanosoma species enzootic to Thailand are extremely rare worldwide (Johnson, 1933; Joshi et al., 2005; Shrivastava & Shrivastava, 1974). Diagnosis of trypanosomiasis often involves examination of stained blood smears, and identification of Trypanosoma species is often based on parasite morphology; however, molecular diagnostic tests to distinguish Trypanosoma species are advantageous because morphological biometry can be problematic. A PCR assay has been developed to detect Trypanosoma lewisi and Trypanosoma vivax in rats (Desquesnes et al., 2002). This assay utilizes primers conserved to 18S and 5.8S rRNA genes (rDNA) that flank the relatively polymorphic internal transcribed spacer 1 (ITS1). Thus, this assay can generate amplicons from divergent Trypanosoma species and allow parasite identification through ITS1 sequence analysis.
Case Report
Patient history
On August 28 2005, a 45-day-old male infant was admitted to Lampang Provincial Hospital in northern Thailand (approx. 500 km from Bangkok, Thailand) with coughing, fever, anorexia and depression. The patient was preliminarily diagnosed with dengue based on the high fever, and anti-pyrexia treatment was prescribed. The patient was readmitted on September 4 2005 due to continued fever, anorexia and depression. Blood was collected for a complete haemogram during the second visit, and trypanosomes were observed in a peripheral blood smear. The patient’s haemogram indicated 15 100 leukocytes µl−1 (45% polymorphonuclear cells, 8% band forms, 40% lymphocytes and 7% monocytes), 11.5 g dl−1 haemoglobin and a haematocrit of 31 %. The patient was treated with 25 mg gentamicin in 30 ml sterile water in an intravenous drip for 1 h. Supportive treatment included 5% glucose, vitamins and amino acids. The patient recovered after antibiotic treatment, and parasite identification was undertaken to determine likely sources of this infection. Trypanocidal drugs were avoided due to their toxicity and the patient’s positive response to treatment.
Trypanosome biometry
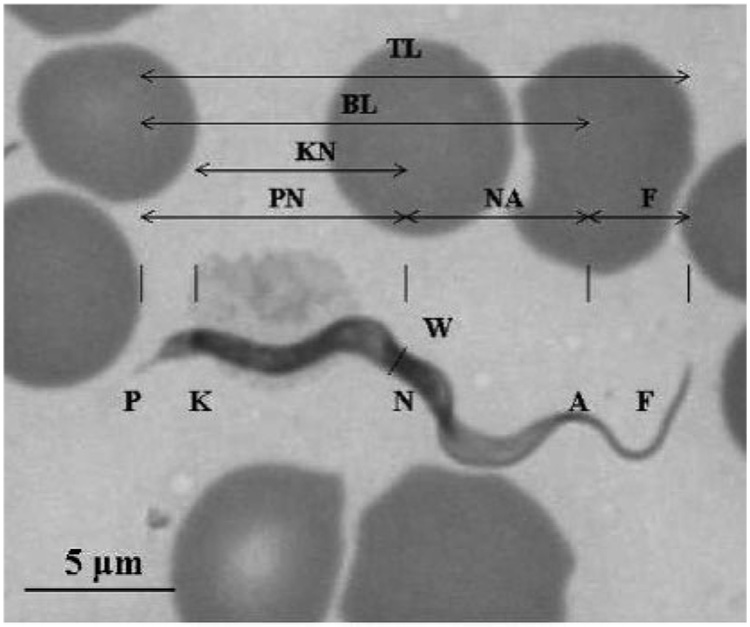
Two trypomastigote morphology types were observed: (1) a slender form with tapered ends, a distinct subterminal kinetoplast, a thin, straight posterior end and a nucleus at the anterior end, and (2) a broad trypomastigote with a large, granular body. Eight morphological parameters of the slender forms were measured to compare with other trypanosomes enzootic to Thailand (Fig. 1). Morphological features were compared to values reported for T. lewisi-like and Trypanosoma evansi parasites in naturally infected indigenous rats and pigs, respectively (Table 1) (Sarataphan et al., 1986; Natheewattana et al., 1973). One-way analysis of variance and the Tukey posthoc procedure were used to compare the above parameters. Average values for PK, KN and TL differed between trypanosomes in the infant’s blood and those in the rat infection. These same values did not differ from those of T. evansi. Conversely, the nuclear index (NI) values were 1.4, 1.4 and 0.9, respectively, for trypomastigotes from the infant, rat (Herpetosoma) and pigs (T. evansi) from Thailand. However, statistical analysis of the kinetoplast index (KI) and NI was not performed due to unavailability of data for these measurements in earlier reports. PN for the infant infection was different from that of T. evansi, but this value was not available for T. lewisi-like parasites in the Thai rat. No differences were observed for W or F among the three trypanosomes.
Fig. 1. Morphological parameters of trypanosomes from a Thai infant.
Locations of different features and organelles are indicated by letters. Distances measured were: PN, posterior end to nucleus centre; NA, nucleus centre to the anterior end; PK, posterior end to kinetoplast centre; KN, kinetoplast centre to nucleus centre; W, parasite maximum width; F, free flagellum length; BL, posterior to anterior end; TL, total parasite length. These values were also used to calculate the NI (NI5PN/NA) and KI (KI5PN/KN). Bar, 5 µm.
Table 1.
Known morphological parameters of trypanosomes enzootic to Thailand
| Infection | Morphological parameter measurements (mean±SD)* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PN (µm) | NA (µm) | PK (µm) | KN (µm) | W (µm) | F (µm) | TL (µm) | BL (µm) | NI | KI | |
| Infant (n=20) | 10.0±1.3† | 7.5±2.0† | 2.3±0.9† | 7.8±1.3† | 1.9±1.3 | 6.4±0.7† | 23.9±1.4† | 17.5±2.0 | 1.4 | 1.3 |
| Rat (n=11) | NR‡ | 9.7±2.9§ | 4.2±2.8§ | 9.5±2.8§ | 2.7±3.4 | 7.8±4.5†§ | 31.8±6.4§ | NR | 1.4 | 1.4 |
| T. evansi (n=60) | 8.0±0.3§ | 9.2±0.6†§ | 1.9±0.3† | 6.1±0.1† | 1.5±0.3 | 4.2±1.1§ | 21.0±0.6† | 16.7±0.6 | 0.9 | 1.3 |
Parameters correspond to those described in Fig. 1.
For each parameter, values marked with different symbols differ (P<0.05) according to Tukey posthoc analysis.
Not reported (Natheewattana et al., 1973).
Molecular diagnostics
Trypomastigote biometry suggested that these parasites were different from the T. lewisi-like parasites described in a naturally infected Thai rat, and there was no morphological evidence of differences between the trypanosomes found in the Thai infant and T. evansi enzootic to Thailand. However, the small sample sizes of these reports compelled us to confirm this observation; thus, PCR was used to amplify the region containing the ITS1 sequence between the 18S and 5.8S rRNA genes. This assay facilitates subgenus identification by amplicon size and species identification with ITS1 sequence homology. Heparinized blood from the patient was frozen and stored at −20 °C prior to DNA isolation with a commercial kit (FlexiGene, Qiagen) according to the manufacturer’s protocol. PCR was carried out with primers TRYP1S (CGTCCCTGCCATTTGTACACAC) and TRYP1R (GGAAGCCAAGTCATCCATCG) for amplification of the ITS1 fragment (Desquesnes et al., 2002). The reactions consisted of 50 µl volumes containing 10 mM Tris/HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 µM each dNTP, 1 µM each primer, 0.25 U Taq polymerase (Qiagen), 5% (v/v) DMSO and 5 µl DNA template. Amplicons were purified and directly sequenced in both directions with the same primers used to amplify the DNA template. Sequences were determined and used for multiple-sequence alignment with ITS1 sequences reported for T. lewisi in an experimentally infected rat (Desquesnes et al., 2002) and T. evansi from Taiwan (GenBank accession no. D89527).
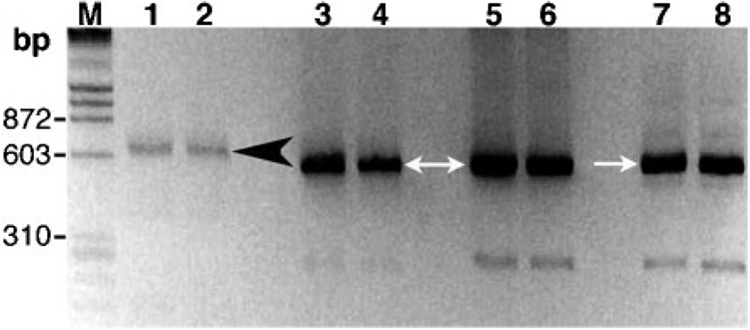
The PCR assay of the infant’s blood resulted in amplicons of ~623 bp, which was consistent with the size reported for the stercorcarian T. lewisi (Fig. 2). Controls included two salivarians, T. evansi and Trypanosoma brucei rhodesiense, which produced smaller amplicons of ~520 bp. Lengths of the ITS1 sequences were 416, 420 and 340 bp from the Thai infant infection, T. lewisi and T. evansi, respectively. The ITS1 sequence from the Thai infant was 98% identical to T. lewisi, which was due to deletion of 4 bases and mismatches between 5 bases of the respective ITS1 sequences. Conversely, the amplicon generated from the infant’s blood was 49% identical to corresponding sequences reported for T. evansi from Taiwan. According to a BLAST search of the National Center for Biotechnology Information (NCBI) database, sequences from T. lewisi, Trypanosoma otospermophili (accession nos AB175625 and AB190228), Trypanosoma grosi (accession nos AB175622–AB175624) and an unclassified Trypanosoma species (accession no. AB175626) were the closest matches to ITS1 from this patient’s blood.
Fig. 2. PCR of trypanosomes in the patient’s blood.
Amplicons that included ITS1 of trypanosome-specific rDNA were generated with primers TRYP1S and TRYP1R and visualized on an agarose gel stained with ethidium bromide. The molecular size standard (M) was λ phage DNA digested with HindIII and ϕX DNA digested with HaeIII. Lanes: 1–2, blood sample from the Thai infant in duplicate assays; 3–4, T. evansi (Thailand); 5–6, T. evansi (Taiwan); 7–8, T. brucei rhodesiense (IL1501). The 520 and 623 bp amplicons are indicated by white arrows and a black arrowhead, respectively.
Discussion
Parasite morphology alone suggested that these trypanosomes were indistinguishable from T. evansi and dissimilar to a T. lewisi-like species, both of which are enzootic to Thailand. However, T. lewisi-like trypomastigotes have only been described from a single naturally infected rat (Natheewattana et al., 1973); thus, it is plausible that the rat trypanosome infection was a different species from the one described in this report. Amplicon size and ITS1 sequence analysis both suggested that the infection was T. lewisi or a closely related species of the stercorcarian subgenus Herpetosoma. Thus, these results indicated that the patient was infected with a trypanosome that utilizes fleas and rodents as natural hosts, which was corroborated by the identification of Ctenocephalides felis fleas in the infant’s dwelling. Although Herpetosoma species are thought to be specific to a single vertebrate host genus, they are reported to infect a relatively broad range of flea vectors (Hoare, 1972; Linardi & Botelho, 2002; Molyneux, 1969). Fleas are often opportunistic parasites of available mammalian hosts, thus the patient described in this report could have been exposed to infective fleas from a rodent reservoir other than rats.
The case reported here is similar to a report of human trypanosomiasis associated with a diagnosed T. lewisi infection of a 4-month-old Malaysian infant with a 3-week history of lassitude and loss of appetite and a 10-day history of fever (Johnson, 1933). Notably, the infant described in the present report was also found to be feverish and anaemic with a heavy trypanosome infection upon admission. Interestingly, attempts to culture or to infect rats with trypanosomes from the Malaysian child’s blood were unsuccessful, suggesting that the infection might not have been T. lewisi sensu stricto. T. lewisi has since become the type species for Herpetosoma, a homogeneous subgenus of several dozen named species that are morphologically indistinguishable, but with various host specificities (Hoare, 1964). Another group has reported a serendipitous diagnosis of ‘T. lewisi-like’ (i.e. Herpetosoma) infections in a febrile married couple during the Indian malaria eradication program (Shrivastava & Shrivastava, 1974). Taken together, these case reports suggest that these three ‘T. lewisi (-like)’ human infections were likely due to unidentified Herpetosoma.
In conclusion, to our knowledge, this is the first report of human trypanosomiasis in Thailand. Although an identification based on biometry was inconclusive, due to the limited morphological data for trypanosomes enzootic to Thailand, molecular diagnostics were indicative of a T. lewisi-like infection in this baby. Thus, rodents and fleas appear to be environmental risks for exposure to the parasites described in this report, rather than the presence of livestock and biting flies associated with exposure to T. evansi, such as in a case recently reported from India (Joshi et al., 2005). Further work is warranted to survey potential vertebrate and invertebrate hosts of both Trypanosoma subgenera in Thailand.
Acknowledgements
The authors gratefully acknowledge the generous assistance of staff members from Lampang Hospital and The Fifth Bureau of Animal Hygiene and Sanitation, Huaykaew, Chiangmai, for providing records and essential information. We also thank Professor Ikuo Igarashi, Director of the National Research Center for Protozoan Disease, Obihiro University of Agriculture and Veterinary Medicine, Japan, who kindly provided control DNA samples from T. evansi (Tansui, Taiwan isolate, D89527) and T. brucei rhodesiense (IL1501). This report was supported by Kasetsart University Research Development Institution (S. J.) and National Institutes of Health AI47932 (R. W. S.).
Abbreviations
- ITS1
internal transcribed spacer 1
- KI
kinetoplast index
- NI
nuclear index.
Footnotes
The GenBank/EMBL/DDBJ accession no. for the 560 bp ITS1 sequence of the T. lewisi-like trypanosome is DQ345394.
A table showing a multiple sequence alignment of ITS1 sequences is available with the online version of this paper.
References
- Desquesnes M, Ravel S, Cuny G. PCR identification of Trypanosoma lewisi, a common parasite of laboratory rats. Kinetoplastid Biol Dis. 2002;1:2. doi: 10.1186/1475-9292-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare CA. Morphologic and taxonomic studies on mammalian trypanosomes. X. Revision of the systematics. J Protozool. 1964;11:200–207. doi: 10.1111/j.1550-7408.1964.tb01741.x. [DOI] [PubMed] [Google Scholar]
- Hoare CA. The Trypanosomes of Mammals. A Zoological Monograph. Oxford and Edinburgh: Blackwell Scientific Publications; 1972. [Google Scholar]
- Johnson PD. A case of infection by Trypanosoma lewisi in a child. Trans R Soc Trop Med Hyg. 1933;26:467. [Google Scholar]
- Joshi PP, Shegokar VR, Powar RM, Herder S, Katti R, Salkar HR, Dani VS, Bhargava A, Jannin J, Truc P. Human trypanosomiasis caused by Trypanosoma evansi in India: the first case report. Am J Trop Med Hyg. 2005;73:491–495. [PubMed] [Google Scholar]
- Linardi PM, Botelho JR. Prevalence of Trypanosoma lewisi in Rattus norvegicus from Belo Horizonte, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2002;97:411–414. doi: 10.1590/s0074-02762002000300024. [DOI] [PubMed] [Google Scholar]
- Molyneux DH. Intracellular stages of Trypanosoma lewisi in fleas and attempts to find such stages in other trypanosome species. Parasitology. 1969;59:737–744. [PubMed] [Google Scholar]
- Natheewattana N, Hongsbhanich L, Khamboonruang C, Thitasut P. Preliminary study of a Trypanosoma lewisi-like parasite of rats in Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 1973;4:322–323. [PubMed] [Google Scholar]
- Sarataphan N, Siriwan C, Punyahotra R, Mekamol C, Meephuch Y, Assavamahasakda C. Trypanosomiasis in pigs; The Sixth Annual Conference of Thailand Livestock Development; Bangkok, Thailand: Ministry of Agriculture; 1986. [Google Scholar]
- Shrivastava KK, Shrivastra GP. Two cases of Trypanosoma species infection of man in India. Trans R Soc Trop Med Hyg. 1974;68:3–4. doi: 10.1016/0035-9203(74)90217-x. [DOI] [PubMed] [Google Scholar]