Abstract
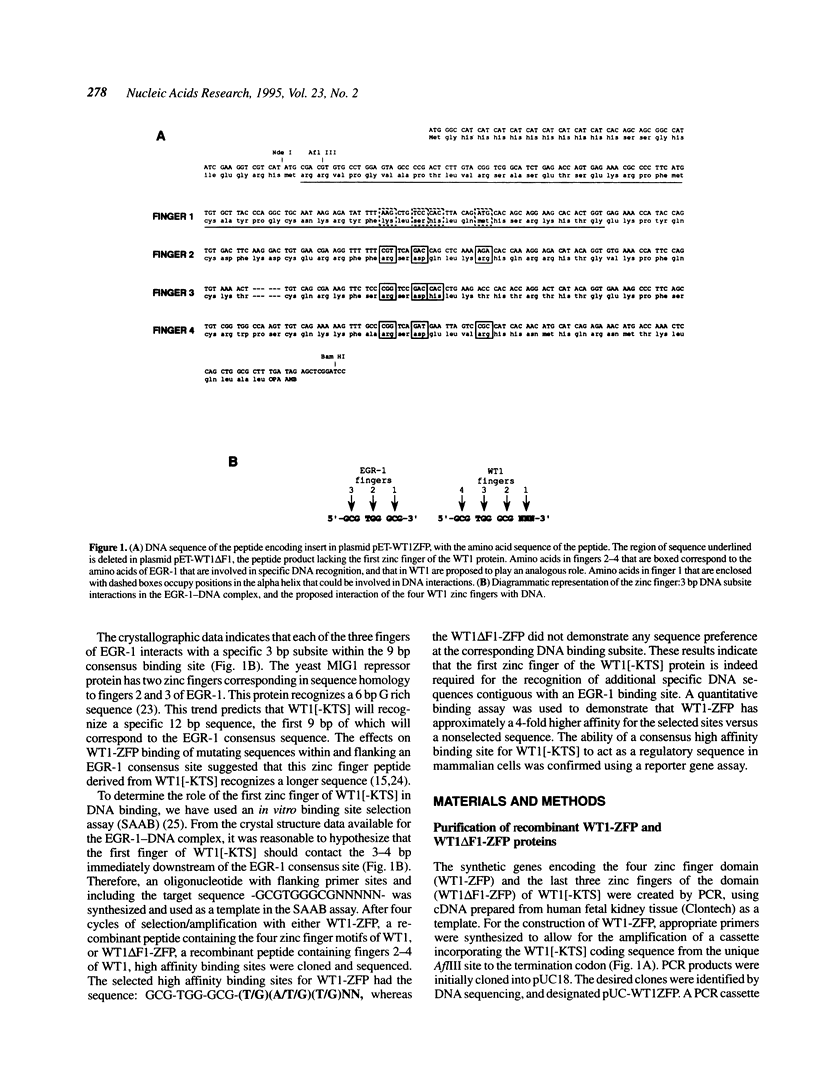
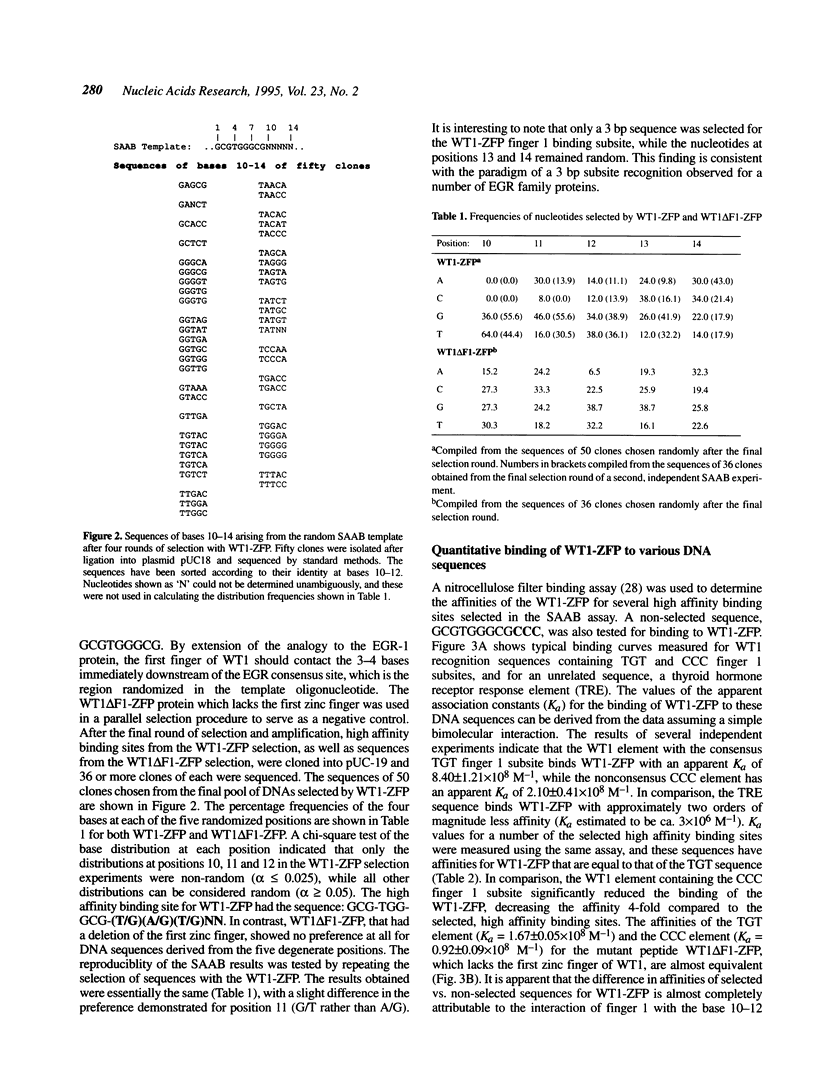
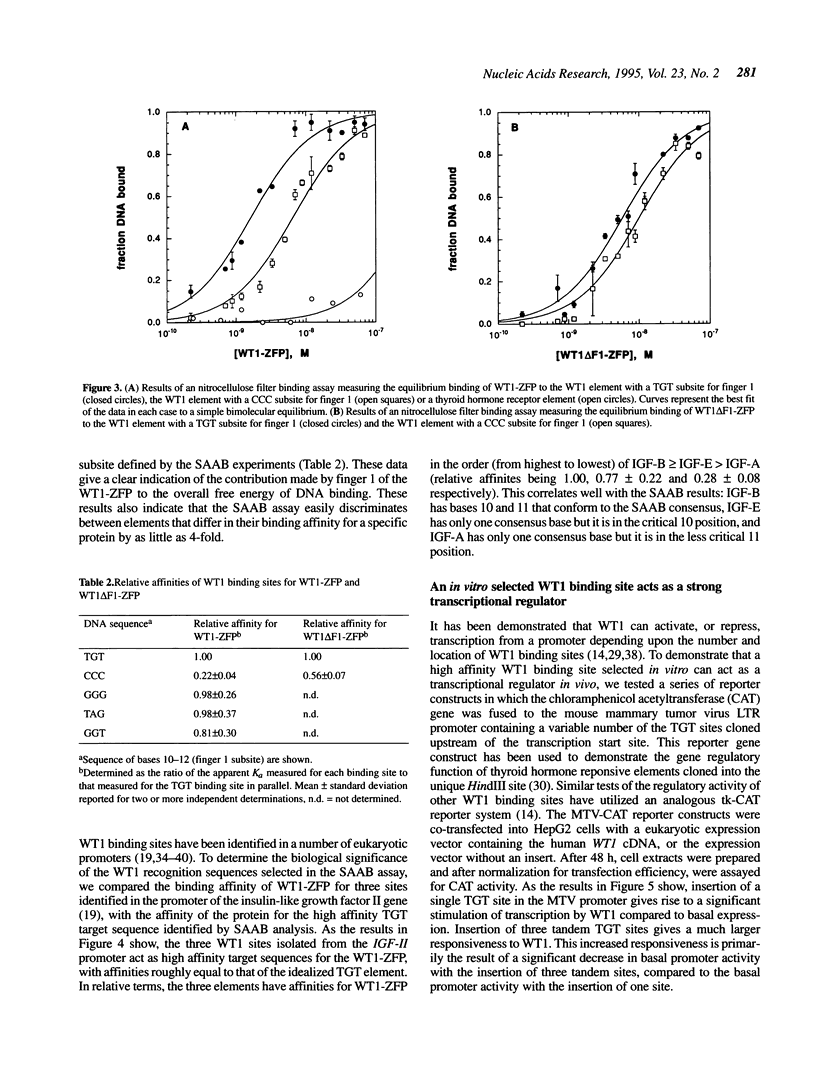
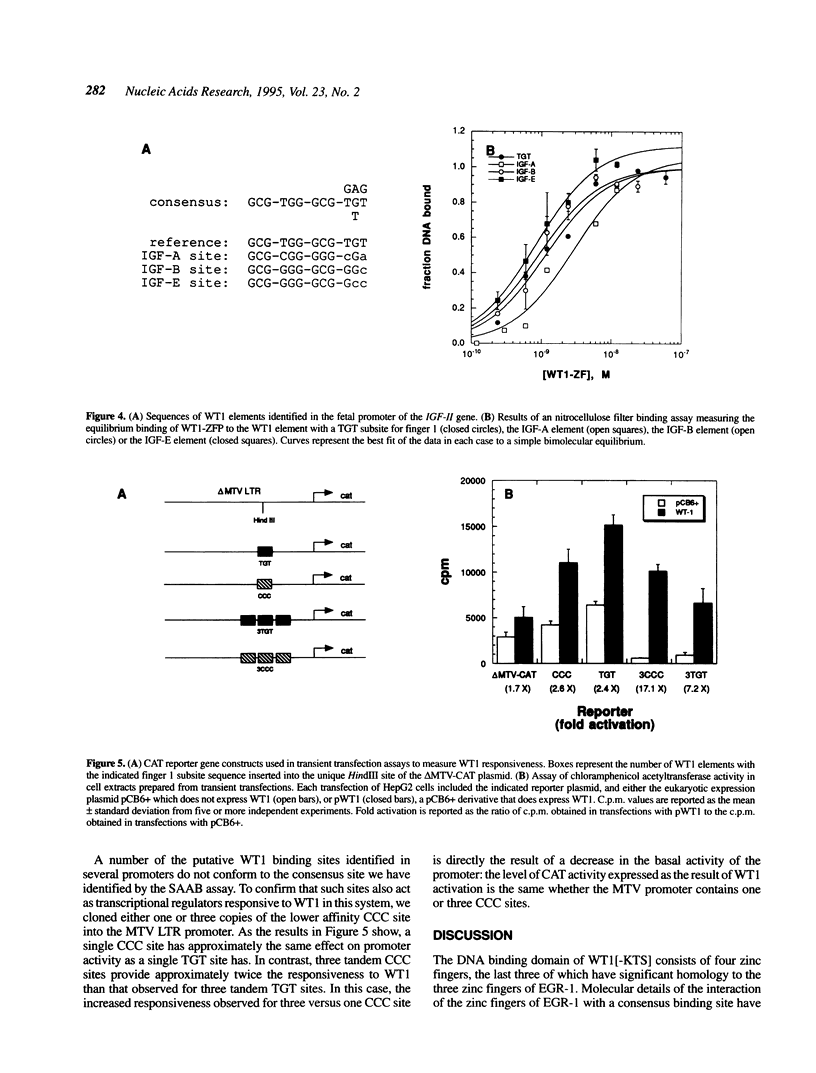
The Wilms' tumour suppressor protein (WT1) is a putative transcriptional regulatory protein with four zinc fingers, the last three of which have extensive sequence homology to the early growth response-1 (EGR-1) protein. Although a peptide encoding the zinc finger domain of WT1[-KTS] can bind to a consensus 9 bp EGR-1 binding site, current knowledge about the mechanisms of zinc finger-DNA interactions would predict a more extended recognition site for WT1. Using a WT1[-KTS] zinc finger peptide (WT1-ZFP) and the template oligonucleotide GCG-TGG-GCG-NNNNN in a binding site selection assay, we have determined that the highest affinity binding sites for WT1[-KTS] consist of a 12 bp sequence GCG-TGG-GCG-(T/G)(G/A/T)(T/G). The binding of WT1-ZFP to a number of the selected sequences was measured by a quantitative nitrocellulose filter binding assay, and the results demonstrated that these sequences have a 4-fold higher affinity for the protein than the nonselected sequence GCG-TGG-GCG-CCC. The full length WT1 protein regulates transcription of reporter genes linked to these high affinity sequences. A peptide lacking the first zinc finger of WT1[-KTS], but containing the three zinc fingers homologous to EGR-1 failed to select any specific sequences downstream of the GCG-TGG-GCG consensus sequence in the binding site selection assay. DNA sequences in the fetal promoter of the insulin-like growth factor II gene that confer WT1 responsiveness in a transient transfection assay bind to the WT1-ZFP with affinities that vary according to the number of consensus bases each sequence possesses in the finger 1 subsite.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. L., Minden A. G., Williams G. T., Bobonis C., Yeung C. Y. Functional significance of an overlapping consensus binding motif for Sp1 and Zif268 in the murine adenosine deaminase gene promoter. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7523–7527. doi: 10.1073/pnas.88.17.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B. R., Sukhatme V. P., Roberts A. B., Sporn M. B., Rauscher F. J., 3rd, Kim S. J. Repression of the transforming growth factor-beta 1 gene by the Wilms' tumor suppressor WT1 gene product. Mol Endocrinol. 1994 May;8(5):595–602. doi: 10.1210/mend.8.5.8058069. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Rupprecht H. D., Rohwer-Nutter P., Lopez-Guisa J. M., Madden S. L., Rauscher F. J., 3rd, Sukhatme V. P. DNA recognition by splicing variants of the Wilms' tumor suppressor, WT1. Mol Cell Biol. 1994 Jun;14(6):3800–3809. doi: 10.1128/mcb.14.6.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairall L., Schwabe J. W., Chapman L., Finch J. T., Rhodes D. The crystal structure of a two zinc-finger peptide reveals an extension to the rules for zinc-finger/DNA recognition. Nature. 1993 Dec 2;366(6454):483–487. doi: 10.1038/366483a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Glaser T., Lewis W. H., Bruns G. A., Watkins P. C., Rogler C. E., Shows T. B., Powers V. E., Willard H. F., Goguen J. M., Simola K. O. The beta-subunit of follicle-stimulating hormone is deleted in patients with aniridia and Wilms' tumour, allowing a further definition of the WAGR locus. 1986 Jun 26-Jul 2Nature. 321(6073):882–887. doi: 10.1038/321882a0. [DOI] [PubMed] [Google Scholar]
- Gupta M. P., Gupta M., Zak R., Sukhatme V. P. Egr-1, a serum-inducible zinc finger protein, regulates transcription of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1991 Jul 15;266(20):12813–12816. [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Harrington M. A., Konicek B., Song A., Xia X. L., Fredericks W. J., Rauscher F. J., 3rd Inhibition of colony-stimulating factor-1 promoter activity by the product of the Wilms' tumor locus. J Biol Chem. 1993 Oct 5;268(28):21271–21275. [PubMed] [Google Scholar]
- Joseph L. J., Le Beau M. M., Jamieson G. A., Jr, Acharya S., Shows T. B., Rowley J. D., Sukhatme V. P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Vesque C., Schmitt J., Stunnenberg H., Frank R., Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990 Jul;10(7):3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Matsunaga E. Genetics of Wilms' tumor. Hum Genet. 1981;57(3):231–246. doi: 10.1007/BF00278936. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Madden S. L., Tournay O. E., Cook D. M., Sukhatme V. P., Rauscher F. J., 3rd Characterization of the zinc finger protein encoded by the WT1 Wilms' tumor locus. Oncogene. 1991 Dec;6(12):2339–2348. [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science. 1993 Sep 24;261(5129):1701–1707. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J. Characterization of the equilibrium binding of Xenopus transcription factor IIIA to the 5 S RNA gene. J Biol Chem. 1990 Oct 15;265(29):17593–17600. [PubMed] [Google Scholar]
- Rupprecht H. D., Drummond I. A., Madden S. L., Rauscher F. J., 3rd, Sukhatme V. P. The Wilms' tumor suppressor gene WT1 is negatively autoregulated. J Biol Chem. 1994 Feb 25;269(8):6198–6206. [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Human tumor suppressor genes. Annu Rev Genet. 1990;24:615–657. doi: 10.1146/annurev.ge.24.120190.003151. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Takimoto Y., Wang Z. Y., Kobler K., Deuel T. F. Promoter region of the human platelet-derived growth factor A-chain gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1686–1690. doi: 10.1073/pnas.88.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Evans R. M. Trans-activation by thyroid hormone receptors: functional parallels with steroid hormone receptors. Proc Natl Acad Sci U S A. 1989 May;86(10):3494–3498. doi: 10.1073/pnas.86.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Madden S. L., Deuel T. F., Rauscher F. J., 3rd The Wilms' tumor gene product, WT1, represses transcription of the platelet-derived growth factor A-chain gene. J Biol Chem. 1992 Nov 5;267(31):21999–22002. [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Deuel T. F. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. J Biol Chem. 1993 May 5;268(13):9172–9175. [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Enger K. T., Deuel T. F. A second transcriptionally active DNA-binding site for the Wilms tumor gene product, WT1. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8896–8900. doi: 10.1073/pnas.90.19.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen V., Boyd P. A., Seawright A., Fletcher J. M., Fantes J. A., Buckton K. E., Spowart G., Porteous D. J., Hill R. E., Newton M. S. Molecular analysis of chromosome 11 deletions in aniridia-Wilms tumor syndrome. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8592–8596. doi: 10.1073/pnas.82.24.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]


