Abstract
1. Cytochrome P450 (CYP) 2Js have been studied in various mammals, but not in sheep, as an animal model used to test veterinary drug metabolism.
2. Sheep CYP2J was cloned from liver messenger RNA (mRNA) by RACE. The cDNA, after modification at its N- and C-terminals, was expressed in Escherichia coli and the sheep CYP2J protein, purified by chromatography, was 80% homologous to human and monkey CYP2J2.
3. Reverse transcriptase-polymerase chain reaction (RT-PCR) experiments showed that CYP2J mRNA was expressed in liver, cortex, respiratory and olfactory mucosa, heart, bronchi, lung, spleen, small intestine and kidney.
4. The purified enzyme was catalytically active towards aminopyrine, all-trans-retinoic acid, and particularly arachidonic acid forming 20-HETE, 19-HETE, and 18-HETE (about 86% of the total) and 14,15-, 11,12-, 8,9-, and 5,6-EETs (cis-epoxyeicosatrienoic acids; about 14% of total), with a regioselectivity similar to that shown by the mammalian CYP2J2s.
Keywords: CYP2J, sheep, CYP2J expression
Introduction
Cytochrome P450 (CYP) is an enzyme superfamily that catalyses the oxidation of a wide variety of endogenous and exogenous substrates. Over the years, various authors have discovered that the major epoxygenase enzymes of arachidonic acid belong to the CYP2 subfamily, namely CYP2C and CYP2J (Daikh et al. 1994; Wu at al. 1996). In particular, CYP2J members produce cis-epoxyeicosatrienoic acids (EETs) and mid-chain cis–trans-conjugated dienols (HETEs), and ω-terminal alcohols (Scarborough et al. 1999). These compounds play an important role in many endogenous functions such as the regulation of hormone secretion, vascular tone (Campbell & Harder 1999), and renal microtubular excretion (Wu et al. 1997). Moreover, a perturbation of the arachidonic acid metabolism and eicosanoid biosynthesis seem to be involved in numerous pathophysiological states, such as vascular disease, neoplastic phenotype (Jiang et al. 2005), inflammation (Spiecker & Liao 2005), the alteration of hormonal secretion (Capdevilla et al. 2000), and the failure of renal microtubular filtration (Wu et al. 1997). Other important compounds are substrates of CYP2J such as all-trans-retinal that is oxidized into all-trans-retinoic acid. The importance of this oxidation derives from the involvement of this metabolite in embryo development during pregnancy. In fact, retinoic acid and its metabolites are responsible for accelerated organ development and maturation (Zhang et al. 1998; Wang et al. 2007). In addition to their role in the metabolism of endogenous compounds, CYP2J enzymes are active in oxidation of xenobiotics such as aminopyrine, benzphetamine, diclofenac, ebastine, and astemizole (Scarborough et al. 1999; Hashizume et al. 2002; Matsumoto et al. 2003).
Many members of this subfamily are described in mammals: one in rabbit (CYP2J1) (Kikuta et al. 1991), two (CYP2J3, CYP2J4) in rat (Wu et al. 1997; Zhang et al. 1997), four (CYP2J5, CYP2J6, CYP2J9, CYP2J11) in mouse (Ma et al. 1999; Scarborough et al. 1999; Qu et al. 2001), and only one (CYP2J2) in human, monkeys, dog, and Bos taurus (see http://drnelson.utmem.edu/cytochromeP450.html). The expression of P4502J is documented in many tissues such as liver, kidney, heart, intestine, lung, and brain and the biochemical features are described using purified recombinant enzymes. However, the enzymatic properties of CYP2Js and their tissue-specific distribution differ across the species (Scarborough et al. 1999). Thus, it appears useful to investigate the feature of CYP2J in a large animal such as sheep. Sheep is an animal model used to test veterinary drug metabolism (Ioannides 2006) and to study vascular process such as heart blood circulation and ductus arteriosus closure at birth (Coceani et al. 1996).
The aim of this work was to isolate a sheep liver cDNA which encodes CYP2J, expresses this enzyme in the Escherichia coli heterologous system, purifies it, and, subsequently, characterizes its catalytic activities.
Materials and methods
Materials
Tripure isolation reagent, restriction endonuclease NdeI and SalI, T4 DNA Ligase were obtained from Roche Molecular Biochemicals (Indianapolis, IA, USA). DNA primers were purchased from Sigma-Aldrich Genomics (Milan, Italy). Gene Racer Kit, Thermo Script III Reverse Transcriptase, Platinum Pfx Taq polymerase, pCR4Blunt vector, Ni-NTA Agarose resin, 3 M imidazole solution were obtain from Invitrogen Life Technologies (Carlsbad, CA, USA). β-Nicotinamide adenine dinucleotide phosphate, reduced form (NADPH), ampicillin, isopropyl-β-D-thiogalactopyranoside (IPTG), δ-aminolevulenic acid 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfate (CHAPS), dilauroyl-L-3-phosphatidyl choline (DLPC), ethyldiaminotetraacetic acid (EDTA), dithiothreitol (DTT), glycerol, arachidonic acid, all-trans retinal, all-trans retinoic acid, 7-ethoxycoumarin, astemizole, diclofenac, aniline, aminopyrine and peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) were from Sigma-Aldrich (St. Louis, MO, USA). Master Mix PCR, Wizard® Plus SV gel extraction kit and Wizard® Plus SV MiniPrepsDNA Purification System were purchased from Promega (Madison, WI, USA). Testosterone and its metabolites were obtained as previously reported (Longo et al. 1991). The ECL kit was purchased from Amersham (Amersham, UK). 8,9-EET-d8 (internal standard) was obtained from Biomol International (Exeter, UK).
Animals
Tissues of untreated sheep (Apuana) weighing 30–40 kg were obtained from San Pietro a Grado breeding section of the Veterinary University of Pisa (Pisa, Italy). All tissues were stored at −80°C until preparation of total RNA.
RNA isolation and sheep CYP2J cloning
Total RNA from sheep liver was isolated with Tripure Isolation Reagent and modified by a Gene Racer Kit according with the manufacturer's instructions. First, a CYP2J cDNA central fragment of 353 bp was cloned with two primers S and AS (Table 1) obtained by a multi-alignment of rat, rabbit, human, dog, and Bos taurus sequences of CYP2J present in Gene Bank database (Table 1). Polymerase chain reaction (PCR) was performed using 40 cycles including 30 s at 94°C, 30 s at 50°C, and 1 min at 68°C and a proof-reading Platinum Pfx Taq Polymerase according to the Invitrogen Gene Racer Kit. The full-length cDNA was obtained employing for the isolation of the missing 5′-end, the two reverse primers: 5′GRP and 5′GNP (Table 1); and for the isolation of the missing 3′-end, the following two forward primers: 3′GRP and 3′GNP (Table 1). The Gene Racer PCR products were sub-cloned in pCR4Blunt vector and sequenced by BMR Genomics Sequencing Core service (Padua, Italy). The CYP2J homologies were performed with NCBI-BLAST database.
Table 1.
Oligonucleotide sequence and size of fragment obtained with PCR primers mix.
| CYP2J fragment | ||
|---|---|---|
| S | 5’-CCC TCA XTT CAA GAT CAA CA-3’ | |
| AS | 5’-GCA GAT GAG GTT TTC TTC AT-3’ | 353 bp |
| Gene Racer Primers | ||
| 5’GRP | 5’-GAA TAG CCT TTG GTG TGG ACC CGG A-3’ | |
| 5’GNP | 5’-TAG GTG ACC TCA TCC AGC AGC CTC A-3’ | 700 bp |
| 3’GRP | 5’ TTG GGG AGC GGT TTG ACT ACC AGG A-3’ | |
| 3’GNP | 5’-TCC GGG TCC ACA CCA AAG GCT ATT C-3’ | 1300 bp |
| CYP2J Modification Primers | ||
| MALLLV | 5’-GCCATGGCTCTGTTATTAGCAGTTTTTGCGGGCTGCCCTCTGG-3’ | |
| RSP | 5’-CTTTGCTCTTTCCATATGTG-3’ | 454 bp |
| FSP | 5’ -CACATATGGAAAGAGCAAAG-3’ | |
| His-tag | 5’-CAGCTGTCAGTGATGGTGATGGGCCCGAGGAACAGC-3’ | 1100 bp |
| ß-actin | ||
| S | 5’-GCTCGTCGTCGACAACGGCTC-3’ | |
| AS | 5’-CAAACATGATCTGGGTCATCTTCTC-3’ | 353 bp |
CYP2J subcloning in pCWOri+
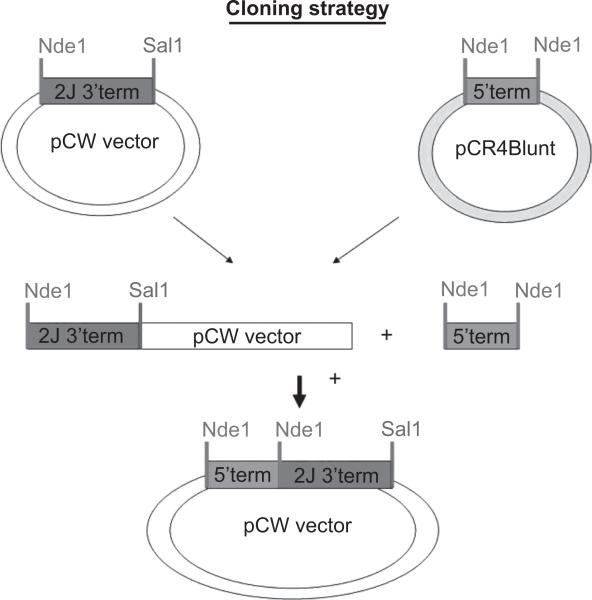
Heterologous expression of CYP2J required its truncation in two parts as the gene contains an internal NdeI site. This expedience was performed since the expression vector used in the studies was the pCWOri+ vector that requires the introduction of the NdeI site in the N-terminal of the CYP gene to establish a right coding frame as described by other workers (Guo et al. 1994; Gillam et al. 1995). Furthermore, the nucleotide sequence encoding its wild-type N-terminal region was changed to include the MALLLV amino acids sequence and the NdeI site, as previously reported (Fisher et al. 1992). These two modifications required the truncation of sheep CYP2J nucleotides sequence in two fragments, because sheep CYP2J cDNA contains an internal NdeI site. The cloning scheme was presented in Figure 1.
Figure 1.
Scheme of sheep CYP2J subcloning in pCWOri vector.
N-terminal modification was introduced with a PCR amplification on native the CYP2J sequence using MALLLV and RSP primers and 40 PCR cycles including 30 s at 94°C, 30 s at 55°C, and 45 s at 68°C. The 540 bp fragment was cloned in pCR4Blunt vector and sequenced by BMR Genomics Sequencing Core service (Padua, Italy).
The nucleotide sequence encoding a wild-type C-terminal region is changed to include the His-tag sequence (4 His) to facilitate protein purification and a SalI restriction site for the cloning in pCWOri+ vector, as previously reported (Guo et al. 1994; Gillam et al. 1995). The used strategy and the PCR program were the same for the N-terminal modification but using FSP and His-tag primers (Table 1). The 1100 pb fragment was cloned in pCR4Blunt vector and sequenced by BMR.
Genomics
Finally, recombinant sheep CYP2J cDNA was recomposed in pCWOri+ vector and introduced in E. coli cells.
Expression of sheep CYP2J pCWOri+ plasmid
Plasmid was transformed in E. coli DH5α cells. A single resistant colony of DH5α cells transformed with the plasmid was grown overnight a 37°C in Luria–Bertani medium containing 100 μg ml−1 of ampicillin. A 10 ml aliquot of Luria–Bertani pre-culture was inoculated into 1 L of Terrific Broth (TB). The TB medium was supplemented with ampicillin (100 μg ml−1), 1 mM thiamine, 0.5 mM δ-aminolevulenic acid and trace elements. The culture was incubated at 30°C in a bath with a vigorous shaking; after 2.5 h, isopropyl β-D-thiogalactopyranoside (1 mM) was added to induce the tac promoter and culture was maintained 24 h for the expression of sheep CYP2J. Cells were kept at −20°C for 1 h and centrifuged at 5000g for 15 min. The TB was discarded and bacterial pellets were resuspended in 100 mM Tris-acetate buffer (pH 7.6) containing 500 mM sucrose and 0.5 mM EDTA (15 ml buffer g−1). Lysozyme was added (to 0.3 mg g−1 cells) and the suspension was diluted two-fold with water. After gentle mixing at 4°C for 30 min, the resulting spheroplasts were collected by centrifugation at 10 000g for 15 min and the pellet was resuspended in 100 mM phosphate buffer (pH 7.4) containing 20% glycerol, 6 mM magnesium acetate, 0.1 mM DTT and protease inhibitors (0.2 mM phenylmethanesulphonyl fluoride (PMSF), 1 μg ml−1 each of aprotinin, leupeptin, bestatin). Spheroplasts were lysed by sonication three times with cycles of 15 s ON and 5 s OFF. The resulting lysate was centrifuged at 10 000g for 20 min and the supernatant was collected and centrifuged at 100 000g for 2.5 h. The soluble fraction was discarded and the red–orange membrane pellet was resuspended and used for CYP2J purification.
Protein purification
Membranes were solubilized for 2 h in potassium phosphate buffer 300 mM (pH 7.4) containing 20% glycerol (v/v) and 1% CHAPS. The solution was centrifuged at 100 000g for 30 min to remove the insoluble fraction. The supernatant containing His-tagged sheep CYP2J was purified by an affinity chromatography column containing 2 ml of Ni-NTA Agarose resin. The column was washed with 30 ml of 300 mM potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v), 1% CHAPS and 20 mM imidazole. Red-coloured fraction containing CYP2J was eluted by 300 mM potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v) and 1% CHAPS and 200 mM imidazole. The removal of imidazole and CHAPS from CYP2J solution was achieved by dialysis in 100 vols of 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA, 0.1 mM DTT and 20% glycerol (v/v) (Wu et al. 2007). The purity was assessed by sodium dodecylsulphate (SDS) (7.5% w/v)-PAGE, according to the method of Laemmli (1970), and by Western blot analysis (Towbin et al. 1979) performed using anti-human CYP2J2 antibodies. Protein content was measured by method of Lowry et al. (1951).
RT-PCR analysis for CYP2J mRNA distribution
Total RNA was isolated from cortex, respiratory mucosa, olfactory mucosa, aorta, pulmonary artery, heart, bronchi, lung, liver, spleen, small intestine and kidney. Total RNA (1 μg) integrity was checked by a formaldehydeagarose gel. Then 2.5 μg were reversed transcribed using oligo-dT primers and cDNAs obtained were used for RT-PCR with a program and primers for 378 bp fragment previously described (Table 1). The PCR products were analysed on agarose gel, purified and sequenced. β-Actin was used as housekeeping gene to test the cDNA quality (Table 1).
Immunoblot analysis
Tissue microsomes were subjected to SDS-PAGE using the method of Laemmli (1970) in a Bio-Rad mini Protean II apparatus. Proteins were transferred from slab gel to nitrocellulose filters following the method of Towbin et al. (1979). Immunodetection was performed with anti-human CYP2J2 polyclonal antibodies (generously supplied by Dr Zeldin). Immunoreactive proteins were visualized with a chemiluminescence reaction kit (Amersham).
Enzymatic assays
CYP content was determined using Omura & Sato (1964) method. Enzyme activities of the purified CYP were performed in a reconstituted system containing 100 pmol of CYP2J, 200 pmol of NADPH-cytochrome P450 reductase, 30 μg of lipids (DLPC) and 1 mM NADPH or NADPH-regenerating system which had been pre-incubated for 30 min at room temperature (Reed et al. 2006). Then, after the addition of G6PDH (1 U) and the substrate in a volume of 1 ml, the complete system was generally incubated for 30 min at 37°C. Hydroxylation of aniline and demethylation of aminopyrine were assayed according to Ko et al. (1987) and Tu & Yang (1983), respectively. Ethoxycumarin-O-deethylase was assayed by the fluorimetric determination of 7-hydroxycumarin (Aitio 1978). Testosterone hydroxylase (Longo et al. 1991) and the all-trans-retinal oxidase (Zhang et al. 1998; Wang et al. 2007) were determined by HPLC as previously reported. Astemizole O-demethylation and diclofenac hydroxylation were performed as previously described by Matsumoto et al. (2003) and Tang et al. (2000), respectively.
Arachidonic acid incubations
The reaction volume was 1 ml and it contained 100 mM phosphate buffer (pH 7.4), 10 μM arachidonic acid (AA), 1 mM NADPH, and a CYP2J reconstituted system which consisted of 60 μg DLPC, 100 pmol of purified sheep CYP2J and 300 pmol of NADPH CYP reductase. The CYP2J reductase system was reconstituted by leaving the mixture at room temperature for 40 min before the addition of arachidonic acid and NADPH. Reactions were incubated for 30 or 60 min. at 37°C. The above conditions were repeated with boiled purified sheep CYP2J to use as a blank. For a positive control, incubations were repeated as above except 500 μg of sheep liver microsomes were used instead of the CYP2J reconstituted system and using boiled liver microsomes as a blank.
All reactions were stopped by acidifying to pH 4.5 with acetic acid followed by the addition of 500 pg of 11(12)-EET-d8, 12-HETE-d8, and 11,12-DiHETrE-d11 to be used as internal standards. Each was then extracted with 2 vols of ethyl acetate twice, dried under nitrogen, reconstituted in 200 μl methanol, and stored at −80 C until LC/MS/MS analysis.
LC/MS/MS analysis
Eicosanoid identification and quantification was performed with a Q-trap 3200 linear ion trap quadrupole LC/MS/MS equipped with a Turbo V ion source operated in negative electrospray mode (Applied Biosystems, Foster City, CA, USA). Extracted samples were suspended in 10 μl of methanol and injected into the HPLC via an Agilent 1200 standard series autosampler equipped with a thermostat set at 4°C (Agilent Technologies, Santa Clara, CA, USA). The HPLC component consisted of an Agilent 1100 series binary gradient pump equipped with an Eclipse plus C18 (50 × 4.6 mm, 1.8 μm) column (Agilent Technologies). The column was eluted at a flow rate of 0.500 ml min−1 with 100% mobile phase A (methanol/water/acetic acid (60:40:0.01, v/v/v) from zero to 2 min and a gradient increasing to 100% B (100% methanol) at 13 min. Multiple reaction monitoring (MRM) was used with a dwell time of 25 or 50 ms for each compound, with source parameters: ion spray voltage, −4500 V; curtain gas, 40 U; ion source gas flow rate 1, 65 U and 2, 50 U; and temperature of 600°C. Synthetic standards were used to obtain standard curves (5–500 pg) for each eicosanoid (linear regression R2 > 0.99) and internal standard.
Eicosanoid values are expressed at pmol min−1 for the CYP2J reactions, and pmol mg−1 protein min−1 for liver reactions. Corresponding blank value averages (n = 2) were subtracted from incubation values. The arachidonic acid starting material was also analysed by LC/MS/MS for auto-oxidative products and these values were subtracted from all incubations including the blanks.
Results and discussion
Gene cloning and sequence analysis
Using a PCR approach followed by RACE, a full-length CYP2J cDNA of 1628 bp was isolated from the sheep liver. The identified cDNA contained an open reading frame of 1506 bp, flanked by initiation (ATG) and termination (TGA) codons. That encoded a 502 amino acid polypep-tide, as reported for the human CYP2J2 and rat CYP2J3 (Scarborough et al. 1999), with a calculated molecular mass of 57 857 Da (Figure 2). The amino acid sequence of sheep CYP2J contained a putative heme-binding peptide (F S I G K R M C L G E Q L A) positioned between 441 and 454 amino acid residues. The invariant cysteine responsible for heme binding, at position 448, and the other conserved residues for any CYP are underlined. Moreover, in this putative amino acid sequence, it was possible to identify a proline-rich motif between positions 41 and 51, which are important to anchor the protein to the endoplasmic reticular membrane. A comparison with the deduced amino acid sequence of the sheep CYP2J with that of other CYP2 families showed a homology of 45–50%, whereas with respect to members of the CYP2J subfamily reported in the Gene Bank database (CYP2J1, various CYP2J2, CYP2J3, CYP2J4, CYP2J5, CYP2J6, CYP2J9) it was of 68–82% (Table 2). The highest homology was with Macaca fascicularis CYP2J2 (82%), Bos taurus and Homo sapiens CYP2J2 (80%). Gotoh (1992) predicted that the amino acid sequences of substrate recognition sites (SRSs) within CYPs should be more variable than the sequence of the rest of protein due to adaptive evolution. A comparison of the sheep CYP2J SRSs, as a guide to examine the function with those of other CYP2Js, revealed a comprehensively high homology and a complete sequence share (100%) with the SRS2 and SRS4 of Canis familiaris, the SRS5 of Rattus norvegicus, and with the heme region of Bos taurus (Table 3). The alignment of SRS amino acid sequences also demonstrated that the SRS1 of sheep CYP2J has, as expected (Scarborough et al. 1999), the lowest amino acid homology among SRSs of other CYP2J.
Figure 2.
Nucleotide and deduced amino acid sequence of sheep CYP2J. The putative initial ATG start codon and the TGA stop codon are shown; the heme region is boxed; and the six putative SRS regions are underlined.
Table 2.
Nucleotide and amino acid sequence identity between sheep CYP2J and CYP2J of other mammals.
| Nucleic acid |
Aminoacid |
|
|---|---|---|
| Species | % identity | % identity |
| 2J2 Macaca Fascicularis | 82 | 82 |
| 2J2 Bos taurus | 85 | 80 |
| 2J2 Homo sapiens | 80 | 80 |
| 2J2 Canis familiaris | 83 | 79 |
| 2J1 Oryctolagus cuniculus | 79 | 74 |
| 2J4 Rattus norvegicus | 76 | 72 |
| 2J6 Mus musculus | 76 | 73 |
| 2J3 Rattus norvegicus | 76 | 69 |
| 2J5 Mus musculus | 76 | 68 |
| 2J9 Mus musculus | 75 | 69 |
Table 3.
Sequence comparison (%) between sheep CYP2J and other mammalian orthologues at the substrate recognition sites (SRS 1-6) and heme binding domain.
| Domain | Ovis aries aa residues in CYP2J | 2J2 Homo s. | 2J2 Canis f. | 2J2 Bos t. | 2J2 Macaca f. | 2J4 Rattus n. |
|---|---|---|---|---|---|---|
| Srs 1 | 109-131 | 72 | 86 | 50 | 77 | 50 |
| Srs 2 | 211-219 | 88 | 100 | 88 | 88 | 88 |
| Srs 3 | 247-254 | 78 | 66 | 66 | 88 | 66 |
| Srs 4 | 301-318 | 94 | 100 | 88 | 94 | 94 |
| Srs 5 | 373-383 | 91 | 91 | 82 | 91 | 100 |
| Srs 6 | 481-488 | 62 | 75 | 62 | 75 | 62 |
| Heme | 441-454 | 93 | 86 | 100 | 93 | 86 |
Percentage of identity with the sheep sequence.
Tissue distribution of CYP2J mRNA
RT-PCR experiments and specific primers examined the tissue distribution of sheep CYP2J mRNA. As shown in Figure 3, a cDNA fragment of the predicted size (378 bp) was amplified from the RNA extracted from twelve tissues of adult sheep (cortex, olfactory mucosa, respiratory mucosa, bronchi, lung, spleen, small intestine, heart, liver, kidney, aorta, and pulmonary artery). The sequences of all the fragments isolated from the extrahepatic tissues showed a perfect identity with that of liver. Thus, the CYP2J2 gene is expressed in all the tissues examined and particularly in the liver, where a more intense band was observed.
Figure 3.
RT-PCR analysis of CYP2J mRNA in various organs of control sheep: cortex (Cx), olfactory mucosa (OM), respiratory mucosa (RM), bronchi (Br), lung (Lu), heart (H), spleen (Spl), small intestine (SI), liver (Li), kidney (K), aorta (A), and pulmonary artery (PA). PCR products were separated by electrophoresis on agarose gel and stained with ethidium bromide.
Heterologous expression of CYP2J in Escherichia coli
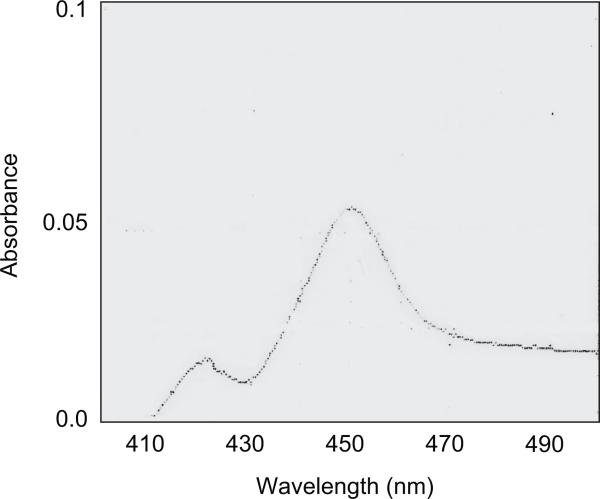
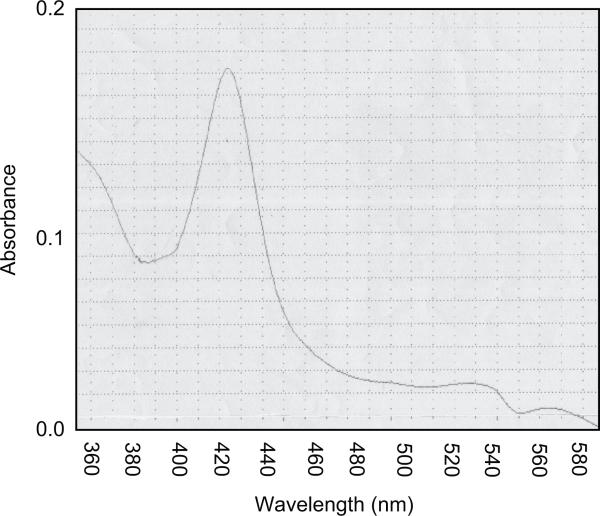
The native coding region of sheep CYP2J was inserted in the pCR4Blunt vector and used as a template for the introduction of the MALLLV and His-tag modification. The two fragments were sub-cloned in the pCR4Blunt vector. Restriction digestions accurately screened the many clones obtained and the positive clones were sequenced to test the correct insertion of the N-terminal and C-terminal modifications. The complete sequence of the modified CYP2J was recomposed directly in the pCWOri+ expression vector, which was transfected in E. coli DH5α as a host for the expression experiments. The membranes obtained from E. coli showed the presence of a functional cytochrome P450 as demonstrated by the CO-difference spectra that showed a maximum at 450 nm and a minor peak at 420 nm (Figure 4). The expression level of CYP2J was about 17 nmol l−1. Membranes were solubilized in a buffer containing the detergent CHAPS (1% v/v) and charged on an Ni-NTA agarose resin that presented high affinity for the four histidine tail, as described in the Materials and Methods section. The CYP2J was eluted as a single protein band, dialysed and collected for enzymatic characterization. As shown in Figure 5, this simple chromatographic step produced a substantially purified protein with a specific P450 content of 5.3 nmol mg−1 protein and a total yield of about 34% (Table 4). The absolute spectrum (Figure 6) of CYP2J had a Soret maximum at 422 nm and two minor peaks at 540 and 576 nm, indicative of a dominant low spin state, as reported for the rabbit CYP2J1 (Ichihara et al. 1985).
Figure 4.
CO-reduced difference spectra of CHAPS-solubilized sheep CYP2J from Escherichia coli. The spectra was recorded in 300 mM potassium phosphate buffer, pH 7.4, 20% glycerol, 1% CHAPS.
Figure 5.
Gel electrophoresis of Escherichia coli membranes at various stages of CYP2J purification. Lanes 1, membrane fraction; 2, CHAPS-solubilized membranes; and 3, fraction eluted from an Ni-NTA agarose column. Gel was visualized by Coomassie Brilliant Blue staining.
Table 4.
Purification of sheep CYP2J from E. coli membranes.
| Fraction | Protein Content (mg/L) | Total CYP (nmol/L) | Specific content (nmol/mg prot) | Yield (%) |
|---|---|---|---|---|
| E. coli membranes | 119 | 17.5 | 0.14 | 100 |
| Solubilized membranes | 42 | 14 | 0.33 | 81 |
| Ni-NTA Agarose eluate | 1.1 | 5.9 | 5.3 | 34 |
Figure 6.
Absolute spectra of purified CYP2J in 100 mM potassium phosphate buffer, pH 7.2, 20% glycerol, 0.1 mM EDTA.
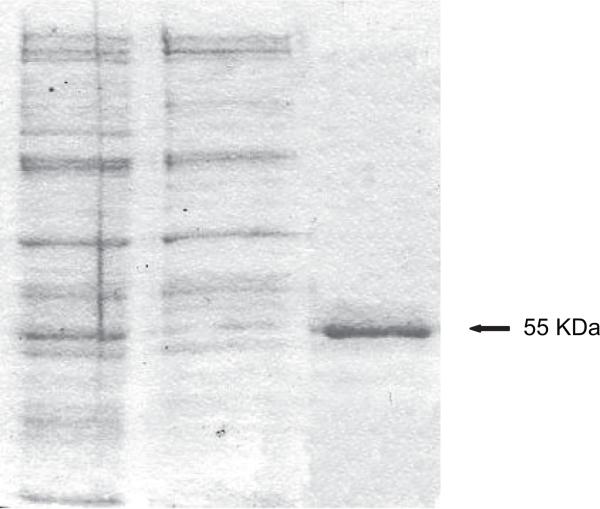
Tissue expression of CYP2J protein
Western blot assay (Figure 7) using polyclonal antibodies anti-human CYP2J2 showed a cross-reactive band with a molecular weight of about 55 kDa, in agreement with the calculated molecular weight of the purified recombinant protein (56 727 Da), indicative of isolation of the sheep CYP2J.
Figure 7.
Immunoblotting analysis of CYP2J in sheep tissues. Purified recombinant CYP2J (0.5 pmol) and CHAPS-solubilized microsomes from 5 μg of liver (Li), 40 μg of cortex (Cx), heart (H), spleen (Spl), kidney (K), and small intestine (SI) were subjected to SDS-PAGE, transferred electrophoretically to a nitrocellulose membrane, and probed with polyclonal antibodies for anti-human CYP2J2.
Anti-human CYP2J2 IgG recognized a discrete band, although at a different extent, in CHAPS-solubilized microsomes from cortex, intestine, and spleen of sheep. At variance, these antibodies recognized in the liver and kidney solubilized microsomes at least two protein bands indicative of cross-reactivity with other CYPs or the presence of more than one CYP2J. Further work will be necessary to determine if these sheep tissues contain multiple CYP2J isoforms.
Enzymatic characterization
Many known substrates of mammalian CYP2Js (Scarborough et al. 1999) were tested to determine the catalytic properties of the purified enzyme. The activities of CYP2J were carried out using a reconstituted system containing P450 reductase and cofactors as described in the Materials and Methods section. The purified CYP2J actively catalysed the oxidation of all-trans-retinal with a rate of 40 pmol min−1 nmol−1 CYP in agreement with the data of rat CYP2J4 (Zhang et al. 1998) and N-demethylation of aminopyrine with a rate of 2.54 nmol min−1 nmol−1 CYP, as reported for the CYP2J1 (Ichihara et al. 1985). On the contrary, despite multiple attempts and prolonged incubations, the recombinant sheep enzyme, unlike human CYP2J2 (Scarborough et al. 1999; Matsumoto et al. 2003), was inactive in the oxidation of aniline, testosterone, 7-ethoxycoumarin, diclofenac, and astemizole.
Arachidonic acid metabolism
To ascertain further the catalytic properties of recombinant CYP2J, this enzyme was incubated with arachidonic acid in a reconstituted system, as described in the Material and Methods section. CYP2J metabolized AA with a catalytic turnover of 10.3 pmol of products formed nmol−1 CYP min−1. The major products formed were 20-HETE, 19-HETE, and 18-HETE, which represented 86.1% of total metabolites. The EETs (14,15-EET, 11,12-EET, 8,9-EET, and 5,6-EET) were formed in lower amounts (13.9% of the total). Thus, sheep CYP2J resulted to be predominantly an AA hydroxylase rather than an AA epoxygenase in keeping with mouse CYP2J9 (Qu et al. 2001) and rat CYP2J3 (Wu et al. 1997), but not with human CYP2J2 (Wu et al. 1996).
Regiochemical analysis of CYP2J-derived EETs (Table 5) revealed a preference for epoxidation at 14,15-double bond (44% of total EETs) followed by epoxidation at 8,9-, 11,12-, and 5,6-double bonds (32.2%, 17.2%, and 6.3% of the total EET products, respectively). This EET regioselectivity profile of sheep CYP2J is quite similar to that of human CYP2J2 (Wu et al. 1996) rather than that of mouse CYP2J9 (Qu et al. 2001).
Table 5.
Regiochemical composition of EETs and HETEs produced by recombinant CYP2J and sheep liver microsomes.
| Distribution (% of total EETs / DHETs) |
||
|---|---|---|
| Regioisomer | CYP2J | microsomes |
| 14,15-EET | 44 | 51 |
| 11,12-EET | 17.5 | 21.2 |
| 8,9-EET | 32.2 | 21.8 |
| 5,6-EET | 6.3 | 6 |
| Distribution (% of total EETs / DHETs) |
||
|---|---|---|
| Regioisomer | CYP2J | microsomes |
| 20-HETE | 16.9 | 34.9 |
| 19-HETE | 53.9 | 30.4 |
| 18-HETE | 29.2 | 34.7 |
Considering the regiochemical analysis of CYP2J-derived HETEs (Table 5), in addition to the prominent formation of 19-HETE following by a minor amount of 20-HETE as found with mouse CYP2J9 and rat CYP2J3 (Qu et al. 2001; Wu et al. 1997), a significant production of 18-HETE was also observed which was not previously reported for other CYP2J enzymes.
Like the recombinant CYP2J, sheep liver microsomes showed both AA epoxygenase and hydroxylase activities. However, with microsomes, unlike the recombinant CYP2J, the formation rate of 14,15-, 11,12-, 8,9-, and 5,6-diydroxyeicosatrienoic acids (DHETs), as stable hydration products of corresponding EETs, was higher (46.7 pmol min−1 mg−1 protein) than that (33.2 pmol min−1 mg−1 protein) of HETEs (20-HETE, 19-HETE, and 18-HETE) in agreement with what has been found in the human liver (Zeldin et al. 1996). In addition to this difference, between the AA oxidation by recombinant CYP2J and microsomes, a different distribution of DHETs and HETEs was also observed (Table 5). These results indicate that CYP isoforms, other than CYP2J, present in the sheep liver microsomes, especially the CYP1A, CYP2C, and CYP4A abundant and catalytically active towards AA (Capdevilla et al. 1990; Zeldin et al. 1996; Scarborough et al. 1999), may contribute substantially to establish this different regioselectivity.
In conclusion, we have cloned the sheep CYP2J gene, have described its expression in E. coli, and isolated the protein in a functional form by a simplified chromatographic technique. The purified CYP2J enzyme was active in the metabolism, among others, of AA leading to the regioselective formation of four EETs and three HETEs.
Given the important CYP2J functional role in the vascular tissues of humans and rodents, the obtained information on sheep CYP2J may be of utility for the study of cardiovascular disease and hypertension in this large animal model.
Acknowledgements
The authors acknowledge Dr Silvia Burchielli of Fondazione Toscana “G. Monasterio” for experimental support.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aitio A. A single sensitive assay of ethoxycoumarin deethylation. Anal Biochem. 1978;85:488–91. doi: 10.1016/0003-2697(78)90245-2. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. Endothelium-derived hyper-polarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circulation Res. 1999;84:484–8. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- Capdevilla JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxyganase. J Lipid Res. 2000;41:163–81. [PubMed] [Google Scholar]
- Capdevilla JH, Karara A, Waxman DJ, Martin MV, Falck JR, Guengerich FP. Cytochrome P450 enzyme-specific control of regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990;265:10865–71. [PubMed] [Google Scholar]
- Coceani F, Kelsey L, Seidlitz E, Korzekwa K. Inhibition of the contraction of the ductus arteriosus to oxygen by 1-aminobenzotriazole and mechanism based inactivation of cytochrome P450. Br J Pharmacol. 1996;117:1586–92. doi: 10.1111/j.1476-5381.1996.tb15325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikh BE, Lasker JM, Raucy JL, Koop DR. Regio- and stereoselective epoxidation of arachidonic acid by human cytochrome P450 2C8 and 2C9. J Pharmacol Exp Ther. 1994;271:1427–33. [PubMed] [Google Scholar]
- Fisher CV, Caudle DL, Martin-Wixtrom C, Quattrocchi LC, Tukey RH, Waterman MR, Estabrook RW. High-level expression in Escherichia coli of enzymatically active fusion proteins containing the domains of mammalian cytochrome P450 and NADPH-P450 reductase flavoprotein. FASEB J. 1992;6:759–64. doi: 10.1073/pnas.89.22.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam EMJ, Guo Z, Martin MV, Jarkins CM, Guengerich FP. Expression of cytochrome P450 2D6 in Escherichia coli, purification, spectral and catalytic characterization. Arch Biochem Biophys. 1995;319:540–50. doi: 10.1006/abbi.1995.1329. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analysis of amino acid and coding nucleotide sequences. J Biol Chem. 1992;267:83–90. [PubMed] [Google Scholar]
- Guo Z, Gillam EMJ, Ohmori S, Turkey RH, Guengerich FP. Expression of modified human cytochrome P4501A1 in Escherichia coli: effect of 5′ substitution, stabilization, purification, spectral characterization and catalytic properties. Archiv Biochem Biophys. 1994;312:436–46. doi: 10.1006/abbi.1994.1330. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Imaoka S, Mise M, Terauchi Y, Fujii T, Miyazaki H, Kamataki T, Funae Y. Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes. J Pharmacol Exp Ther. 2002;300:298–304. doi: 10.1124/jpet.300.1.298. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Kusunose E, Kaku M, Yamamoto S, Kusunose M. Separation of two constitutive forms of cytochrome P-450 active in aminopyrine N-demethylation from rabbit intestinal mucosa microsomes. Biochem Biophys Acta. 1985;831:99–105. doi: 10.1016/0167-4838(85)90155-4. [DOI] [PubMed] [Google Scholar]
- Ioannides C. Cytochrome P450 expression in the liver of food producing animals. Curr Drug Metab. 2006;7:335–48. doi: 10.2174/138920006776873544. [DOI] [PubMed] [Google Scholar]
- Jiang J- G, Chen C- L, Card JW, Yang S, Chen J- X, Fu X- N, Ning Y- G, Xiao X, Zeldin DC, Wang DW. Cytochrome P4502J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–15. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- Kikuta Y, Sogawa K, Haniu M, Kinosaki P, Kusunose E, Nojima Y, Yamamoto S, Ichihara K, Kusunose M, Fujii-Kuriyama Y. A novel species of cytochrome P450 (P-450ib) specific for the small intestine of rabbit: cDNA cloning and its expression in COS cells. J Biol Chem. 1991;266:17821–5. [PubMed] [Google Scholar]
- Ko IY, Park SS, Song BJ, Patten C, Tan Y, Han YC, Yang CS, Gelboin HV. Monoclonal antibodies to ethanol-induced rat liver cyto-chrome P450 that metabolize aniline and nitrosamines. Cancer Res. 1987;47:3101–9. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longo V, Mazzaccaro A, Naldi F, Gervasi PG. Drug metabolizing enzymes in liver, olfactory and respiratory epithelium of cattle. J Biochem Toxicol. 1991;6:123–8. doi: 10.1002/jbt.2570060206. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Manropot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression and cellular localization of mouse cytochrome P450 highly expressed in Kidney. J Biol Chem. 1999;274:17777–88. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hirama T, Kim HJ, Nagata K, Yamazoe Y. In vitro inhibition of human small intestinal and liver microsomal astemizole O-demethylation. Differ contribution of CYP2J2 in the small intestine and liver. Xenobiotica. 2003;33:615–23. doi: 10.1080/0049825031000105778. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II solubilisation, purification and properties. J Biol Chem. 1964;239:2379–85. [PubMed] [Google Scholar]
- Qu W, Bradbury JA, Tsao C- C, Maronpot R, Harry GJ, Parker CE, Davis LS, Breyer MD, Waalkes MP, Falck JR, Chen J, Rosenberg RL, Zeldin DC. Cytochrome P450 CYP2J9, a new mouse arachidonic acid w-1 hydroxylase predominantly expressed in brain. J Biol Chem. 2001;276:25467–79. doi: 10.1074/jbc.M100545200. [DOI] [PubMed] [Google Scholar]
- Reed JR, Kelley RW, Backes WL. An evaluation of methods for the reconstitution of cytochrome P450 and NADPH P450 reductase into lipid vesicles. Drug Metab Dispos. 2006;34:660–8. doi: 10.1124/dmd.105.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough PE, Ma J, Qu W, Zeldin DC. P450 subfamily CYP2J and their role in the bioactivation of arachidonic acid in extrahepatic tissues. Drug Metab Rev. 1999;31:205–34. doi: 10.1081/dmr-100101915. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Liao JK. Vascular protective effect of cytochrome P450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–20. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Tang C, Shou M, Rodrigues AD. Substrate-dependent effect of acetonitrile on human liver microsomal cytochrome P4502C9 (CYP2C9) activity. Drug Metab Dispos. 2000;28:567–72. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YY, Yang CS. High-affinity nitrosamine dealkylase system in rat liver microsomes and its induction by fasting. Cancer Res. 1983;43:623–9. [PubMed] [Google Scholar]
- Wang L, Yao J, Chen L, Chen J, Xue J, Jia W. Expression and possible functional roles of cytochrome P4502J1 (zf Cyp2J1) in zebrafish. Biochem Biophys Res Comm. 2007;352:850–5. doi: 10.1016/j.bbrc.2006.11.129. [DOI] [PubMed] [Google Scholar]
- Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. Molecular cloning, expression and functional significance of a cytochrome P450 highly expressed in rat miocytes. J Biol Chem. 1997;272:12551–9. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–8. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- Wu Z- L, Sohl CD, Shimada T, Guengerich FP. Recombinant enzyme overexpressed in bacteria show broad catalytic specificity of human cytochrome P4502W1 and limited activity of human cytochrome P450 2S1. Mol Pharmacol. 2007;69:2007–13. doi: 10.1124/mol.106.023648. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Moomaw CR, Jesse N, Tomer KB, Beetham J, Hammok BD, Wu S. Biochemical characterization of the human liver cytochrome P450 arachidonoic acid epoxygenase pathway. Arch Biochem Biophys. 1996;330:87–96. doi: 10.1006/abbi.1996.0229. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Ding X, Kaminsky LS. cDNA cloning, heterologous expression and characterization of rat intestinal CYP2J4. Arch Biochem Biophys. 1997;340:270–8. doi: 10.1006/abbi.1997.9922. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Raner G, Ding X, Dunbar D, Coon MJ, Kaminsky LS. Characterization of the cytochrome P450 CYP2J4: Expression in rat small intestine and role in the retinoic acid biotransformation from retinal. Arch Biochem Biophys. 1998;353:257–64. doi: 10.1006/abbi.1998.0654. [DOI] [PubMed] [Google Scholar]