Abstract
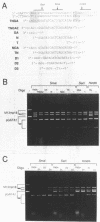
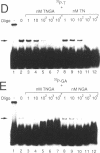
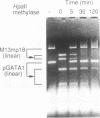
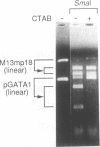
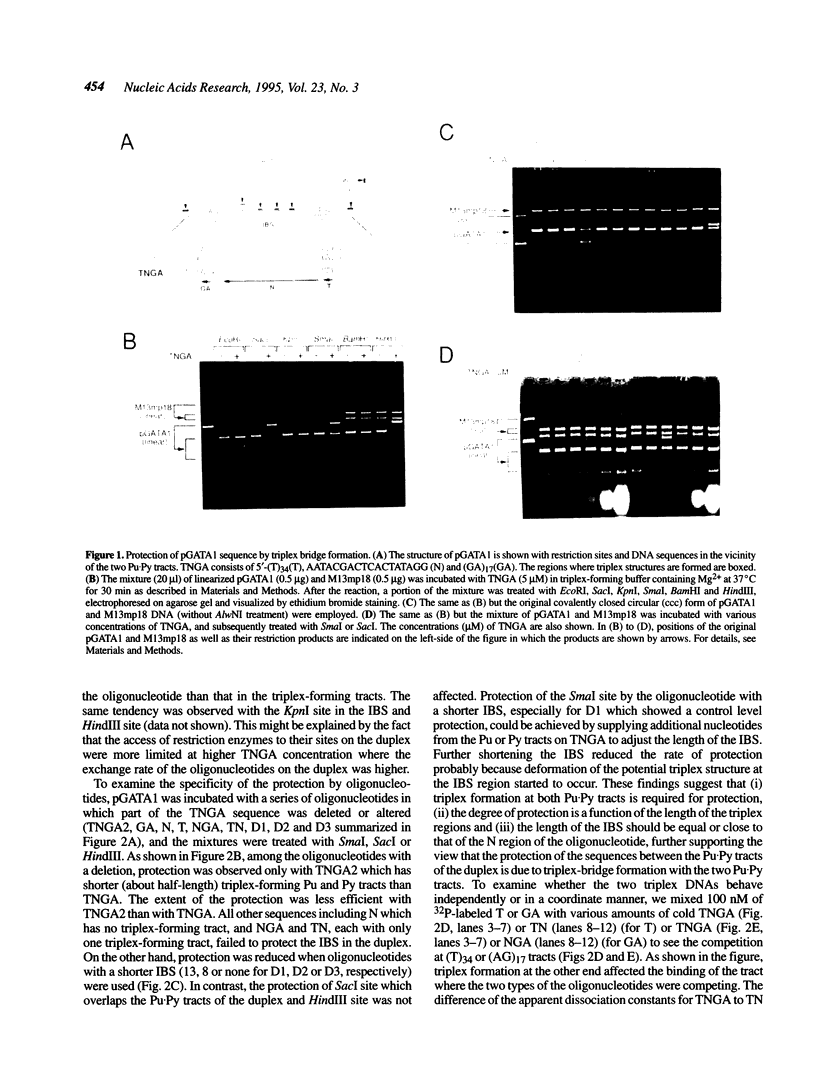
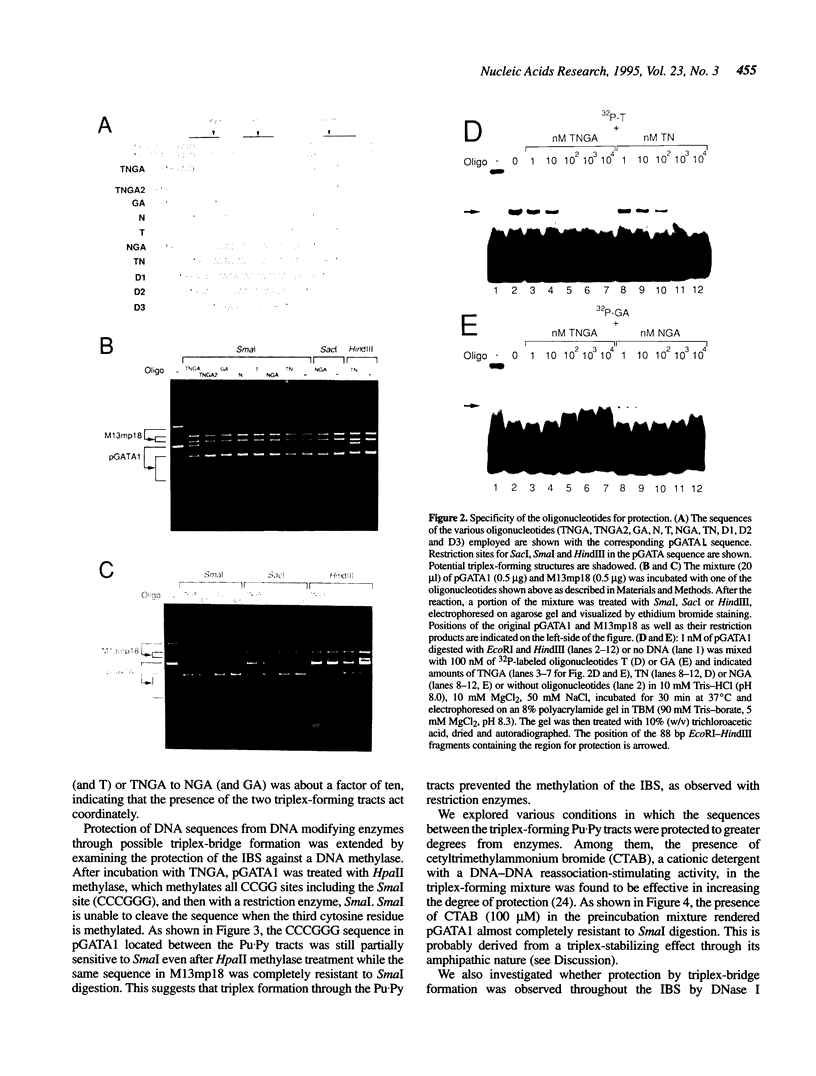
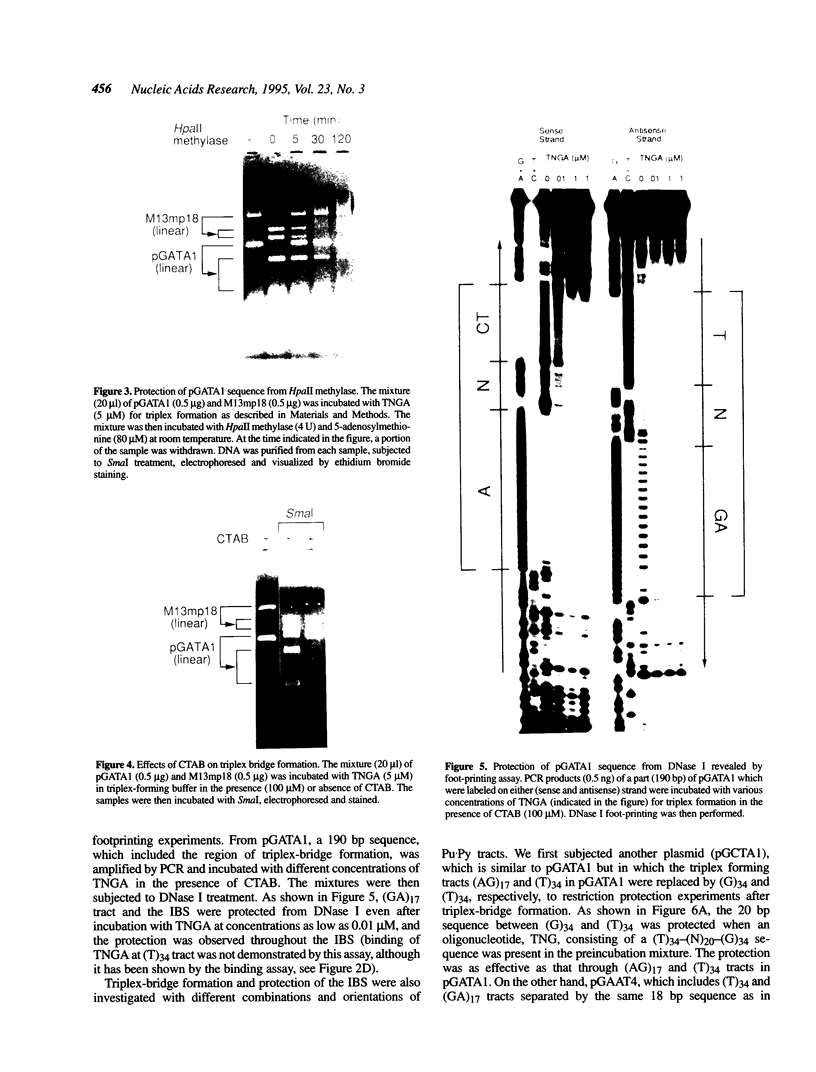
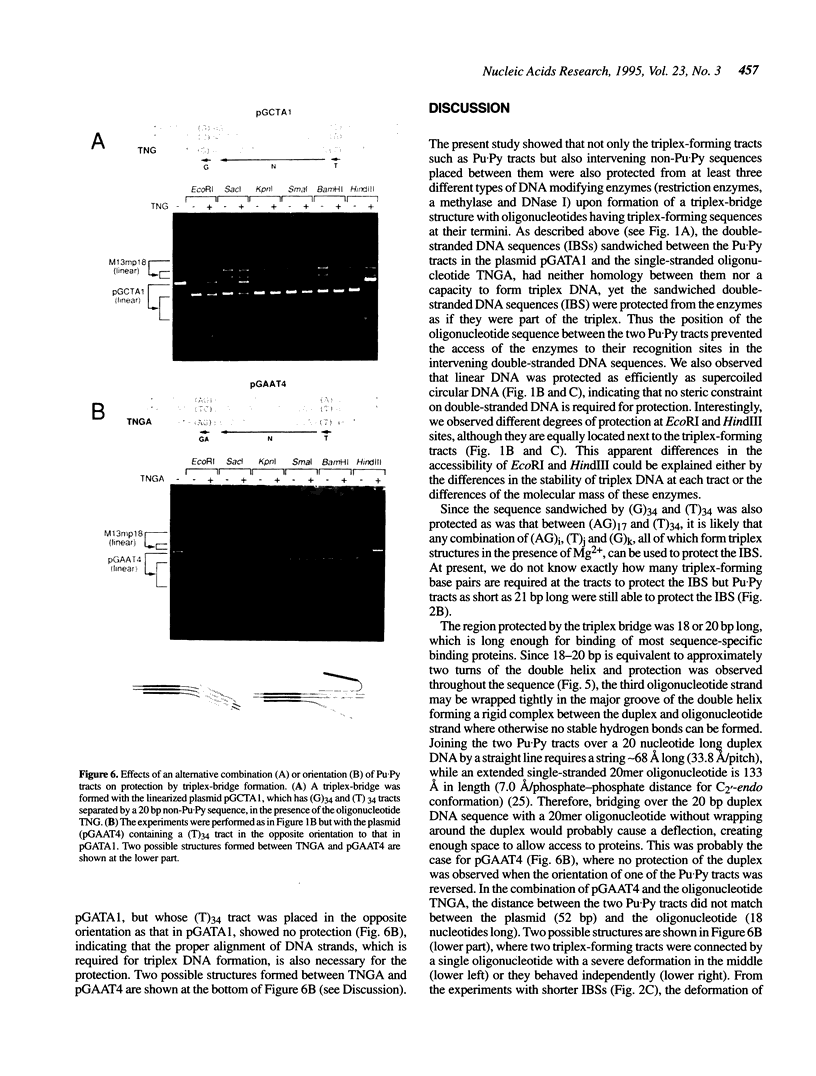
We have demonstrated that the DNA sequence between two triplex-forming polypurine.polypyrimidine (Pu.Py) tracts was protected from DNA modifying enzymes upon formation of triplex DNA structures with an oligodeoxyribonucleotide in which two triplex-forming Pu or Py tracts were placed at the termini (triplex-bridge formation). In model experiments, when two triplex structures were formed between double-stranded DNA with the sequence (AG)17-(N)18-(T)34, and an oligodeoxyribonucleotide, (T)34-(N)18-(GA)17, not only the Pu.Py tracts but also the 18 bp non-Pu.Py sequence in the duplex DNA between the tracts was protected from restriction enzymes, HpaII methylase and DNase I. This protection occurred only when both of the Pu.Py tracts were involved as triplexes. The length of the tracts could be as short as 21 bp, while the difference in length between the non-Pu.Py sequences on the duplex and the oligodeoxyribonucleotide should be within 10 nucleotides. The efficiency of protection was enhanced in the presence of a cationic detergent, cetyltrimethylammonium bromide, during triplex formation. Protection was also observed with another type of the triplex bridge formed between (G)34 and (T)34 tracts with an oligodeoxyribonucleotide, (T)34-(N)20-(G)34. These findings suggest that the protection of specific DNA sequences from enzymes by triplex-bridge formation can be applied to any DNA sequence by placing it between two triplex-forming sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrin L. J., Camerini-Otero R. D. Selective cleavage of human DNA: RecA-assisted restriction endonuclease (RARE) cleavage. Science. 1991 Dec 6;254(5037):1494–1497. doi: 10.1126/science.1962209. [DOI] [PubMed] [Google Scholar]
- Griffin L. C., Dervan P. B. Recognition of thymine adenine.base pairs by guanine in a pyrimidine triple helix motif. Science. 1989 Sep 1;245(4921):967–971. doi: 10.1126/science.2549639. [DOI] [PubMed] [Google Scholar]
- Havre P. A., Gunther E. J., Gasparro F. P., Glazer P. M. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7879–7883. doi: 10.1073/pnas.90.16.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Kessler D. J., Murphy M., Jayaraman K., Zendegui J. G., Hogan M. E., O'Malley B. W., Tsai M. J. In vivo transcription of a progesterone-responsive gene is specifically inhibited by a triplex-forming oligonucleotide. Nucleic Acids Res. 1993 Jun 25;21(12):2789–2796. doi: 10.1093/nar/21.12.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena S. D., Johnston B. H. Oligonucleotide-directed triple helix formation at adjacent oligopurine and oligopyrimidine DNA tracts by alternate strand recognition. Nucleic Acids Res. 1992 Oct 25;20(20):5279–5288. doi: 10.1093/nar/20.20.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. J., Pettitt B. M., Cheng Y. K., Smith S. R., Jayaraman K., Vu H. M., Hogan M. E. Triple helix formation at distant sites: hybrid oligonucleotides containing a polymeric linker. Nucleic Acids Res. 1993 Oct 11;21(20):4810–4815. doi: 10.1093/nar/21.20.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Camerini-Otero R. D. A triplex DNA-binding protein from human cells: purification and characterization. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10450–10454. doi: 10.1073/pnas.88.23.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob M., Szybalski W. Cleaving yeast and Escherichia coli genomes at a single site. Science. 1990 Oct 12;250(4978):271–273. doi: 10.1126/science.2218529. [DOI] [PubMed] [Google Scholar]
- Krawczyk S. H., Milligan J. F., Wadwani S., Moulds C., Froehler B. C., Matteucci M. D. Oligonucleotide-mediated triple helix formation using an N3-protonated deoxycytidine analog exhibiting pH-independent binding within the physiological range. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3761–3764. doi: 10.1073/pnas.89.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Pontius B. W., Berg P. Rapid renaturation of complementary DNA strands mediated by cationic detergents: a role for high-probability binding domains in enhancing the kinetics of molecular assembly processes. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8237–8241. doi: 10.1073/pnas.88.18.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S. A., Dervan P. B. Single-site enzymatic cleavage of yeast genomic DNA mediated by triple helix formation. Nature. 1991 Mar 14;350(6314):172–174. doi: 10.1038/350172a0. [DOI] [PubMed] [Google Scholar]
- Strobel S. A., Doucette-Stamm L. A., Riba L., Housman D. E., Dervan P. B. Site-specific cleavage of human chromosome 4 mediated by triple-helix formation. Science. 1991 Dec 13;254(5038):1639–1642. doi: 10.1126/science.1836279. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Malek S., Feigon J. Structure of a G.T.A triplet in an intramolecular DNA triplex. Biochemistry. 1992 May 26;31(20):4838–4846. doi: 10.1021/bi00135a015. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Yoon K., Hobbs C. A., Koch J., Sardaro M., Kutny R., Weis A. L. Elucidation of the sequence-specific third-strand recognition of four Watson-Crick base pairs in a pyrimidine triple-helix motif: T.AT, C.GC, T.CG, and G.TA. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3840–3844. doi: 10.1073/pnas.89.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]