Abstract
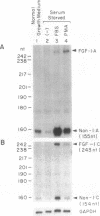
We have previously isolated the human FGF-1 gene in order to elucidate the molecular basis of its gene expression. The gene spans over 100 kbp and encodes multiple transcripts expressed in a tissue- and cell-specific manner. Two variants of FGF-1 mRNA (designated FGF-1.A and 1.B), which differ in their 5' untranslated region, were identified in our laboratory. Recently, two novel variants of FGF-1 mRNA (designated FGF-1.C and 1.D) have been isolated. In this study we used RNase protection assays to demonstrate expression of FGF-1.D mRNA in human fibroblasts and vascular smooth muscle cells and to show that promoter 1D has multiple transcription start sites. A single-strand nuclease-sensitive region has also been identified in the promoter 1D region that may have implications in chromatin conformation and transcriptional regulation of this promoter. Using Northern blot hybridization analyses, a previous study demonstrated a significant increase of FGF-1 mRNA levels in cultured saphenous vein smooth muscle cells in response to serum and phorbol ester. Here we confirm these results by RNase protection analysis and show that FGF-1.C mRNA is significantly increased in response to these stimuli. RNase protection assays indicate that promoter 1C has one major start site. The phorbol ester effect suggests that a protein kinase C-dependent signalling pathway may be involved in this phenomenon. Our results point to a dual promoter usage of the FGF-1 gene in vascular smooth muscle cells. Thus, normal growing cells primarily utilize promoter 1D. In contrast, quiescent cells, when exposed to serum or phorbol ester, utilize a different FGF-1 promoter, namely promoter 1C. Overall, these phenomena suggest mechanisms for increased production of FGF-1 that may play a role in inflammatory settings, wound healing, tissue repair, and neovascularization events and processes via autocrine and paracrine mechanisms. Our findings suggest that different FGF-1 promoters may respond to different physiological conditions and stimuli, in reference to the cell type or tissue milieu, resulting in ultimate production of the FGF-1 protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benitz W. E., Kelley R. T., Anderson C. M., Lorant D. E., Bernfield M. Endothelial heparan sulfate proteoglycan. I. Inhibitory effects on smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1990 Jan;2(1):13–24. doi: 10.1165/ajrcmb/2.1.13. [DOI] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogi E., Winkles J. A., Underwood R., Clinton S. K., Alberts G. F., Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest. 1993 Nov;92(5):2408–2418. doi: 10.1172/JCI116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burkholder G. D., Latimer L. J., Lee J. S. Immunofluorescent staining of mammalian nuclei and chromosomes with a monoclonal antibody to triplex DNA. Chromosoma. 1988 Nov;97(3):185–192. doi: 10.1007/BF00292959. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Pukac L. A., Caleb B. L., Wright T. C., Jr, Karnovsky M. J. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J Cell Biol. 1989 Dec;109(6 Pt 1):3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Wang W. P., Lehtoma K. Alternative splicing generates two forms of mRNA coding for human heparin-binding growth factor 1. Oncogene. 1990 May;5(5):755–762. [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Cuevas P., Carceller F., Ortega S., Zazo M., Nieto I., Giménez-Gallego G. Hypotensive activity of fibroblast growth factor. Science. 1991 Nov 22;254(5035):1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- Davis T. L., Firulli A. B., Kinniburgh A. J. Ribonucleoprotein and protein factors bind to an H-DNA-forming c-myc DNA element: possible regulators of the c-myc gene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9682–9686. doi: 10.1073/pnas.86.24.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen S. J., Heckel J. L., Reich E., Degen J. L. The murine urokinase-type plasminogen activator gene. Biochemistry. 1987 Dec 15;26(25):8270–8279. doi: 10.1021/bi00399a038. [DOI] [PubMed] [Google Scholar]
- Engelmann G. L., Dionne C. A., Jaye M. C. Acidic fibroblast growth factor and heart development. Role in myocyte proliferation and capillary angiogenesis. Circ Res. 1993 Jan;72(1):7–19. doi: 10.1161/01.res.72.1.7. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect Biol Med. 1985 Autumn;29(1):10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Interleukin 1 regulates heparin-binding growth factor 2 gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):296–300. doi: 10.1073/pnas.88.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M. K. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell. 1990 Jun 29;61(7):1165–1166. doi: 10.1016/0092-8674(90)90676-6. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Isolation and characterization of acidic and basic fibroblast growth factor. Methods Enzymol. 1987;147:106–119. doi: 10.1016/0076-6879(87)47102-4. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Miwa T., Boxer L. M., Kedes L. Interaction of nuclear proteins with muscle-specific regulatory sequences of the human cardiac alpha-actin promoter. Mol Cell Biol. 1988 Oct;8(10):4110–4119. doi: 10.1128/mcb.8.10.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Intramolecular DNA triplexes in supercoiled plasmids. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6292–6296. doi: 10.1073/pnas.85.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Murphy M., George D. L. An S1 nuclease-sensitive homopurine/homopyrimidine domain in the c-Ki-ras promoter interacts with a nuclear factor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2705–2709. doi: 10.1073/pnas.87.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Johnson A. C., Jinno Y., Merlino G. T. Modulation of epidermal growth factor receptor proto-oncogene transcription by a promoter site sensitive to S1 nuclease. Mol Cell Biol. 1988 Oct;8(10):4174–4184. doi: 10.1128/mcb.8.10.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. H. The S1-sensitive form of d(C-T)n.d(A-G)n: chemical evidence for a three-stranded structure in plasmids. Science. 1988 Sep 30;241(4874):1800–1804. doi: 10.1126/science.2845572. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Garbisa S., Glaser B., Liotta L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983 Jul 15;221(4607):281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J., Morgan A. R. Poly(pyrimidine) . poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984 Aug 24;12(16):6603–6614. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D. Structures of homopurine-homopyrimidine tract in superhelical DNA. J Biomol Struct Dyn. 1986 Feb;3(4):667–669. doi: 10.1080/07391102.1986.10508454. [DOI] [PubMed] [Google Scholar]
- Maciag T., Kadish J., Wilkins L., Stemerman M. B., Weinstein R. Organizational behavior of human umbilical vein endothelial cells. J Cell Biol. 1982 Sep;94(3):511–520. doi: 10.1083/jcb.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem. 1986 Jan;34(1):85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- Mansson P. E., Malark M., Sawada H., Kan M., McKeehan W. L. Heparin-binding (fibroblast) growth factors type one and two genes are co-expressed in proliferating normal human vascular endothelial and smooth muscle cells in culture. In Vitro Cell Dev Biol. 1990 Feb;26(2):209–212. doi: 10.1007/BF02624114. [DOI] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Miyamoto M., Naruo K., Seko C., Matsumoto S., Kondo T., Kurokawa T. Molecular cloning of a novel cytokine cDNA encoding the ninth member of the fibroblast growth factor family, which has a unique secretion property. Mol Cell Biol. 1993 Jul;13(7):4251–4259. doi: 10.1128/mcb.13.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D. E., Rifkin D. B. Stimulation of motility in cultured bovine capillary endothelial cells by angiogenic preparations. J Cell Physiol. 1984 May;119(2):247–254. doi: 10.1002/jcp.1041190216. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Gustafson T. A., Kedes L. A common factor regulates skeletal and cardiac alpha-actin gene transcription in muscle. Mol Cell Biol. 1988 Oct;8(10):4120–4133. doi: 10.1128/mcb.8.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. L., Payson R. A., Chotani M. A., Deaven L. L., Chiu I. M. Gene structure and differential expression of acidic fibroblast growth factor mRNA: identification and distribution of four different transcripts. Oncogene. 1993 Feb;8(2):341–349. [PubMed] [Google Scholar]
- Nabel E. G., Yang Z. Y., Plautz G., Forough R., Zhan X., Haudenschild C. C., Maciag T., Nabel G. J. Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature. 1993 Apr 29;362(6423):844–846. doi: 10.1038/362844a0. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Bae K. S., Chin K., Wada Y., Schechter A. N., Hankins W. D. Cloning of the human erythropoietin receptor gene. Blood. 1991 Nov 15;78(10):2548–2556. [PubMed] [Google Scholar]
- Payson R. A., Canatan H., Chotani M. A., Wang W. P., Harris S. E., Myers R. L., Chiu I. M. Cloning of two novel forms of human acidic fibroblast growth factor (aFGF) mRNA. Nucleic Acids Res. 1993 Feb 11;21(3):489–495. doi: 10.1093/nar/21.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Mango S. E., Flint S. J. A nuclease-hypersensitive element of the human c-myc promoter interacts with a transcription initiation factor. Mol Cell Biol. 1989 Nov;9(11):5123–5133. doi: 10.1128/mcb.9.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukac L. A., Ottlinger M. E., Karnovsky M. J. Heparin suppresses specific second messenger pathways for protooncogene expression in rat vascular smooth muscle cells. J Biol Chem. 1992 Feb 25;267(6):3707–3711. [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi J. A., Mori H., Fukumoto M., Igarashi K., Jaye M., Oda Y., Kikuchi H., Hatanaka M. Gene expression of fibroblast growth factors in human gliomas and meningiomas: demonstration of cellular source of basic fibroblast growth factor mRNA and peptide in tumor tissues. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5710–5714. doi: 10.1073/pnas.87.15.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Miyamoto K., Minamino N., Takeda M., Sato B., Matsuo H., Matsumoto K. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8928–8932. doi: 10.1073/pnas.89.19.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Lyall R. M., Hic S., Friesel R., Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985 Dec;101(6):2330–2334. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wang W. P., Lehtoma K., Varban M. L., Krishnan I., Chiu I. M. Cloning of the gene coding for human class 1 heparin-binding growth factor and its expression in fetal tissues. Mol Cell Biol. 1989 Jun;9(6):2387–2395. doi: 10.1128/mcb.9.6.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. P., Myers R. L., Chiu I. M. Single primer-mediated polymerase chain reaction: application in cloning of two different 5'-untranslated sequences of acidic fibroblast growth factor mRNA. DNA Cell Biol. 1991 Dec;10(10):771–777. doi: 10.1089/dna.1991.10.771. [DOI] [PubMed] [Google Scholar]
- Wang W. P., Quick D., Balcerzak S. P., Needleman S. W., Chiu I. M. Cloning and sequence analysis of the human acidic fibroblast growth factor gene and its preservation in leukemia patients. Oncogene. 1991 Sep;6(9):1521–1529. [PubMed] [Google Scholar]
- Weich H. A., Iberg N., Klagsbrun M., Folkman J. Expression of acidic and basic fibroblast growth factors in human and bovine vascular smooth muscle cells. Growth Factors. 1990;2(4):313–320. doi: 10.3109/08977199009167026. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Gay C. G. Serum, phorbol ester, and polypeptide mitogens increase class 1 and 2 heparin-binding (acidic and basic fibroblast) growth factor gene expression in human vascular smooth muscle cells. Cell Growth Differ. 1991 Nov;2(11):531–540. [PubMed] [Google Scholar]
- Yagil G. Paranemic structures of DNA and their role in DNA unwinding. Crit Rev Biochem Mol Biol. 1991;26(5-6):475–559. doi: 10.3109/10409239109086791. [DOI] [PubMed] [Google Scholar]
- Yavachev L. P., Georgiev O. I., Braga E. A., Avdonina T. A., Bogomolova A. E., Zhurkin V. B., Nosikov V. V., Hadjiolov A. A. Nucleotide sequence analysis of the spacer regions flanking the rat rRNA transcription unit and identification of repetitive elements. Nucleic Acids Res. 1986 Mar 25;14(6):2799–2810. doi: 10.1093/nar/14.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Winkel J. G., Ernst L. K., Anderson C. L., Chiu I. M. Gene organization of the human high affinity receptor for IgG, Fc gamma RI (CD64). Characterization and evidence for a second gene. J Biol Chem. 1991 Jul 15;266(20):13449–13455. [PubMed] [Google Scholar]