Abstract
The expression of DCC (deleted in colorectal cancer) is often markedly reduced in colorectal and other cancers. However, the rarity of point mutations identified in DCC coding sequences and the lack of a tumor predisposition phenotype in DCC hemizygous mice have raised questions about its role as a tumor suppressor. DCC also mediates axon guidance and functions as a dependence receptor; such receptors create cellular states of dependence on their respective ligands by inducing apoptosis when unoccupied by ligand. We now show that DCC drives cell death independently of both the mitochondria-dependent pathway and the death receptor/caspase-8 pathway. Moreover, we demonstrate that DCC interacts with both caspase-3 and caspase-9 and drives the activation of caspase-3 through caspase-9 without a requirement for cytochrome c or Apaf-1. Hence, DCC defines an additional pathway for the apoptosome-independent caspase activation.
Vogelstein and his colleagues (1) have shown that the development of colonic carcinoma from normal colonic epithelium is associated with the mutation of a specific set of genes. Allelic deletions (loss of heterozygosity) on chromosome 18q in more than 70% of primary colorectal tumors prompted the search for a tumor suppressor gene at that locus. This search led to the cloning of a putative cell-surface receptor, DCC (deleted in colorectal cancer) (1). DCC expression was then shown to be markedly reduced in more than 50% of colorectal tumors. Moreover, the loss of DCC expression is not restricted to colon carcinoma but has been observed in other tumor types, including carcinoma of the stomach, pancreas, esophagus, prostate, bladder, breast, male germ cell tumors, neuroblastomas, gliomas, and some leukemias (2, 3). However, proof that DCC is a tumor suppressor gene remains inconclusive (4, 5).
DCC encodes an approximately 200-kDa type I membrane protein of 1,447 amino acids, which displays homology in its extracellular domain with cell adhesion molecules (2), suggesting that DCC may play a role in cell–cell or cell–matrix interactions (6). However, DCC-mediated cell aggregation has not been firmly established (7). Recently, Tessier-Lavigne and collaborators (8, 9) have suggested that DCC may function as a component of a receptor complex that mediates the effects of the axonal chemoattractant netrin-1. The role of DCC in mediating growth cone extension has been supported by the analysis of the DCC knockout mice, which display abnormal brain development (4). However, the signaling transduction of netrin-1 through DCC that results in axon outgrowth is mainly unknown. In response to netrin-1 binding, DCC has been shown to interact with other netrin-1 receptors like UNC5H (i.e., three members UNC5H1, -2, and -3) (10) or the adenosine A2b receptor shown to transduce cAMP production upon netrin-1 binding (11). Recently, it also has been proposed that Frazzled, the Drosophila ortholog of DCC, is not, in certain circumstances, a transducing receptor but rather a carrier for the cue netrin-1 that allows netrin-1 distribution in specific regions of the nervous system (12).
The link between the putative role of DCC as a tumor suppressor and the ability of DCC to bind netrin-1 and to mediate axon guidance was, however, not at all clear. Recently, we have shown that DCC is a dependence receptor (13) and therefore functionally related to other dependence receptors such as p75NTR, the common neurotrophin receptor, the androgen receptor, and RET (14–17). Such receptors create cellular states of dependence on their respective ligands by inducing apoptosis when unoccupied by ligand but inhibiting apoptosis in the presence of ligand (13–16). Hence, we have shown that the expression of DCC induces apoptosis in the absence of netrin-1, but in the presence of netrin-1, DCC is antiapoptotic. Furthermore, DCC was demonstrated to be a caspase substrate, with the major site of cleavage at Asp-1290. The caspase cleavage of DCC was shown to be required for DCC to exert its proapoptotic effect, just as it has been shown for the androgen receptor and RET (13, 16, 17). Functionally, therefore, DCC serves as a caspase amplifier in the absence of ligand via exposure of a proapoptotic domain lying in the amino-terminal region of the intracellular domain, proximal to the cleavage site.
We investigated the mechanism by which DCC induces cell death when cleaved by caspases. We demonstrate that DCC induces apoptosis in a caspase-9-dependent pathway, yet by a mechanism that is independent of the intrinsic (mitochondria-dependent) apoptotic pathway. We also show that DCC recruits caspase-3 and caspase-9, resulting in the activation of caspase-3 via caspase-9. Hence, DCC defines an additional pathway for the apoptosome-independent caspase activation.
Methods
Cells, Transfection Procedures, and Constructs.
The neuroblastoma cell line IMR32 constitutively expressing DCC was from ECA cell lines. The Apaf1 −/− SAK-2 cells were obtained from P. Gruss, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany, and transient transfection was performed by using Lipofectamine+ (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The caspase-9 −/− cells were obtained from R. Flavell, Yale Univ. School of Medicine, New Haven, CT, and transient transfection was performed by using Lipofectamine+ (Life Technologies). The 293-EBNA-netrin-1 producing netrin-1 were obtained from M. Tessier-Lavigne, Univ. of California San Francisco. Transient transfection of colorectal cell line REGb or of human embryonic kidney 293T cells was performed as described (13) by using the following plasmids. pDCC-CMV-S, pDCC-CMV.D1290N, and pGNET1myc have been described (13). pBabe-p35 was a gift of P. Friesen, Univ. of Wisconsin, Madison. pcDNA-Crma was a gift of David Pickup, Duke Univ. Medical Center, Durham, NC. pcDNA-Bcl2 was obtained by cloning the 1,855-bp cDNA fragment of Bcl-2 (18) into pcDNA-3.1. pcDNA-casp3, pcDNA-casp6, pcDNA-casp7, pcDNA-casp8, and pcDNA-casp9 were gifts of G. S. Salvesen, Burnham Institute, La Jolla, CA. Dominant negative caspase-encoding plasmids were obtained by mutating the active site cysteines to alanine via a Quikchange strategy (Stratagene). pcDNA-DNcasp3, pcDNA-DNcasp6, pcDNA-DNcasp7, pcDNA-DNcasp8, and pcDNA-DNcasp9 encode, respectively, the dominant negative mutants for caspase-3, -6, -7, -8, and -9.
Immunoblotting and Immunoprecipitation.
One-dimensional immunoblots by using antibodies raised against DCC (Oncogene Research Products, Cambridge, MA), Flag M2 epitope (Sigma), and caspase-3 and caspase-9 (PharMingen) were performed as described (19). Coimmunoprecipitations were performed on cotransfected 293T or IMR32 lysed in 50 mM Hepes, pH 7.6/125 mM NaCl/5 mM EDTA/0.1% Nonidet P-40, by using a caspase-3, caspase-9, or Flag M2 antibody and protein-A Sepharose (Sigma). DCC interaction with caspases was monitored by immunoblot by using anti-DCC antibody.
Cell Death Analysis and Caspase Activity Measurement.
Cell death was analyzed by using trypan blue as described in Rabizadeh et al. (20). Briefly, 48 h after transfection, all of the cells contained in a 35-mm dish were washed and resuspended in DMEM. The percentage of cell death was then obtained by enumerating the trypan blue positive cells versus the total number of cells. Caspase-8 and caspase-3 activity were measured by using the ApoAlert caspase-8, ApoAlert caspase-3 assays from CLONTECH and the caspase-3 activity assay from Boehringer Mannheim. These assays use the Ac-IETD-AFC and Ac-DEVD-AFC substrates, respectively. These activities were determined according to the manufacturer's instructions, and caspase activation is presented as the ratio between the caspase activity of the sample and that measured in 293T cells transfected with pCMV. Terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) assays also were performed by using the ApoAlert DNA fragmentation assay (CLONTECH) according to manufacturer's instructions on either adherent SAK-2 or cytospun 293T and IMR32 cells. TUNEL reactivity was examined and photographed with a Zeiss Axioscope photomicroscope. The number of TUNEL-positive cells was estimated by counting at least 100 TUNEL-positive cells from six separate fields.
Two-Hybrid Analysis.
The two-hybrid system Matchmaker II, developed by CLONTECH, was used according to the manufacturer's instructions. As bait, the coding sequence of DCC-IC (1121–1447) from pSVK3-HA-DCC-IC (13) was inserted in pAS21 through NdeI–SalI insertion. The human brain library was also from CLONTECH. Y190 yeast strain was cotransformed with the DCC-IC fused to the DNA binding domain of GAL4 and the brain library fused to the activating domain of GAL4. Cells were then allowed to grow in the absence of leucine, tryptophan, and histidine and in the presence of 55 mM 3-amino-1,2,4-triazole. Forty-five positive clones representing nine unique cDNAs were isolated from 107 screened.
In Vitro Translation, S100 293T Lysate, and Caspase Cleavage Reactions.
Plasmids pCR-DCC-IC, pCR-DCC-IC.D1290N, pCR-DCC-IC/1121–1290, or pCR-DCC-IC/1290–1447 described in ref. 13 were transcribed by using T7 polymerase, then translated by using the TNT system (Promega) for 2 h at 30°C. The S100 lysate was prepared as described by Li et al. (21) from 108 293T, L929, or SAK-2 cells. To immunodeplete S100 lysate of cytochrome c, 1 μg of anti-cytochrome c (PharMingen) was added to 100 μl of lysate, and protein-A Sepharose was used to pull them out. A similar immunodepletion of caspase-9 was performed by using caspase-9 antibody (PharMingen).
Results
DCC-Induced Apoptosis via Caspase-9.
As DCC cleavage by caspases has been shown to be required for DCC proapoptotic activity (13), we first assessed whether caspase inhibition is sufficient to block DCC-induced cell death. Baculovirus protein p35, a potent and general caspase inhibitor (22), was expressed in the presence of DCC in human embryonic kidney 293T cells, and cell death was measured by trypan blue staining. Fig. 1A shows the complete suppression of DCC-induced cell death in the presence of p35, confirming the role of caspases in DCC-induced apoptosis. A similar block of cell death was observed when the caspase inhibitor zVAD-fmk was used (Fig. 1C). Hence, caspase activation is required for DCC-induced cell death.
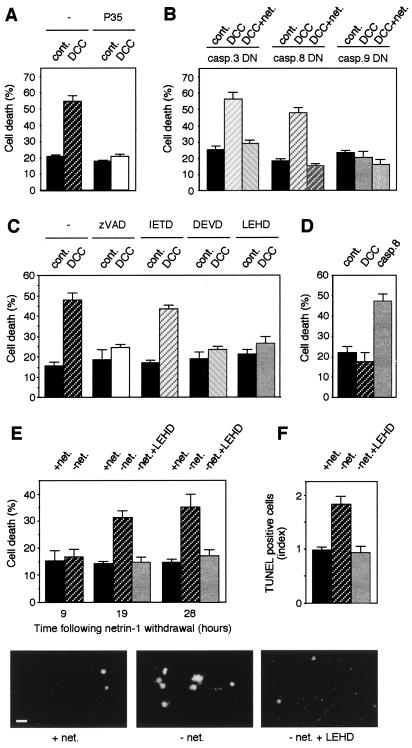
Figure 1.
Cell death induction by DCC requires caspase-9. Human embryonic kidney 293T cells were transiently transfected as described (13) with the pCMV control plasmid (cont.), the DCC expression plasmid pDCC-CMV-S (DCC), or the DCC expression plasmid and the netrin-1-encoding plasmid pGNET1myc (DCC + net.) in the presence of the p35 expression construct pBabe-p35 (A), or of the dominant negative caspase-3, -8, or -9 expression construct (B). Cell death was analyzed by using trypan blue as described in ref. 13. (C) Mock (cont.) or DCC (DCC) transfected 293T cells were incubated or not with 20 μM Zvad-fmk (Zvad), IETD-fmk (IETD), DEVD-fmk (DEVD), or LEHD-fmk (LEHD) 24 h after the beginning of the transfection. Cell death was then analyzed by using trypan blue (as in A and B) 24 h later. (D) Caspase-9 −/− cells were transfected with a mock plasmid (cont.), DCC-expressing plasmid (DCC), or the caspase-8-expressing plasmid pcDNA-casp.8 (casp.8). (E and F) IMR32 cells were cultured for 48 h in the presence of culture medium issued of 293-EBNA-netrin-1 cells producing extracellular netrin-1. After this conditioned growth, cells were either further cultured with netrin-1-containing medium (+ net.) or with medium devoid of netrin-1 (− net.). In the latter case, cells were incubated or not with 20 μM LEHD-fmk (+LEHD). Cell death was then measured either by trypan blue staining at the indicated time following netrin-1 withdrawal (E) or by TUNEL reactivity after 24 h of netrin-1 withdrawal (F).
To specify what caspase was involved, we either coexpressed DCC with dominant negative (catalytic) mutants of caspases or expressed DCC in the presence of caspase inhibitors. Coexpression of the dominant negative mutant of caspase-8 failed to block DCC-induced apoptosis (Fig. 1B). Similarly, the addition of the caspase-8 inhibitor IETD-fmk showed no effect on cell death induction by DCC (Fig. 1C). The caspase-8 inhibitor CrmA was also unable to block DCC-induced cell death (not shown). Hence, caspase-8 is not required for DCC-induced cell death. The coexpression of the dominant negative mutants of caspase-6 and -7 also failed to block DCC-induced cell death (not shown), suggesting that caspase-6 and -7 are not required either. The expression of the dominant negative mutant of caspase-3 also had no influence on cell death induction by DCC (Fig. 1B), but the treatment with the caspase-3 inhibitor DEVD-fmk blocked DCC-induced cell death (Fig. 1C). This observation suggested that either caspase-3 is required but can be replaced in certain circumstances by another caspase-3-like caspase or that the proapoptotic activity of DCC required a caspase-3-like caspase displaying an affinity for the DEVD motif.
Of interest, coexpression of the dominant negative mutant of caspase-9 completely blocked cell death induced by DCC (Fig. 1B). An inhibition of DCC-induced cell death by the dominant negative mutant of caspase-9 also was observed in the colorectal REGb cell line (not shown). Similarly, the addition of the caspase-9 inhibitor LEHD-fmk suppressed the cell death induction by DCC (Fig. 1C). To determine whether DCC-induced cell death requires caspase-9 per se or another caspase that could be inhibited by the dominant negative mutant caspase-9 and by the LEHD-fmk inhibitor, we analyzed the DCC effect in caspase-9 knockout cells. Remarkably, whereas, as a positive control, a caspase-8 transfection induced cell death, DCC transfection was unable to drive caspase-9 knockout cell death (Fig. 1D).
Finally, to investigate whether in cells endogenously expressing DCC, netrin-1 withdrawal led to a caspase-9-dependent cell death, we first “conditioned” the neuroblastoma cell line IMR-32 endogenously expressing DCC by allowing their growth in the presence of netrin-1. After such “conditioning”, netrin-1 withdrawal led to an increased number of apoptotic cells measured either by trypan blue staining (Fig. 1E) or TUNEL reactivity (Fig. 1F). Of interest, inhibition of caspase-9 with the LEHD-fmk inhibitor blocked cell death induced by netrin-1 withdrawal. Hence, apoptosis induced by DCC required caspase-9, and because the only known apoptotic pathway by using caspase-9 involves apoptosome formation with caspase-9/Apaf-1/cytochrome c/(d)ATP, these data suggested the involvement of this mitochondria-dependent apoptotic pathway in DCC-induced cell death (23).
DCC-Induced Apoptosis Is Independent of the Mitochondria-Dependent Apoptotic Pathway.
We therefore analyzed the ability of DCC to induce cell death in the presence of inhibitors of this mitochondria-dependent apoptotic pathway. 293T cells were transfected with DCC in the presence or absence of Bcl-2 (18). Fig. 2A shows that although Bcl-2 expression was sufficient to block staurosporine-induced cell death, Bcl-2 had no effect on the proapoptotic activity of DCC. A similar effect was observed in colorectal REGb (not shown) and DLD cells (24). Bcl-2 has been shown to block the cell-death pathway involving cytochrome c release and cytochrome c-dependent activation of caspase-9 (23, 25). Hence, we analyzed the ability of an inhibitor of cytochrome c release to modulate DCC proapoptotic activity. DCC-transfected cells then were incubated in the presence of cyclosporin A, an inhibitor of the mitochondrial membrane permeability transition (26). As shown in Fig. 2B, although cyclosporin A blocked staurosporine-induced cell death, it had no effect on DCC-induced apoptosis. Moreover, comparing control and DCC-transfected cells, no DCC-induced specific release of cytochrome c was observed, at least during the 36 h following transfection (not shown). To further analyze whether the apoptosome was actually required for cell death induction by DCC, we studied the effect of DCC expression in Apaf1 −/− knockout cells. As shown in Fig. 2C, in Apaf1 −/− cells, although a staurosporine treatment had no effect on cell death, DCC expression induced an increased trypan blue staining (Fig. 2C) and TUNEL reactivity (not shown). Hence, these data strongly suggest that, although DCC required caspase-9, it did not require the mitochondria-dependent apoptotic pathway that involves cytochrome c release and apoptosome formation, which is the only known pathway for caspase-9 activation.
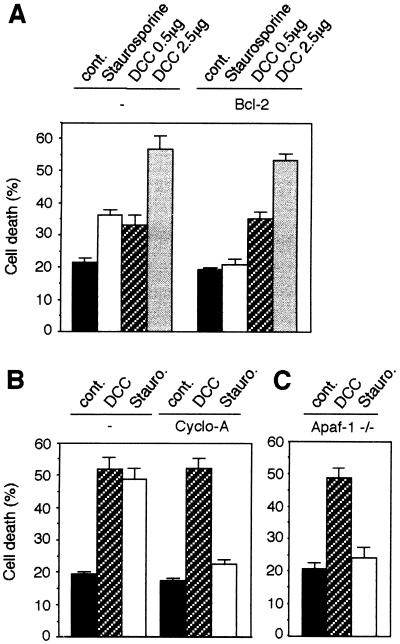
Figure 2.
DCC-induced cell death does not require a mitochondria-dependent apoptotic pathway. (A) 293T cells were transiently transfected as described in Fig. 1 with either the DCC expression plasmid pDCC-CMV-S (DCC) or pCMV control plasmid (cont.) in the presence or absence of the Bcl-2 expression construct pcDNA-Bcl2. Cell death was then monitored 48 h after transfection as described in Fig. 1. Two tested quantities of DCC plasmid (0.5 and 2.5 μg/60-mm dish) are shown; 0.5 μg/60-mm dish is the lowest quantity of DCC-expressing plasmid tested that was able to induce a significant increase of cell death. Bcl-2 activity was controlled by measuring death of Bcl-2-transfected cell after addition of 5 μM staurosporine for 4 h. (B) Control or DCC-transfected cells were incubated the last 18 h with 5 μM cyclosporin A. Cell death was then monitored 48 h after transfection. As a control assay, pCMV-transfected cells were treated for 4 h with 5 μM staurosporine. (C) Apaf-1 −/− SAK-2 cells were transiently transfected with either the pCMV control plasmid (cont.) or the DCC expression plasmid pDCC-CMV-S (DCC). 48 h after transfection, the mock-transfected cells were then treated (Stauro.) or not for 4 h with 2 μM staurosporine. Cell death was then monitored at 48 h as described in Fig. 1.
DCC Interacts with Caspase-3 and Caspase-9.
A search for DCC-interacting proteins that may explain this apparent paradox, by using two-hybrid screening of a human brain library with the DCC intracellular domain (DCC-IC) as bait, revealed the interaction of DCC-IC with a polypeptide whose sequence corresponds to that of caspase-3 (Fig. 3A). A coimmunoprecipitation study confirmed the interaction of caspase-3 with DCC (Fig. 3B). However, the caspase-3–DCC interaction was detected only when DCC was expressed in the presence of a ligand or when the caspase cleavage site of DCC (Asp-1290) was mutated, both of which are conditions in which DCC does not induce cell death (13). As a control experiment, when a similar coimmunoprecipitation was performed with caspase-6, no interaction was detected (Fig. 3C). In a search of other caspases interacting with DCC, we observed an interaction of DCC with caspase-9 (Fig. 3C and ref. 13). However, the interaction was visualized predominantly when DCC was in its proapoptotic state; only weak binding was detected when DCC was expressed with netrin-1 or when DCC D1290N was used. Caspase-9–DCC interaction was monitored either from a pull-down with caspase-9 (Fig. 3C) or from a pull-down with DCC (not shown). Moreover, interaction of DCC with caspase-9 was visualized in the neuroblastoma cell line IMR-32 endogenously expressing DCC and caspase-9 (Fig. 3D).
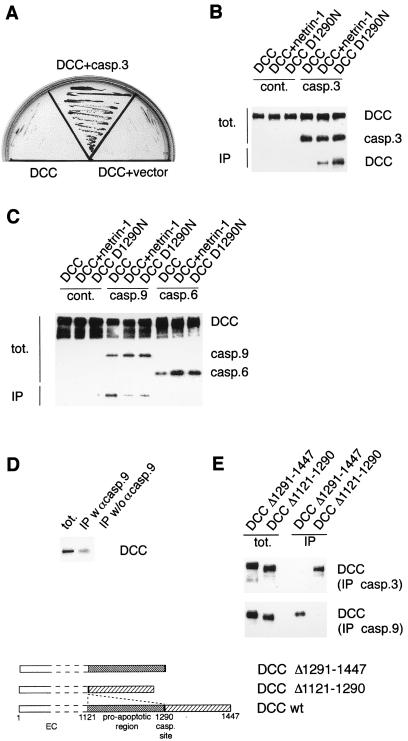
Figure 3.
Interaction of caspase-3 and caspase-9 with the intracytoplasmic domain of DCC. (A) Two-hybrid screening of DCC-IC revealed interaction with caspase-3. Y190 yeast transformed with a GAL4 DNA binding domain-DCC-IC fusion (DCC) were transformed with either a mock plasmid containing the GAL4 transcriptional activation domain AD (DCC + vector) or a similar construct fused to a brain cDNA library. Cells were then allowed to grow in the absence of leucine, tryptophan, and histidine and in the presence of 55 mM 3-amino-1,2,4-triazole. The clone corresponding to caspase-3 is shown (DCC + caspase-3). (B) Coimmunoprecipitations were performed on 293T cells cotransfected with pcDNA-DNcasp3 (casp.3) or pcDNA-3 (cont.) and pDCC-CMV-S, pDCC-CMV.D1290N, or pDCC-CMV-S + pGNET1myc, the netrin-1-encoding plasmid. To obtain a similar expression of the protein DCC in the different sample, half of the plasmid pDCC-CMV-S was used when cotransfected with the netrin-1-expressing vector because the presence of DCC ligand leads to increased stability of DCC (13). Anti-caspase-3 antibody was used to immunoprecipitate caspase-3, and binding was revealed by Western blot by using anti-DCC antibody. (C) Same as in B except that pcDNA-DNcasp9 and pcDNA-DNcasp6 were used. In this case, anti-Flag antibody was used to immunoprecipitate DNcaspase-9 and DNcaspase-6. (D) Immunoprecipitation of DCC by caspase-9 was performed by using IMR-32 cells. The pull-down was performed with or without anti-caspase-9 antibody. (E) Coimmunoprecipitations were performed, respectively, as in B and C on 293T cotransfected with pcDNA-DNcasp3 or pcDNA-DNcasp9 in the presence of either pDCC-CMV-Δ1121–1290 or pDCC-CMV-Δ1290–1447. tot., immunoblots performed on the lysate before addition of the antibodies allowing the pull out; IP, immunoblots by using an anti-DCC antibody on samples derived from the pull-downs. In B–E, dominant negative mutants of caspases were chosen instead of tagged wild-type because overexpression of wild-type caspase drove massive 293T cell death.
In a search of the interacting domain of DCC, the proapoptotic region of DCC (amino acids 1121–1290, ref. 13) lying upstream of its main caspase cleavage site was found to be crucial for caspase-9 interaction, whereas the region distal to the caspase site was required for caspase-3 interaction (Fig. 3E). Such an interaction of DCC with caspase-3 and -9 is reminiscent of the apoptosome complex Apaf-1, caspase-9, caspase-3, cytochrome c, and (d)ATP (21).
In Vitro Activation of Caspase-3 by DCC.
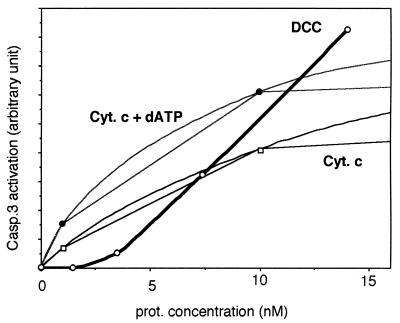
Because the caspase-9 apoptosome requires cytochrome c to activate caspase-9, which then activates caspase-3 (21), whereas DCC induced apoptosis independently of cytochrome c release, we established an in vitro assay to determine whether the DCC complex could form a direct activator of caspase-3 in the absence of cytochrome c. The combination of in vitro-translated DCC-IC, caspase-9, and caspase-3 was not sufficient to induce caspase-3 activation (not shown). However, the addition of an S100 cell lysate depleted of cytochrome c showed a significant activation of caspase-3 by DCC-IC monitored either by the cleavage of purified pro-caspase-3 (Fig. 4A) or of Ac-DEVD-AFC (Fig. 4B). Dose-dependence analysis revealed that DCC-IC and cytochrome c display different kinetics for caspase-3 activation (Fig. 5). Indeed, the minimum required concentration of cytochrome c for pro-caspase-3 activation was found to be 3.5 times less than the one for DCC-IC. Moreover, the addition of exogenous dATP further increased cytochrome c-induced caspase-3 activation at a low concentration of cytochrome c, hence suggesting that, at low concentration, cytochrome c is a better activator than DCC-IC (Fig. 5). However, the limiting factors present in the cell lysate were reached more rapidly with cytochrome c, as the maximal caspase-3 activation was observed with DCC-IC. Hence, in a physiological cellular context, DCC might conceivably be a good activator of caspase activation. DCC was able to mediate caspase-3 activation in the absence of cytochrome c and in an S100 lysate of Apaf-1 −/− cells (Fig. 6A), strongly suggesting that DCC-induced caspase-3 activation is apoptosome-independent. Moreover, DCC-induced caspase-3 activation was not accompanied with caspase-9 cleavage that is very often associated with caspase-9 activation (not shown). However, caspase-9 was required, as immunodepletion of caspase-9 in the S100 cell lysate led to an inhibition of the DCC-induced caspase-3 activation (Fig. 6B).
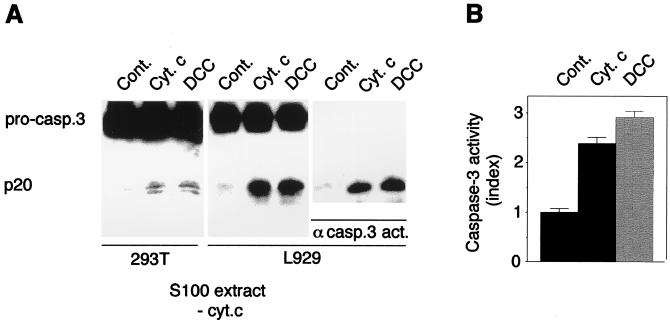
Figure 4.
DCC-IC activation of caspase-3 in vitro. Cytochrome c-immunodepleted S100 extract from 293T or L929 cells (19) was incubated with 0.2 μM of purified pro-caspase-3 in the presence of an in vitro translation of DCC-IC (DCC) or of an empty vector (cont.) for 1 h (293T) or 24 h (L929) at 30°C. As a positive control, 1 μM cytochrome C was added. (A) Immunoblots developed with anti-caspase-3 (active and pro-form of caspase-3) or anti-active caspase-3 antibodies (PharMingen) are shown. (B) Caspase-3 activity was also monitored as described in Methods by using the ApoAlert caspase-3 activity assay.
Figure 5.
Comparison of caspase-3 activation by DCC-IC versus cytochrome c. A dose-dependence analysis of DCC-induced caspase-3 activation was performed. In a similar in vitro assay as in Fig. 4, different quantities of DCC-IC or cytochrome c in the presence or not of 1 mM dATP were added. In the case of DCC-IC, the concentration was estimated based on comparison with purified protein (L.G. and P.M., unpublished data). Purified cytochrome c was used as attempts to activate caspase-3 in vitro with in vitro-translated cytochrome c were unsuccessful, probably as a result of the absence of the cytochrome c-associated heme (C.F. and P.M., unpublished results). The p20 cleavage fragment of caspase-3 was then detected by immunoblot and quantified by densitometry. A graph of the level of caspase-3 activation is presented.
Figure 6.
DCC-IC activation of caspase-3 required caspase-9 and DCC cleavage. The same in vitro assay described in Fig. 4A was performed, but in this case, the S100 lysate was generated from Apaf-1 −/− SAK-2 cells (A), the 293T S100 lysate was immunodepleted in caspase-9 (B), or in vitro-translated DCC-IC D1290N, DCC-IC/1121–1290, DCC-IC/1291–1447 were used instead of DCC-IC (C).
Thus, DCC interacts with caspases-3 and -9 and mediates the caspase-9-dependent activation of caspase-3 in a cytochrome c-independent manner. Therefore, DCC participates in an alternative pathway of caspase activation in which cytochrome c is not required but DCC cleavage is. Indeed, when a similar in vitro assay was performed by using DCC-IC D1290N, the intracellular domain of DCC mutated in its main caspase site, no activation of caspase-3 was observed (Fig. 6C). Hence, cleavage of DCC by caspases is a necessary step for further caspase-3 activation. Furthermore, the proapoptotic region of DCC that binds to caspase-9 (1121–1290) was sufficient to activate pro-caspase-3, whereas the C-terminal IC region (1290–1447) had no comparable activity. Taken together, these results suggest that the caspase-activating complex lies in the proapoptotic region of DCC, a region released or exposed via the caspase cleavage in D1290.
Discussion
Here, we present evidence that DCC-induced cell death requires caspase-9 but does not involve cytochrome c release and subsequent apoptosome-associated caspase-9 activation. DCC-induced cell death may thus be related to the ability of the intracellular domain of DCC to (i) recruit pro-caspase-9 and pro-caspase-3 and (ii) mediate caspase-9-dependent caspase-3 activation in a cell-free extract. Moreover, this mechanism of activation of caspase-3, which is not only independent of mitochondria, cytochrome c release, and apoptosome formation but also independent of caspase-8 activation, represents a novel mechanism for the induction of apoptosis. As with the Fas-FADD(Mort-1)/pro-caspase-8/FLASH (27) and cytochrome c/Apaf-1/pro-caspase-9 pathways (21), DCC induces caspase activation and apoptosis through the recruitment of caspases into an activating complex. Of interest, DCC sequence does not show any death domain, CARD motif, or death effector domain, suggesting either an indirect interaction (i.e., DCC with caspase-9) via an adaptor molecule or a direct interaction (i.e., DCC with caspase-3) via an unknown motif of interaction. The observation that the DCC, caspase-9, and caspase-3 combination is not sufficient to activate in vitro caspase-3 but required the addition of a S100 lysate strongly suggests that other proteins present in the lysate are required to allow a functional DCC-associated caspase-activating complex. The nature of this complex, and whether it includes additional proteins similar to FADD or Apaf-1, remains to be determined.
There exists an interesting difference between the DCC-associated caspase-activating complex and the apoptosome complex. Indeed, within the apoptosome complex, caspase-9 once recruited is cleaved and displays an increased activity (21), whereas caspase-9 recruitment into the DCC complex is apparently not accompanied by caspase-9 processing. However, caspase-9 zymogen has been described to have proteolytic activity similar to its cleaved form because the zymogenicity (i.e., ratio of the processed protease activity to the nonprocessed protease activity) of caspase-9 is approximately 10, whereas the zymogenicity of caspase-3 is greater than 10,000 (28). Moreover, as described recently (29), the proteolytic processing of pro-caspase-9 has only a minor effect on the enzyme's catalytic activity, whereas the key requirement for caspase-9 activation is its association with a dedicated protein cofactor Apaf-1. Because DCC still allowed caspase-3 activation in Apaf1 −/− knockout cell lysate, another caspase-9 cofactor may be present in the DCC-associated caspase-activating complex. An alternative explanation may be that caspase-9 is required for DCC-induced cell death and caspase activation not because it functions as a protease but rather because it displays another unknown function (e.g., adaptor). Along this line, it has been recently shown that in certain circumstances, caspase-9 zymogen but not its LEHD-cleaving activity is needed for cell death induction (30).
It is tempting to speculate that the ligand withdrawal leads to the formation of the DCC-associated caspase-activating complex, as ligand withdrawal leads to DCC pro-apoptotic activity. The nature of the DCC change allowing caspase-3 activation is probably related to a change of conformation of the receptor similar to the one described in the case of p75ntr (15). This change could then affect DCC proapoptotic activity at two levels: (i) the caspase cleavage of DCC and (ii) the recruitment of caspase-9. Both of these events are necessary to allow further caspase-3 activation. In favor of the second effect, we indeed showed that caspase-9 binds DCC predominantly when expressed in the absence of netrin-1, although we cannot exclude the possibility that this preferential recruitment is a subsequent effect of the DCC caspase cleavage. The fact that DCC cleavage is blocked by ligand presence is, however, mostly speculative, as we have been unable to detect the caspase cleavage of full-length DCC (ref. 13, C.F. and P.M., unpublished results). Two models could thus be proposed. A first model would be that the presence of netrin-1 blocks DCC caspase cleavage (e.g., via a closed conformation), which allows the recruitment of pro-caspase-3. Ligand withdrawal would then lead to the caspase cleavage of DCC and the subsequent exposure and/or release of the pro-apoptotic region of DCC. This would then lead to the recruitment of a caspase-activating complex. The second model would then postulate that DCC is, whether the netrin-1 is present or not, cleaved by active caspase, a preliminary but not sufficient event for further pro-apoptotic activity. Along this line, RET, another dependence receptor, was shown to be cleaved both in the absence and in the presence of its ligand (17). The presence of the ligand would then inhibit caspase-9 recruitment and the further formation of a caspase-activating complex. In both models, an apparent paradox would emerge because caspase cleavage releases the C-terminal domain of DCC shown here to bind pro-caspase-3. The caspase-activating complex would then appear in the N-terminal region IC of DCC when the pro-caspase-3 is no longer present to be activated. To reconcile this apparent paradox, two possible scenarios can be proposed: (i) the cleavage of DCC brings together the pro-caspase-3 interacting with the C-terminal IC domain and the caspase-activating complex interacting with the N-terminal IC domain of DCC and allows further caspase-3 activation, or (ii) the DCC-associated caspase-activating complex based on the presence of caspase-9 activates other effector caspases (e.g., caspase-7 or caspase-3 not associated with DCC) and pro-caspase-3 interaction with DCC is independent of the caspase-activating complex. Thus, the pro-caspase-3 interaction with DCC observed in the presence of netrin-1 then could represent a reservoir of unavailable caspase-3, contributing to the antiapoptotic activity of DCC bound to its ligand (13).
DCC and its associated caspase-activating complex may thus play a critical role in the regulation of axon guidance during development of the nervous system (4, 8, 9) and in tumor suppression. Recently, Yee et al. (9) demonstrated that netrin-1 knock-out mice show not only a loss in axonal guidance but also in neural pontine cell migration, the latter of which is related to an increased cell death (31). This result provides strong support for the notion that DCC can function in vivo to induce cell death. We also observed that in rat embryonic dorsal spinal cord explants, the presence of netrin-1 not only allows axonal projections but also blocks a caspase-9-dependent death of the explant known to express DCC (unpublished work), suggesting that axonal guidance and cell death could represent two dependent mechanisms. Whether netrin-1-mediated inhibition of caspase activation has a role in directing axon projections in the nervous system, however, remains to be shown. Finally, with respect to the potential function of DCC as a tumor suppressor, these results have suggested that at least one role of DCC may be to induce apoptosis in expressing cells that grow or metastasize beyond the limitation of ligand availability, by using a mechanism that requires a DCC-associated caspase-activating complex.
Acknowledgments
We thank G. S. Salvesen for the gift of caspase cDNAs, M. Tessier-Lavigne for the 293-EBNA netrin-1, P. Gruss for the Apaf-1 knockout cells, and R. Flavell for the caspase-9 knockout cells. L.G. and V.C. were supported by Association pour la Recherche sur le Cancer Grant 9036. This work was supported by the Ligue Contre le Cancer, the Fondation pour la Recherche Médicale, Association pour la Recherche sur le Cancer Grants 9036 and 9340, the Emergence Program, and National Institutes of Health Grants NS33376 and CA69381.
Abbreviation
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fearon E R, Cho K R, Nigro J M, Kern S E, Simons J W, Ruppert J M, Hamilton S R, Preisinger A C, Thomas G, Kinzler K W, et al. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 2.Cho K R, Fearon E R. Curr Opin Genet Dev. 1995;5:72–78. doi: 10.1016/s0959-437x(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 3.Fearon E R. Biochim Biophys Acta. 1996;1288:M17–M23. doi: 10.1016/0304-419x(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 4.Fazeli A, Steen R G, Dickinson S L, Bautista D, Dietrich W F, Bronson R T, Bresalier R S, Lander E S, Costa J, Weinberg R A. Nature (London) 1997;386:796–804. [Google Scholar]
- 5.Tanaka K, Oshimura M, Kikuchi R, Seki M, Hayashi T, Miyaki M. Nature (London) 1991;349:340–342. doi: 10.1038/349340a0. [DOI] [PubMed] [Google Scholar]
- 6.Hedrick L, Cho K R, Fearon E R, Wu T C, Kinzler K W, Vogelstein B. Genes Dev. 1994;8:1174–1183. doi: 10.1101/gad.8.10.1174. [DOI] [PubMed] [Google Scholar]
- 7.Chuong C M, Jiang T X, Yin E, Widelitz R B. Dev Biol. 1994;164:383–397. doi: 10.1006/dbio.1994.1208. [DOI] [PubMed] [Google Scholar]
- 8.Keino-Masu K, Masu M, Hinck L, Leonardo E D, Chan S S, Culotti J G, Tessier-Lavigne M. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 9.Serafini T, Colamarino S A, Leonardo E D, Wang H, Beddington R, Skarnes W C, Tessier-Lavigne M. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 10.Hong K, Hinck L, Nishiyama M, Poo M-M, Tessier-Lavigne M, Stein E. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 11.Corset V T, Nguyen-Ba-Charvet K, Forcet C, Moyse E, Chédotal A, Mehlen P. Nature (London) 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 12.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. Nature (London) 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 13.Mehlen P, Rabizadeh S, Snipas S J, Assa-Munt N, Salvesen G S, Bredesen D E. Nature (London) 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 14.Rabizadeh S, Oh J, Zhong L T, Yang J, Bitler C M, Butcher L L, Bredesen D E. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 15.Bredesen D E, Ye X, Tasinato A, Sperandio S, Wang J J, Assa-Munt N, Rabizadeh S. Cell Death Differ. 1998;5:365–371. doi: 10.1038/sj.cdd.4400378. [DOI] [PubMed] [Google Scholar]
- 16.Ellerby L M, Hackam A S, Propp S S, Ellerby H M, Rabizadeh S, Cashman N R, Trifiro M A, Pinsky L, Wellington C L, Salvesen G S, et al. J Neurochem. 1999;72:185–195. doi: 10.1046/j.1471-4159.1999.0720185.x. [DOI] [PubMed] [Google Scholar]
- 17.Bordeaux M C, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen D E, Edery P, Mehlen P. EMBO J. 2000;19:4056–4063. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 19.Mehlen P, Kretz-Remy C, Preville X, Arrigo A-P. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 20.Rabizadeh S, Ye X, Sperandio S, Wang J L, Ellerby H M, Ellerby L M, Giza C, Andrusiak R L, Frankowski H, Yaron Y, Moayen N N, et al. J Mol Neurosci. 2000;15:215–230. doi: 10.1385/JMN:15:3:215. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A J, Cruz W D, Zoog S J, Schneider C L, Friesen P D. EMBO J. 1999;18:2031–2039. doi: 10.1093/emboj/18.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y Q, Hsieh J T, Yao F, Fang B, Pong R C, Cipriano S C, Krepulat F. Oncogene. 1999;18:2747–2754. doi: 10.1038/sj.onc.1202629. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 26.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature (London) 1999;397:441–445. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 28.Stennicke H R, Salvesen G S. Cell Death Differ. 1999;6:1054–1059. doi: 10.1038/sj.cdd.4400599. [DOI] [PubMed] [Google Scholar]
- 29.Hengartner M O. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 30.Sperandio S, de Belle I, Bredesen D E. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee K T, Simon H H, Tessier-Lavigne M, O'Leary D D M. Neuron. 1999;24:607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]