Abstract
Background & Aims
Studies of hepatitis C virus (HCV) infection, immunopathogenesis, and resulting liver diseases have been hampered by the lack of a small animal model. We developed humanized mice with human immune system and liver tissues to improve the studies of hepatitis C pathogenesis and treatment.
Methods
To promote engraftment of human hepatocytes, we expressed a fusion protein of the FK506 binding protein (FKBP) and caspase 8 under control of the albumin promoter (AFC8), which induces liver cell death, in Balb/C Rag2-/- γC-null mice. Co-transplantation of human CD34+ human hematopoietic stem cells (HSC) and hepatocyte progenitors into the transgenic mice led to efficient engraftment of human leukocytes and hepatocytes. We then infected these humanized mice (AFC8-hu HSC/Hep) with primary HCV isolates and studied HCV-induced immune responses and liver diseases.
Results
HCV-infected livers of AFC8-hu HSC/Hep mice generated a human immune T-cell response against HCV. HCV infection induced liver inflammation, hepatitis, and fibrosis, which correlated with activation of stellate cells and expression of human fibrogenic genes.
Conclusions
AFC8-hu HSC/Hep mice are a useful model of HCV infection, the immune response, and liver disease, because they contain human immune system and liver cells. These mice become infected with HCV, generate a specific immune response against the virus, and develop liver diseases that include hepatitis and fibrosis. This model might also be used to develop therapeutics for HCV infection.
Keywords: animal model of hepatitis, human immunology, fibrosis, virology
Introduction
Over 175 million people are chronically infected by hepatitis C virus (HCV), often resulting in hepatitis, liver fibrosis, cirrhosis and development of hepatocellular carcinoma (HCC)1. The liver consists of unique subsets of antigen presenting cells and lymphocytes2, 3. It is postulated that HCV infection in the liver leads to impaired immune response that contributes to its chronic infection in 80% of infected patients. Chronic inflammation and impaired T cell responses in the liver are proposed to lead to liver disease4-7. Due to the difficulty of studying HCV infection in human patients, very little is known about how HCV infects patients and evades host immunity to establish chronic infection in the liver.
HCV infection triggers robust CD4 and CD8 cytotoxic T cell (CTL) responses in infected patients. However, the majority of HCV patients fail to resolve HCV infection and become chronically infected, associated with impaired CD4 and CD8 T cell functions4, 7. Sustained CD4+ T helper activity in the blood is a hallmark of infections that are cleared spontaneously in 10-20% of HCV-infected patients4, 6, 8. While chimpanzees that resolve infection have strong CTL response, those with chronic infection show reduced CTL response8, 9. The CTL response in chronically infected HCV patients is also reduced8, 10, 11. During chronic HCV infection in humans and chimps, regulatory T cells (Treg) are also induced to subdue the anti-viral immune responses12, 13. Thus, both CD4 and CD8 T cells are involved in HCV immunopathogenesis. However, we understand very little of human immune response to HCV infection, due to lack of a robust model to study HCV infection and immuno-pathogenesis14.
Although chimpanzees have played a critical role in studying HCV infection15, there are several drawbacks, including low chronic infection rate and lack of liver fibrosis, as well as costs and ethical concerns, that limit their utility. A mouse engrafted with human liver cells and a functional human immune system will be an excellent model to study the virus14. A number of human-mouse chimeric liver models have been developed, but allow analyses of only limited aspects of HCV infection and pathogenesis16, 17. The Alb-uPA/SCID transgenic mouse contains the uPA transgene under control of an albumin promoter, and homozygous animals are unhealthy and die due to profound hypo-fibrinogenemia and accelerated hepatocyte death, which can be rescued by transplantation of mouse or human hepatocytes17, 18. The Alb-uPA/SCID mouse with efficient human hepatocyte engraftment can be infected with HCV17. It is reported recently that Fah-Rag2- γC-null mouse can also be highly engrafted with human hepatocytes to support HCV infection19-21. However, due to lack of a functional immune system, it is not possible to study HCV immuno-pathogenesis and no liver diseases are observed in Alb-uPA/SCID or Fah-Rag2-γC-null model17, 21.
The Balb/C Rag2-γC-null mouse supports development of a functional human immune system after injecting CD34+ human hematopoietic stem cells (HSC)22. To facilitate engraftment of human liver cells in Balb/C Rag2-γC-null mice, we expressed the active Caspase 8 fused with FK506 binding domain (FKBP) with inducible suicidal activity23. We co-transplanted human hepatocyte progenitor cells and CD34+ HSC into the transgenic mice and treated them with the FKBP dimerizer. Human immune cells and human hepatocytes were both efficiently developed, thereby generating a mouse model containing both a human immune system and human liver cells (AFC8-hu HSC/Hep). AFC8-hu HSC/Hep mice supported HCV infection in the liver and generated human T cell response to HCV. In addition, HCV infection induced liver inflammation and fibrosis, correlated with activation of stellate cells and expression of human fibrogenic genes.
Results
AFC8 mice can be efficiently repopulated with both human liver and immune cells
We constructed the FKBP-Caspase 8 fusion gene23 driven by the albumin enhancer/promoter (AFC8 gene)24. Dimerization of the active Caspase 8 by AP20187 induced apoptosis of cells in which it is expressed but not of bystander cells (Fig. 1A and Supplementary Figure 1). We then generated transgenic Balb/C Rag2-γC-null mice with the AFC8 transgene, and confirmed the liver-specific expression of the AFC8 transgene (Supplementary Figure 2).
Figure 1. AFC8 mice support engraftment of human hepatocytes in the liver.
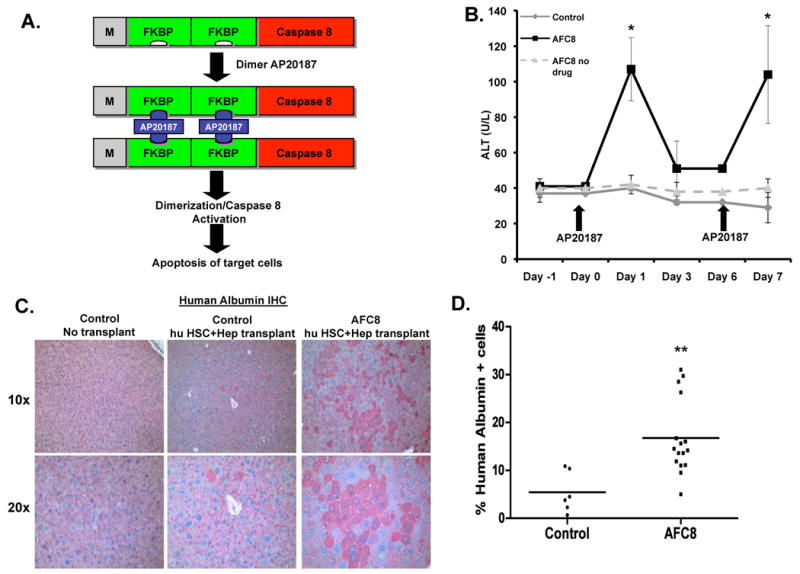
(A) Inducible activation of Caspase 8 through dimerization of FKBP-Caspase 8. The chemical dimerizer AP20187 leads to activation of Caspase 8 and apoptosis. (B) Mouse liver injury can be repeatedly induced in AFC8 transgenic mice. AFC8 or littermate control mice (4-6 weeks old) were injected with AP20187 at day 0 and day 6. ALT was measured in serum at -1, 0, 1, 3, 6 and 7 days post-drug treatment. Data are representative of three experiments with n = 4 per group. Data represent means ± s.d. *, P < .05. (C) Liver sections from control mice without transplant (left) and both control (middle) and AFC8 (right) transplanted with human HSC/Hep (AFC8-hu HSC/Hep) were stained with anti-human albumin antibody. Human hepatocytes were present around the veins and dispersed throughout all lobes of the liver. (D) Human albumin positive cells and total nucleated cells in the liver of humanized AFC8 and non-transgenic littermate control mice were counted and the percentage of Alb+ positive cells was calculated (n = 16 mice per group, **, P < .01).
When AFC8+ mice were treated with AP20187, we observed a repeated induction of ALT in AFC8 mice but not in littermate control mice (Figure 1B). To test the ability of the AFC8 mice to engraft human hepatocytes as well as human immune cells, human CD34+ cells (HSC) and hepatocyte progenitor cells (Hep) from the same human fetal liver tissue were injected into the liver of irradiated newborn AFC8 mice. We observed a significant increase of human albumin+ hepatocytes in the AFC8-hu chimeric liver (Figure 1C). About 15% (n>10 mice) of total nucleated cells in the chimeric liver expressed human albumin in AFC8-hu HSC/Hep mice from multiple human fetal liver tissues between 5-16 weeks post transplant (Figure 1D and Table 1). Using human gene-specific PCR primers (Supplementary Figure 3A), we also detected expression of human albumin and human hepatocyte genes CYP2E1, CYP2C9 and UGT2B7 in the liver of AFC8-hu HSC/Hep mice (Supplementary Figure 3B). As expected, AFC8-hu HSC/Hep mice also generated human immune cells in all lymphoid organs including the liver (Supplementary Figure 4 and data not shown). Therefore, we established a humanized mouse model with both human immune and human liver cells.
Table 1. Reconstitution of AFC8 mice with both human blood and liver cells.
| Donor | Mouse # | Sex | AFC8a | Cells Transplantedb | % CD45+ cellsc | % Albumin+d |
|---|---|---|---|---|---|---|
| n/a | 1 | F | - | none | - | |
| n/a | 2 | F | - | none | - | |
| 1 | 108 | M | + | 4e5 HSC + 1e6 HPC | 1.23 | 28.5 |
| 1 | 109 | M | + | 4e5 HSC + 1e6 HPC | 13.14 | 29.7 |
| 2 | 112 | M | + | 4.7e5 HSC + 1.5e6 HPC | - | 10.7 |
| 2 | 116 | F | - | 4.7e5 HSC + 1.5e6 HPC | 11.4 | 2.3 |
| 2 | 117 | F | + | 4.7e5 HSC + 1.5e6 HPC | 0.92 | 9.5 |
| 2 | 114 | M | + | 4.7e5 HSC + 1.5e6 HPC | 1.7 | 15.6 |
| 2 | 115 | F | + | 4.7e5 HSC + 1.5e6 HPC | 1.8 | 16.7 |
| 3 | 56*4 | F | - | 1e5HSC + 5e5 HPC | 1.4 | 4.5 |
| 3 | 57*4 | F | - | 1e5HSC + 5e5 HPC | 0.8 | 3.8 |
| 3 | 58*4 | F | + | 1e5HSC + 5e5 HPC | 0.3 | 16.0 |
| 3 | 59*4 | M | + | 1e5HSC + 5e5 HPC | - | n/a |
| 4 | 98*4 | M | - | 1e6 HSC + 1e6 HPC | 17.9 | 11 |
| 4 | 107*4 | M | - | 1e6 HSC + 1e6 HPC | 7.9 | n/a |
| 4 | 99*4 | M | + | 1e6 HSC + 1e6 HPC | 14 | 14 |
| 4 | 110*4 | F | + | 1e6 HSC + 1e6 HPC | 19.9 | 14 |
| 4 | 100*4 | M | + | 1e6 HSC + 1e6 HPC | 24.8 | 11 |
| 4 | 92*4 | F | + | 1e6 HSC + 1e6 HPC | 28.3 | |
| 4 | 108*4 | F | + | 1e6 HSC + 1e6 HPC | 34.4 | 26 |
| 5 | 56 | F | + | 1e6 HSC + 1e6 HPC | 31.1 | 14 |
| 5 | 58 | M | + | 1e6 HSC + 1e6 HPC | 32.3 | 15 |
| 5 | 54 | F | + | 1e6 HSC + 1e6 HPC | 34.9 | 11 |
| 5 | 57 | F | + | 1e6 HSC + 1e6 HPC | 64.9 | 12 |
| 5 | 581 | F | - | 1e6 HSC + 1e6 HPC | 10.2 | 1 |
+ = AFC8 transgenic mouse, - = non-transgenic littermates.
Number of cells transplanted into the mice. HSC = CD34+ hematopoietic stem/progenitor cells. HPC = Hepatocyte stem/progenitor cells.
% human CD45+ cells in the blood. -, <0.1%.
Liver sections were stained for human albumin. Human albumin positive cells were counted in >four 4× fields and averaged. The percentage was calculated relative to total nucleated cells in the liver.
HCV infection leads to liver infiltration and hepatitis in AFC8-hu HSC/Hep mice
We inoculated AFC8-hu mice with patient HCV isolates (genotype 1a) or control human serum (Table 2). We detected HCV genomic RNA in the liver of about 50% HCV-infected AFC8-hu mice (Figure 2A and Table 2) at termination (1-4 months post infection), but were unable to detect HCV viremia in the blood of infected mice (data not shown). As controls, we did not detect HCV RNA in mock-infected AFC8-hu HSC/Hep mice or in HCV-infected AFC8 mice with no human cell transplant (Figure 2A).
Table 2. HCV infection and liver pathology in AFC8-hu mice.
| Mouse # | HCV | HCV particles inoculated | days p.i.a | Metavir scoreb | Viral load in liver tissue (×103 copies/ug RNA)c |
|---|---|---|---|---|---|
| 1 | PBS | none | 80 | 0 | n/d |
| 2 | PBS | none | 110 | 0 | n/d |
| 87 | PBS | none | 110 | 1 | n/d *1 |
| 3 | HCV patient 1 | ∼9e5 | 110 | 1 | n/d |
| 4 | HCV patient 1 | ∼9e5 | 110 | 1 | n/d |
| 110d | HCV patient 1 | ∼9e5 | 110 | 1 | 18 *5 |
| 136d,e | HCV patient 1 | ∼9e5 | 93 | 2 | 87 *6 |
| 137d,e | HCV patient 1 | ∼9e5 | 89 | 2 | 180 *8 |
| 138d | HCV patient 1 | ∼9e5 | 110 | 1 | n/d *2 |
| 139d | HCV patient 1 | ∼9e5 | 80 | 1 | n/d *3 |
| 108 | HCV patient 1 | ∼9e5 | 32 | 3 | 164 *7 |
| 109 | HCV patient 1 | ∼9e5 | 80 | 3 | 14 *4 |
| 116f | Human sera | none | 62 | 1 | n/d |
| 117 | Human sera | none | 48 | 1 | n/d |
| 88*4 | Human sera | none | 97 | 1 | n/d |
| 89*4 | Human sera | none | 97 | 1 | n/d |
| 99*4 | Human sera | none | 69 | 1 | n/d |
| 110*4 | Human sera | none | 69 | 1 | n/d |
| 90*4 | HCV patient 2 | ∼5e5 | 34 | 3 | 323 |
| 91*4 | HCV patient 2 | ∼5e5 | 57 | 2 | 168 |
| 92*4 | HCV patient 2 | ∼5e5 | 97 | 4 | n/d |
| 98*4 | HCV patient 2 | ∼5e5 | 69 | 1 | n/d |
| 100*4 | HCV patient 2 | ∼5e5 | 69 | 2 | 347 |
| 108*4 | HCV patient 2 | ∼5e5 | 97 | 2 | n/d |
mice sacrificed due to experimental endpoints or poor health on days post HCV injection.
determined by analysis of Sirius Red stained liver sections. 0 = no fibrosis; 1 = only portal fibrosis; 2 = portal fibrosis with few septa; 3 = numerous septa without cirrhosis; 4 = cirrhosis.
HCV viral load in the liver tissue. *(1-8) mouse # from figure 2a. n/d = not detectable.
AFC8 mice reconstituted with human adult hepatocytes.
Mice found dead (analyzed between 12-24 hr after death) on the indicated days post infection.
Mouse #116, a non-transgenic mouse.
Figure 2. HCV infection in AFC8-hu HSC/Hep mice leads to human immune infiltration and liver injury.
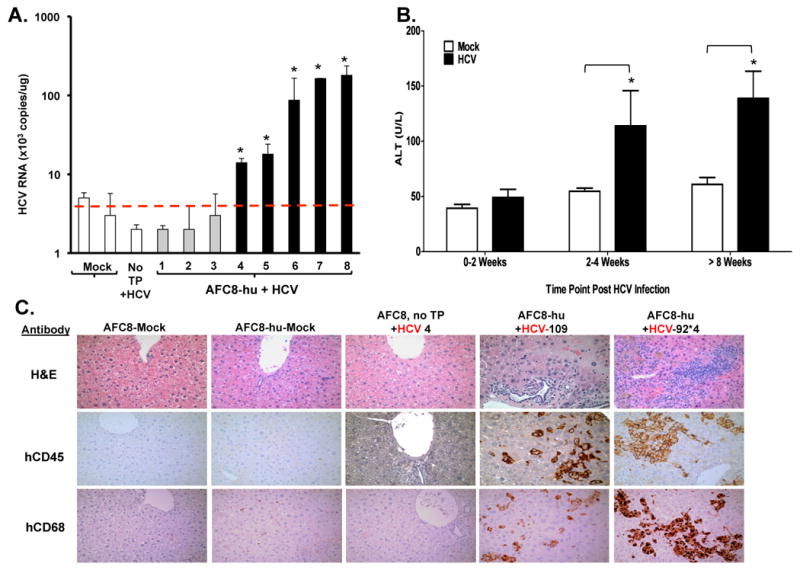
AFC8-hu HSC/Hep mice or control mice were infected with HCV patient isolates (genotype 1a, 1×10e5 i.u./mouse) or control serum. Blood samples were collected at various times after infection. Liver samples were collected at termination time points. (A) HCV genomic RNA was detected in the liver of HCV-infected AFC8-hu mice. Liver tissues were harvested from HCV-infected AFC8-hu or control mice at 70-80 days post-infection. Values represent HCV RNA copy#/ug liver-derived RNA in triplicates. * P <.05. (B) Increased ALT in sera from HCV-infected AFC8-hu or control mice. Data represent mean ± s.e.m (n = 6 mice per group). *, P < .05. (C) Representative liver sections from control/mock, AFC8-hu/mock, control (no transplant)/HCV, and two AFC8-hu/HCV mice were stained to detect leukocyte infiltration with H&E (top panels), or with anti-human CD45 (middle panels) or human CD68 (bottom panels).
When ALT levels were measured at various times after HCV infection, significant induction of ALT was detected in HCV-infected AFC8-hu mice after 2-4 weeks post infection, but not in control mice (Figure 2B). Consistent with elevated ALT levels, we observed significant leukocyte infiltration into the liver in HCV infected AFC8-hu HSC/Hep mice (Figure 2C). We observed an increase of human CD45+ leukocyte, including CD68+ macrophages and CD3+ T cells, in the liver of HCV infected mice (Figure 2C lower panel and Supplementary Figure 5A). When total intrahepatic leukocytes were analyzed, we detected an increase of multiple human leukocyte subsets including CD3+CD4+ and CD3+CD8+ T cells, CD3-CD56+ natural killer cells, and plasmacytoid dendritic cells (pDC, CD123+CD4+CD3-) in the liver of HCV infected mice (Supplementary Figure 5B). Similar infiltrations have been reported in HCV-infected humans25-27. Thus AFC8-hu HSC/Hep mice were able to support HCV infection in the liver, leading to liver infiltration and injury.
HCV infection induces HCV-specific human T cell immune response
To characterize the human T cell response in HCV-infected AFC8-hu HSC/Hep mice, we stimulated (PHA for 12 hrs) spleen and lymph nodes (LN) cells from mock- or HCV-infected mice and measured human effector cytokines (IL2, IFNγ, and TNFα) in human CD4+ and CD8+ T cells. We observed a significant increase of all three cytokines in both CD4+ (Figure 3A,B) and CD8+ (Figure 3C) T cells from HCV-infected AFC8-hu HSC/Hep mice. Therefore, HCV infection primed human T cells in AFC8-hu HSC/Hep mice.
Figure 3. HCV infection induces human T cell response in AFC8-hu mice.
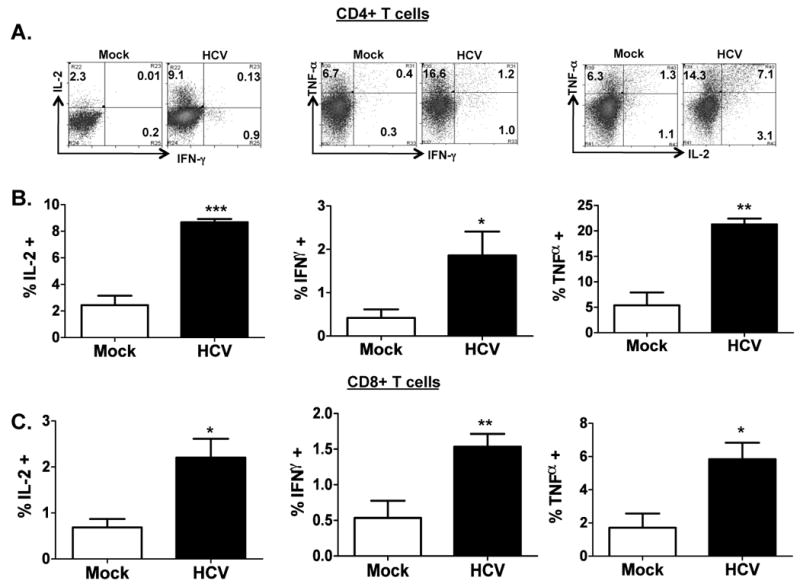
Spleen and lymph node cells from mock- and HCV-infected AFC8-hu mice at 6-8 weeks post infection were stimulated for 12-16 hours with PHA. Cells were stained with antibodies for human T cells (CD3, CD4 and CD8) and for intracellular human cytokines (IL-2, IFN-γ and TNF-α). (A) Human CD3+ CD4+ T cells were analyzed for expression of effector cytokines. Representative FACS plots are shown for each cell population. Numbers represent the % cytokine+ of CD4 or CD8 T cells. (B) Increase of human CD4 effector T cells in HCV infected AFC8-hu mice. Summarized data (n=4 mice/group) are shown. (C) Increase of human CD8 effector T cells in HCV infected AFC8-hu mice. Human CD3+ CD8+ T cells were similarly analyzed for expression of effector cytokines. Data represent mean ± s.e.m from two cohorts of mice with n = 4 per group. *, P <.05; **, P <.01; ***, P <.001.
To detect HCV-specific T cell response, we stimulated the spleen/LN cells from mock and HCV infected mice with an overlapping peptide pool from the HCV core region in the presence of anti-CD28 mAb. After 8-10 days in culture, we observed a 4-5 fold preferential expansion of human CD3+ T cells from HCV-infected mice relative to mock control mice (Figure 4A). When restimulated with HCV core peptides, both human CD4+ (Figure 4B,C) and CD8+ (Figure 4D,E) T cells from HCV-infected mice also responded to produce IFNγ, IL2 and TNFα. Thus HCV infection in AFC8-hu HSC/Hep mice induced specific human T cell response to HCV antigens.
Figure 4. HCV infection induces HCV-specific human T cell response in AFC8-hu mice.
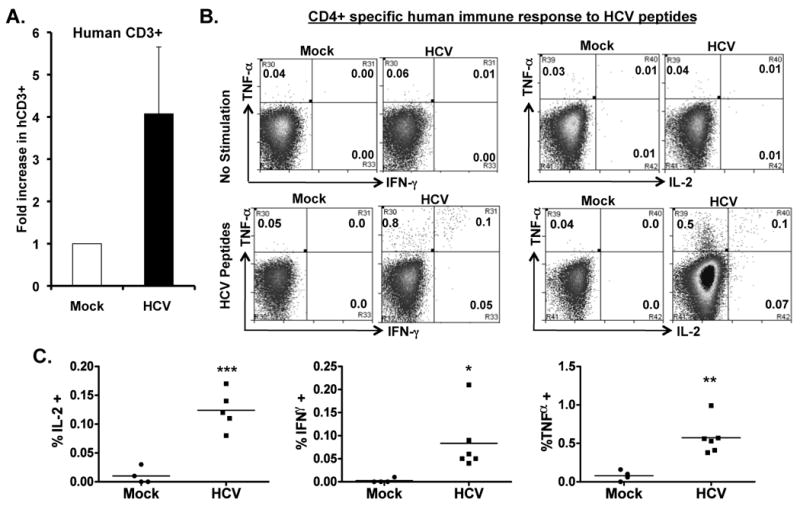
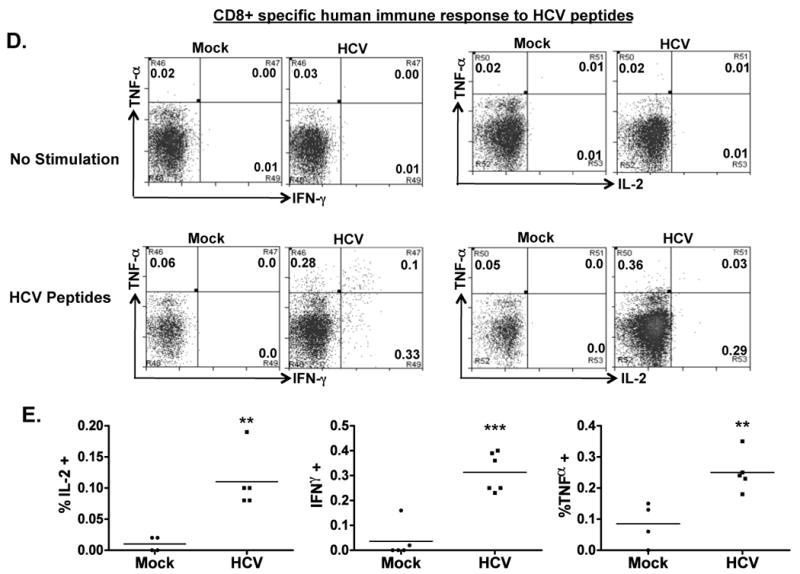
(A) Preferential expansion of human T cells from HCV-infected AFC8-hu mice after stimulation with HCV Core peptides. Spleen and LN cells from mock and HCV-infected AFC8-hu mice were stimulated with a pool of HCV Core peptides + anti-CD28 mAb, and cultured for 8-10 days. Fold increase of human CD45+CD3+ cells from HCV infected samples (n=4) was calculated relative to mock samples (n=3). P <.05. (B-E) The expanded T cells were re-stimulated with HCV peptides and stained for intracellular human cytokines (IL-2, IFN-γ and TNF-α). (B) Human CD4+ T cells are analyzed for expression of the cytokines. No stimulation controls show background. (C) Summarized results of individual mice are shown. (D) Human CD8+ T cells are analyzed for expression of the cytokines. Representative FACS plots are shown for each cell population. Numbers in FACS plots represent the % cytokine+ of CD4 or CD8 T cells. (E) Shown are summarized data of individual mice from two cohorts of mice (n = 4 per group). Lines indicate the mean values. *, P <.05; **, P <.01; ***, P < .001.
AFC8-hu HSC/Hep mice develop liver fibrosis after HCV infection
We then assessed liver pathology in HCV-infected AFC8-hu HSC/Hep mice. About 50% of HCV-infected AFC8-hu HSC/Hep mice had severe fibrosis throughout the liver parenchyma (Figure 5A, top panels and Table2), whereas mock-inoculated AFC8-hu HSC/Hep mice and HCV-inoculated AFC8 mice without human transplants did not develop fibrosis. Infiltrated human CD45+ cells in the liver were detected in the fibrotic regions (Figure 2). In the absence of a functional immune system, uPA/SCID or Fah-Rag-γC-null mice transplanted with only human hepatocytes supported HCV infection but no significant liver fibrosis17, 21. We analyzed HCV infection and liver pathology in five AFC8-hu mice transplanted with only human adult hepatocytes, and did not detect significant liver fibrosis (Table 2). The two AFC8-hu Hep mice with low-grade fibrosis were analyzed 12-24 hrs after unexpected death.
Figure 5. AFC8-hu HSC/Hep mice develop liver fibrosis after HCV infection.
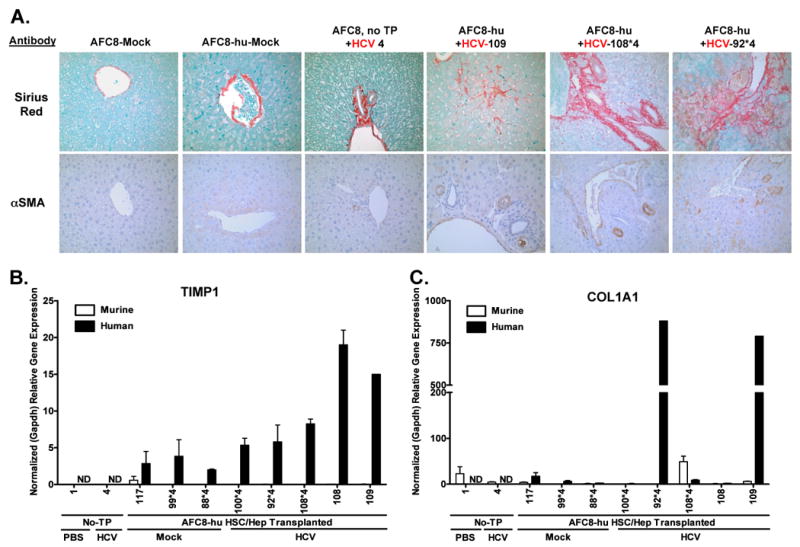
(A) Representative liver sections from AFC8/mock, AFC8-hu/mock, AFC8 (no transplant)/HCV, and three AFC8-hu/HCV mice were stained with Sirius Red/Fast Green (top panels) to visualize liver fibrosis. Liver sections were also stained with a mouse monoclonal antibody against human α-smooth muscle actin (αSMA, bottom panels) to visualize activated stellate cells. Human or murine specific gene expression of TIMP1 (B) and COL1A1 (C) in the liver was measured using species-specific PCR primers. Values shown are relative gene expression normalized to human or mouse GAPDH, respectively. Data represent mean ± s.e.m.
In human patients, HCV infection leads to activation of hepatic stellate cells, which contributes to liver fibrosis28. We stained liver sections with the mouse anti-human α-smooth muscle actin (αSMA) mAb to detect activated stellate cells. We observed activated stellate cells in the liver of HCV-infected AFC8-hu HSC/Hep mice (Figure 5, A bottom panels). Activated hepatic stellate cells also express extracellular matrix protein Col1A1 and TIMP1 which inhibits degradation of extracellular matrix and fibrosis resolution29. We thus analyzed the expression of these genes in the liver with human-specific or mouse-specific PCR primers (Supplemental Figure 6). Interestingly, we detected specific induction of human TIMP1 (Figure 5B) and human COL1A1 (Figure 5C), but not mouse fibrosis genes, in the liver of some HCV-infected AFC8-hu HSC/Hep mice. These results suggest that HCV infection activated human stellate cells and induced expression of human fibrogenic genes in the chimeric liver of AFC8-hu HSC/Hep mice.
Discussion
We report here a novel mouse model that supports efficient engraftment of both human immune cells and human liver cells. The Balb/C Rag2-γC-null mouse with the AFC8 transgene enabled us to inducibly deplete murine hepatocytes. In addition to human immune cells in lymphoid and liver organs, AFC8-hu HSC/Hep mice were also efficiently repopulated with human albumin+ liver cells. AFC8-hu HSC/Hep mice supported HCV infection, which induced HCV-specific human immune response, liver infiltration, hepatitis and fibrosis.
In the absence of a functional immune system, uPA/SCID or Fah-Rag-γC-null mice transplanted with only human hepatocytes supported HCV infection but no significant liver fibrosis17, 21. Immuno-deficient mice expressing the uPA transgene in the liver or carrying the Fah mutation allow human adult hepatocytes to efficiently re-populate the liver17-20. These mice have poor health including neonatal death and, most importantly, the lack a human immune system. The severe liver injury in these mice may impair the development and function of human liver and immune cells. To overcome these deficiencies, the AFC8 mouse enabled us to inducibly deplete murine hepatocytes through a programmed cell death mechanism without bystander cell killing. In addition, the Rag2-γC-null mouse in Balb/C background permits efficient engraftment of human immune cells. Therefore, AFC8-hu HSC/Hep mice provide the first humanized mouse model with both human immune and liver cells.
We inoculated AFC8-hu HSC/Hep mice with HCV genotype 1a clinical isolates. We were able to detect HCV genomic RNA in the liver of AFC8-hu HSC/Hep mice (Figure 2A and Table 2), but we did not detect significant HCV viremia in the blood of HCV-inoculated AFC8-hu HSC/Hep mice. This may be due to the relative low level of human hepatocyte engraftment (∼15%). To detect HCV viremia in the blood, the adult hepatocyte-engrafted uPA or Fah models support >50% engraftment of human hepatocytes17, 19-21. In the future we will improve the AFC8-hu HSC/Hep model by optimizing the death induction conditions with different doses and times of dimer injections. It is also reported that the NOD-Scid-γC-/- (NSG) mouse is more permissive in accepting human cells30. It is likely that the introduction of the AFC8 transgene into NSG mice will create a better host for engrafting human immune and liver cells. To get different sources of human HSC and liver progenitor cells, the AFC8-hu mouse model will be useful to study functional differentiation of human embryonic stem (hES) or induced plural-potent (iPS) cells to the human liver lineages. The construction of AFC8-hu HSC/Hep mouse with HSC and liver progenitor cells derived from ES or iPS cells will be an exciting future direction.
HCV infection appeared to induce human immune responses in AFC8-hu SHC/Hep mice. We observed significant infiltration into the liver of HCV-infected AFC8-hu HSC/Hep mice, including human T cells, macrophages, DC and NK cells. Not only did we observe a preferential expansion of human T cells from HCV-infected AFC8-hu HSC/Hep mice with HCV-derived peptides, the expanded T cells also expressed IL2, TNFα and IFNγ in response to HCV peptide re-stimulation (Figure 4). Therefore, HCV infection induced HCV-specific human T cell responses in AFC8-hu HSC/Hep mice. It will be interesting to test if depletion of human T cells (CD4 and/or CD8) will affect HCV infection and pathogenesis in AFC-hu HSC/Hep mice. It will also be of interest to characterize and study human T cell subsets such as regulatory T cells and NKT cells in future experiments. Consistent with poor B cell response has been reported in all humanized mouse models, we failed to detect HCV-specific human antibodies in HCV-infected AFC8-hu HSC/Hep mice (data not shown).
In human patients, immune responses against HCV are implicated as mediators of liver diseases31, 32. Remarkably, HCV-infected AFC8-hu mice developed human leukocyte infiltration, hepatitis and liver fibrosis throughout the liver parenchyma with bridging septa (Figure 5). Human CD3+ T cells and CD68+ macrophages, as well as human albumin+ hepatocytes and αSMA+ activated stellate cells, were detected in and near the fibrotic region (supplementary Figure 7), suggesting contribution of human leukocytes to liver fibrosis. We also showed that HCV infection led to increased level of activated human stellate cells in fibrotic livers. Human extracellular matrix protein Col1A1 and inhibitor of matrix degradation TIMP1 were induced in HCV-infected AFC8-hu HSC/Hep mouse livers. It is of interest that the corresponding mouse fibrosis-associated genes were not induced by HCV infection. The lack of liver fibrosis in chronically infected chimpanzees also suggests a species-specific nature of HCV-induced liver fibrosis33. The AFC8-hu HSC/Hep mouse will provide an excellent model to elucidate the mechanisms of HCV-induced human liver fibrosis.
Since HIV co-infection occurs in nearly 25% of HCV patients and often leads to accelerated end-stage liver disease32, 34, 35, there is a significant need for a model system to study HCV/HIV co-infection. It will be of interest to test how HIV-1 infection will affect HCV infection and pathogenesis in the AFC8-hu HSC/Hep mouse. In addition to HCV infection and immuno-pathogenesis, AFC8-hu HSC/Hep mice will also be useful to study other liver-tropic pathogens such as HBV and malaria.
Methods
Plasmids
The FKBP-Caspase 8 gene was generated by cloning active human Caspase 8 (fragment Ser217-Asp479, kindly provided by Dr. Terry Combs, UNC-Chapel Hill) into the pC4M-Fv2E vector (Ariad Pharmaceuticals) to express the FKBP-Caspase 8 (FC8) fusion protein23. The transgenic construct was generated with the Albumin enhancer/promoter24 (kindly provided by Dr. Snorri Thorgessons, NIH) controlling expression of the FKBP-Caspase 8 gene.
Generation of AFC8 transgenic mice
Animal procedures were obtained approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC). Balb/C Rag2-/-γC-/- mutant female mice were super-ovulated and the fertilized eggs were isolated to generate transgenic mice by standard procedures in the UNC transgenic core facility. AFC8 mice were bred and handled under specific pathogen free conditions in the UNC DLAM facility.
Induction of murine hepatocyte death in AFC8 mice
We injected mice ip or iv with AP20187 (Ariad Pharmecuticals) or vehicle only (4% ethanol, 10% PEG-400, 2% Tween-20 in water) at a dose of 5 ug/g body weight at indicated days post transplant of human cells23. ALT levels were measured in the blood of treated mice.
Human hepatocyte progenitor cells and CD34+ HSC from human fetal liver
Human hepatocyte progenitor and CD34+ cells were isolated from 15-18 weeks old fetal liver tissue essentially as described 36-38. Parenchyma cells (30% EpCAM+ hepatoblasts/progenitors) were separated from the non-parenchyma cells (including CD34 HSC cells). CD34+ cells were isolated by magnetic-activated cell sorting (MACS), and the purity was greater than 95%.
Transplantation of AFC8 mice with human CD34 HSC and liver progenitor cells
CD34+ HSCs (0.5 -1×106) and Hep progenitor cells (0.5-1×106) from the same donor were co-injected into the liver of 1 to 5 days old newborn AFC8 and littermate control mice, previously irradiated at 400 rad36, 38. We then injected AFC8-hu mice with AP20187 (Ariad Pharmecuticals) at a dose of 5ug/g body weight as described above.
Blood and tissue analysis
AFC8-hu HSC/Hep mice were bled to measure human leukocyte reconstitution at 12-16 weeks post transplant. At termination, liver tissue was snap-frozen in RNAlater (Qiagen) or fixed in 10% formalin. Liver sections were stained with hematoxylin and eosin (H&E), Sirius Red and Fast Green, or with antibodies: mouse monoclonal anti-human α smooth muscle actin (1A4, Dako), mouse monoclonal anti-human CD45 (2B11 + PD7/26, Dako), and anti-human albumin (Dako). Immunoreactivity was determined by incubation with DAB substrate (Pierce) or Vulcan red (Dako), and counterstained with hematoxilin36, 38.
Human-specific gene expression
Human or mouse specific quantitative real time PCR primers were designed using NCBI primer design program and Blast database (see supplemental Figures 3 & 6). Gene expression analysis was examined using Thermo Scientific SYBR Green Realtime PCR reagents. Values shown are relative gene expression normalized to specific GAPDH.
HCV infection of AFC8-hu HSC/Hep mice
AFC8-hu HSC/Hep mice or control mice were inoculated iv with 100ul of clinical isolates of HCV genotype 1a (1∼5×107 genome copies/ml) or control human sera. Mouse blood and liver tissues were harvested to prepare RNA for measuring HCV genomic RNA as reported39.
T cell response analysis
Leukocytes from various tissues of AFC8-hu mice were analyzed by FACS for human surface markers. Total spleens and lymph node cells containing 1 × 105 human CD45+ cells were stimulated for 20 hours with 10ug/ml PHA in IMDM + 10% FBS media (Gibco). The cells were then stained for human T cells (CD45, CD3, CD4, CD8), and for intracellular human IL-2, TNF-α, and IFN-γ. To detect HCV-specific human T cell response, human T cells were expanded by stimulating spleen/LN cells with a pool of 19 HCV core peptides (20mer overlapping by 10) at 10ug/ml each + 1ug/ml anti-CD28 mAb. The cells were then cultured for 8-10 days with expansion medium (IMDM, 10% FBS, 10U/mL human IL-2 and 125ng/mL IL-7). The cells were re-stimulated with the same HCV core peptide pool for 18 hours and stained as above.
Statistical analysis
We used unpaired two-tailed Student's t-tests for all comparisons. p<0.05 is considered significant. All data are reported as means ± standard deviation (s.d.) or standard error (s.e.m) as indicated.
Supplementary Material
Acknowledgments
We thank Drs. F. Bai and A. Rogers for help with liver pathology analysis; L. Chi, T.A. Curtis and L. Chen for technical support; Drs. M. Fried and J. Darling for providing the patient HCV sera (UNC Division of Gastroenterology and Hepatology), Dr. L Arnold for FACS support and UNC CFAR for virology support.
Grant Support- This work was supported in part by grants from a UNC UCRF innovation grant; from NIH (AI076142, AA018009 to L.S.), an immunology training grant (T32 AI007273 to M.L.W) and UNC Lineberger Comprehensive Cancer Center Postdoctoral Training Grant (M.T.B); and a grant from LCRF (JAF). This work was also supported by the Greenberg Medical Research Institute, the Ellison Medical Foundation, the Starr Foundation, the Ronald A. Shellow Memorial Fund, the Richard Salomon Family Foundation, and by a grant from the Foundation NIH through the Grand Challenges in Global Health initiative (C.M.R. and A.P.) and a grant from the Center for Clinical and Translational Research (RR024143 to A.P.).
Abbreviations
- HCV
Hepatitis C Virus
- HSC
hematopoietic stem cells
- SCID
severe combined immunodeficiency
- Alb
albumin
- ALT
alanine aminotransferase
- LN
lymph nodes
- TIMP1
tissue inhibitor of matrix metalloproteinases 1
- Col1A1
Collagen 1A1
- NK
natural killer cells
- pDC
plasmacytoid dendritic cells
- PHA
phytohaemagglutinin
- Treg
regulatory T cells
- uPA
urokinase-type plasminogen activator
- Fah
fumarylacetoacetate hydrolase
- AFC8
FKBP-Caspase 8 gene driven by the albumin promoter
- AFC8-hu HSC/Hep
AFC8 transgenic mouse transplanted with human CD34+ hematopoietic stem cells and hepatocyte progenitor cells
- αSMA
α-smooth muscle actin
Footnotes
Disclosures- The authors declare no competing financial interests.
Author Contributions- M.L.W. contributed to experimental design, performance, data analysis and wrote the manuscript. L.Z. contributed to initial experimental design, performance and data analysis, M.T.B. contributed to apoptosis and liver gene expression assays, G.I.K. contributed to animal experimental and T cell results; A.B. and J.A.F. to HCV-specific T cell immune responses, and W.B., A.P. and C.M.R. to measuring HCV viral load and data analysis. L.S. conceived the research project, planned and designed the experiments, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruno S, Facciotto C. The natural course of HCV infection and the need for treatment. Ann Hepatol. 2008;7:114–9. [PubMed] [Google Scholar]
- 2.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 3.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 4.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 5.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 6.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 7.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 8.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–49. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, Shintani AK, Walker CM, Kalams SA. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J Exp Med. 2004;200:307–19. doi: 10.1084/jem.20040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–44. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 11.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeg MH, Ulsenheimer A, Gruner NH, Jung MC, Gerlach JT, Raziorrouh B, Schraut W, Horster S, Kauke T, Spannagl M, Diepolder HM. FOXP3(+) Expression in Hepatitis C Virus-Specific CD4(+) T Cells During Acute Hepatitis C. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–48. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP, Eisenbarth S, Eynon E, Flavell RA, Guzman CA, Huntington ND, Kremsdorf D, Manns MP, Manz MG, Mention JJ, Ott M, Rathinam C, Rice CM, Rongvaux A, Stevens S, Spits H, Strick-Marchand H, Takizawa H, van Lent AU, Wang C, Weijer K, Willinger T, Ziegler P. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukh J. A critical role for the chimpanzee model in the study of hepatitis C. Hepatology. 2004;39:1469–75. doi: 10.1002/hep.20268. [DOI] [PubMed] [Google Scholar]
- 16.Maeda N, Watanabe M, Okamoto S, Kanai T, Yamada T, Hata J, Hozumi N, Katsume A, Nuriya H, Sandhu J, Ishii H, Kohara M, Hibi T. Hepatitis C virus infection in human liver tissue engrafted in mice with an infectious molecular clone. Liver Int. 2004;24:259–67. doi: 10.1111/j.1478-3231.2004.0909.x. [DOI] [PubMed] [Google Scholar]
- 17.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–33. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 18.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–56. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 19.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–10. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissig KD, Le TT, Woods NB, Verma IM. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci U S A. 2007;104:20507–11. doi: 10.1073/pnas.0710528105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–30. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 23.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 24.Conner EA, Lemmer ER, Omori M, Wirth PJ, Factor VM, Thorgeirsson SS. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene. 2000;19:5054–62. doi: 10.1038/sj.onc.1203885. [DOI] [PubMed] [Google Scholar]
- 25.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2009;138:325–35. e1. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulsenheimer A, Gerlach JT, Jung MC, Gruener N, Wachtler M, Backmund M, Santantonio T, Schraut W, Heeg MH, Schirren CA, Zachoval R, Pape GR, Diepolder HM. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–51. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 27.Yoshizawa K, Abe H, Kubo Y, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y. Expansion of CD4(+)CD25(+)FoxP3(+) regulatory T cells in hepatitis C virus-related chronic hepatitis, cirrhosis and hepatocellular carcinoma. Hepatol Res. 2010 doi: 10.1111/j.1872-034X.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 28.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–40. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–60. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 30.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Burzenski L, Gott B, Foreman O, Kavirayani A, Herlihy M, Rossini AA, Shultz LD, Greiner DL. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y, Fuller L, Carreno M, Zucker K, Roth D, Esquenazi V, Karatzas T, Swanson SJ, 3rd, Tzakis AG, Miller J. The immune reactivity role of HCV-induced liver infiltrating lymphocytes in hepatocellular damage. J Clin Immunol. 1997;17:140–53. doi: 10.1023/a:1027326415164. [DOI] [PubMed] [Google Scholar]
- 32.Kim AY, Chung RT. Coinfection with HIV-1 and HCV--a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigger CB, Guerra B, Brasky KM, Hubbard G, Beard MR, Luxon BA, Lemon SM, Lanford RE. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78:13779–92. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Sierra C, Arizcorreta A, Diaz F, Roldan R, Martin-Herrera L, Perez-Guzman E, Giron-Gonzalez JA. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–8. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 35.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Q, Zhang L, Wang R, Jeffrey J, Washburn ML, Brouwer D, Barbour S, Kovalev GI, Unutmaz D, Su L. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2-/-gammaC-/- mice in vivo. Blood. 2008;112:2858–68. doi: 10.1182/blood-2008-03-145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–8. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109:2978–81. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G, Rice CM. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103:3805–9. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


