Abstract
Formation of intramolecular cross links by addition of C(5') deoxyribose radicals to the C(8)-N(7) double bond of an attached adenine base was analyzed by ab initio quantum-chemical methods. Conformational preferences that influence the stereospecificity of the reaction were investigated. A good correlation was found between the ratio of experimental yields of R and S stereoisomers of 8,5'-cyclodeoxyadenosine and the relative energy of conformations of the C(5') radical that are precursors to these isomers. Molecular mechanics based on the AMBER force field was used to model the effect of 8,5'-cyclodeoxyadenosine on the conformation of the DNA dodecamer d(CGCGAATTCGCG)2 with the lesion at the A6 position. The R and S stereoisomers of the intrastrand cross link cause comparable levels of DNA distortion with the major conformational changes occurring in backbone torsional angles at the site of the lesion.
Full text
PDF
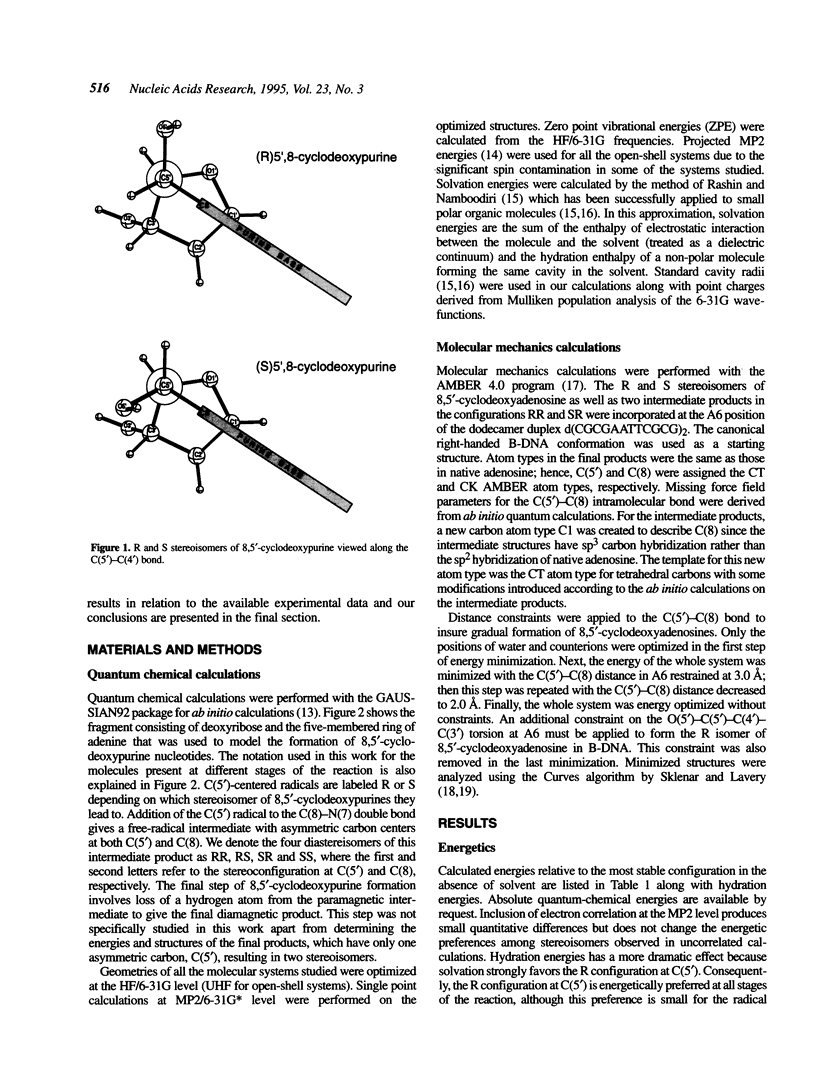
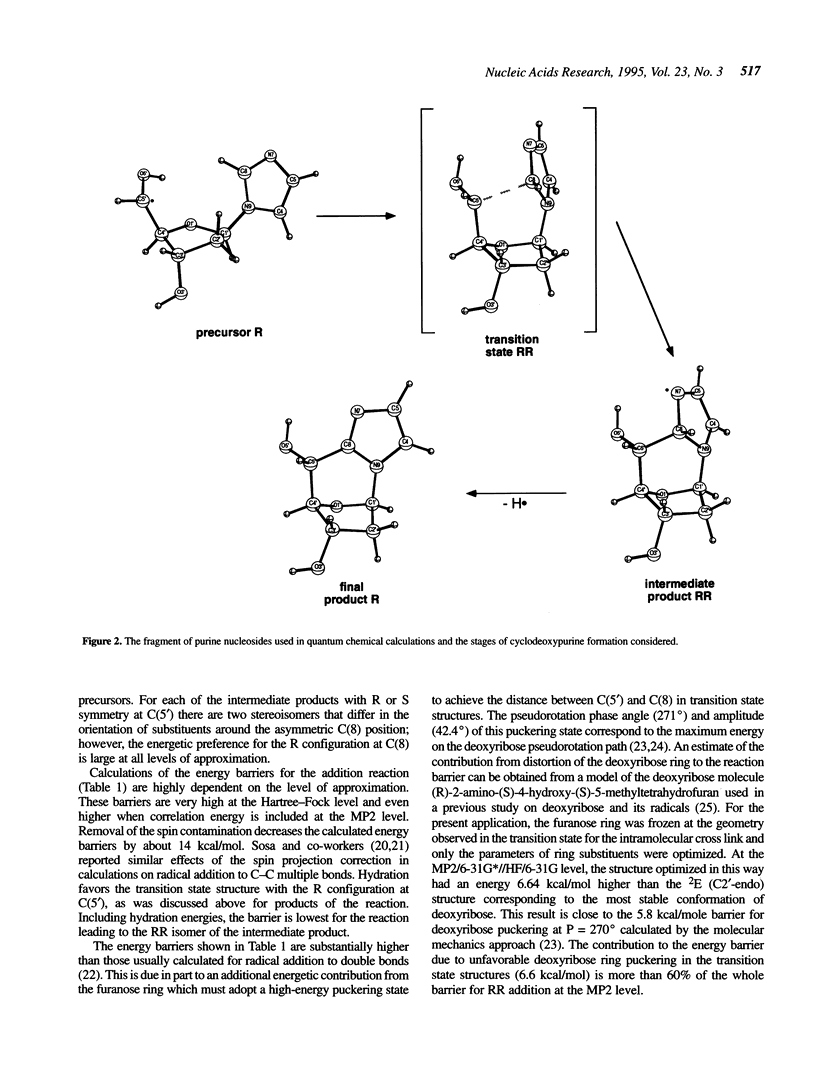
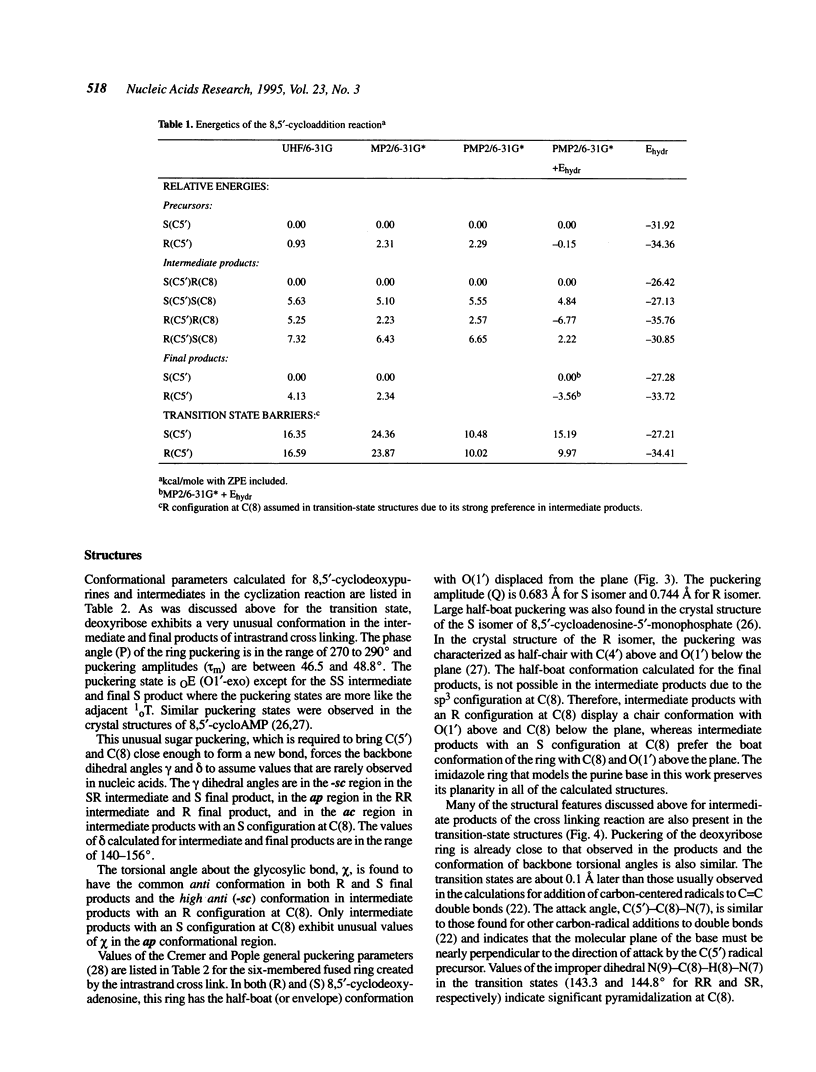
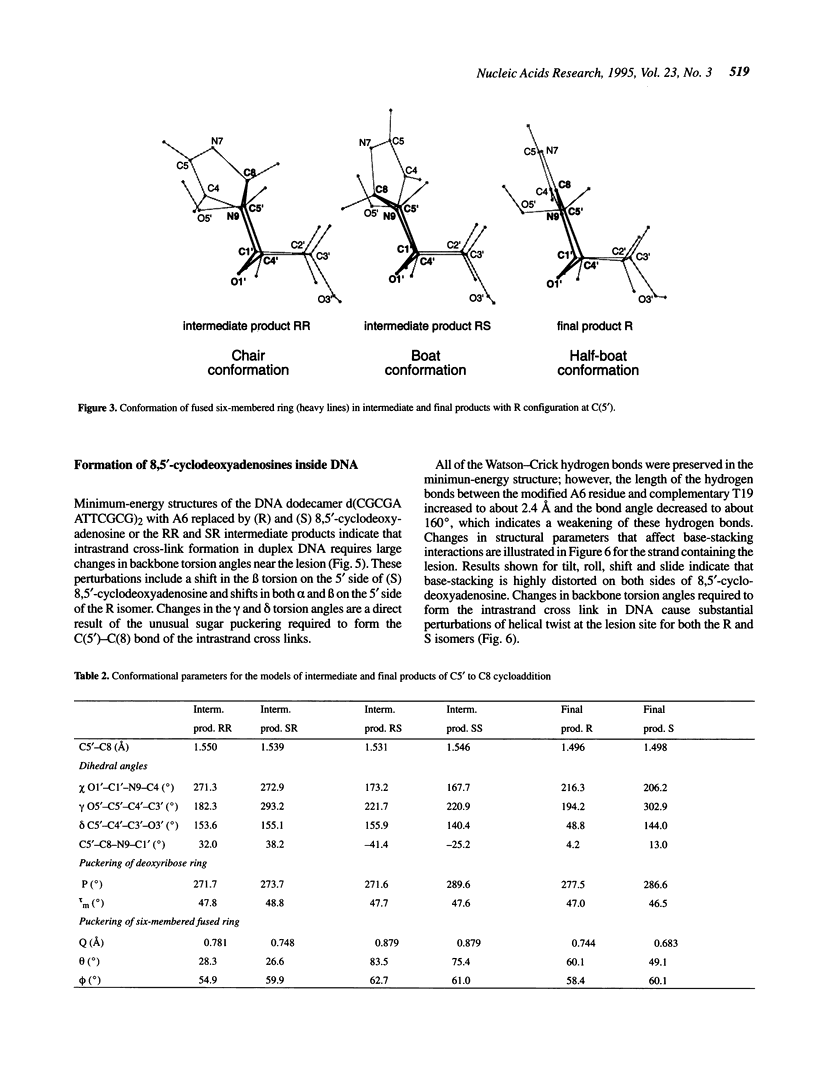

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum G. I., Cygler M., Dudycz L., Stolarski R., Shugar D. Comparison of solid state and solution conformations of R and S epimers of 8,5'-cycloadenosine and their relevance to some enzymatic reactions. Biochemistry. 1981 May 26;20(11):3294–3301. doi: 10.1021/bi00514a048. [DOI] [PubMed] [Google Scholar]
- Dirksen M. L., Blakely W. F., Holwitt E., Dizdaroglu M. Effect of DNA conformation on the hydroxyl radical-induced formation of 8,5'-cyclopurine 2'-deoxyribonucleoside residues in DNA. Int J Radiat Biol. 1988 Aug;54(2):195–204. doi: 10.1080/09553008814551631. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Dirksen M. L., Jiang H. X., Robbins J. H. Ionizing-radiation-induced damage in the DNA of cultured human cells. Identification of 8,5-cyclo-2-deoxyguanosine. Biochem J. 1987 Feb 1;241(3):929–932. doi: 10.1042/bj2410929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M. Free-radical-induced formation of an 8,5'-cyclo-2'-deoxyguanosine moiety in deoxyribonucleic acid. Biochem J. 1986 Aug 15;238(1):247–254. doi: 10.1042/bj2380247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuciarelli A. F., Koch C. J., Raleigh J. A. Oxygen dependence of product formation in irradiated adenosine 5'-monophosphate. Radiat Res. 1988 Mar;113(3):447–457. [PubMed] [Google Scholar]
- Fuciarelli A. F., Mele F. G., Raleigh J. A. Interaction of nitroaromatic radiosensitizers with irradiated polyadenylic acid as measured by an indirect immunochemical assay with specificity for the 8,5'-cycloadenosine moiety. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):629–639. doi: 10.1080/09553008414552161. [DOI] [PubMed] [Google Scholar]
- Fuciarelli A. F., Shum F. Y., Raleigh J. A. Intramolecular cyclization in irradiated nucleic acids: correlation between high-performance liquid chromatography and an immunochemical assay for 8,5'-cycloadenosine in irradiated poly(A). Radiat Res. 1987 Apr;110(1):35–44. [PubMed] [Google Scholar]
- Fuciarelli A. F., Shum F. Y., Raleigh J. A. Stereoselective intramolecular cyclization in irradiated nucleic acids: R- and S-8,5'-cycloadenosine in polyadenylic acid. Biochem Biophys Res Commun. 1986 Jan 29;134(2):883–887. doi: 10.1016/s0006-291x(86)80502-2. [DOI] [PubMed] [Google Scholar]
- Haromy T. P., Raleigh J., Sundaralingam M. Enzyme-bound conformations of nucleotide substrates. X-ray structure and absolute configuration of 8,5'-cycloadenosine monohydrate. Biochemistry. 1980 Apr 15;19(8):1718–1722. doi: 10.1021/bi00549a031. [DOI] [PubMed] [Google Scholar]
- Keck K. Bildung von Cyclonucleotiden bei Bestrahlung vässriger Lösungen von Purinnucleotiden. Z Naturforsch B. 1968 Aug;23(8):1034–1043. [PubMed] [Google Scholar]
- Lavery R., Sklenar H. Defining the structure of irregular nucleic acids: conventions and principles. J Biomol Struct Dyn. 1989 Feb;6(4):655–667. doi: 10.1080/07391102.1989.10507728. [DOI] [PubMed] [Google Scholar]
- Lavery R., Sklenar H. The definition of generalized helicoidal parameters and of axis curvature for irregular nucleic acids. J Biomol Struct Dyn. 1988 Aug;6(1):63–91. doi: 10.1080/07391102.1988.10506483. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Kremers W., Whitehouse R. Radiation chemistry of nucleotides: 8,5'-cyclonucleotide formation and phosphate release initiated by hydroxyl radical attack on adenosine monophosphates. Radiat Res. 1976 Mar;65(3):414–422. [PubMed] [Google Scholar]


