Abstract
Plasmodium falciparum causes the most severe form of malaria in humans and invades erythrocytes using multiple ligand-receptor interactions. Two important protein families involved in erythrocyte binding are the erythrocyte binding-like (EBL) and the reticulocyte binding-like (RBL or P. falciparum Rh [PfRh]) proteins. We constructed P. falciparum lines lacking expression of EBL proteins by creating single and double knockouts of the corresponding genes for eba-175, eba-181, and eba-140 and show that the EBL and PfRh proteins function cooperatively, consistent with them playing a similar role in merozoite invasion. We provide evidence that PfRh and EBL proteins functionally interact, as loss of function of EBA-181 ablates the ability of PfRh2a/b protein antibodies to inhibit merozoite invasion. Additionally, loss of function of some ebl genes results in selection for increased transcription of the PfRh family. This provides a rational basis for considering PfRh and EBL proteins for use as a combination vaccine against P. falciparum. We immunized rabbits with combinations of PfRh and EBL proteins to test the ability of antibodies to block merozoite invasion in growth inhibition assays. A combination of EBA-175, PfRh2a/b, and PfRh4 recombinant proteins induced antibodies that potently blocked merozoite invasion. This validates the use of a combination of these ligands as a potential vaccine that would have broad activity against P. falciparum.
Plasmodium falciparum causes the most severe form of malaria in humans (43). The asexual blood stage multiplies in erythrocytes and is responsible for the disease manifestations of malaria (29). Merozoites are released from erythrocytes every 48 h, and these rapidly invade new red blood cells (RBCs) in a complex process involving multiple steps and a cascade of ligand-receptor interactions (reviewed in reference 8).
While the function of different parasite ligands in merozoite invasion is not fully understood, the erythrocyte binding-like (EBL) and reticulocyte binding-like (RBL or P. falciparum Rh [PfRh]) homologues have been shown to play a central role (9, 10, 12-14, 24, 36, 40, 44, 47, 50-52). Five genes that potentially encode EBL proteins have been identified, and this group includes the erythrocyte binding antigen 175 (EBA-175; MAL7P1.176) (33, 40), EBA-181 (also known as JESEBL; PFA0125c) (16, 28), and EBA-140 (also known as BAEBL; MAL13P1.60) (24, 27, 30, 48). EBL-1 (PF13_0115) is not expressed in some parasite lines, as it has missense mutations within the coding region. However, it is functional in some lines and may play an important role in P. falciparum invasion in the field (26). The gene encoding EBA-165 (PFD1155w) also has missense mutations, suggesting that it is a pseudogene, but its expression has yet to be detected in any parasite line (53). The EBL proteins consist of the F region (region II), which is made up of two related domains (domains F1 and F2) that are involved in receptor binding (33, 39, 40). EBA-175 and EBA-140 bind to glycophorins A and C, respectively, and these interactions are sialic acid dependent (1, 21, 24, 27, 32, 39, 48). The receptor for EBA-181 is still unknown, although its physical properties show that it is neuraminidase sensitive, trypsin resistant, and chymotrypsin sensitive (16). EBA-181 has been shown to bind to erythrocyte protein 4.1, although this is unlikely to be its host receptor (19).
EBA-175 is polymorphic in region II, the binding region of this ligand, and appears to be under diversifying selection (6). In contrast, EBA-140 and EBA-181 have few polymorphisms in the equivalent domain (28). The polymorphisms in the binding regions of EBA-140 and EBA-181 have been suggested to alter their respective receptor specificities (28). In contrast, other studies have shown that these polymorphisms affect EBA-140 and EBA-181 receptor-binding affinity but not their receptor specificity (22). A correlation between decreased EBA-140 binding and its functional contribution in merozoite invasion has been shown (22, 24). This suggests that sequence variability in individual EBL proteins is driven by immune selection but not by receptor selectivity.
The PfRh family consists of PfRh1 (PFD0110w), PfRh2a (PF13_0198), PfRh2b (MAL13P1.176), PfRh3 (PFL2520w), PfRh4 (PFD1150c), and PfRh5 (PFD1145c). PfRh3 appears to be a transcribed pseudogene, at least for the P. falciparum lines analyzed (45). PfRh1 undergoes protease cleavage events (51), and the N-terminal region has been shown to bind to erythrocytes in a sialic acid-dependent and protease-sensitive manner (36, 52). Genetic disruption of the PfRh1 gene in P. falciparum resulted in a decrease in the sialic acid dependence of merozoite invasion, showing that this ligand plays a direct role in this process (50). Additionally, antibodies raised to the PfRh1 binding region inhibit merozoite invasion (13). In contrast, PfRh4 binds to erythrocytes in a sialic acid-independent and protease-sensitive manner, suggesting that it binds directly to a protein receptor (14, 18, 44) and antibodies against PfRh4 can directly inhibit the function of this ligand in invasion (47). PfRh2a and PfRh2b share a region comprising over 80% of the protein and differ only in the C-terminal sequence. PfRh2b has not been shown to directly bind to erythrocytes, but disruption of the gene and inhibition of merozoite invasion using specific antibodies have shown that it plays an important role in invasion through a candidate receptor, Z (9, 10, 12). PfRh2a has also not been demonstrated to bind to erythrocytes, and at this stage it is not clear if it plays an important role in merozoite invasion (10, 12). PfRh5 is an atypical member of the PfRh family; it is a much smaller protein and does not have a transmembrane region (3, 17). It binds to erythrocytes in a sialic acid-independent manner, although its receptor has yet to be identified. While the receptors for EBA-175 and EBA-140 have been characterized, for the PfRh family of proteins, only the receptor for PfRh4 has been identified, with complement receptor 1 (CR1) shown to be the receptor mediating a specific invasion pathway (46).
EBL and PfRh proteins are located in the apical organelles of the merozoite and are released onto the surface during invasion of erythrocytes (12, 41, 51). Current evidence suggests that the EBA and PfRh proteins are targets of human invasion-inhibitory antibodies and important components of acquired protective immunity (35). It has been shown that differential expression and activation of PfRh proteins provide a mechanism for phenotypic variation in invasion by P. falciparum (12, 35, 44). Disruption of the EBA-175 gene in parasite line W2mef selects for parasites that have activated a normally silenced PfRh4 gene (4, 11, 14, 44). P. falciparum strains can differentially express PfRh2a and PfRh2b, with some lacking any detectable protein, despite the presence of intact genes, suggesting that they too are silenced and under appropriate selection could be activated. Phenotypic variation of the PfRh and EBL families is a mechanism to vary use of invasion pathways (11, 14, 44), creating a hierarchy of invasion ligands that may allow parasites to escape antibody-mediated host immune responses (35) or adapt to variation in erythrocyte surface receptors (4, 12). These studies have provided important insights into the function of the EBA and PfRh proteins and raised them to be possible vaccine candidates. However, in order to block the broad array of receptor-ligand interactions that the parasite has available, a vaccine that targets a combination of the PfRh and EBA proteins would be required.
In order to better understand the role of the EBA and PfRh family of proteins in erythrocyte invasion, we have constructed single- and double-gene-knockout strains in P. falciparum and tested these parasites for growth and invasion. This study shows that loss of function of EBL proteins can be compensated for by increased expression of PfRh ligands. Additionally, we have evaluated a combination of EBL and PfRh proteins for potential use as a vaccine by testing their ability to induce antibodies that block a broad array of ligand-receptor interactions and thereby effectively inhibit merozoite invasion.
MATERIALS AND METHODS
Parasite culture, plasmid vectors, and transfection.
P. falciparum asexual stages were maintained in human type O-positive erythrocytes and synchronized by standard methods (49). 3D7 is a cloned line derived from NF54, supplied by David Walliker, Edinburgh University. The vectors used for construction of the gene-knockout strains were pCC1 and pCC4 (25). For disruption of EBA-175, pCC1 was used, and the 5′ flank was 565 bp starting at −14 bp from the ATG site and was cloned into the SpeI/BglII sites. The 3′ flank was 1,486 bp starting at 2,254 bp and was cloned into ClaI and AvrII. For disruption of EBA-181, pCC1 and pCC4 were used, and for both vectors, the 5′ flank was 775 bp starting at −10 bp from the ATG site and was cloned using the restriction enzymes SacII/SpeI. The 3′ flank was 1,257 bp starting at 1,516 bp and was cloned using ClaI and AvrII. For disruption of EBA-140, pCC4 was used, and the 5′ flank was 938 bp starting at −17 bp from the ATG site and was cloned using the restriction enzymes SpeI/BglII. The 3′ flank was 945 bp starting at 2,433 bp and was cloned using EcoR I and AvrII.
Transfection with 80 μg of purified plasmid DNA (Qiagen, Hilden, Germany) and selection for stable transfectants were performed as described previously (23). Successful disruption of the targeted genes was confirmed by Southern blot analysis, using the Roche digoxigenin (DIG) system, according to the manufacturer's instructions. Each transfected line was cloned by limiting dilution, and all analyses were performed on these parasites.
Coculture assay.
Cultures of P. falciparum 3D7, 3D7ΔEBA-175, 3D7ΔEBA-181, 3D7ΔEBA-140, 3D7ΔEBA-175/181, and 3D7ΔEBA-175/140 parasite lines were synchronized with 5% sorbitol twice 16 h apart. The parasitemia was determined by fluorescence-activated cell sorter (FACS; FACSCalibur; BD) analysis and was adjusted to 0.5% parasitemia and 4% hematocrit. Trophozoite-infected RBCs were used to set up the assay. Once the parasitemia had been adjusted, the parasitemia was then rechecked by FACS analysis to confirm the correct parasitemia. Assays were set up in 10-ml petri dishes containing 5 ml of 3D7 parasites and 5 ml from the different knockout parasite lines in the absence of WR99210 and/or blasticidin. The mixed cultures were maintained at about 1% parasitemia and 4% hematocrit by adding fresh erythrocytes every 2 days, and on every fourth day infected erythrocytes were collected to make genomic DNA (gDNA). The assay was performed over 5 weeks. Real-time PCR was performed on a LightCycler 480 real-time PCR system (Roche) in 10-μl reaction mixtures using the LightCycler 480 SYBR green I master mix (Roche). The PCR conditions consisted of an initial incubation at 95°C for 10 min and then 45 cycles at 95°C for 10 s, 58°C for 10 s, and 72°C for 10 s. Fluorescence was acquired at the end of each extension phase, and melting curve analysis was performed on each reaction mixture to determine the specificity of amplification. Two microliters of genomic DNA was used per PCR, and all samples were tested in duplicate. Water was used as a negative control, and serial dilutions of gDNA (five dilutions, 1-, 10-, 100-, 1,000-, and 10,000-fold concentrations) were used to generate standard curves for each gene. The amount of each target gene was estimated using the standard curve. The absolute quantification method was used to measure the copy number of genes for each sample. The primers (sequences) for target genes were as follows: eba-175 forward primer (5′-TTCGTGATGAGTGGTGGAAA-3′), eba-175 reverse primer (5′-GGCAATAATCATCACCCCATT-3′), eba-181 forward primer (5′-GATTCTGGAATGGGGGAAAT-3′), eba-181 reverse primer (5′-TACATCCGGTTGAGCAGTCA-3′), eba-140 forward primer (5′-CGCGGAAGAACCTCAAATTA-3′), eba-140 reverse primer (5′-GGAAGCCTACATCCACCAAA-3′), hdhfr forward primer (5′-CTCAAGGAACCTCCACAAGG-3′), hdhfr reverse primer (5′-GTTTAAGATGGCCTGGGTGA-3′), bsd forward primer (5′-TTTTACTGGGGGACCTTGTG-3′), and bsd reverse primer (5′-CAAGATGCCCCTGTTCTCAT-3′).
Primers for ebl genes were designed to amplify in 3D7 parasites only, and primers for blasticidin deaminase (bsd) and human dihydrofolate reductase (hDHFR) genes were designed to amplify the gene-knockout mutant parasite lines. The relative concentration of target genes was normalized to that of the aldolase gene at the initial time point (time zero). The normalized data were then used to generate a ratio when they were compared to those for aldolase. Using the normalized data for the target gene over those for aldolase generated the relative concentration of the copy number for the different parasite lines. Two-sided t tests were used to test for differences in gene copy numbers between 3D7 and ebl-knockout mutant lines.
Antibody generation.
Rabbit antibodies were raised to Escherichia coli-expressed fusion proteins PfRh4 and PfRh2a/b and were named PfRh4-9 (47) and Rh2a9 (12), respectively. Antibodies to EBA-175 were to regions III to V (EBA-175RIII-V), corresponding to amino acids 761 to 1298 (37). Antibodies to EBA-181 were to regions III to V, corresponding to amino acids 755 to 1339 (16). Antibodies to EBA-140 were to the F2 domain, corresponding to amino acids 746 to 1043 (24, 48). Rabbits were injected with combinations of each antigen (75 μg) in Freund's complete adjuvant, followed by two immunizations (75 μg) in Freund's incomplete adjuvant. This was followed by three immunizations (50 μg) in phosphate-buffered saline (PBS). The first blood sample (bleed) was taken after injection three, the second bleed after injection four, and the third bleed after injection six.
Immunoblotting.
Proteins were separated on 3 to 8% Tris acetate SDS-polyacrylamide gels (Invitrogen). Western blotting onto nitrocellulose (Invitrogen) was performed according to standard protocols, and blots were processed with an enhanced chemiluminescence system (ECL; Amersham).
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (35). Ninety-six-well flat-bottom plates (Maxisorp; Nunc) were coated with recombinant fusion protein at a concentration of 1 μg/ml in human tonicity PBS (HT-PBS) overnight at 4°C. Plates were incubated with 10% skim milk-0.05% Tween 20 for 2 h at 37°C to block unspecific binding. After the plates were washed, samples were applied diluted in 5% skim milk-0.05% Tween 20. Plates were incubated for 1 h at room temperature before serum was removed by washing. Secondary antibody (horseradish peroxidase [HRP]-conjugated goat anti-human antibody; Chemicon) was used at a 1:5,000 dilution in 5% skim milk-0.05% Tween 20. Plates were incubated for 1 h at room temperature. Azino-bis-3-ethylbenthiazoline-6-sulfonic acid (liquid substrate system; Sigma-Aldrich) was used to detect HRP activity. The reaction was stopped with 1% SDS, and the optical density (OD) was measured at 405 nm. All washes were done in 1× HT-PBS-0.05% Tween 20. All samples were tested in duplicate. The OD from wells incubated with PBS instead of serum was considered the background level and was deducted from the ODs of all samples.
Growth inhibition assay.
Growth inhibition assays (GIAs) were performed as described previously (34). Briefly, late-trophozoite-stage parasites were added to erythrocytes to give a parasitemia of 0.2% and an hematocrit of 1% in 96-well round-bottom microtiter plates (Becton Dickinson, Franklin Lakes, NJ). Five microliters of purified rabbit IgG was added to a final concentration of 2 mg/ml. For the antibody titrations, inhibitory antibodies were added to final concentrations of 0.125, 0.25, 0.5, 1, and 2.0 mg/ml. Cultures were supplemented with 10 μl of culture medium after 48 h (one cycle) of growth. After incubation with antibodies for two cycles of parasite growth (80 to 96 h), the parasitemia of each well was counted by flow cytometry using ethidium bromide (10 μg/ml; Bio-Rad, Hercules, CA) to stain trophozoite-stage parasites using a FACSCalibur apparatus with a plate reader (Becton Dickinson). For each well, more than 40,000 cells were counted; all samples were tested in triplicate. Growth was expressed as a percentage of the parasitemia of the mean parasitemia of six rabbit preimmunization IgG samples. Three independent assays were performed for the parasite lines, each with triplicate wells.
RNA extraction and reverse transcription.
Highly synchronized cultures at late schizont stage were used to extract RNA. Parasites were in 30-ml cultures at ∼5% parasitemia and 4% hematocrit. Schizont-stage parasites were resuspended in ice-cold saponin, and RNA was extracted from saponin pellets using a Qiagen RNeasy kit (Qiagen), according to the manufacturer's instructions. RNA was stored in RNase-free water at −80°C. RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific).
One microgram of total RNA was reverse transcribed using a Superscript III first-strand reverse transcription kit (Invitrogen), according to the manufacturer's instructions. The resultant cDNA was used for subsequent analysis by quantitative real-time PCR.
Quantitative real-time PCR.
Real-time PCR was performed on a LightCycler 480 real-time PCR system (Roche) in 10-μl reaction mixtures using LightCycler 480 SYBR green I master mix (Roche) (31). The PCR conditions consisted of an initial incubation at 95°C for 10 min and then 45 cycles at 95°C for 10 s, 58°C for 10 s, and 72°C for 10 s. Fluorescence was acquired at the end of each extension phase, and melting curve analysis was performed on each reaction mixture to determine specificity. Two microliters of cDNA was used per PCR, and all samples were tested in duplicate. Water was used as a negative control, and serial dilutions of cDNA (five dilutions, 1-, 10-, 100-, 1,000-, and 10,000-fold concentrations) were used to generate standard curves for each gene. The amount of each transcript was estimated using the standard curve. The relative quantification method was used to determine the differential gene expression and to calculate the concentrations of each sample. The primers (sequences) for target genes were as follows: EBA-175 forward primer (5′-TTCGTGATGAGTGGTGGAAA-3′), EBA-175 reverse primer (5′-GGCAATAATCATCACCCCATT-3′), EBA-181 forward primer (5′-GATTCTGGAATGGGGGAAAT-3′), EBA-181 reverse primer (5′-TACATCCGGTTGAGCAGTCA-3′), EBA-140 forward primer (5′-CGCGGAAGAACCTCAAATTA-3′), EBA-140 reverse primer (5′-GGAAGCCTACATCCACCAAA-3′), MTRAP forward primer (5′-TGCACATGGTCACTTTGAAGA-3′), MTRAP reverse primer (5′-TCCTTTCCCTTGAGAGCATCA-3′), actin forward primer (5′-AGCAGCAGGAATCCACACA-3′), actin reverse primer (5′-TGATGGTGCAAGGGTTGTAA-3′), Rh1 forward primer (5′-ATGTATTTTGCCAGTGGAAT-3′), Rh1 reverse primer (5′-TTGTGTGCTTTTATCATCCA-3′), Rh2a forward primer (5′-CACAGCAGGAAGTGTAGCTTT-3′), Rh2a reverse primer (5′-GAACATCATCATTCGGTTCAA-3′), Rh2b forward primer (5′-ATGGAAACACATGGTCCAAA-3′), Rh2b reverse primer (5′-AACAAACAACTCCTCCAGCA-3′), Rh4 forward primer (5′-AGGAAAATCTTCAGGGAAAG-3′), Rh4 reverse primer (5′-TCTGAATGTGCATTATCTGC-3′), RON5 forward primer (5′-GCACTTCGCAATATGGGACT-3′), RON5 reverse primer (5′-ATACCCAAGCAGATCCATCG-3′), Rh5 forward primer (5′-ACGAAGAATCAAGAAAATAATCTGACGTTACT-3′), Rh5 reverse primer (5′-TGTTGAATGATCTTTAGCATTATTTGTTTTTATATTCTCTTT-3′), AMA1 forward primer (5′-GAAAAGGAAATGCTGAAAAA-3′), and AMA1 reverse primer (5′-TGGTGTTGTATGTGATGCTC-3′).
The levels of expression of target genes were normalized to the level of expression of ama1 as described previously (31). The relative concentration of transcript level for each target gene was then examined as a ratio of the transcript levels for the EBL-knockout lines to the level for 3D7. Two-sided t tests were used to test for differences in gene expression levels between 3D7 and EBL-knockout parasite lines.
RESULTS
Disruption of ebl genes in P. falciparum.
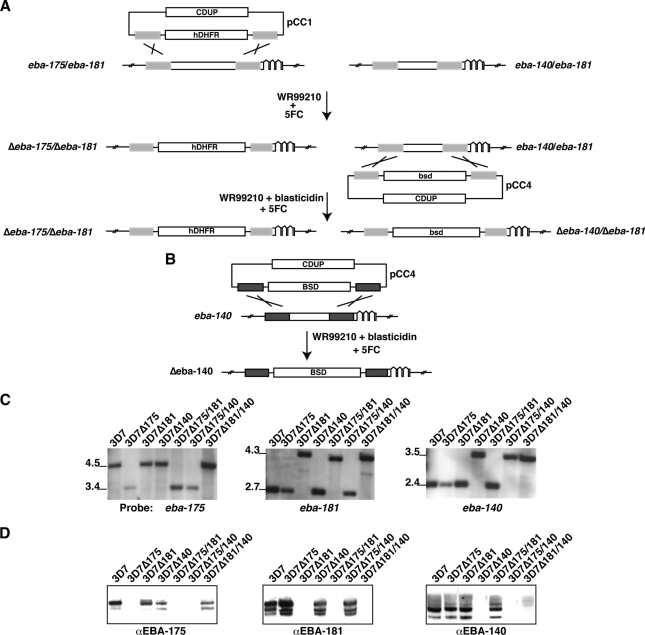
To test the role of the EBL and PfRh protein families, we constructed P. falciparum 3D7 lines in which the genes encoding EBA-175, EBA-181, and EBA-140 were disrupted singly or in combination (Fig. 1) (25). This was done using the vectors pCC1 and pCC4, which contained the genes for human dihydrofolate reductase (hDHFR) or blasticidin deaminase (bsd), to enable the generation of parasites that had two genes disrupted by consecutive transfection of each vector by targeted double-crossover homologous recombination (Fig. 1A and B) (25). All transfectants were analyzed by Southern blotting to confirm that the expected targeting event had occurred (Fig. 1C). We generated the parasite lines 3D7Δ175, 3D7Δ181, and 3D7Δ140, each of which had a single gene ablated (eba-175, eba-140, and eba-181, respectively; Fig. 1C). We then used these for a second round of transfection to generate parasite lines 3D7Δ175/181, 3D7Δ175/140, and 3D7Δ181/140, each of which had two genes disrupted (Fig. 1C). To confirm that the 3D7 P. falciparum lines with specific gene knockouts lacked expression of the expected proteins, we performed immunoblotting with specific antibodies (Fig. 1D). This showed that 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, 3D7Δ175/140, and 3D7Δ181/140 did not express the expected ligands. Analysis of these lines showed that 3D7Δ181/140 had deleted the PfRh2b gene, and consequently, this line was not further studied.
FIG. 1.
Genetic disruption of the eba-175, eba-181, and eba-140 genes in P. falciparum. (A) The strategy for construction of 3D7 P. falciparum parasites with single- and double-gene knockouts of the ebl family. The vectors pCC1 and pCC4 were used, allowing selection for hDHFR (WR99210) and blasticidin deaminase (blasticidin), respectively. Flucytosine (5FC) was used for negative selection to obtain parasites with a double-crossover recombination event with Saccharomyces cerevisiae cytosine deaminase/uracil phosphoribosyltransferase. The eba-175 and eba-181 genes were disrupted first using pCC1 and then using eba-140 or eba-181 with pCC4. (B) The eba-140 gene was disrupted in 3D7 using pCC4 to make a single knockout by double-crossover recombination. (C) Southern blot analysis of 3D7, 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, 3D7Δ175/140, and 3D7Δ181/140 to show that pCC1 and pCC4 had integrated into the expected genes by double-crossover recombination. For the first panel, the restriction enzymes used were EcoRV and MfeI (EBA-175), while for the second panel they were EcoRI and NcoI (eba-181) and for the third panel they were AgeI, HincII, and BamHI (EBA-140). (D) Immunoblots using specific antibodies to EBA-175, EBA-181, and EBA-140 to show that the different knockout strains lacked expression of the expected proteins. Shown are supernatants harvested from 3D7, 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, 3D7Δ175/140, and 3D7Δ181/140 that were probed with anti-EBA175, anti-EBA-181, or anti-EBA-140 antibodies.
The EBL and PfRh proteins function cooperatively.
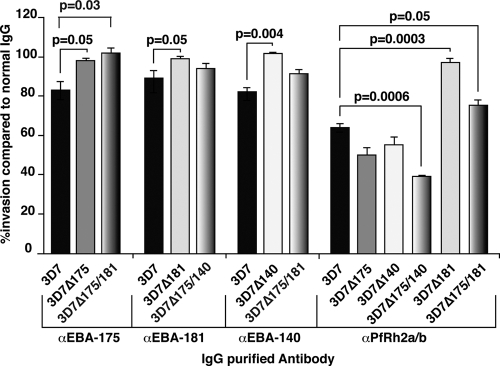
Having constructed a set of P. falciparum cloned lines lacking expression of specific EBL proteins, we performed GIAs to test the function of specific ligands in merozoite invasion (35). Antibodies to EBA-175 (anti-EBA-175RIII-V), EBA-181 (anti-EBA-181RIII-V), and EBA-140 (anti-EBA-140F2) (Fig. 2) inhibited growth of 3D7 at 16.5% (P = 0.05), 10.8% (P = 0.05), and 17.6% (P = 0.004), respectively, compared to the level of inhibition with control antibodies (Fig. 3), which was consistent with previous results (11, 14, 44). This was shown to be specific because the inhibition was not observed in 3D7Δ175, 3D7Δ181, and 3D7Δ140, in which each of these ligands was not expressed (Fig. 3). Antibodies to PfRh2a/b (anti-Rh2a9) inhibited growth of 3D7 at 35.6%, and in 3D7Δ175 (49%) and 3D7Δ140 (44.3%), in which the EBA-175 and EBA-140 proteins were absent, inhibition was increased to a small but not statistically significant extent. This was more evident for 3D7Δ175/140, where growth inhibition in the presence of anti-Rh2a9 was increased to 60.5% (P = 0.004) compared to the level of inhibition of parental line 3D7. This strongly suggested that PfRh2a and/or PfRh2b had compensated for the loss of EBA-175 and EBA-140 function in 3D7, consistent with these protein families having overlapping roles in merozoite invasion.
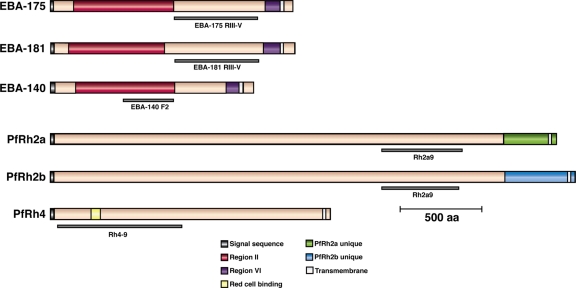
FIG. 2.
Structures of the EBA-175, EBA-181, EBA-140, PfRh2a, PfRh2b, and PfRh4 proteins and the positions to which recombinant proteins were made for use as immunogens to raise antibodies. The red region in EBA-175, EBA-181, and EBA-140 corresponds to region II, which encompasses the receptor-binding domain. PfRh2a and PfRh2b are identical for most of the N-terminal region and differ only at the C terminus (green for PfRh2a and blue for PfRh2b). The red cell binding region defined for PfRh4 is shown in yellow, toward the N terminus. The proteins are drawn to scale, with the sizes shown in amino acids (aa).
FIG. 3.
Antibodies to EBL and PfRh2a/b proteins inhibit invasion of P. falciparum into human erythrocytes. The results of GIAs for 3D7, 3D7Δ175, 3D7Δ175/181, 3D7Δ181, 3D7Δ175/140, 3D7Δ140, 3D7Δ175/140, and 3D7Δ175/181 are shown. The antibodies used are IgG purified from rabbits, used at 1 mg/ml. Shown are the results of three independent experiments, with each done in triplicate, using anti-EBA-175, anti-EBA-181, anti-EBA-140, and anti-PfRh2a/b antibodies which were made to the regions of each protein, as shown in Fig. 2. The histogram represents 3 independent experiments, performed in triplicate. The error bars show the standard errors of the means.
Surprisingly, when we tested the anti-Rh2a9 antibodies with 3D7Δ181 and 3D7Δ175/181 parasites, little inhibition was observed, which was in contrast to inhibition by these antibodies with 3D7, 3D7Δ175, 3D7Δ140, and 3D7Δ175/140 (Fig. 3). Both 3D7Δ181 and 3D7Δ175/181 lack EBA-181 expression (Fig. 1D). To test that PfRh2a and PfRh2b expression was retained in 3D7Δ181 and 3D7Δ175/181, we performed immunoblotting with anti-PfRh2a/b antibodies, and the protein was detected in all parasite lines (data not shown). Both PfRh2a and PfRh2b proteins were detected in the parental 3D7 line as well as 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, and 3D7Δ175/140. Therefore, abrogation of the inhibitory effect of anti-PfRh2a/b antibodies on the growth of parasites lacking EBA-181 was not due to silencing of PfRh2a and PfRh2b expression. Moreover, 3D7Δ181 and 3D7Δ175/181 represent independent eba-181 gene disruption events (Fig. 1), making it unlikely that mutation of PfRh2a or PfRh2b during ongoing culture or transfection had affected the function of these ligands or the ability of Rh2a9 antibodies to inhibit. Our previous data have suggested that PfRh2a was not functional in the 3D7 parental parasite (12), suggesting that the inhibition by Rh2a9 antibodies, which are raised against the common region of PfRh2a/b (Fig. 2), was principally affecting PfRh2b function. These data suggest that PfRh2b functions cooperatively with EBA-181 in merozoite invasion. Experiments to show a direct interaction of PfRh2b and EBA-181 by immunoprecipitation were not successful; this may be due to the interaction occurring transiently during invasion or the functional cooperation not requiring direct interaction of the proteins.
Antibodies against both PfRh and EBL proteins together increased growth inhibition of P. falciparum.
The EBL and PfRh proteins may have potential as vaccine candidates when they are used in specific combinations that generate antibodies to block a broad array of ligands required for merozoite invasion, thereby overcoming functional redundancy among invasion ligands and the capacity for immune evasion (35, 47). To test this hypothesis, we expressed regions of EBL and PfRh proteins that we knew generated invasion-inhibitory antibodies (Fig. 2) and vaccinated rabbits with single proteins as well as specific combinations.
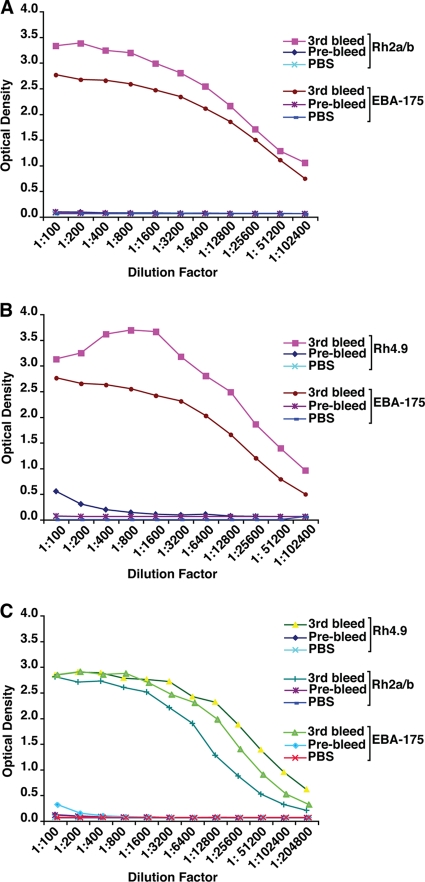
The combinations that we tested were EBA-175RIII-V/Rh2a9, EBA-175RIII-V/Rh4.9, and EBA-175RIII-V/Rh2a9/Rh4.9 (Fig. 2) because of the ability to express large amounts of soluble protein and previous data suggesting that they were functionally important members of the protein families involved in merozoite invasion (11, 12, 37, 44, 47). The recombinant proteins from each combination were used to immunize two rabbits, and IgG was purified from serum for use in assays. We confirmed the specificity and titer of the antibodies by ELISA with the same fusion protein used to immunize rabbits (Fig. 4). The ELISAs showed strong reactivity for each combination that included EBA-175RIII-V with Rh2a9 (Fig. 4A), EBA-175RIII-V with Rh4.9 (Fig. 4B), and EBA-175RIII-V with both Rh2a9 and Rh4.9 (Fig. 4C). No inhibition was observed for IgG purified from rabbit serum collected before immunization (prebleed). The titer of antibody was approximately the same for each antigen, suggesting that the immune responses were similar and that antigenic competition was not a major problem. The titer of antibodies was determined for the second bleed, and they were at levels very similar to those observed after the third bleed (data not shown). Similar results were obtained for serum from a second rabbit immunized with each combination (data not shown). The titers of IgG antibodies from rabbits injected with EBA-175RIII-V, Rh2a9, or Rh4.9 for the single second bleed were determined, and they were similar to those induced by the immunogens. This showed that the antibody response to these antigens was higher than that when the antigens were used in combinations. Therefore, any increased inhibitory effect of the antibodies from rabbits injected with the combination of immunogens is likely to be due to the combination of antigens used rather than just a reflection of different antibody titers.
FIG. 4.
Antibody titers for the IgG antibodies purified from rabbits immunized with the EBA-175, PfRh2a/b, and PfRh4 combinations. (A) PfRh2a/b and EBA-175 recombinant proteins were coated on separate plates, and antibodies from rabbits were tested for reactivity. Prebleed antibodies and PBS were used as negative controls. (B) PfRh4 and EBA-175 recombinant proteins were coated on separate plates, and antibodies from rabbits were tested for reactivity. Prebleed antibodies and PBS were used as negative controls. (C) PfRh4, PfRh2a/b, and EBA-175 recombinant proteins were coated on separate plates, and antibodies from rabbits were tested for reactivity. Prebleed antibodies and PBS were used as negative controls.
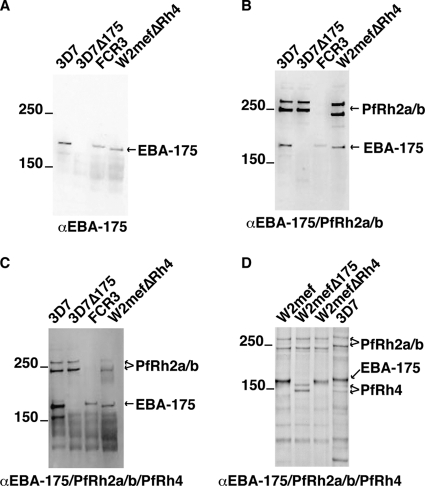
Additionally, we showed that the antibodies reacted to the native parasite proteins in immunoblots of parasite lines 3D7, 3D7Δ175, FCR3, W2mefΔ175, and W2mefΔRh4 (Fig. 5). The anti-EBA-175RIII-V antibodies specifically detected EBA-175 in 3D7, FCR3, and W2mefΔRh4 but not in 3D7Δ175 (or W2mefΔ175) (Fig. 5A). Similarly, antibodies obtained from the rabbits immunized with EBA-175RIII-V/Rh2a9 reacted with EBA-175 and both PfRh2a and PfRh2b (Fig. 5B). Neither PfRh2a nor PfRh2b is expressed in FCR3, and the antibodies detected only EBA-175. The antibodies from the triple-combination immunization (EBA-175RIII-V/Rh2a9/Rh4.9) detected EBA-175, PfRh2a, and PfRh2b; however, it was not possible to identify PfRh4 in parasite culture supernatant material because of the presence of nonspecific bands (44, 47) (Fig. 5C). Consequently, we performed similar immunoblotting assays using schizont-stage material with the parasite lines 3D7, W2mefΔRh4, W2mefΔ175, and W2mef (Fig. 5D). This gave similar results but, importantly, showed that the antibodies detected the expected doublet of approximately 155 kDa for the PfRh4 protein in 3D7 (faint reactivity) and W2mefΔ175 but not in W2mefΔRh4 and W2mef, which lack expression of PfRh4 (44).
FIG. 5.
Antibodies react with the corresponding EBL and PfRh proteins in immunoblots of supernatants or schizont preparations made from 3D7, 3D7Δ175, FCR3, W2mefΔRh4, W2mef, and W2mefΔ175. (A) Supernatants from 3D7, 3D7Δ175, FCR3, and W2mefΔRh4 were separated by SDS-PAGE, and filters were probed with antibodies from rabbits immunized with EBA-175. 3D7Δ175 lacks expression of EBA-175. (B) Supernatants from 3D7, 3D7Δ175, FCR3, and W2mefΔRh4 were separated by SDS-PAGE, and filters were probed with antibodies from rabbits immunized with EBA-175 and PfRh2a/b. (C) Supernatants from 3D7, 3D7Δ175, FCR3, and W2mefΔRh4 were separated by SDS-PAGE, and filters were probed with antibodies from rabbits immunized with EBA-175, PfRh2a/b, and PfRh4. FCR3 lacks expression of PfRh2a and PfRh2b, while 3D7Δ175 does not express EBA-175. W2mefΔRh4 does not express PfRh4. (D) Schizonts from W2mef, W2mefΔ175, W2mefΔRh4, and 3D7 were separated by SDS-PAGE, and filters were probed with antibodies from rabbits immunized with EBA-175, PfRh2a/b, and PfRh4. W2mefΔ175 does not express EBA-175 but does express PfRh4. W2mefΔRh4 does not express PfRh4 but does express EBA-175. W2mef does not express PfRh4.
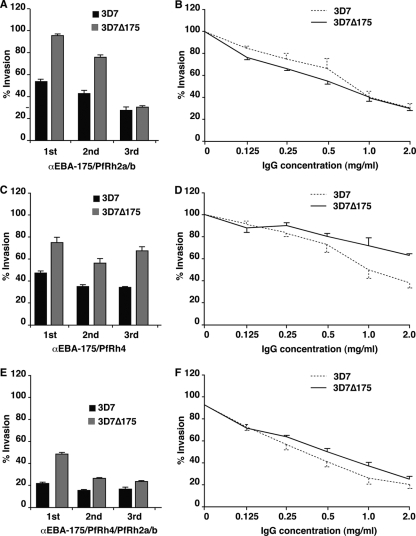
As shown above, anti-EBA-175RIII-V antibodies were able to inhibit 3D7 parasites by 16.5% (Fig. 3); however, when EBA-175RIII-V and Rh2a9 were coimmunized, growth inhibition was significantly greater (Fig. 6A and B). The IgG antibodies from the first bleed tested inhibited at 46%, and this increased significantly for the second (57%) and third (72%) bleeds. In order to dissect the contribution of antibodies to each protein in inhibition of invasion, we tested the ability of antibodies to inhibit 3D7Δ175. This showed that antibodies to EBA-175 played a significant role in the inhibitory effect, as evidenced by the reduced inhibition of 3D7Δ175. However, IgG from the third bleed inhibited 3D7 and 3D7Δ175 equally, suggesting that PfRh2a/b antibodies also played an important role, particularly in the later stages of the immunization schedule. Titration of antibodies from the third bleed showed that the inhibitory effect was still significant at lower antibody concentrations and that 3D7 and 3D7Δ175 were inhibited at equivalent levels (Fig. 6B). These data show that EBA-175RIII-V and Rh2a9, as a vaccine combination, were able to raise antibodies that are more potent in blocking merozoite invasion than either protein used alone.
FIG. 6.
Antibodies from rabbits immunized with EBA-175, PfRh2a/b, and PfRh4 inhibit growth of P. falciparum. (A) Results of GIAs of 3D7 and 3D7Δ175 using IgG antibodies from rabbits immunized with a combination of EBA-175 and PfRh2a/b. (B) Titration of antibodies from rabbits immunized with a combination of EBA-175 and PfRh2a/b tested in GIAs with 3D7 and 3D7Δ175 parasites. (C) Results of GIAs of 3D7 and 3D7Δ175 using IgG antibodies from rabbits immunized with a combination of EBA-175 and PfRh4. (D) Titration of antibodies from rabbits immunized with a combination of EBA-175 and PfRh4 tested in GIAs with 3D7 and 3D7Δ175 parasites. (E) Results of GIAs of 3D7 and 3D7Δ175 using IgG antibodies from rabbits immunized with a combination of EBA-175/PfRh4 and PfRh2a/b. (F) Titration of antibodies from rabbits immunized with a combination of EBA-175, PfRh4, and PfRh2a/b tested in GIAs with 3D7 and 3D7Δ175 parasites.
Similarly, we tested the ability of a combination of EBA-175RIII-V and Rh4.9 to raise invasion-inhibitory antibodies (Fig. 6C). This gave results similar to those for the EBA-175RIII-V/Rh2a9 combination, and IgG from each of the bleeds tested was able to inhibit invasion at a significantly greater level than antibodies raised against each of the ligands alone. Testing for inhibition of 3D7 and 3D7Δ175 suggested that antibodies to both EBA-175 and PfRh4 played a significant role in the overall inhibitory effect; however, the former antigen was clearly more important. Titration of IgG antibodies from the third bleed showed that inhibition was significant at lower concentrations (Fig. 6D).
Finally, we tested antibodies generated by immunization with the EBA-175RIII-V, Rh2a9, and Rh4.9 proteins for invasion-inhibitory activity (Fig. 6E). The three bleeds tested from rabbits immunized showed considerably greater levels of inhibition of both 3D7 and 3D7Δ175 compared to those achieved by immunization with two antigens (Fig. 6). This was apparent from the first bleed tested, which showed 78% inhibition of 3D7, and this increased to a maximum of 84% for the second bleed. Inhibition of 3D7Δ175 again showed that antibodies against both EBA-175 and the PfRh proteins were important for the overall level of inhibition. Additionally, in the second and third bleeds, the anti-PfRh response appeared to have been significantly boosted, as there was a very small difference in inhibition of 3D7 and 3D7Δ175 (Fig. 6E). This was also evident in the titration of IgG from the third bleed, for which there was only a small difference in the inhibition of 3D7 and 3D7Δ175 (Fig. 6F). Importantly, the inhibitory effect could be titrated, and even at a final concentration of 0.125 mg/ml of total IgG, the level of inhibition was significant (Fig. 6F). Overall, these data show that a combination of EBA-175RIII-V, Rh2a9, and Rh4.9 was able to generate an antibody response that was more potent than that obtained with immunization using a double-antigen combination. This strongly supports the idea that an effective immune response that targets different merozoite invasion pathways can significantly reduce the growth potential of P. falciparum and validates these antigens as a potential combination vaccine.
Loss of EBA protein function decreases the fitness of P. falciparum and selects for increased transcription of some PfRh genes.
To determine if loss of EBL function decreased the ability of P. falciparum to invade erythrocytes, we used coculture assays in which we mixed equal numbers of the mutant parasites with the parental line 3D7 and monitored their ability to grow and expand using quantitative PCR (qPCR) over a 5-week period that encompassed approximately 17 generations (Fig. 7). Comparison of the growth rate of the single-knockout strains 3D7Δ175, 3D7Δ181, and 3D7Δ140 showed that loss of function of the corresponding ligand had a small fitness cost, reflected by a reduced growth rate compared to that for parental line 3D7, suggesting that they were not able to invade as efficiently. Interestingly, similar levels of growth rate reduction were obtained for 3D7Δ175/140 and 3D7Δ175/181 when the growth rates were compared directly with the 3D7 growth rate. Therefore, loss of EBL function in 3D7 parasites decreases the fitness of the parasite most likely by decreasing the efficiency of merozoite invasion.
FIG. 7.
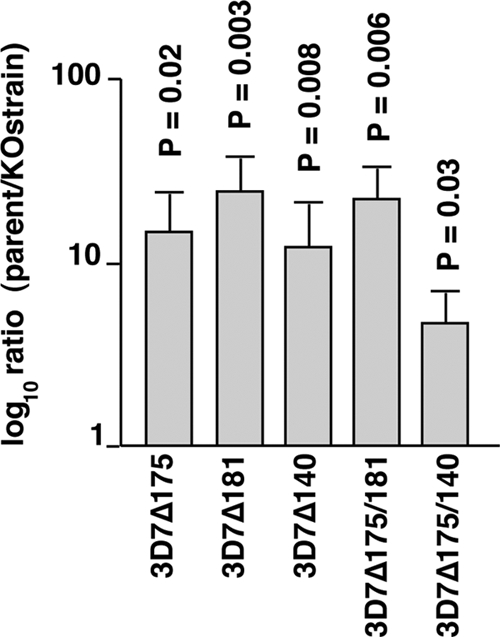
P. falciparum lines lacking expression of specific EBL proteins are less fit than the parental line 3D7, as shown in growth competition assays. The parasite lines 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, and 3D7Δ175/140 were mixed in approximately equal amounts with the 3D7 parental line, and the growth of each was monitored by qPCR over a 5-week period. The log10 ratio of the parent/knockout (KO) strain was calculated. The P values shown represent the probability of a difference in growth rate compared to the 3D7 growth rate and were calculated using a Student t test.
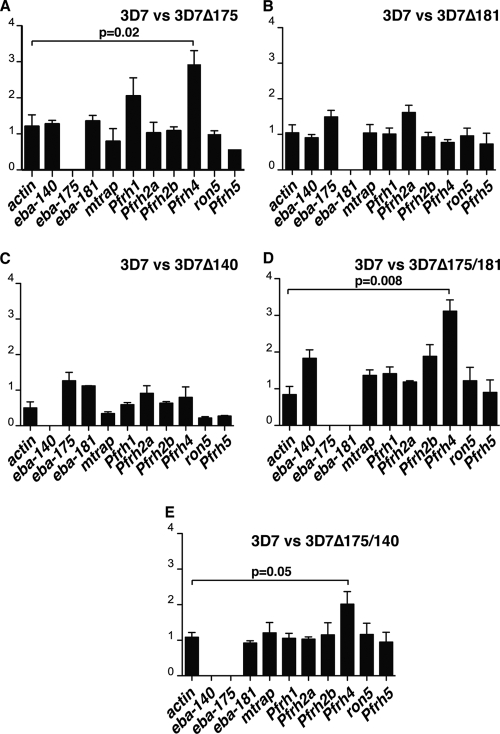
We determined if there were transcriptional differences between 3D7 and the different parasite strains lacking expression of specific ebl genes (Fig. 8). Initially, Affymetrix arrays were used, and the only significant transcriptional changes detected, for genes likely to be involved in merozoite invasion, were in the PfRh gene family (data not shown). To analyze this in more detail, we used qPCR on cDNA made from 3D7, 3D7Δ175, 3D7Δ181, 3D7Δ140, 3D7Δ175/181, and 3D7Δ175/140 (Fig. 8). As controls, we also determined the level of transcription for actin and two genes involved in merozoite invasion, i.e., mtrap and ron5 (5, 38). The most significant change in transcription of the PfRh family occurred in the 3D7 lines in which the eba-175 gene had been disrupted either singly or in combination with other ebl genes, where mRNA levels from PfRh4 were increased 2- to 3-fold (Fig. 8A, D, and E). The significant increase in transcription of PfRh4 in some parasite lines lacking expression of eba-175 suggests that a specific loss function selects for increased transcription of the PfRh4 family to functionally compensate for its role in merozoite invasion.
FIG. 8.
3D7 ebl gene-knockout lines vary in the relative expression of PfRh4 in late-stage schizonts. The genes whose transcriptional levels were compared included actin, eba-140, eba-175, eba-181, mtrap, PfRh1, PfRh2a, PfRh2b, PfRh4, ron5, and PfRh5. The histograms show the relative concentration (knockout strain/3D7) as a ratio for the 11 genes used. Values are given as means ± standard errors of the means. The statistical analysis used was the Student t test. The bars show the results of three independent experiments done in triplicate. (A) Comparison of transcriptional levels between 3D7 and 3D7Δ175 (which lacks expression of EBA-175); (B) comparison of transcriptional levels between 3D7 and 3D7Δ181 (which lacks expression of EBA-181); (C) comparison of transcriptional levels between 3D7 and 3D7Δ140 (which lacks expression of EBA-140); (D) comparison of transcriptional levels between 3D7 and 3D7Δ175/181 (which lacks expression of EBA-175 and EBA-181); (E) comparison of transcriptional levels between 3D7 and 3D7Δ175/140 (which lacks expression of EBA-175 and EBA-140).
DISCUSSION
Invasion of P. falciparum merozoites into human erythrocytes involves multiple ligand-receptor interactions. The EBL and PfRh families are central to host cell recognition and are responsible for mediating distinct invasion pathways (for reviews, see references 8 and 15). We have used single- and double-gene knockouts to analyze the role of these proteins in invasion and explore their potential for use as vaccine candidates in a combination that would block a broad array of invasion pathways (4, 25, 47). Our findings suggest that the role of these two protein families is overlapping, as deletion of EBL members can be functionally compensated for by other PfRh proteins in merozoite invasion. Additionally, our data suggest that PfRh2b interacts functionally with EBA-181, providing evidence that these proteins act cooperatively in merozoite invasion.
The possibility that EBA-175 and PfRh4 have a complementary function has been suggested previously (44). Disruption of the eba-175 gene or selection of W2mef or Dd2 parasites by growth in neuraminidase-treated erythrocytes (which removes the sialic acid-dependent receptor) resulted in parasites in which PfRh4 transcription was activated (14, 44). This suggested that PfRh4 expression compensated for the loss of EBA-175 function in merozoite invasion. The ability of anti-PfRh2a/b antibodies to more efficiently inhibit invasion in parasites lacking EBA-175 and/or EBA-140 function is consistent with a similar compensatory mechanism existing between these two ligands in 3D7 parasites. Interaction of EBA-175 with its receptor, glycophorin A, on the erythrocyte restores basal cytosolic calcium levels in the merozoite, resulting in release of the rhoptry contents, and it is possible that PfRh proteins perform the same function (42). Thus, it is likely that EBL and PfRh proteins bind the erythrocyte via specific receptors, confirming identification of the appropriate cell for invasion and then activation of signals for subsequent events, such as rhoptry release, for entry of the parasite. The differential expression and function of these two protein families provides a mechanism of phenotypic variation in the P. falciparum population for evasion of host responses and to overcome the polymorphic nature of the erythrocyte surface in the human population (12).
PfRh2b antibodies do not inhibit merozoite invasion when EBA-181 is not expressed, suggesting that these two proteins in some way cooperate and that loss of one ablates function in the other. Interestingly, when we disrupted the eba-181 and eba-140 genes, the resulting parasites had deleted the PfRh2b gene. This may have occurred as PfRh2b was no longer functional with the loss of EBA-181 expression and was therefore easily lost from the genome by recombination between the closely linked and highly homologous gene PfRh2a (12). We were unable to coimmunoprecipitate PfRh2b and EBA-181 from 3D7 to demonstrate a direct interaction. It is possible that PfRh2b and EBA-181 interact transiently during merozoite invasion and immunoprecipitation experiments before or after this rapid event using schizonts or culture supernatants, making capture of these interacting proteins very difficult. The interaction of apical membrane antigen 1 (AMA-1) and the RON complex in both T. gondii and P. falciparum occurs only during tachyzoite or merozoite invasion, and they are located in different subcellular locations before egress of the invasive forms (2, 20, 38). Interestingly, the EBL proteins are located in micronemes, while PfRh proteins appear to be in the neck of the rhoptries so that they could associate when the micronemal proteins are released (12, 33, 40). It is likely that other EBL and PfRh proteins besides PfRh2b and EBA-181 also interact functionally during merozoite invasion, and it may be possible to demonstrate these interactions using purified viable merozoites in the process of erythrocyte invasion (7).
The cooperative function of EBL and PfRh proteins in merozoite invasion has important implications with respect to their potential as vaccine candidates. We chose to test EBA-175 in combination with other PfRh proteins because previous studies have suggested that it plays a more important role than EBA-181 and EBA-140 in at least some P. falciparum lines (22). Additionally, the eba-175 gene is more polymorphic in P. falciparum populations than other members of the EBL family, consistent with it being under strong diversifying selective pressure and therefore an important target of immunity (6). Furthermore, recent studies suggest that EBA-175 is a target of acquired invasion-inhibitory antibodies in humans (35). We have shown that the small number of polymorphisms identified in EBA-181 and EBA-140 can decrease the ability of these ligands to bind their receptor and reduce their function such that, in some strains, they do not contribute measurably to invasion (22, 24). The lack of diversifying selection for EBA-140 and EBA-181 and accumulation of polymorphisms that decrease their function are consistent with them playing a lesser role than EBA-175 in merozoite invasion in the P. falciparum population. Our demonstration that PfRh2b and EBA-181 function cooperatively suggests that only one of these needs to be included in a vaccine combination to block the invasion pathways mediated by these proteins. Antibodies to the PfRh4 receptor binding site can inhibit merozoite invasion, suggesting that this may be an important component of an EBL/PfRh combination vaccine. It would be challenging and expensive to include all EBL and PfRh proteins in a vaccine combination, and we therefore chose to test EBA-175 in combination with PfRh2a/b and/or PfRh4, as it appears to be functionally the most important member of the EBL family.
While we have tested EBA-175, PfRh2a/b, and PfRh4 as combinations for their ability to raise invasion-inhibitory antibodies, it has not yet been possible to include PfRh5 (3, 17). It is likely that PfRh5 is potentially important as a candidate vaccine molecule in combination with other members of the PfRh and EBL families. It has not been possible to disrupt the gene for PfRh5 in any parasite line that we have so far tested, suggesting that as a member of this family of proteins it plays a key function in invasion (3, 17). However, it is difficult to express this protein in a recombinant form that enables production of efficient invasion-inhibitory antibodies, and at this stage its importance as a vaccine candidate in combination with other EBL and PfRh proteins remains to be determined.
Comparison of growth rates for the different EBL-knockout strains showed that loss of function of this family of proteins results in a measurable decrease in fitness compared to that of the parental strain. However, we also observed increased transcription of the PfRh4 gene, suggesting that this has to some degree complemented the eba-175 function in merozoite invasion. Consistent with previous data, in the P. falciparum lines in which we ablated EBA-175 function, PfRh4 transcription was significantly increased. These results are similar to previous results of studies in which either EBA-175 function has been blocked by gene disruption or, alternatively, the corresponding receptor has been removed by neuraminidase treatment of the red blood cell receptor (14, 44). This resulted in selection of parasites in which the PfRh4 gene was activated to express the corresponding parasite ligand that mediated an invasion pathway that we have subsequently identified through the host receptor CR1 (46). Thus, it is apparent that P. falciparum strains that can utilize a larger number of EBL and PfRh proteins may be more efficient at invading the host erythrocyte and therefore have a survival advantage. This may be because a larger array of ligands provides an increased affinity of binding to the host erythrocyte and therefore increases the likelihood of successful invasion through delivery of the appropriate signal (42), a property that may also be related to virulence potential (4, 12).
The combination of EBA-175, PfRh2a/b, and PfRh4 proteins generated antibodies that were potent inhibitors of merozoite invasion, and this raises interest in their potential as combination vaccine candidates. Antibodies to the region of EBA-175 tested appeared to be most important in the overall inhibitory effect; however, antibodies to the PfRh2a/b and PfRh4 domains used were also highly inhibitory in combination with EBA-175 antibodies, particularly for rabbit bleeds taken later in the immunization schedule, suggesting that the latter antigens may be less immunogenic than EBA-175. Nevertheless, these results have shown that a combination of EBL and PfRh proteins can be used as a combination vaccine to raise antibodies that are capable of efficiently blocking merozoite invasion.
Acknowledgments
We thank Kaye Wycherley and the Monoclonal Services Group at the Walter and Eliza Hall Institute of Medical Research for performing the ELISAs and purifying IgG preparations. We thank the Red Cross Blood Service (Melbourne, Victoria, Australia) for the supply of red cells and serum.
This work was supported by the National Health and Medical Research Council of Australia (NHMRC), the PATH Malaria Vaccine Initiative, and a Victorian State Government Operational Infrastructure Support grant. A.F.C. is a Howard Hughes International Scholar and an Australia fellow of the NHMRC. J.G.B. was supported by a career development award from NHMRC and the Miller Fellowship from the Walter and Eliza Hall Institute of Medical Research. A.G.M. is an ARC Australian Research Fellow.
Editor: J. H. Adams
Footnotes
Published ahead of print on 13 December 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Adams, J. H., et al. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. U. S. A. 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, D. L., J. Mital, G. E. Ward, P. Bradley, and J. C. Boothroyd. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum, J., et al. 2009. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39:371-380. [DOI] [PubMed] [Google Scholar]
- 4.Baum, J., A. G. Maier, R. T. Good, K. M. Simpson, and A. F. Cowman. 2005. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog. 1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum, J., et al. 2006. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J. Biol. Chem. 281:5197-5208. [DOI] [PubMed] [Google Scholar]
- 6.Baum, J., A. W. Thomas, and D. J. Conway. 2003. Evidence for diversifying selection on erythrocyte-binding antigens of Plasmodium falciparum and P. vivax. Genetics 163:1327-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle, M. J., et al. 2010. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl. Acad. Sci. U. S. A. 107:14378-14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowman, A. F., and B. S. Crabb. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755-766. [DOI] [PubMed] [Google Scholar]
- 9.DeSimone, T. M., A. K. Bei, C. V. Jennings, and M. T. Duraisingh. 2009. Genetic analysis of the cytoplasmic domain of the PfRh2b merozoite invasion protein of Plasmodium falciparum. Int. J. Parasitol. 39:399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSimone, T. M., et al. 2009. Cooperativity between Plasmodium falciparum adhesive proteins for invasion into erythrocytes. Mol. Microbiol. 72:578-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duraisingh, M. T., A. G. Maier, T. Triglia, and A. F. Cowman. 2003. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. U. S. A. 100:4796-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duraisingh, M. T., et al. 2003. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 22:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, X., et al. 2008. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog. 4:e1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaur, D., et al. 2006. Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. Mol. Biochem. Parasitol. 145:205-215. [DOI] [PubMed] [Google Scholar]
- 15.Gaur, D., D. C. Mayer, and L. H. Miller. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413-1429. [DOI] [PubMed] [Google Scholar]
- 16.Gilberger, T. W., et al. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278:14480-14486. [DOI] [PubMed] [Google Scholar]
- 17.Hayton, K., et al. 2008. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 4:40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, L., S. Duriseti, P. Sun, and L. H. Miller. 2009. Molecular basis of binding of the Plasmodium falciparum receptor BAEBL to erythrocyte receptor glycophorin C. Mol. Biochem. Parasitol. 168:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanzillotti, R., and T. L. Coetzer. 2006. The 10 kDa domain of human erythrocyte protein 4.1 binds the Plasmodium falciparum EBA-181 protein. Malaria J. 5:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrun, M., et al. 2005. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell. Microbiol. 7:1823-1833. [DOI] [PubMed] [Google Scholar]
- 21.Lobo, C. A., M. Rodriguez, M. Reid, and S. Lustigman. 2003. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 6:6. [DOI] [PubMed] [Google Scholar]
- 22.Maier, A. G., J. Baum, B. Smith, D. J. Conway, and A. F. Cowman. 2009. Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infect. Immun. 77:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier, A. G., J. A. Braks, A. P. Waters, and A. F. Cowman. 2006. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150:118-121. [DOI] [PubMed] [Google Scholar]
- 24.Maier, A. G., et al. 2003. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier, A. G., et al. 2008. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134:48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer, D. C., et al. 2009. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc. Natl. Acad. Sci. U. S. A. 106:5348-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer, D. C., O. Kaneko, D. E. Hudson-Taylor, M. E. Reid, and L. H. Miller. 2001. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. U. S. A. 98:5222-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer, D. C., et al. 2004. Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc. Natl. Acad. Sci. U. S. A. 101:2518-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, L. H., and B. Greenwood. 2002. Malaria—a shadow over Africa. Science 298:121-122. [DOI] [PubMed] [Google Scholar]
- 30.Narum, D. L., S. R. Fuhrmann, T. Luu, and B. K. Sim. 2002. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 119:159-168. [DOI] [PubMed] [Google Scholar]
- 31.Nery, S., et al. 2006. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. Mol. Biochem. Parasitol. 149:208-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlandi, P. A., F. W. Klotz, and J. D. Haynes. 1992. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha2-3)Gal-sequences of glycophorin A. J. Cell Biol. 116:901-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlandi, P. A., K. L. Sim, J. D. Chulay, and J. D. Haynes. 1990. Characterization of the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 40:285-294. [DOI] [PubMed] [Google Scholar]
- 34.Persson, K. E., C. T. Lee, K. Marsh, and J. G. Beeson. 2006. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J. Clin. Microbiol. 44:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson, K. E., et al. 2008. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 118:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayner, J. C., E. Vargas-Serrato, C. S. Huber, M. R. Galinski, and J. W. Barnwell. 2001. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J. Exp. Med. 194:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, M. B., et al. 2000. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid independent pathway of invasion. Proc. Natl. Acad. Sci. U. S. A. 97:7509-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard, D., et al. 2010. Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J. Biol. Chem. 285:14815-14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sim, B. K. L., C. E. Chitnis, K. Wasniowska, T. J. Hadley, and L. H. Miller. 1994. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264:1941-1944. [DOI] [PubMed] [Google Scholar]
- 40.Sim, B. K. L., et al. 1990. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J. Cell Biol. 111:1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh, A. P., et al. 2005. Targeted deletion of Plasmodium knowlesi Duffy binding protein confirms its role in junction formation during invasion. Mol. Microbiol. 55:1925-1934. [DOI] [PubMed] [Google Scholar]
- 42.Singh, S., M. M. Alam, I. Pal-Bhowmick, J. A. Brzostowski, and C. E. Chitnis. 2010. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 6:e1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stubbs, J., et al. 2005. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309:1384-1387. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, H. M., et al. 2001. Plasmodium falciparum homologue of the genes for Plasmodium vivax and Plasmodium yoelii adhesive proteins, which is transcribed but not translated. Infect. Immun. 69:3635-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tham, W.-H., et al. 2010. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc. Natl. Acad. Sci. U. S. A. 107:17327-17332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tham, W. H., et al. 2009. Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect. Immun. 77:2427-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, J. K., T. Triglia, M. B. Reed, and A. F. Cowman. 2001. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 41:47-58. [DOI] [PubMed] [Google Scholar]
- 49.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 50.Triglia, T., M. T. Duraisingh, R. T. Good, and A. F. Cowman. 2005. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol. Microbiol. 55:162-174. [DOI] [PubMed] [Google Scholar]
- 51.Triglia, T., W. H. Tham, A. Hodder, and A. F. Cowman. 2009. Reticulocyte binding protein homologues are key adhesins during erythrocyte invasion by Plasmodium falciparum. Cell. Microbiol. 11:1671-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triglia, T., et al. 2001. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect. Immun. 69:1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Triglia, T., J. K. Thompson, and A. F. Cowman. 2001. An EBA175 homologue which is transcribed but not translated in erythrocytic stages of Plasmodium falciparum. Mol. Biochem. Parasitol. 116:55-63. [DOI] [PubMed] [Google Scholar]