Abstract
Lectin-like oxidized LDL receptor-1 (LOX-1) is an endothelial receptor for oxidized LDL (oxLDL) and plays multiple roles in the development of cardiovascular diseases. We screened more than 400 foodstuff extracts for identifying materials that inhibit oxLDL binding to LOX-1. Results showed that 52 extracts inhibited LOX-1 by more than 70% in cell-free assays. Subsequent cell-based assays revealed that a variety of foodstuffs known to be rich in procyanidins such as grape seed extracts and apple polyphenols, potently inhibited oxLDL uptake in Chinese hamster ovary (CHO) cells expressing LOX-1. Indeed, purified procyanidins significantly inhibited oxLDL binding to LOX-1 while other ingredients of apple polyphenols did not. Moreover, chronic administration of oligomeric procyanidins suppressed lipid accumulation in vascular wall in hypertensive rats fed with high fat diet. These results suggest that procyanidins are LOX-1 inhibitors and LOX-1 inhibition might be a possible underlying mechanism of the well-known vascular protective effects of red wine, the French Paradox.
Keywords: LOX-1, cardiovascular diseases, lipid accumulation, procyanidin, French Paradox
Introduction
Atherosclerosis is a leading burden of cardiovascular diseases such as coronary artery diseases and stroke. It is a chronic, multifactorial disease of vascular wall involving lipid accumulation, inflammation, cell death, and thrombosis. The changes in the vascular wall, which precede by far earlier the appearance of coronary artery diseases.1,2) Environmental factors such as diet and cigarette smoking together with such pathological conditions as hyperlipidemia, hypertension and diabetes greatly influence the disease progression over time. Therefore, lifestyle changes such as diet control and smoking cessation are strongly encouraged in patients with high risk of cardiovascular diseases.
Lectin-like oxidized receptor-1 (LOX-1) was originally identified as an endothelial receptor for oxidized LDL (oxLDL), a critical player involved in the initiation of atherosclerosis.3) OxLDL binds to LOX-1 and increases expressions of adhesion molecules and inflammatory cytokines, and decreases nitric oxide release, resulting in endothelial dysfunction.4–6) LOX-1 also recognizes other ligands such as platelets and C-reactive protein, and mediates platelet adhesion and vascular hyperpermeability, respectively.7,8) LOX-1 is expressed not only in endothelial cells but in macrophages, vascular smooth muscle cells, platelets and cardiomyocytes; and the expression is highly induced under atherogenic settings such as hyperlipidemia, hypertension and diabetes.9–11) In vivo evidence has also demonstrated that LOX-1 contributes to the initiation and development of a wide range of cardiovascular diseases. For instance, LOX-1 gene-deficient mice display less atherosclerotic lesions on high fat diet and less myocardial injury after ischemia-reperfusion.12,13) Conversely, LOX-1 overexpressing mice display increased atheroma-like lesions and impaired endothelium-dependent vasorelaxation on high fat diet.14,15) Importantly, we have recently demonstrated that high LOX Index, which is calculated by multiplying circulating concentrations of soluble LOX-1 and LOX-1 ligand LDL, associates with an increased risk of coronary heart diseases and stroke in Japanese population.16) These lines of evidence suggest that inhibition of LOX-1 could be a strategy for the prevention and/or treatment of cardiovascular diseases.
Although statin therapy has made a success to reduce the risk of cardiovascular events, multiple lines of evidence have suggested that daily intake of certain foods or beverages could be an effective strategy to prevent the development of cardiovascular diseases. For instance, fish oil and red wine have been long postulated to possess cardioprotective actions.17,18) Moreover, a case–control study involving participants from 52 countries reported an inverse association between the risk of myocardial infarction and intake of prudent diet (high in fruit and vegetables).19) However, the molecular targets and/or the active ingredients of those foods and beverages are largely unknown although a number of health food supplements are available in the market. This study was undertaken to identify materials that inhibit oxLDL binding to LOX-1 from foodstuff extracts.
Materials and methods
Preparation of lipoproteins.
Serum was isolated from healthy volunteers and LDL (density: 1.019–1.063 g/mL) was prepared by sequential ultracentrifugation. Isolated LDL was oxidized with 7.5 µM CuSO4 for 16 h and labeled with 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen, Carisbad, CA, USA) (DiI-oxLDL) as described previously.3)
Preparation of procyanidins.
Procyanidins were separated and purified from apple polyphenols as previously described20,21) and lyophilized until use.
Primary screening in LOX-1 ELISA.
437 foodstuff extracts and 35 test reagents stored in Asahi Breweries, Ltd. were used for the screening. Powdery foodstuff extracts were collected by micro spatula, dissolved in 1 mL DMSO and centrifuged to remove the unsolved fraction. The solutions were diluted 50-fold in 10 mM HEPES buffer containing 5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid (EDTA). They were mixed with oxLDL to the final concentration of oxLDL at 1 µg/mL and were added to 384-well plate (Greiner, Frickenhausen, Germany) coated with human LOX-1 (61-273, aa). OxLDL binding to LOX-1 was determined using horseradish peroxide (HRP)-conjugated sheep anti-human apoB (The Bindingsite, Birmingham, UK) as previously reported.16) OxLDL binding was expressed as a ratio of the binding in the presence of foodstuff extracts to that in the presence of vehicle alone. The final concentration of DMSO was less than 0.5% of total volume.
Secondary screening in CHO cells expressing LOX-1.
Tetracycline-inducible human LOX-1 (tagged with V5-6×His at C-terminus) expressing CHO-K1 (LOX-1-CHO) cells were maintained as previously described.8) The cells were seeded in 96-well plate at 104 cells/well in the presence of doxycycline (1 µg/mL) (Calbiochem, La Jolla, CA, USA) and were incubated in Ham’s F-12 medium containing 10% FBS at 37 ℃ for 24 h. After being washed with the medium without FBS, the cells were treated with foodstuff extracts or an anti-LOX-1 antibody at the final concentration of 10 µg/mL for 1 h. The cells were washed again and incubated with DiI-oxLDL (10 µg/mL) for 2 h. After washing, the cells were fixed with 10% formalin, and were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO, USA). The binding of DiI-oxLDL to LOX-1-CHO cells were determined similarly to the uptake assay, except that the incubation of DiI-oxLDL was performed at 4 ℃ for 45 min. The samples were subjected to fluorescence microscopic analysis (Axiovert 200M, Zeiss, Oberkochen, Germany) or quantitative fluorescence analysis using the IN Cell analyzer system (GE Healthcare, Fairfield, CT, USA). Cell-associated DiI-oxLDL was determined as a ratio of DiI-oxLDL fluorescence intensity per cell in the presence of foodstuff extracts to that in the absence of extracts. All experiments were conducted in more than triplicates. For the determination of fluorescence quenching by procyanidins, the fluorescence was also measured before washing out unbound DiI-oxLDL (Spectramax, Molecular Devices, Sunnyvale, CA, USA). The final concentration of DMSO was less than 0.1% of total volume.
Animal study.
All protocols were approved by the Institutional Animal Care and Use Committee of the National Cerebral and Cardiovascular Center. Animals were individually housed at 23 ± 2 ℃ with 12 h light-dark cycles (7:00–19:00 light-on). Eight-week-old male stroke-prone spontaneously hypertensive rats (SHR-SP) (CLEA Japan, Tokyo, Japan) were divided into two groups (n = 8 each) with similar blood pressure, heart rates and body weights (tail cuff and pulse transducer system, BP-98A, Softron, Tokyo, Japan). They were given high fat diet without cholic acid or vitamin E (CLEA Japan, Tokyo, Japan) and physiological saline as drinking water for 2 weeks. Half of the rats received physiological saline containing 0.5%(w/v) oligomeric procyanidins (OPC) with various chain lengths purified from apples. At the end of the study, the rats were euthanized with isoflurane inhalation and blood was collected from vena cava between 11:00–14:00. The rats were perfused systemically with physiological saline for Oil Red O staining. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, and thiobarbituric acid reactive substance (TBARS) were determined enzymatically using commercially available kits (Wako Pure Chemical Industries, Osaka, Japan; Zeptometrix corporation, Buffalo, NY, USA). In a separate experiment, rats (n = 4) were given 0.5% OPC for two days and similarly sacrificed between 7:00–8:00 for the determination of plasma concentrations of epicatechin(4β-8)epicatechin (procyanidin B2) and epicatechin(4β-8)epicatechin(4β-8)epicatechin (procyanidin C1) as previously reported.20)
Oil Red O staining of accumulated lipid in mesenteric arteries.
Oil Red O staining was used for the detection of lipid accumulation in mesenteric arteries as previously reported.22) In brief, the intestine was excised out and the enteric canal was removed. Branches of the mesenteric artery were isolated from adipose tissue and fixed with 10% formalin. The mesenteric arteries were incubated in 60% isopropanol for five minutes followed by staining with 0.18% Oil Red O (Merck KGaA, Darmstadt, Germany). Lipid deposits were manually enumerated under stereomicroscope in the posterior 8 branches of the artery (Stemi 2000, Carl Zeiss, Oberkochen, Germany).
Anti-LOX-1 antibody.
A monoclonal antibody against human LOX-1 (TS92) was used for blocking ligand binding to LOX-1. Characteristics of the antibody were described in previous reports.7,23)
Statistics.
One-way ANOVA followed by Dunnett or Student’s t-test was performed for multiple or paired comparison, respectively. P < 0.05 was considered statistically significant and indicated by *.
Results
Screening.
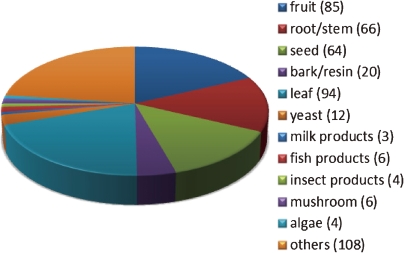
472 products were investigated to identify LOX-1 inhibitors. They included 437 foodstuff extracts and 35 test reagents such as amino acids. Almost all the products were commercially available and many of them have been utilized for healthcare as folk medicine or health food supplements. The assayed products were categorized as shown in Fig. 1.
Figure 1.
Products assayed in ELISA were categorized according to the origins of foodstuff; plant-derived products, yeast extracts, milk products, fish products, insect products, mushroom, algae and others. The plant-derived products were further classified by the parts used. The number in parenthesis indicates the number of the assayed products.
First we employed a sandwich ELISA using oxLDL as a ligand for LOX-1. Results showed that 52 products inhibited oxLDL binding by more than 70% (data not shown). The products were further examined using a cell-based assay whether they inhibited oxLDL uptake in LOX-1-CHO cells. Control CHO cells did not uptake DiI-labeled oxLDL (DiI-oxLDL) at detectable levels, indicating the specific uptake of DiI-oxLDL by LOX-1 (data not shown). In LOX-1-CHO cells, we found 26 products that inhibited DiI-oxLDL uptake by more than 70% (Table 1 ). These included a number of foodstuff extracts that are known to contain a large amount of procyanidins such as grape seed extracts, pine bark extracts, peanuts skin and apple polyphenols (highlighted in bold, Table 1). We considered the possibility that procyanidins may inhibit oxLDL binding to LOX-1 and conducted the subsequent studies.
Table 1.
Foodstuff extracts (10 µg/mL) that showed more than 70% inhibition in DiI-oxLDL uptake in LOX-1-CHO cells. Similar foodstuff extracts sold by multiple suppliers were assayed as different extracts since the extraction methods are different or are not specified. As a result, similar extracts are listed in the table such as grape seed extract. Those extracts are attached with superscript numbers and the suppliers are shown at the bottom. Products known to be rich in procyanidins are shown in bold.
| Extract/Product Name | Inhibition (%) |
|---|---|
| Black Soybean Hull | 99 |
| Propolis | 98 |
| Oligomoeric Proanthocyanidins from Grape Seed*1 | 98 |
| Grape Seed*2 | 98 |
| Peanut Seed Coat*3 | 98 |
| Apple Condensed Tannin | 97 |
| Grape Seed*4 | 97 |
| Peanut Seed Coat*5 | 97 |
| French Maritime Pine Bark | 96 |
| Proanthocyanidins | 95 |
| New Zealand Pine Bark | 95 |
| Grape Seed*6 | 91 |
| Apple Polyphenol | 88 |
| Pomegranate Skin | 86 |
| Rooibos Tea | 86 |
| Mangosteen Fruit Skin | 84 |
| Wasabi Leaf | 83 |
| Gingko Leaf | 80 |
| Bilberry*7 | 80 |
| Bilberry*8 | 78 |
| Guava Leaf | 76 |
| Vitis Coignetiae | 76 |
| Quercus Salicina | 76 |
| Pine Bark | 75 |
| Hop Polyphenol | 73 |
| Cat’s Claw | 72 |
*1Kikkoman, *2Sun Globe Food, *3Kishimoto Sangyo, *4Hoechst Marion Roussel, *5Tokiwa Phytochemical, *6Chika Industries, *7Indena Japan, *8Tokiwa Phytochemical.
Characterization of apple polyphenols.
In the next series of experiments, we characterized the profiles of apple polyphenols as a representative containing a large amount of procyanidins. Apple polyphenols dose-dependently and completely inhibited the DiI-oxLDL (10 µg/mL) binding to LOX-1-CHO cells with an IC50 value of 102 ng/mL (Fig. 2A ). As apple polyphenol contains catechin/epicatechin ((epi)catechin), phenolcarboxylic acids, and other ingredients as well as procyanidins (Fig. 2B), we examined which of the ingredients inhibited DiI-oxLDL binding to LOX-1. As shown in Fig. 2C, the fractions containing procyanidins and (epi)catechin significantly inhibited the DiI-oxLDL binding while phenolcarboxylic acids and others did not. These results clearly indicated that procyanidins and/or (epi)catechin were active ingredients for the inhibition of oxLDL binding to LOX-1. Neither apple polyphenol nor procyanidins displayed cytotoxic effects up to 10 µg/mL (data not shown).
Figure 2.
DiI-oxLDL (10 µg/mL) binding in LOX-1-CHO cells in the presence of apple polyphenols (A) and each ingredient of apple polyphenols (C, 100 ng/mL each). Figure 2B represents the relative contents of each ingredient in apple polyphenols. The graph was created according to the reference.20) * represents statistical significance from control.
In vitro profiles of procyanidins.
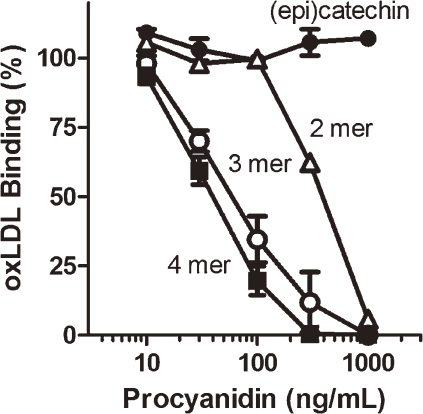
We next analyzed the activities of procyanidins according to the polymerization levels. (Epi)catechin and oligomeric procyanidins from dimer to heptamer were separated from apple polyphenols as previously reported.20,21) Purified procyanidins were used for the analysis. The results showed that procyanidins of ≥dimer displayed a concentration-dependent inhibition of DiI-oxLDL (10 µg/mL) binding to LOX-1-CHO cells. In contrast, (epi)catechin was without effect up to 1 µg/mL (Fig. 3 ). Moreover, the efficacy was much more potent with procyanidin of ≥trimer than dimer procyanidin. The IC50 of dimer procyanidin was 330 ng/mL and that of oligomeric procyanidins ranged from 61 ng/mL (trimer) to 27 ng/mL (heptamer). Oligomeric procyanidins with chain length of more than eight also similarly inhibited oxLDL binding (data not shown). These results clearly indicated that oligomeric procyanidins inhibited oxLDL binding to LOX-1.
Figure 3.
DiI-oxLDL (10 µg/mL) binding in LOX-1-CHO cells in the presence of different levels of polymerized procyanidins. The results of procyanidins ≥ pentamer are omitted from the graph for clarity.
It has been reported that trimer apple procyanidins comprises six isoforms.21) In the next study, we prepared major four isomers of apple procyanidins, epicatechin(4β-8)epicatechin(4β-8)epicatechin (known as procyanidin C1), epicatechin(4β-8)epicatechin(4β-8)catechin, epicatechin(4β-6)epicatechin(4β-8)catechin and epicatechin(4β-6)epicatechin(4β-8)epicatechin, and examined their activities. As shown in Fig. 4A , the fluorescence of DiI-oxLDL (10 µg/mL) in LOX-1-CHO cells was significantly attenuated by procyanidin C1 (300 ng/mL). The fluorescence represents the specific binding to LOX-1 since it was disrupted by anti-LOX-1 antibody (LOX-1 Ab). These results indicated that procyanidin C1 inhibited oxLDL binding to LOX-1. Quantitative analysis showed that all of the trimer procyanidins similarly inhibited DiI-oxLDL binding to LOX-1-CHO cells with IC50s of 51, 41, 47 and 73 ng/mL, respectively (Fig. 4B). The fluorescence quenching of DiI-oxLDL by procyanidin C1 (300 ng/mL) was less than 25% (data not shown). The IC50 of procyanidin C1 decreased to 7 ng/mL and 26 ng/mL when DiI-oxLDL was used at the concentrations of 1 and 3 µg/mL, respectively (data not shown).
Figure 4.
DiI-oxLDL (10 µg/mL) binding in LOX-1-CHO cells in the presence of various isomers of trimer procyanidins. Fluorescent images of DiI-oxLDL (red) binding to LOX-1-CHO cells in the absence or presence of procyanidin C1 (300 ng/mL) or in the presence of anti-LOX-1 antibody (A). Nuclei were stained by DAPI (blue). DiI-oxLDL binding in LOX-1-CHO cells in the presence of various concentrations of trimer procyanidin isomers (B). Chemical structures of the trimer procyanidin isomers used in the experiments are shown. Difference in the hydroxyl groups indicated by red jagged lines causes stereoisomers of catechin and epicatechin.
In vivo effects of oligomeric procyanidins.
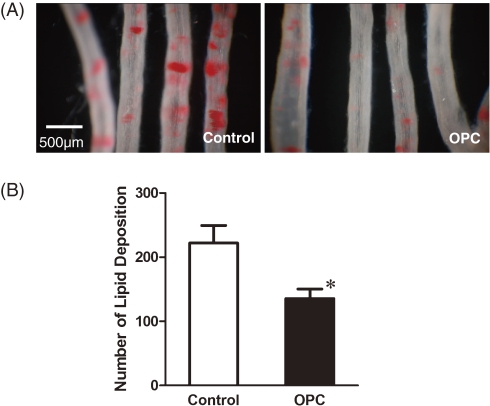
Finally, we addressed whether procyanidins inhibited lipid accumulation in vascular wall in vivo. Oligomeric procyanidins (OPC), a mixture containing procyanidins with various chain lengths purified from apple, was used for in vivo study. SHR-SP rats are well-known disease-model animals for hypertension and stroke, and accumulate lipids in arterial wall in response to high fat diet.22,24) In this study, 8 week old SHR-SP rats were given high fat diet and physiological saline containing 0.5% OPC (OPC rats) for 2 weeks. OPC did not affect the daily food intake and body weight during the experimental period. Systolic and diastolic blood pressure and heart rates were also unaffected (Table 2 ). At termination, the mesenteric artery isolated from the control rats displayed numerous Oil Red O-positive dots while that from the OPC-treated rats displayed significantly less dots, indicating that OPC suppressed arterial lipid deposition (Fig. 5 ). Plasma total cholesterol (TC), HDL-cholesterol, triglyceride and TBARS did not differ between the groups (Table 2). The plasma concentrations of procyanidin B2 and C1 in OPC rats were 7.9 ± 1.8 ng/mL and 4.9 ± 1.9 ng/mL, respectively.
Table 2.
Body weight, cardiovascular parameters, food intake and plasma parameters of SHR-SP rats before and after the administration of procyanidins (OPC).
| Parameters | Control | OPC | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Body Weight (g) | 198 ± 5 | 247 ± 4 | 199 ± 5 | 237 ± 4 |
| Systolic Blood Pressure (mmHg) | 173 ± 7 | 191 ± 6 | 174 ± 4 | 197 ± 3 |
| Diastolic Blood Pressure (mmHg) | 119 ± 6 | 137 ± 3 | 121 ± 3 | 142 ± 4 |
| Heart Rate (/min) | 351 ± 7 | 348 ± 5 | 364 ± 8 | 364 ± 7 |
| Food Intake (g/day) | 18.0 ± 0.4 | 17.1 ± 0.3 | ||
| Plasma Triglyceride (mg/dL) | 404.8 ± 45.3 | 378.2 ± 46.1 | ||
| Total Cholesterol (mg/dL) | 157.6 ± 8.4 | 152.3 ± 6.4 | ||
| HDL-cholesterol (mg/dL) | 36.1 ± 1.6 | 35.6 ± 0.9 | ||
| TBARS (nmol/mL) | 0.89 ± 0.05 | 0.84 ± 0.04 | ||
Figure 5.
Arterial lipid deposition in SHR-SP rats on high fat diet. Oil Red-O image (A) and the number of lipid deposition (B) in control and OPC rats. * represents statistical significance from control.
Discussion
We successfully identified several foodstuff extracts that inhibit LOX-1 using ELISA and DiI-oxLDL uptake assay. The results obtained with the cell assays suggested that procyanidins might be one of the components that inhibit oxLDL uptake since nearly half of the potent hit extracts are known to contain a large amount of procyanidins. Indeed, purified procyanidins inhibited oxLDL binding in LOX-1-CHO cells. Moreover, OPC suppressed lipid accumulation in vascular wall of SHR-SP rats in which an anti-LOX-1 antibody was also effective.22) To date, several natural products with cardioprotective effects have been identified to reduce the biological actions of oxLDL and/or the expression of LOX-1 such as flavonoids from seabuckthorn, bergamot oil and mulberry leaf aqueous fractions.25–27) However, their molecular targets are not known. To our knowledge, this is the first report demonstrating a direct antagonistic action of natural products to LOX-1.
LOX-1-inhibiting properties were almost identical among procyanidins ≥ trimer and the dimer also potently inhibited LOX-1. Moreover, four different isomers of trimer procyanidins almost equally inhibited oxLDL binding to LOX-1. These results implicate that intake of procyanidin-rich foods potentially inhibits LOX-1, regardless of food source since the polymerization levels of procyanidins significantly differ among foods.28) In support to this, OPC, a mixture of various chain lengths of procyanidins, inhibited vascular lipid accumulation in SHR-SP rats. We have previously demonstrated that an anti-LOX-1 antibody inhibited vascular lipid accumulation in this animal model,22) suggesting that procyanidins function as a LOX-1 inhibitor in in vivo as well. A wide range of in vivo effects of procyanidin-rich extracts such as anti-oxidative, anti-hypertensive and anti-hyperlipidemic actions have been reported.29–32) These actions potentially reduce vascular lipid accumulation, since in this animal model vascular lipid accumulation is increased by high fat feeding and decreased by the treatment with anti-oxidant or hypotensive agents22) (unpublished observation). It may be less likely, however, since OPC did not affect cardiovascular parameters, plasma lipids, or TBARS, a marker of oxidative state, in the present study. Different experimental conditions such as dosage and experimental period may have affected the readouts. Shoji et al. have demonstrated that procyanidin B2 and C1 are orally available.20) In agreement to this, we were able to detect procyanidin B2 and C1 in the plasma of rats treated with orally administered OPC. However, the plasma levels of procyanidins were small and close to the detection limits. These results may argue against that OPC suppressed vascular lipid accumulation by inhibiting LOX-1 on vascular wall. Yet, the plasma level may be sufficient to inhibit LOX-1 since plasma oxLDL level in SHR-SP rats is about 200 ng/mL22) and procyanidin C1 halved the oxLDL binding in LOX-1-CHO cells even at 7 ng/mL when 1 µg/mL oxLDL was used. Alternatively, plasma procyanidin levels may have reached higher when rats actively intake OPC during light-off period. It is also possible that OPC may inhibit the function of LOX-1 expressed on the apical membrane of intestinal epithelium, in which LOX-1 mediates the transcytosis of bile salt-dependent lipase.33) Since intestinal lumen is exposed to a significant amount of OPC during the absorption process, OPC may have inhibited intestinal LOX-1 and thereby modulated lipid metabolism leading to less lipid accumulation. Further studies are definitely called for to reach positive evidences to determine whether OPC inhibits LOX-1 in vivo or other unknown factors might be involved.
Out of more than 400 foodstuff extracts derived from various sources, more than half of those displaying potent LOX-1 inhibition are known to contain a large amount of procyanidin. In addition, not well-known compared to apples or grape seeds, procyanidins are also contained in other extracts listed in Table 1 such as pomegranate skin,34) mangosteen fruit skin,35) bilberry,36) Quercus salicina37) and cat’s claw.38) These results implicate that LOX-1 inhibition may underlie the health benefits of these extracts. Of particular interest, procyanidins as well as resveratrol are considered to be one of the bioactive ingredients in red wine for the cardioprotective effects, known as “French Paradox”.39–41) The present findings implicate that LOX-1 is a molecular target of procyanidins as is sirtuin 1 for resveratrol. Furthermore, Corder et al. identified procyanidins from red wine as the molecule that suppressed endothelin-1 release from vascular endothelial cells.42,43) They also found that the concentrations of procyanidins were high in wines from certain geographic areas, which correlated with longevity in such regions.42,43) As LOX-1 activation leads to endothelin-1 release from endothelial cells,44) these results may further support the hypothesis that LOX-1 inhibition is an underlying mechanism of the cardiovascular protective effects of red wine.
In conclusion, we demonstrate that LOX-1-inhibiting property is rich in foodstuffs containing a large amount of procyanidins and that procyanidins are potent inhibitors of LOX-1. These results implicate the role of LOX-1 in the French Paradox. Moreover, the LOX-1 ELISA and CHO cell assays employed in this study are likely to make powerful methods to identify LOX-1 inhibitors not only from foodstuffs but also from chemical compounds for future drug discovery.
Acknowledgement
The authors thank Mrs. Shohei Nakashima and Motoyuki Tagashira for purifying apple procyanidins. This study was supported in part by the grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Ministry of Health, Labour and Welfare of Japan; the National Institute of Biomedical Innovation; Japan Science and Technology Agency; the New Energy and Industrial Technology Development Organization; Japan Vascular Disease Research Foundation and the Institute of Seizon and Life Sciences.
Abbreviations
- LOX-1
lectin-like oxidized LDL receptor-1
- oxLDL
oxidized LDL
- OPC
oligomeric procyanidins
- CHO cell
Chinese hamster ovary cell
References
- 1).Velican D., Velican C. (1980) Atherosclerotic involvement of the coronary arteries of adolescents and young adults. Atherosclerosis 36, 446–460 [DOI] [PubMed] [Google Scholar]
- 2).Tuzcu E.M., Kapadia S.R., Tutar E., Ziada K.M., Hobbs R.E., McCarthy P.M., Young J.B., Nissen S.E. (2001) High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 103, 2705–2710 [DOI] [PubMed] [Google Scholar]
- 3).Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y., Tanaka T., Miwa S., Katsura Y., Kita T., Masaki T. (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73–77 [DOI] [PubMed] [Google Scholar]
- 4).Li D., Mehta J.L. (2000) Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 101, 2889–2895 [DOI] [PubMed] [Google Scholar]
- 5).Cominacini L., Rigoni A., Pasini A.F., Garbin U., Davoli A., Campagnola M., Pastorino A.M., Lo Cascio V., Sawamura T. (2001) The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 276, 13750–13755 [DOI] [PubMed] [Google Scholar]
- 6).Li D., Liu L., Chen H., Sawamura T., Mehta J.L. (2003) LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler. Thromb. Vasc. Biol. 23, 816–821 [DOI] [PubMed] [Google Scholar]
- 7).Kakutani M., Masaki T., Sawamura T. (2000) A platelet–endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc. Natl. Acad. Sci. USA 97, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Fujita Y., Kakino A., Nishimichi N., Yamaguchi S., Sato Y., Machida S., Cominacini L., Delneste Y., Matsuda H., Sawamura T. (2009) Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin. Chem. 55, 285–294 [DOI] [PubMed] [Google Scholar]
- 9).Nagase M., Hirose S., Sawamura T., Masaki T., Fujita T. (1997) Enhanced expression of endothelial oxidized low-density lipoprotein receptor (LOX-1) in hypertensive rats. Biochem. Biophys. Res. Commun. 237, 496–498 [DOI] [PubMed] [Google Scholar]
- 10).Chen M., Nagase M., Fujita T., Narumiya S., Masaki T., Sawamura T. (2001) Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem. Biophys. Res. Commun. 287, 962–968 [DOI] [PubMed] [Google Scholar]
- 11).Chen H., Li D., Sawamura T., Inoue K., Mehta J.L. (2000) Upregulation of LOX-1 expression in aorta of hypercholesterolemic rabbits: modulation by losartan. Biochem. Biophys. Res. Commun. 276, 1100–1104 [DOI] [PubMed] [Google Scholar]
- 12).Mehta J.L., Sanada N., Hu C.P., Chen J., Dandapat A., Sugawara F., Satoh H., Inoue K., Kawase Y., Jishage K., Suzuki H., Takeya M., Schnackenberg L., Beger R., Hermonat P.L., Thomas M., Sawamura T. (2007) Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 100, 1634–1642 [DOI] [PubMed] [Google Scholar]
- 13).Hu C., Chen J., Dandapat A., Fujita Y., Inoue N., Kawase Y., Jishage K., Suzuki H., Li D., Hermonat P.L., Sawamura T., Mehta J.L. (2008) LOX-1 abrogation reduces myocardial ischemia-reperfusion injury in mice. J. Mol. Cell. Cardiol. 44, 76–83 [DOI] [PubMed] [Google Scholar]
- 14).Inoue K., Arai Y., Kurihara H., Kita T., Sawamura T. (2005) Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ. Res. 97, 176–184 [DOI] [PubMed] [Google Scholar]
- 15).Eichhorn B., Muller G., Leuner A., Sawamura T., Ravens U., Morawietz H. (2009) Impaired vascular function in small resistance arteries of LOX-1 overexpressing mice on high-fat diet. Cardiovasc. Res. 82, 493–502 [DOI] [PubMed] [Google Scholar]
- 16).Inoue N., Okamura T., Kokubo Y., Fujita Y., Sato Y., Nakanishi M., Yanagida K., Kakino A., Iwamoto S., Watanabe M., Ogura S., Otsui K., Matsuda H., Uchida K., Yoshimoto R., Sawamura T. (2010) LOX Index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin. Chem. 56, 550–558 [DOI] [PubMed] [Google Scholar]
- 17).St. Leger A.S., Cochrane A.L., Moore F. (1979) Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet 1, 1017–1020 [DOI] [PubMed] [Google Scholar]
- 18).Lavie C.J., Milani R.V., Mehra M.R., Ventura H.O. (2009) Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54, 585–594 [DOI] [PubMed] [Google Scholar]
- 19).Iqbal R., Anand S., Ounpuu S., Islam S., Zhang X., Rangarajan S., Chifamba J., Al-Hinai A., Keltai M., Yusuf S., on behalf of the INTERHEART Study Investigators (2008) Dietary patterns and the risk of acute myocardial infarction in 52 countries: Results of the INTERHEART study. Circulation 118, 1929–1937 [DOI] [PubMed] [Google Scholar]
- 20).Shoji T., Masumoto S., Moriichi N., Akiyama H., Kanda T., Ohtake Y., Goda Y. (2006) Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the Porter method and high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 54, 884–892 [DOI] [PubMed] [Google Scholar]
- 21).Shoji T., Masumoto S., Moriichi N., Kanda T., Ohtake Y. (2006) Apple (Malus pumila) procyanidins fractionated according to the degree of polymerization using normal-phase chromatography and characterized by HPLC-ESI/MS and MALDI-TOF/MS. J. Chromatogr. A 1102, 206–213 [DOI] [PubMed] [Google Scholar]
- 22).Nakano A., Inoue N., Sato Y., Nishimichi N., Takikawa K., Fujita Y., Kakino A., Otsui K., Yamaguchi S., Matsuda H., Sawamura T. (2010) LOX-1 mediates vascular lipid retention under hypertensive state. J. Hypertens. 28, 1273–1280 [DOI] [PubMed] [Google Scholar]
- 23).Sugimoto K., Ishibashi T., Sawamura T., Inoue N., Kamioka M., Uekita H., Ohkawara H., Sakamoto T., Sakamoto N., Okamoto Y., Takuwa Y., Kakino A., Fujita Y., Tanaka T., Teramoto T., Maruyama Y., Takeishi Y. (2009) LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc. Res. 84, 127–136 [DOI] [PubMed] [Google Scholar]
- 24).Yamori Y., Hamashima Y., Horie R., Handa H., Sato M. (1975) Pathogenesis of acute arterial fat deposition in spotaneously hypertensive rats. Jpn. Circ. J. 39, 601–609 [DOI] [PubMed] [Google Scholar]
- 25).Bao M., Lou Y. (2006) Flavonoids from seabuckthorn protect endothelial cells (EA.hy926) from oxidized low-density lipoprotein induced injuries via regulation of LOX-1 and eNOS expression. J. Cardiovasc. Pharmacol. 48, 834–841 [DOI] [PubMed] [Google Scholar]
- 26).Shibata Y., Kume N., Arai H., Hayashida K., Inui-Hayashida A., Minami M., Mukai E., Toyohara M., Harauma A., Murayama T., Kita T., Hara S., Kamei K., Yokode M. (2007) Mulberry leaf aqueous fractions inhibit TNF-α-induced nuclear factor κB (NF-κB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 193, 20–27 [DOI] [PubMed] [Google Scholar]
- 27).Mollace V., Ragusa S., Sacco I., Muscoli C., Sculco F., Visalli V., Palma E., Muscoli S., Mondello L., Dugo P., Rotiroti D., Romeo F. (2008) The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J. Cardiovasc. Pharmacol. Ther. 13, 120–129 [DOI] [PubMed] [Google Scholar]
- 28).Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Gebhardt S., Prior R.L. (2004) Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 134, 613–617 [DOI] [PubMed] [Google Scholar]
- 29).Del Bas J.M., Fernandez-Larrea J., Blay M., Ardevol A., Salvado M.J., Arola L., Blade C. (2005) Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. 19, 479–481 [DOI] [PubMed] [Google Scholar]
- 30).Busserolles J., Gueux E., Balasińska B., Piriou Y., Rock E., Rayssiguier Y., Mazur A. (2006) In vivo antioxidant activity of procyanidin-rich extracts from grape seed and pine (Pinus maritima) bark in rats. Int. J. Vitam. Nutr. Res. 76, 22–27 [DOI] [PubMed] [Google Scholar]
- 31).Magos G.A., Mateos J.C., Páez E., Fernández G., Lobato C., Márquez C., Enríquez R.G. (2008) Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J. Ethnopharmacol. 117, 58–68 [DOI] [PubMed] [Google Scholar]
- 32).Del Bas J.M., Ricketts M.L., Vaqué M., Sala E., Quesada H., Ardevol A., Salvadó M.J., Blay M., Arola L., Moore D.D., Pujadas G., Fernandez-Larrea J., Bladé C. (2009) Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol. Nutr. Food Res. 53, 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Bruneau N., Richard S., Silvy F., Verine A., Lombardo D. (2003) Lectin-like ox-LDL receptor is expressed in human INT-407 intestinal cells: involvement in the transcytosis of pancreatic bile salt-dependent lipase. Mol. Biol. Cell 14, 2861–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).de Pascual-Teresa S., Santos-Buelga C., Rivas-Gonzalo J.C. (2000) Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 48, 5331–5337 [DOI] [PubMed] [Google Scholar]
- 35).Fu C., Loo A.E.K., Chia F.P.P., Huang D. (2007) Oligomeric proanthocyanidins from Mangosteen pericarps. J. Agric. Food Chem. 55, 7689–7694 [DOI] [PubMed] [Google Scholar]
- 36).Maatta-Riihinen K.R., Kahkonen M.P., Torronen A.R., Heinonen I.M. (2005) Catechins and procyanidins in berries of vaccinium species and their antioxidant activity. J. Agric. Food Chem. 53, 8485–8491 [DOI] [PubMed] [Google Scholar]
- 37).Nonaka G., Nishimura H., Nishioka I. (1982) Tannins and related compounds. IV. Seven new phenol glucoside gallates from Quercus stenophylla Makino (1). Chem. Pharm. Bull. (Tokyo) 30, 2061–2067 [Google Scholar]
- 38).Miyake M., Sasaki K., Ide K., Matsukura Y., Shijima K., Fujiwara D. (2006) Highly oligomeric procyanidins ameliorates experimental autoimmune encephalomyelitis via suppression of Th1 immunity. J. Immunol. 176, 5797–5804 [DOI] [PubMed] [Google Scholar]
- 39).Opie L.H., Lecour S. (2007) The red wine hypothesis: from concepts to protective signalling molecules. Eur. Heart J. 28, 1683–1693 [DOI] [PubMed] [Google Scholar]
- 40).Rasmussen S.E., Frederiksen H., Krogholm K.S., Poulsen L. (2005) Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 49, 159–174 [DOI] [PubMed] [Google Scholar]
- 41).Brown L., Kroon P.A., Das D.K., Das S., Tosaki A., Chan V., Singer M.V., Feick P. (2009) The biological responses to resveratrol and other polyphenols from alcoholic beverages. Alcohol. Clin. Exp. Res. 33, 1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Corder R., Douthwaite J.A., Lees D.M., Khan N.Q., Viseu dos Santos A.C., Wood E.G., Carrier M.J. (2001) Health: Endothelin-1 synthesis reduced by red wine. Nature 414, 863–864 [DOI] [PubMed] [Google Scholar]
- 43).Corder R., Mullen W., Khan N.Q., Marks S.C., Wood E.G., Carrier M.J., Crozier A. (2006) Oenology: Red wine procyanidins and vascular health. Nature 444, 566. [DOI] [PubMed] [Google Scholar]
- 44).Sakurai K., Cominacini L., Garbin U., Fratta Pasini A., Sasaki N., Takuwa Y., Masaki T., Sawamura T. (2004) Induction of endothelin-1 production in endothelial cells via co-operative action between CD40 and lectin-like oxidized LDL receptor (LOX-1). J. Cardiovasc. Pharmacol. 44, S173–S180 [DOI] [PubMed] [Google Scholar]