Abstract
Chlamydia trachomatis genital infection is a worldwide public health problem, and considerable effort has been expended on developing an efficacious vaccine. The murine model of C. muridarum genital infection has been extremely useful for identification of protective immune responses and in vaccine development. Although a number of immunogenic antigens have been assessed for their ability to induce protection, the majority of studies have utilized the whole organism, the major outer membrane protein (MOMP), or the chlamydial protease-like activity factor (CPAF). These antigens, alone and in combination with a variety of immunostimulatory adjuvants, have induced various levels of protection against infectious challenge, ranging from minimal to nearly sterilizing immunity. Understanding of the mechanisms of natural infection-based immunity and advances in adjuvant biology have resulted in studies that are increasingly successful, but a vaccine licensed for use in humans has not yet been brought to fruition. Here we review immunity to chlamydial genital infection and vaccine development using the C. muridarum model.
Chlamydia trachomatis, a Gram-negative obligate intracellular bacterium with a tropism for mucosal epithelial cells, is the most common cause of bacterial sexually transmitted disease in both developed and developing countries, with more than 90 million new cases occurring each year (113-115). More than 4 million new cases of C. trachomatis infection occur each year in the United States, where costs associated with treating those infections and associated complications are in excess of $2 billion annually (107). In the genital tract, infection with C. trachomatis is propagated within the single-cell columnar layer of the epithelium in the urethra of men and the endocervix of women. Within the epithelial cells, C. trachomatis undergoes a unique biphasic developmental cycle consisting of an infectious, but metabolically inert elementary body (EB) and a noninfectious, but metabolically active reticulate body (RB). After completion of the developmental cycle, the EBs are released and infect neighboring epithelial cells, thereby spreading the infection.
Infection can result in acute inflammation characterized by redness, edema, and mucosal discharge and is diagnosed clinically as mucopurulent cervicitis in women and nongonococcal urethritis in men (10, 85). In women, infection can manifest as abnormal vaginal discharge and/or postcoital bleeding, while the infection is limited to the lower genital tract, and irregular uterine bleeding and/or pelvic discomfort once the infection ascends to the upper genital tract (85). Symptoms in males are generally limited to dysuria and moderate clear-to-whitish discharge (85). While these symptoms signify an infection, the absence of such symptoms does not necessarily indicate the absence of infection. It is estimated that >70% of women and 50% of men experience asymptomatic infections (15, 113). Without symptoms providing the impetus, asymptomatic individuals may not seek diagnostic testing and the infection will go untreated. Untreated C. trachomatis infection can wreak havoc on the reproductive organs, profoundly affecting fertility in women. Taken together, the high rate of asymptomatic infections and the severity of the infection-related pathology indicate that, despite the availability of very effective antimicrobial therapy, control of chlamydial infections will most likely require a vaccine.
HISTORICAL VACCINE STUDIES
Almost immediately following the 1957 isolation of the etiologic agent of trachoma by T'ang, human vaccine trials were initiated in areas of trachoma endemicity (5). The outcomes of these trials were mixed, and the results ranged from considerable protection against infection and pathology to partial, short-lived protection (5). In one notable study, researchers vaccinated children with formalin-fixed chlamydial EBs and followed them for 3 years. Vaccination conferred only partial, serovar-specific, short-lived immunity, and compared to their nonvaccinated counterparts a small, but significant, portion of vaccinated individuals experienced an increase in the incidence and severity of infection upon exposure to chlamydiae (5, 94). The exacerbated disease and pathology in these individuals were postulated to be a result of delayed-type hypersensitivity. These experiments were repeated in nonhuman primates, and the results mimicked what was seen in the human trials, leading researchers away from the use of whole organisms in immunization and back into animal models (5, 10, 11, 30). To date, no other C. trachomatis human vaccine studies targeting ocular or genital infection have been published.
Animal models are instrumental in the study of chlamydial genital infection and essential in characterizing the host response to C. trachomatis in females. Mouse, guinea pig, nonhuman primate, nonprimate monkey, rat, and pig models have all been established (30). However, the availability of inbred mouse lines, transgenic and gene knockout mice, and immunological reagents has made the mouse the preferred model to study chlamydial genital infection. Mice have been used extensively to study acute genital infection, protective immune responses, and vaccine development.
Chlamydia muridarum, formerly the C. trachomatis agent of mouse pneumonitis (MoPn), is a murine pathogen that was originally isolated from the lungs of mice and later used to establish a mouse model of genital infection (4, 70). The genomes of C. muridarum and C. trachomatis serovar D share remarkable similarity in the content and order of genes, with the exception of a region ∼50 kb from the origin of termination deemed the plasticity zone (PZ) (92, 98). Within the PZ are genes coding for cytotoxins and a tryptophan operon. Analysis of the cytotoxin genes in C. muridarum and C. trachomatis has revealed similarities in putative virulence factors (6). The presence of a tryptophan operon in the genome of C. trachomatis and the absence of one in the C. muridarum genome are important differences between the two biovars and are responsible for their differential sensitivity to gamma interferon (IFN-γ) (69, 87).
In addition to genetic similarities, murine genital infection with C. muridarum mimics many aspects of acute genital infection with C. trachomatis infection in women. Intravaginal inoculation of C. muridarum produces a self-limiting infection of the vaginal and cervical epithelial cells that subsequently ascends to the upper genital tract via the epithelial surfaces of the uterine horns and oviducts (57, 58). Resolution of the infection takes approximately 4 weeks and results in long-lived adaptive immunity that protects markedly against reinfection (4, 58). Histopathological analysis has revealed that early infection is characterized by an abundant cellular infiltrate of genital tract tissues predominated by polymorphonuclear neutrophils (4, 58, 60, 102). As the infection resolves, those cells are replaced by macrophages and populations of lymphocytes, including B cells, CD4+ T cells, and CD8+ T cells. CD4+ T cells are present throughout the course of infection, and it has been observed that aggregates of these cells remain in the genital tract submucosae after the infection has cleared (45, 57, 60). Upon resolution of infection, more than 60% of mice are solidly resistant to reinfection with the homologous Chlamydia strain, and mice that are susceptible experience infections of considerably shorter duration and lower bacterial burden than primary infection (57). Postinfection sequelea, such as infertility, are observed in humans as well as in the murine model, providing further support for this model.
C. muridarum infects various strains of mice nearly equally (23, 56, 89). Some strains have a higher propensity for the development of hydrosalpinx and shed somewhat greater numbers of bacteria, but overall rates of infection are more or less equivalent. In contrast, infection of mice with C. trachomatis is highly dependent on mouse strain. For example, C57BL/6 and C57BL/10 mice are highly resistant to genital infection, even when high challenge doses of C. trachomatis are used, and the infection is characterized by low bacterial shedding, minimal inflammation, and no hydrosalpinx, and infectious bacteria are only detected for a few days following challenge (23, 88, 89, 116). C3H mice, however, are more susceptible to infection with C. trachomatis. When challenged with C. trachomatis, genital infection in C3H mice is characterized by moderate shedding of bacteria (102 to 104 inclusion-forming units [IFU]) for 2 to 5 weeks and minimal to moderate inflammation (23, 89, 100). Postinfection sequelae (i.e., hydrosalpinx) are less common when mice are challenged vaginally with C. trachomatis, which necessitates the direct inoculation of the uterine horns and/or ovarian bursa with large doses of chlamydiae (109, 110). Strong adaptive responses are generated when mice are infected with C. trachomatis serovars (10, 11, 30), but studies have shown that these infections can clear in the absence of adaptive immunity (87), suggesting that innate immune responses alone can resolve infection.
The observation that C. trachomatis infection can resolve in the absence of adaptive immunity should not be used per se to invalidate the use of C. trachomatis serovars in murine studies of genital tract infection or in vaccine development. Knowledge of the natural course of human genital infection and of the protective responses is very limited because diagnosis of infection mandates treatment. It is, therefore, possible that the rather mild infection seen in murine studies utilizing human C. trachomatis biovars may replicate some aspects of human infection; however, no model has been shown to replicate all of the clinical manifestations of human infection. Vaccine studies using C. trachomatis have also demonstrated that induction of adaptive responses against human strains can confer a degree of protection against genital challenge, which supports the validity of murine C. trachomatis infection for vaccine studies (30). However, because innate immune responses alone resolve murine C. trachomatis genital infection (87), it is imperative that all vaccine studies include controls for the contribution of protective innate responses. In contrast to C. trachomatis, the resolution of murine C. muridarum genital infection, and protection against reinfection, is absolutely dependent on the adaptive immune responses. Thus, the C. muridarum genital infection model is more amenable to the study of adaptive immunity and vaccine development. Because the considerable differences between C. muridarum and C. trachomatis murine genital infection make direct comparisons difficult, we have limited our review to focus primarily on the C. muridarum model of infection and immunity, with only mention of notable C. trachomatis studies.
PROTECTIVE IMMUNE RESPONSES TO C. MURIDARUM INFECTION
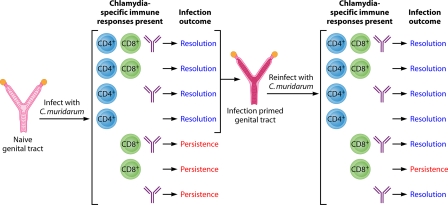
Identification of protective responses is a key component of vaccine development. The responses central to chlamydial immunity, both in terms of resolution of primary infection and immunity to reinfection, have been identified through a variety of experimental approaches in the mouse model of genital infection and have been reviewed previously (57, 93). T cells, particularly major histocompatibility complex (MHC) class II-restricted CD4+ T cells, are required for protective immunity (47, 58, 61, 63, 101). MHC class I-restricted CD8+ T cells, on the other hand, are not necessary for infection resolution or immunity to reinfection (58, 61, 63, 101). The protective role of antibody is less easily discernible than that of the cellular response (59, 63, 90), but important to vaccine development, it is as protective as CD4+ T cells in immunity to reinfection (59, 63). Based on our previous studies (58-63) and a plethora of other studies, we have developed a model depicting immune responses that contribute to the resolution of primary C. muridarum genital infection and resistance to reinfection (Fig. 1). Primary C. muridarum genital infection resolves in the presence of CD4+ T cells, either alone or in concert with CD8+ T cells and/or antibody. The infection persists in the absence of CD4+ T cells. Changes take place during resolution of the primary C. muridarum infection that “prime” the genital tract. Because resolution is dependent on CD4+ T cells, “priming” of the genital tract is facilitated by CD4+ T cells, although the exact mechanism is unknown. Upon reinfection of a “primed” genital tract, mice are infection resistant if CD4+ T cells and/or antibody is present. CD8+ T cells appear to be unnecessary in resistance to reinfection. Although not depicted in Fig. 1, cytokines of the Th1 lineage also play an important role in the protective response. Specifically, IFN-γ and interleukin-12 (IL-12) are essential for protection (19, 40, 86, 87), while the Th2 cytokine IL-10 has been associated with a pathological response (39). Taken together, natural immunity to C. muridarum genital infection in mice is mediated by CD4+ T cells, Th1-type cytokines, and antibody and the effectiveness of a vaccine will likely depend on its ability to induce such responses.
FIG. 1.
Immunity to murine C. muridarum primary infection and reinfection. Genital infection with C. murdiarum produces robust long-lived adaptive immunity. The immune responses that are elicited during infection resolve primary infection in approximately 4 to 5 weeks, and upon rechallenge, those adaptive immune responses result in an infection of much shorter duration (3 to 10 days), and far fewer infectious bacteria (>104 fewer) are shed. Generally speaking, adaptive immune responses elicited during infection consist of CD4+ T cells, CD8+ T cells, and antibody. CD4+ T cells are absolutely essential to bring about the resolution of primary genital infection, whereas CD8+ T cells and antibody are dispensable. In the absence of CD4+ T cells, primary infection persists. Interestingly, immunity to reinfection is governed by a more complex set of responses. First, as with primary infection, CD4+ T cells protect against reinfection and resolve infection in the absence of CD8+ T cells and/or antibody responses. However, in the context of reinfection/rechallenge, antibody is now protective and resolves secondary infection in the absence of CD4+ and/or CD8+ T cells. An indispensable element of the antibody-mediated protective immunity is the priming of the genital tract tissues by CD4+ T cells (infection-primed genital tract). Once the genital tract has been primed, CD4+ T cells are dispensable. Therefore, while the protective efficacy of antibody is dependent on CD4+ T-cell priming of the genital tract, antibody functions independently of CD4+ T cells. We do not yet understand the precise mechanism(s) by which antibody functions, but it is highly protective against reinfection and is absolutely dependent upon the activation and/or recruitment of another cell type, by CD4+ T cells, that functions with antibody to resolve infection.
UNIQUENESS OF THE GENITAL TRACT IMMUNE SYSTEM
The mucosal immune system, especially that of the genital tract, has many unique properties that must be taken into account when designing an effective vaccine targeting C. trachomatis. The genital tract is unique among mucosal effector sites in that it lacks organized lymphatics (55). Unlike the intestinal tract, which has mucosal inductive sites such as the Peyer's patches, propagation of immune response for the genital tract occurs at distant sites, such as the spleen and iliac lymph nodes, which can result in a delayed systemic response relative to other sites (55). The female genital tract is also subjected to hormonal regulation, and the effectiveness of intravaginal immunization has been shown to be influenced by the phase of the menstrual cycle (41, 46, 55). Disparity in the dominant Ig class is found between the genital tract and other mucosal sites: the dominant immunoglobulin class in the genital tract is IgG, while IgA is the dominant class at other mucosal sites (55). IgG translocation from the serum to secretions accounts for its dominating presence in the genital tract, rather than an increase in local IgG-secreting cells (48, 55). The immunological properties of the genital tract and the tropism of Chlamydia for mucosal epithelial cells emphasize the necessity for a C. trachomatis vaccine to induce both systemic and mucosal protective responses.
As such, mucosal vaccination using a number of antigens and multiple mucosal routes has been employed as a strategy to induce protective responses against C. trachomatis in the genital tract (17, 22, 33, 35, 75, 78, 79). Although mucosal effector sites are separated by substantial distances in the body, the common mucosal immune system homes antigen-specific lymphocytes to mucosal sites other than that of initial exposure (111). Accordingly, immunization at one mucosal site induces generalized mucosal immune responses, although oral immunization induces much more restricted responses than other routes (111). Despite the significant numbers of mucosal pathogens, mucosal vaccination is not widely used. Some mucosal vaccines are currently approved for use in humans, and the pathogens causing diseases such as polio, cholera, typhoid, and tuberculosis are targeted through oral immunization (36, 55, 111). Other mucosally administered vaccines targeting viral pathogens, such as a past rotavirus vaccine, have been recalled due to adverse reactions and demonstrate the difficulty of targeting these sensitive areas (36, 111). There are currently no mucosal vaccines targeting genital tract pathogens approved for use in humans.
VACCINES
Vaccine development in the murine model was initiated in the early 1990s, using both C. trachomatis and C. muridarum. A host of antigens, adjuvants, and delivery systems have been assessed for their ability to induce protective immunity against genital challenge, with only limited success. A list of vaccine studies utilizing the C. muridarum genital infection model published to date is presented in Table 1. The immunogenicity of the vaccines and the protective outcome of the studies are not included in the table due to variations in the methodology that impact the readout and outcome of the studies. Of the studies listed in the table, there are several of particular note because they demonstrated considerable protection against genital challenge. For the purpose of this review, we define protection against genital challenge as a significant decrease (several log10) in IFU recovered from cervicovaginal swabbing. Other attributes of protection such as shortened duration of infection and decreased incidence of infection will be noted separately as these readouts are not included in all studies. Notable studies include the adoptive transfer of dendritic cells pulsed ex vivo with inactivated C. muridarum EBs (103), parenteral vaccination with native C. muridarum major outer membrane protein (nMOMP) with Th1-driving adjuvants (80), and mucosal vaccination with recombinant MOMP (rMOMP) delivered via liposomes or vault nanoparticles (17, 31). Despite the considerable effort that has been focused on vaccine development, no chlamydial vaccine has been tested in humans since the early trachoma vaccine trials.
TABLE 1.
Summary of vaccines developed in the murine model of genital infection with C. muridarum
| Yr | Antigena | Route of vaccinationb | Route of challenge | Adjuvant/delivery systemc | Mouse strain | Reference |
|---|---|---|---|---|---|---|
| 1994 | EBs | i.n | Ovarian bursa | None | BALB/c | 75 |
| 1994 | MOMP, Hsp60 | s.c. | Vagina | IFA | BALB/cByJ | 8 |
| 1996 | EBs | i.n. | Ovarian bursa | None | BALB/c | 78 |
| 1997 | nMOMP, COMC | s.c. | Ovarian bursa | CFA, IFA | BALB/c | 84 |
| 1998 | EBs | Intravaginal | Vagina | Dendritic cells pulsed ex vivo | C57BL/10 | 103 |
| 1999 | pMOMP | i.m. | Ovarian bursa, vagina | None | BALB/c, C3H/HeN, C57BL/6 | 74 |
| 2000 | EBs, RBs | s.c., i.m. | Ovarian bursa | CFA | BALB/c | 82 |
| 2000 | EBs | Intravaginal | Vagina | Oxytetracycline (subclinical infection) | C57BL/10 | 104 |
| 2001 | nMOMP | s.c., i.m. | Ovarian bursa | IFA, CFA | BALB/c | 83 |
| 2002 | rMOMP | Intravaginal | Vagina | Dendritic cells pulsed ex vivo | C57BL/10 | 95 |
| 2003 | rMOMP | i.m. | Vagina | VCG | BALB/c | 27 |
| 2003 | EBs | i.n. | Ovarian bursa | None | C3H/HeN, BALB/c, C57BL/6 | 77 |
| 2003 | nMOMP | s.c., i.m. | Ovarian bursa | OspA | C3H/HeN, BALB/c | 76 |
| 2004 | rMOMP | Transcutaneous | Vagina | CpG-1826, CT | BALB/c | 7 |
| 2005 | EBs | i.n. | Vagina | None | BALB/c (newborn) | 79 |
| 2005 | nMOMP | s.c., i.m. | Ovarian bursa | CpG-1826, Montanide ISA 720 | BALB/c | 80 |
| 2006 | nMOMP | s.c., i.m., i.n. | Ovarian bursa | LT-R72, MF59, LT-K63 | C3H/HeN, BALB/c | 81 |
| 2006 | rMOMP | i.n., intravaginal | Vagina | CTB | BALB/c | 96 |
| 2006 | rCPAF | i.n. | Vagina | IL-12 | HLA-DR4 (transgenic) | 67 |
| 2007 | EBs (plasmid-deficient) | Intravaginal | Vagina | None | C3H/HeouJ | 71 |
| 2007 | DNAexpression library | Abdominal | Vagina | Gene gun | BALB/c | 53 |
| 2007 | rCPAF | i.n. | Vagina | IL-12 | BALB/c | 66 |
| 2007 | rCPAF | i.n. | Vagina | CpG-1826 | BALB/c | 18 |
| 2007 | rCPAF, rMOMP, rIncA | i.n. | Vagina | IL-12 | BALB/c | 49 |
| 2008 | pgp3 | i.n. | Vagina | None | BALB/c | 52 |
| 2008 | rMOMP | s.c. | Vagina | CAF01 | BALB/c, C57BL/6 | 1 |
| 2008 | rMOMP | s.c. | Vagina | CAF01 | C57BL/6 | 31 |
| 2009 | rMOMP | i.n. | Vagina | CTAI-DD | BALB/c | 22 |
| 2009 | rMOMP | i.n. | Vagina | Vault nanoparticles | C57BL/6 | 17 |
| 2009 | rMOMP | Transcutaneous | Vagina | CT, CpG-1826, lipid C | BALB/c | 34 |
| 2009 | rTARP, EBs | i.m. | Vagina | CpG-1826, IFA | BALB/c | 112 |
| 2009 | rCPAF | i.n. | Vagina | CpG-1826 | C57BL/6, μMT | 65 |
| 2009 | rMOMP | i.m., intravaginal, transcutaneous | Vagina | CTA2B, VCG | C57BL/6 | 28 |
| 2009 | RplF, PmpE/F2 | Intravaginal | Vagina | Dendritic cells | C57BL/6 | 118 |
| 2010 | rCPAF, inactivated | i.n. | Vagina | IL-12 | BALB/c | 16 |
| 2010 | rMOMP, PmpG1, RplF, PmpE/F2 | s.c. | Vagina | DDA/TBD, AbISCO, CpG-1826 | C57BL/6, BALB/c | 119 |
| 2010 | rMOMP | Oral | Vagina | Lipid C, CpG-1826, CT | BALB/c | 33 |
| 2010 | CTH1 | s.c. | Vagina | CAF01 | C3H/HeN, CB6F1 | 73 |
| 2010 | rCPAF + EBs (UV inactivated) | i.n. | Vagina | CpG-1826 | BALB/c | 50 |
| 2010 | nMOMP | s.c., i.m. | Vagina | CpG-1826, Montanide ISA 720 | C57BL/6 | 29 |
MOMP, major outer membrane protein (native, recombinant, plasmid); Hsp60, 60-kDa heat shock protein; COMC, outer membrane complex; CPAF, chlamydial protease-like activity factor; IncA, inclusion membrane protein A; pgp3, secreted plasmid protein encoded by pORF5; TARP, putative type III secretion effector protein; RplF, ribosomal protein L6; Pmp, polymorphic membrane protein; CTH1, fusion protein of OmcB and rl16s.
i.n., intranasal; s.c., subcutaneous; i.m., intramuscular.
IFA, incomplete Freund's adjuvant; CFA, complete Freund's adjuvant; VCG, Vibrio cholerae ghosts; OspA, Borrelia burgdorferi outer surface protein A; CT, cholera toxin; CpG-1826, unmethylated bacterial oligodeoxynucleotide; Montanide ISA 720, water in oil adjuvant; LT-R72, mutated heat-labile enterotoxin from Escherichia coli; MF-59, oil in water adjuvant; LT-K63, E. coli heat-labile enteroxin; CAF01 (DDA/TDB), liposomal adjuvant containing the synthetic mycobaterial immunomodulator TDB; CTAI-DD, a toxin-based adjuvant constructed of the cholera toxin A1 subunit directly liked to a dimer of the B cell targeting moiety D from protein A of Staphylococcus aureus; lipid C, a lipid-based matrix composed of purified and fractionated triglycerides; CTA2B, modified cholera toxin consisting of A2, the nontoxic cleavage product of the A subunit, and the nontoxic B subunit; AbISCO, an immune-stimulating complex.
ANTIGENS
A host of chlamydial antigens have been assessed in mice for their immunogenicity in terms of ability to elicit humoral and cellular immune responses, and in vaccine development with both C. muridarum and C. trachomatis. Immunoaccessible antigens, such as components of the outer membrane, have received the most attention, but the availability of the genome sequence has led to the identification of new immunogenic antigens. The construction of complete genomic libraries and expression of specific predicted proteins have led to studies to assess the immunogenicity and protective ability of a number of potential vaccine antigens (30, 94). There is significant overlap of antigens used in vaccine studies targeting C. muridarum and C. trachomatis, but some antigens have been tested in only one system, therefore making comparison difficult. As noted above, the emphasis in this overview will therefore be on antigens tested with C. muridarum infection, with mention of notable antigens used exclusively with C. trachomatis.
EBs.
Despite the negative stigma attached to the use of whole EBs from the human trachoma vaccine trials of the 1950s, a considerable number of studies have used whole C. muridarum EBs to induce protective immunity (Table 1). Intranasal immunization of mice with C. muridarum EBs protects against vaginal challenge and infertility (75, 78, 82). This protection is long lived (78, 79) and protects newborn mice (78, 79), indicating that protection against chlamydial challenge can be conferred at a young age, prior to environmental or sexual exposure. The developmental form of C. muridarum also impacts the conference of immunity; intranasal vaccination with RBs does not protect (82). The lack of protection in animals immunized with RBs in contrast to EBs likely occurs because RBs are noninfectious.
The early human field studies utilized intramuscular vaccination with inactivated EBs, which resulted in increased pathology in some of the vaccinated individuals upon reinfection (5). Likewise, intramuscular immunization of mice with a detergent extract of chlamydiae containing MOMP and heat shock protein 60 (Hsp60), also results in increased inflammation in the genital tract and limited protection upon challenge (8). Conversely, when mice are immunized intramuscularly with whole C. muridarum EBs by using the adjuvants CpG-1826 and incomplete Freund's adjuvant (112), a level of protection is observed. These mice experience a decrease in bacterial shedding, shorter duration of infection, and significantly less inflammation and pathology than nonvaccinated controls (112). The reasons for differences in the outcomes of the human ocular studies and the murine genital studies are not clear but may relate to the differences in the immune responses between ocular and genital tissues or between mice and humans or may be due to the choice of adjuvant.
Striking protection against C. muridarum genital challenge is achieved by the adoptive transfer of dendritic cells pulsed ex vivo with inactivated C. muridarum EBs (103). This type of personalized health care, while effective, has limited real world applications for prevention of an infection like C. trachomatis. The study does, however, support further investigation into the use of EBs as a vaccine antigen and demonstrates the utility of dendritic cells in vaccination strategies.
A live attenuated vaccine is another approach utilizing whole EBs in vaccine strategies. Mice infected with a plasmid-cured strain of C. muridarum have a similar course of infection to mice infected with wild-type C. muridarum but do not develop oviduct pathology (71). These mice are subsequently protected against challenge with fully virulent C. muridarum (71). Since the plasmid-deficient strain of C. muridarum establishes a productive infection, it induces a protective immune response that, like infection with fully virulent C. muridarum, protects against reinfection (57, 71). Induction of a protective response against genital challenge in the absence of pathology is the goal of vaccine development, and this study indicates the usefulness of investigating a live attenuated vaccine. The safety of a live attenuated vaccine was also demonstrated with the attenuated plasmidless C. trachomatis serovar L2R strain (72). Mice infected with C. trachomatis L2R did not develop pathology even after repeated infections. The vaccination, however, failed to protect against pathology when mice were infected with a nonhomologous C. trachomatis serovar D strain (72). Collectively, these studies demonstrate the utility of whole EBs in chlamydial vaccine development despite the negative implications of early studies.
MOMP.
MOMP is an approximately 40-kDa highly disulfide cross-linked surface-exposed protein that comprises about 60% of the outer membrane of chlamydiae (12, 13, 80, 105). Along with other high-molecular-weight proteins, MOMP maintains the structural rigidity of the outer membrane of the chlamydial EB, which lacks the peptidoglycan found in the outer membrane of other bacteria (32, 117). MOMP is an immunodominant antigen in both humans and animals and contains multiple B- and T-cell epitopes, eliciting both neutralizing antibody and T-cell immunity (9, 80, 91). The structure of MOMP is comprised of four variable domains interspersed with five constant domains (2, 99, 105). C. trachomatis serovars are typed according to MOMP, and the uniqueness of each serovar is defined by the amino acid sequence of their variable domains. There are multiple alleles coding for MOMP in C. trachomatis but only a single MOMP allele in C. muridarum (10). Therefore, vaccine studies targeting MOMP in the C. muridarum model of genital infection cannot account for a vaccine's ability to provide cross-serovar protection. However, C. trachomatis serovars belong to one of three serogroups, and it has been shown in vitro that monoclonal antibodies to serovars in the same serogroup are cross protective (120), so serovar-specific immunity may not be a necessary requirement of a chlamydia vaccine.
MOMP was among the antigens used in the early vaccine studies with the murine model and has seen continuous use throughout the years (8, 30, 108). Included as part of an extracted outer membrane complex, as purified native or recombinant protein, in peptide form, in plasmid form, and as DNA, MOMP is the most frequently used vaccine antigen (Table 1). Initial studies with MOMP as part of a chlamydial detergent extract along with Hsp60 were disappointing and, similar to the early human ocular vaccination studies, resulted in increased pathology (8). A later study immunizing with a detergent-extracted outer membrane complex (COMC) coupled with incomplete Freund's adjuvant showed more promising results with the immunized mice shedding fewer chlamydiae and exhibiting a decrease in pathology and infertility relative to the controls (84). In the same study, several preparations of purified MOMP extracted with different detergents did not protect as well as the MOMP-containing COMC (84). The authors hypothesized that the difference in protection was due to the conformation of the MOMP rather than the presence of other, potentially immunogenic, proteins in the COMC (84). The proteins in the COMC likely retained the native, or close to the native, conformation, whereas the purified MOMP may have lost the native conformation during extraction and purification. Further support for this hypothesis comes from a study comparing the protective efficacies of nMOMP and rMOMP, both combined with equal amounts of the adjuvants CpG-1826 and Montanide ISA 720 in a murine model of C. muridarum lung infection (106). The nMOMP resulted in significantly more protection than the rMOMP. It has also been demonstrated that adoptive transfer of dendritic cells pulsed ex vivo with rMOMP does not protect against genital challenge as well as the transfer of dendritic cells pulsed ex vivo with whole C. muridarum EBs (95).
rMOMP has been used in a number of vaccine studies (Table 1) (30). Vaccination with rMOMP, using a variety of adjuvants, has induced a wide range of levels of immune protection (1, 7, 17, 22, 27, 28, 31, 33, 34, 49, 95, 96, 118, 119). This is also true for studies utilizing nMOMP (76, 77, 81, 83, 84). Most MOMP-based vaccines induce at least partial protection against genital challenge, but the eliciting of a protective response that parallels that achieved by intranasal or genital infection with whole C. muridarum EBs is far more infrequent. Two remarkable examples of MOMP-based vaccines that have been shown to protect as well as intranasal infection are a parenteral nMOMP vaccine coupled with the adjuvants CpG-1826 and Montanide ISA 720 (80) and a mucosal vault nanoparticle vaccine with rMOMP as the antigen (17). Both vaccines induce strong Th1 type responses and result in significant protection against challenge. The nMOMP vaccine also provided protection against the development of infertility (80), something which has not yet been demonstrated with the rMOMP nanoparticle vaccine (17). The ability of both an rMOMP vaccine and an nMOMP vaccine to induce such striking immunity may indicate that while the structural conformation is likely important, the correct adjuvant(s) may be able to overcome the conformational defects in rMOMP.
The vaccine preparation containing nMOMP plus CpG-1826 and Montanide ISA 720 has been shown to induce protection not only against upper genital tract challenge in mice (80), but also against vaginal challenge with C. muridarum (29). Further studies into the mechanisms of the nMOMP vaccine-induced protective response revealed that both CD4+ T cells and antibody are required for the nMOMP vaccine-induced protection (29). The passive transfer of the polyclonal anti-MOMP serum that is generated by vaccination was also shown to protect mice in a model of genital tract reinfection better than convalescent-phase serum from infection-immune animals (29). A recent study assessing the ability of antibody to rMOMP to protect against genital challenge found that while the anti-rMOMP decreased the infectious burden, this treatment accelerated the development of pathology (21). This finding is in contrast to the results of the nMOMP study mentioned above as well as other studies looking at the protective efficacy of anti-MOMP responses (17, 31, 80, 81, 83, 84). The protection elicited by the polyclonal anti-MOMP serum (29) has important implications for vaccine design as it provides further evidence that MOMP is a key protective antigen and anti-MOMP responses provide significant protection against infection.
The vaccine preparation containing nMOMP plus CpG-2395 (macaque-specific CpG) and Montanide ISA 720 also protected nonhuman primates against ocular infection with C. trachomatis, although the protection was limited to decreasing bacterial burden and vaccination did not protect against infection-related inflammation and pathology (42). This discrepancy between the findings of the murine and the nonhuman primate studies may be due to differences in the host (mouse versus nonhuman primate), the site of challenge (genital tract versus eye), or the biovar of Chlamydia used (C. muridarum versus C. trachomatis).
CPAF.
The chlamydial protease-like activity factor (CPAF) is a secreted protease conserved among Chlamydia species (37). A variety of host proteins are subject to degradation by CPAF, thereby implicating this molecule in evasion of host defenses and in pathogenesis (18, 37, 66, 67). Among the targets of CPAF degradation are host transcription factors such as upstream stimulation factor 1 (USF-1) and regulatory factor X 5 (RFX5), which are required for MHC expression (37, 68). The proapoptotic BH3-only proteins are also degraded by CPAF, implicating this protein in blocking apoptosis in infected cells (18, 37). CPAF has also been shown to cleave the cytoskeletal protein cytokeratin 8, and this cleavage may be a mechanism involved in expansion of the inclusion (37). It has been hypothesized that CPAF is released into the extracellular environment from the cytosol upon rupture of the cell and is then taken up by neighboring cells and processed by the exogenous MHC II pathway (16). As such, CPAF is a dominant antigen in seropositive humans and has been investigated as a possible candidate for a subunit-based vaccine (16).
CPAF is a relative newcomer to the field of chlamydial vaccine studies, but considerable effort has been invested in studying its potential utility as a candidate vaccine antigen. Vaccination with recombinant CPAF (rCPAF) plus IL-12 or CpG-1826 shortens the duration of infection and decreases the cellular infiltrate and oviduct pathology relative to that in unvaccinated animals (18, 66). CPAF exists in both inactive and active forms, but vaccine-induced protection is independent of CPAF's activity state (16). The mechanism of protection induced by CPAF has been investigated and was found to be dependent on IFN-γ (66) and cell-mediated immunity, but not humoral immunity (67). CPAF vaccination has also been shown to induce protective immunity in an HLA-DR4 transgenic strain of mice, which expresses the predominant allele involved in chlamydial antigen presentation to CD4+ T cells in humans (67), suggesting that CPAF may also induce a protective response in humans.
While vaccination with CPAF results in a decrease in bacterial burden and a shortened duration of infection relative to naïve animals, the impact of vaccination on the infection is not apparent until 1 week postinfection, despite the use of a mucosal route for vaccination (18, 66). CPAF is secreted into the cytosol of the chlamydia-infected cell during growth and replication. Inactive CPAF is detected within the inclusion as early as 12 h postinfection, but activated, secreted CPAF cannot be detected until 24 h postinfection (37). This requirement for a productive infection may explain the delay in the vaccine-induced protective effect when protection is measured by the shedding of fewer infectious chlamydiae following challenge. However, if the measure of protection is prevention of oviduct pathology, then CPAF vaccination provides significant protection (18, 66). Vaccination with CPAF can induce a protective response against vaginal challenge, but due to the delayed induction of protection, CPAF may be best utilized in conjunction with another antigen. As such, mice vaccinated with rCPAF plus UV-inactivated EBs and CpG-1826 have a shorter duration of infection than those vaccinated with rCPAF and CpG-1826 alone (50). These mice also began clearing the infection sooner than those vaccinated with rCPAF and CpG-1826 alone (50).
Other antigens.
Vaccine studies targeting C. muridarum have overwhelmingly favored the use of whole EBs, MOMP, and, more recently, CPAF, but protective responses have also been elicited by vaccination with a number of other antigens, some of which are listed in Table 1. Several polymorphic membrane proteins (Pmps) have been assessed as vaccine candidates after both parenteral vaccination (119) and adoptive transfer of dendritic cells pulsed ex vivo with the proteins (118). Adoptive transfer of the ex vivo-pulsed dendritic cells does not alter the course of infection notably, but liposomal delivery of PmpG leads to a significant decrease in bacterial shedding and a shortened course of infection (118, 119). The putative type III secretion effector protein Tarp induces a C. muridarum-specific immune response and offers some protection against inflammation and pathology, but it has little impact on the course of infection (112). Likewise vaccination with inclusion membrane protein A (IncA) does not induce significant protection against genital challenge and only leads to a slight decrease in inflammation (49). When mice are vaccinated with IncA in addition to rCPAF, the protection is more substantial (49). The chlamydial plasmid encodes the secreted protein pgp3 in the open reading frame 5 (pORF5) that is recognized by serum from infected humans, and in that respect is considered immunodominant. Vaccination with pgp3, however, provides minimal protection against genital challenge with C. muridarum (51). While many of the chlamydial antigens elicit at least some level of protection against infection or inflammation, none have been able to induce considerable protection on their own, and it is likely that they would fair better in a multisubunit vaccine.
Recent vaccine studies with the murine model, including those utilizing human C. trachomatis biovars, were reviewed by Hafner et al. (30). As with the C. muridarum vaccine studies, MOMP is the predominant antigen used in the C. trachomatis studies, although rMOMP is almost exclusively utilized. Outer membrane proteins other than MOMP, such as OmcB, are immunogenic and induce partial protection against genital challenge with C. trachomatis after vaccination (26, 30). Additional Inc proteins as well as putative inclusion proteins have also been assessed for their immunogenicity and protective effect, but the results are not as promising as those obtained with MOMP vaccines (30, 53, 54, 97). Porin protein B (PorB) (43, 44), the small chain of ribonucleoside reductase (NrdB) (3), and PmpD (20) are all recognized by serum from C. trachomatis-infected patients, and immune serum and/or monoclonal antibodies specific to these proteins are able to neutralize infection in vitro (3, 20). Vaccination with PorB results in partial protection against genital challenge, and protection is increased when MOMP is included as part of a subunit vaccine with Vibrio cholerae ghosts (38). Immunization with NrdB induces a robust immune response in mice characterized by high-titer specific antibody in the serum, vaginal lavage, and uterine lavage, as well as high IFN-γ responses by splenoctyes (3). NrdB-specific CD4+ T cells induce partial protection against genital challenge upon adoptive transfer (3), but testing NrdB directly for vaccine efficacy has not yet been reported. PmpD is a species-specific antigen that elicits pan-neutralizing antibodies (20). If found to be a protective vaccine antigen, it could potentially provide cross-serovar protection. Importantly, in vitro antibodies to chlamydial MOMP and lipopolysaccharide (LPS) block the neutralizing effect of anti-PmpD, indicating a possible mechanism of C. trachomatis pathogenesis (20). There have been no published accounts of PmpD used in vaccine studies.
ADJUVANTS AND DELIVERY SYSTEMS
The importance of antigen selection in the design of an effective vaccine is irrefutable, but the choice of adjuvant and/or delivery system is also of utmost importance as this choice can impact the type and strength of the immune response generated. The unique properties of the genital tract also impact vaccine development and indicate that protection requires both mucosal and systemic responses. To address these issues and develop an efficacious vaccine, a variety of adjuvants and delivery systems have been tested (Table 1). The adjuvants tested include the traditional choices of alum, complete Freund's adjuvant (CFA), incomplete Freund's adjuvant (IFA), bacterial proteins, including subunits of cholera toxins (CTA1-DD, CTB, CTA2B, and CT), the outer surface protein of Borrelia burgdorferi (OspA), and several adjuvants that are currently in clinical trials, such as CpG, Montanide ISA 720, LT-R72, MF-59, LT-K63, and IL-12. Novel delivery systems have also been introduced, and these include lipid C, liposomes (CAF01 and DDA/TDB), dendritic cells, gene guns, Vibrio cholerae ghosts, and vault nanoparticles. Vault nanoparticles and liposomes are especially promising as adjuvants for a C. trachomatis vaccine as they have been shown to induce significantly protective responses when used with rMOMP (1, 17, 31). Studies of the protective responses involved in immunity to C. muridarum demonstrate the importance of promoting a strong Th1-type response. This can be seen in the disparity of protection induced by vaccination with nMOMP plus alum, a Th2-targeting adjuvant, and vaccination with nMOMP plus CpG-1826 and Montanide ISA 720, a Th1-targeting adjuvant combination (80). A study comparing the protective efficacy of vaccination of mice with nMOMP and rMOMP with the same combination of adjuvants demonstrated that while significant protection is induced with rMOMP, nMOMP elicits a far more protective response (106). This suggests that using nMOMP with vault nanoparticles or liposomes as the delivery system has the potential to induce better protection than has already been shown with rMOMP and these systems (1, 17, 31). The caveat to these studies, however, lies in their use of mucosal routes for vaccination and the limited utilization of this route in current human vaccination protocols.
IMPORTANT CONSIDERATIONS
A major challenge for the field of Chlamydia vaccine research is the lack of standardization in the procedures used to test Chlamydia vaccines, which leads to difficulty in comparing the efficacy of vaccines relative to each other. Several important differences used by various labs engaged in Chlamydia vaccine research that significantly impact the results of infection and vaccine studies include mouse strain, route of infectious challenge, dose of infectious challenge, adjuvant, and the antigen used to assess cellular and humoral immune responses in vitro. Several studies have made evident that the mouse strain used for infection impacts the outcome of the infection in terms of infection titer, duration of infection, degree of the upper genital tract infection, severity of the infection-induced pathology, and immune responses generated (23-25). Infectious dose has been shown to impact the course of infection and ascension of bacteria to the upper genital tract (14). Humoral responses after vaccination are measured by enzyme-linked immunosorbent assay (ELISA) to either the antigen used in the vaccination or whole chlamydial EBs. While using the vaccine antigen in the ELISA demonstrates the ability of the vaccine to elicit an antigen-specific response, using whole EBs evaluates whether the antibody elicited by the vaccine will recognize the agent of infection. Vaccines utilizing secreted proteins would not produce antibody that recognize whole EBs, thereby necessitating the use of the vaccine antigen in ELISAs. Collectively, the differences in the criteria and testing strategies listed here as well as other methodologies that are used to assess the protective efficacy of vaccines present an added level of variation that confounds comparison across laboratories.
The route of infectious challenge also impacts vaccine assessment. Vaccines targeting genital infection utilize one of two routes of infection: vaginal and ovarian bursa, which provide two very important models to assess the efficacy of vaccines. Challenging mice at the vagina, the natural route of infection, allows a normal progression of the infection through the genital mucosa. When mice are challenged vaginally, the infection propagates in the lower genital tract at the cervix and then ascends naturally to the upper genital tract, where it leads to pathology and infertility in some mice. Vaginal challenge provides a model to study the ability of a vaccine to prevent infection and the resulting pathology. However, not every mouse that is challenged vaginally develops pathology, and thus the model of upper genital tract challenge is useful in that regard. In the model of upper genital tract challenge, mice are infected directly in the ovarian bursa by a minor surgical procedure. Directly infecting the upper genital tract increases the likelihood of a productive infection of these tissues, thereby increasing the incidence of pathology. Both infection models are of great utility in vaccine development, but given that most studies utilize only one of the models, it is difficult to evaluate and compare the efficacies of vaccines.
CONCLUSIONS
Chlamydial genital infection is a worldwide public health concern. The past several decades have seen significant advancements in chlamydial immunobiology that have made the prospect of a chlamydial vaccine more attainable. Animal models, particularly the mouse model of genital infection, have proven to be enormously useful in identifying candidate vaccine antigens and in elucidating immune responses that contribute to protective immunity. These models have shown that several infection/disease outcomes can be used as measures of vaccine efficacy, including reduced bacterial shedding, shortened duration of infection, and diminished tissue damage (i.e., hydrosalpinx). Using those measures, some vaccines such as the noninfectious nMOMP vaccine are protective as assessed by all three measures (29, 80), whereas other vaccines, such as the noninfectious CPAF vaccine, have some effect on bacterial shedding but provide robust protection against upper genital tract disease (18, 64, 66, 67). Further, recent studies have shown that infection of mice with a plasmid-cured strain of C. muridarum produces marked protective immunity without development of deleterious postinfection sequelae (71). These recent advances in chlamydial vaccine biology demonstrate that several options exist for targeting vaccine immunity. A vaccine that produced near-sterilizing immunity would be ideal; however, a vaccine need not necessarily produce sterilizing immunity to be effective. Vaccines that reduced postinfection sequelae, diminished bacterial shedding, and/or shortened the duration of infection would all potentially facilitate the control of chlamydial infections.
The current research activity in the field of chlamydial immunology signifies a renewed interest in vaccine development. Those studies have produced a wealth of new knowledge and research tools, which have made it possible for researchers to home in on and induce the appropriate protective responses with a variety of candidate vaccines. Continued discoveries in defining mechanisms of protection and understanding the pathogenesis of infection will only improve the chance of bringing a licensed chlamydial vaccine to fruition.
Acknowledgments
The authors are supported by National Institutes of Health grant AI-038991.
Biography
 Christina M. Farris, a native of Annapolis, Maryland, completed her Bachelor of Science degree in Biology at Hobart and William Smith Colleges in Geneva, NY. Dr. Farris received her Ph.D. in Microbiology from the University of Alabama at Birmingham, where her graduate studies focused on elucidating the mechanisms of MOMP vaccine-induced protection against Chlamydia muridarum genital infection in mice. She is currently a postdoctoral research associate at Texas A&M Health Science Center, where her studies focus on vaccines and protective immunity to Coxiella burnetii.
Christina M. Farris, a native of Annapolis, Maryland, completed her Bachelor of Science degree in Biology at Hobart and William Smith Colleges in Geneva, NY. Dr. Farris received her Ph.D. in Microbiology from the University of Alabama at Birmingham, where her graduate studies focused on elucidating the mechanisms of MOMP vaccine-induced protection against Chlamydia muridarum genital infection in mice. She is currently a postdoctoral research associate at Texas A&M Health Science Center, where her studies focus on vaccines and protective immunity to Coxiella burnetii.
 Richard P. Morrison, a native of Montana, received his undergraduate education at Montana State University and his Ph.D. in Microbiology from the University of Oklahoma in 1982. After completing postdoctoral training in retroviral immunology at the NIAID Rocky Mountain Laboratory, he remained on staff to study Chlamydia pathogenesis. Professor Morrison held faculty positions at Montana State University and the University of Alabama at Birmingham before being appointed Professor and Chair of Microbiology and Immunology at the University of Arkansas for Medical Sciences in 2007. He also holds the Endowed Chair in Sciences Basic to Medicine at UAMS. For the past 25 years, Professor Morrison's lab has focused on the pathogenesis of Chlamydia infection, with particular emphasis on immunity to genital infection. He is a member of the American Academy of Microbiology, has served as an editor for Infection and Immunity since 2006, and serves on the editorial board of Clinical and Vaccine Immunology.
Richard P. Morrison, a native of Montana, received his undergraduate education at Montana State University and his Ph.D. in Microbiology from the University of Oklahoma in 1982. After completing postdoctoral training in retroviral immunology at the NIAID Rocky Mountain Laboratory, he remained on staff to study Chlamydia pathogenesis. Professor Morrison held faculty positions at Montana State University and the University of Alabama at Birmingham before being appointed Professor and Chair of Microbiology and Immunology at the University of Arkansas for Medical Sciences in 2007. He also holds the Endowed Chair in Sciences Basic to Medicine at UAMS. For the past 25 years, Professor Morrison's lab has focused on the pathogenesis of Chlamydia infection, with particular emphasis on immunity to genital infection. He is a member of the American Academy of Microbiology, has served as an editor for Infection and Immunity since 2006, and serves on the editorial board of Clinical and Vaccine Immunology.
Editor: H. L. Andrews-Polymenis
Footnotes
Published ahead of print on 15 November 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Agger, E. M., et al. 2008. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehr, W., et al. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. U. S. A. 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker, C. J., K. W. Beagley, L. M. Hafner, and P. Timms. 2008. In silico identification and in vivo analysis of a novel T-cell antigen from Chlamydia, NrdB. Vaccine 26:1285-1296. [DOI] [PubMed] [Google Scholar]
- 4.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 5.Bell, S. D., Jr., R. L. Nichols, and N. A. Haddad. 1963. The immunology of the trachoma agent with a preliminary report on field trials on vaccine. Invest. Ophthalmol. 2:471-481. [PubMed] [Google Scholar]
- 6.Belland, R. J., et al. 2001. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc. Natl. Acad. Sci. U. S. A. 98:13984-13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, L. J., et al. 2004. Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect. Immun. 72:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blander, S. J., and A. J. Amortegui. 1994. Mice immunized with a chlamydial extract have no increase in early protective immunity despite increased inflammation following genital infection by the mouse pneumonitis agent of Chlamydia trachomatis. Infect. Immun. 62:3617-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunham, R. C. 1999. Human immunity to chlamydiae, p. 211-238. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology Press, Washington, DC.
- 10.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 11.Brunham, R. C., D. J. Zhang, X. Yang, and G. McClarty. 2000. The potential for vaccine development against chlamydial infection and disease. J. Infect. Dis. 181(Suppl. 3):S538-S543. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell, H. D., and R. C. Judd. 1982. Structural analysis of chlamydial major outer membrane proteins. Infect. Immun. 38:960-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey, A. J., K. A. Cunningham, L. M. Hafner, P. Timms, and K. W. Beagley. 2009. Effects of inoculating dose on the kinetics of Chlamydia muridarum genital infection in female mice. Immunol. Cell Biol. 87:337-343. [DOI] [PubMed] [Google Scholar]
- 15.CDC. 2009. Sexually transmitted disease surveillance, 2008. Centers for Disease Control and Prevention, Atlanta, GA.
- 16.Chaganty, B. K., et al. 2010. Heat denatured enzymatically inactive recombinant chlamydial protease-like activity factor induces robust protective immunity against genital chlamydial challenge. Vaccine 28:2323-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champion, C. I., et al. 2009. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One 4:e5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong, Y., et al. 2007. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine 25:3773-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crane, D. D., et al. 2006. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. U. S. A. 103:1894-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham, K. A., A. J. Carey, L. Hafner, P. Timms, and K. W. Beagley. 2011. Chlamydia muridarum major outer membrane protein-specific antibodies inhibit in vitro infection but enhance pathology in vivo. Am. J. Reprod. Immunol. 65:118-124. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham, K. A., A. J. Carey, N. Lycke, P. Timms, and K. W. Beagley. 2009. CTA1-DD is an effective adjuvant for targeting anti-chlamydial immunity to the murine genital mucosa. J. Reprod. Immunol. 81:34-38. [DOI] [PubMed] [Google Scholar]
- 23.Darville, T., et al. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darville, T., et al. 2001. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect. Immun. 69:7419-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, and R. G. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 69:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eko, F. O., et al. 2004. A novel recombinant multisubunit vaccine against Chlamydia. J. Immunol. 173:3375-3382. [DOI] [PubMed] [Google Scholar]
- 27.Eko, F. O., et al. 2003. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 21:1694-1703. [DOI] [PubMed] [Google Scholar]
- 28.Ekong, E. E., et al. 2009. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunol. Med. Microbiol. 55:280-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farris, C. M., S. G. Morrison, and R. P. Morrison. 2010. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect. Immun. 78:4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hafner, L., K. Beagley, and P. Timms. 2008. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol. 1:116-130. [DOI] [PubMed] [Google Scholar]
- 31.Hansen, J., et al. 2008. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital chlamydial infection in a mouse model. J. Infect. Dis. 198:758-767. [DOI] [PubMed] [Google Scholar]
- 32.Hatch, T. P. 1999. Developmental biology, p. 29-67. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology Press, Washington, DC.
- 33.Hickey, D. K., F. E. Aldwell, and K. W. Beagley. 2010. Oral immunization with a novel lipid-based adjuvant protects against genital Chlamydia infection. Vaccine 28:1668-1672. [DOI] [PubMed] [Google Scholar]
- 34.Hickey, D. K., F. E. Aldwell, and K. W. Beagley. 2009. Transcutaneous immunization with a novel lipid-based adjuvant protects against Chlamydia genital and respiratory infections. Vaccine 27:6217-6225. [DOI] [PubMed] [Google Scholar]
- 35.Hickey, D. K., et al. 2004. Intranasal immunization with C. muridarum major outer membrane protein (MOMP) and cholera toxin elicits local production of neutralizing IgA in the prostate. Vaccine 22:4306-4315. [DOI] [PubMed] [Google Scholar]
- 36.Holmgren, J., C. Czerkinsky, K. Eriksson, and A. Mharandi. 2003. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21(Suppl. 2):S89-S95. [DOI] [PubMed] [Google Scholar]
- 37.Huang, Z., et al. 2008. Structural basis for activation and inhibition of the secreted Chlamydia protease CPAF. Cell Host Microbe 4:529-542. [DOI] [PubMed] [Google Scholar]
- 38.Ifere, G. O., et al. 2007. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J. Microbiol. Immunol. Infect. 40:188-200. [PubMed] [Google Scholar]
- 39.Igietseme, J. U., et al. 2000. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J. Immunol. 164:4212-4219. [DOI] [PubMed] [Google Scholar]
- 40.Ito, J. I., and J. M. Lyons. 1999. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect. Immun. 67:5518-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson, E. L., L. Wassen, J. Holmgren, M. Jertborn, and A. Rudin. 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kari, L., et al. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J. Immunol. 182:8063-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawa, D. E., J. Schachter, and R. S. Stephens. 2004. Immune response to the Chlamydia trachomatis outer membrane protein PorB. Vaccine 22:4282-4286. [DOI] [PubMed] [Google Scholar]
- 44.Kawa, D. E., and R. S. Stephens. 2002. Antigenic topology of chlamydial PorB protein and identification of targets for immune neutralization of infectivity. J. Immunol. 168:5184-5191. [DOI] [PubMed] [Google Scholar]
- 45.Kelly, K. A., and R. G. Rank. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozlowski, P. A., et al. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566-574. [DOI] [PubMed] [Google Scholar]
- 47.Landers, D. V., K. Erlich, M. Sung, and J. Schachter. 1991. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect. Immun. 59:3774-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lencer, W. I., and R. S. Blumberg. 2005. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 15:5-9. [DOI] [PubMed] [Google Scholar]
- 49.Li, W., et al. 2007. Induction of cross-serovar protection against genital chlamydial infection by a targeted multisubunit vaccination approach. Clin. Vaccine Immunol. 14:1537-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, W., et al. 2010. Immunization with a combination of integral chlamydial antigens and a defined secreted protein induces robust immunity against genital chlamydial challenge. Infect. Immun. 78:3942-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li, Z., D. Chen, Y. Zhong, S. Wang, and G. Zhong. 2008. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect. Immun. 76:3415-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, Z., S. Wang, Y. Wu, G. Zhong, and D. Chen. 2008. Immunization with chlamydial plasmid protein pORF5 DNA vaccine induces protective immunity against genital chlamydial infection in mice. Sci. China C Life Sci. 51:973-980. [DOI] [PubMed] [Google Scholar]
- 53.McNeilly, C. L., et al. 2007. Expression library immunization confers partial protection against Chlamydia muridarum genital infection. Vaccine 25:2643-2655. [DOI] [PubMed] [Google Scholar]
- 54.Meoni, E., et al. 2009. CT043, a protective antigen that induces a CD4+ Th1 response during Chlamydia trachomatis infection in mice and humans. Infect. Immun. 77:4168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mestecky, J., Z. Moldoveanu, and M. W. Russell. 2005. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 53:208-214. [DOI] [PubMed] [Google Scholar]
- 56.Molina, D. M., et al. 2010. Identification of immunodominant antigens of Chlamydia trachomatis using proteome microarrays. Vaccine 28:3014-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175:7536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison, S. G., and R. P. Morrison. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 68:2870-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison, S. G., and R. P. Morrison. 2005. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infect. Immun. 73:6183-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphey, C., et al. 2006. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell. Immunol. 242:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murthy, A. K., et al. 2009. A limited role for antibody in protective immunity induced by rCPAF and CpG vaccination against primary genital Chlamydia muridarum challenge. FEMS Immunol. Med. Microbiol. 55:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murthy, A. K., J. P. Chambers, P. A. Meier, G. Zhong, and B. P. Arulanandam. 2007. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 75:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murthy, A. K., et al. 2006. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect. Immun. 74:6722-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murthy, A. K., M. N. Guentzel, G. Zhong, and B. P. Arulanandam. 2009. Chlamydial protease-like activity factor—insights into immunity and vaccine development. J. Reprod. Immunol. 83:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson, D. E., et al. 2005. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc. Natl. Acad. Sci. U. S. A. 102:10658-10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nigg, C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 95:49-50. [DOI] [PubMed] [Google Scholar]
- 71.O'Connell, C. M., R. R. Ingalls, C. W. Andrews, Jr., A. M. Scurlock, and T. Darville. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J. Immunol. 179:4027-4034. [DOI] [PubMed] [Google Scholar]
- 72.Olivares-Zavaleta, N., W. Whitmire, D. Gardner, and H. D. Caldwell. 2010. Immunization with the attenuated plasmidless Chlamydia trachomatis L2(25667R) strain provides partial protection in a murine model of female genitourinary tract infection. Vaccine 28:1454-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olsen, A. W., M. Theisen, D. Christensen, F. Follmann, and P. Andersen. 2010. Protection against chlamydia promoted by a subunit vaccine (CTH1) compared with a primary intranasal infection in a mouse genital challenge model. PLoS One 5:e10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pal, S., et al. 1999. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine 17:459-465. [DOI] [PubMed] [Google Scholar]
- 75.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pal, S., C. J. Luke, A. G. Barbour, E. M. Peterson, and L. M. de la Maza. 2003. Immunization with the Chlamydia trachomatis major outer membrane protein, using the outer surface protein A of Borrelia burgdorferi as an adjuvant, can induce protection against a chlamydial genital challenge. Vaccine 21:1455-1465. [DOI] [PubMed] [Google Scholar]
- 77.Pal, S., E. M. Peterson, and L. M. de la Maza. 2003. Induction of protective immunity against a Chlamydia trachomatis genital infection in three genetically distinct strains of mice. Immunology 110:368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal, S., E. M. Peterson, and L. M. de la Maza. 1996. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect. Immun. 64:5341-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination of newborn mice induces a strong protective immune response against respiratory and genital challenges with Chlamyida trachomatis. Vaccine 23:5351-5358. [DOI] [PubMed] [Google Scholar]
- 80.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pal, S., E. M. Peterson, R. Rappuoli, G. Ratti, and L. M. de la Maza. 2006. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine 24:766-775. [DOI] [PubMed] [Google Scholar]
- 82.Pal, S., J. Rangel, E. M. Peterson, and L. de la Maza. 2000. Immunogenic and protective ability of the two developmental forms of Chlamydiae in a mouse model of infertility. Vaccine 18:752-761. [DOI] [PubMed] [Google Scholar]
- 83.Pal, S., I. Theodor, E. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 65:3361-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peipert, J. F. 2003. Clinical practice. Genital chlamydial infections. N. Engl. J. Med. 349:2424-2430. [DOI] [PubMed] [Google Scholar]
- 86.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-g-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 87.Perry, L. L., et al. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 88.Peterson, E. M., J. Z. You, V. Motin, and L. M. de la Maza. 1999. Intranasal immunization with Chlamydia trachomatis serovar E protects from a subsequent vaginal challenge with the homologous serovar. Vaccine 17:2901-2907. [DOI] [PubMed] [Google Scholar]
- 89.Ramsey, K. H., J. L. DeWolfe, and R. D. Salyer. 2000. Disease outcome subsequent to primary and secondary urogenital infection with murine or human biovars of Chlamydia trachomatis. Infect. Immun. 68:7186-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramsey, K. H., and R. G. Rank. 1991. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 59:925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rank, R. G. 1999. Models of immunity, p. 239-295. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology Press, Washington, DC.
- 92.Read, T. D., et al. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roan, N. R., and M. N. Starnbach. 2008. Immune-mediated control of Chlamydia infection. Cell. Microbiol. 10:9-19. [DOI] [PubMed] [Google Scholar]
- 94.Rockey, D. D., J. Wang, L. Lei, and G. Zhong. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev. Vaccines 8:1365-1377. [DOI] [PubMed] [Google Scholar]
- 95.Shaw, J., V. Grund, L. Durling, D. Crane, and H. D. Caldwell. 2002. Dendritic cells pulsed with a recombinant chlamydial major outer membrane protein antigen elicit a CD4+ type 2 rather than type 1 immune response that is not protective. Infect. Immun. 70:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh, S. R., et al. 2006. Mucosal immunization with recombinant MOMP genetically linked with modified cholera toxin confers protection against Chlamydia trachomatis infection. Vaccine 24:1213-1224. [DOI] [PubMed] [Google Scholar]
- 97.Starnbach, M. N., et al. 2003. An inclusion membrane protein from Chlamydia trachomatis enters the MHC class I pathway and stimulates a CD8+ T cell response. J. Immunol. 171:4742-4749. [DOI] [PubMed] [Google Scholar]
- 98.Stephens, R. S., et al. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 99.Stephens, R. S., R. Sanchez-Pescador, E. A. Wagar, C. Inouye, and M. S. Urdea. 1987. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 169:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sturdevant, G. L., et al. 2010. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect. Immun. 78:3660-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su, H., et al. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su, H., R. Messer, W. Whitmire, S. Hughes, and H. D. Caldwell. 2000. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for the development of live attenuated chlamydial vaccine strains. Infect. Immun. 68:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun, G., et al. 2007. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J. Bacteriol. 189:6222-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun, G., S. Pal, J. Weiland, E. M. Peterson, and L. M. de la Maza. 2009. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 27:5020-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tavakoli, M., A. M. Craig, and M. Malek. 2002. An econometric analysis of screening and treatment of patients with suspected Chlamydia. Health Care Manag. Sci. 5:33-39. [DOI] [PubMed] [Google Scholar]
- 108.Tuffrey, M., F. Alexander, W. Conlan, C. Woods, and M. Ward. 1992. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J. Gen. Microbiol. 138:1707-1715. [DOI] [PubMed] [Google Scholar]
- 109.Tuffrey, M., F. Alexander, and D. Taylor-Robinson. 1990. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J. Exp. Pathol. (Oxf.) 71:403-410. [PMC free article] [PubMed] [Google Scholar]
- 110.Tuffrey, M., P. Falder, J. Gale, and D. Taylor-Robinson. 1986. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br. J. Exp. Pathol. 67:605-616. [PMC free article] [PubMed] [Google Scholar]
- 111.van Ginkel, F. W., H. H. Nguyen, and J. R. McGhee. 2000. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg. Infect. Dis. 6:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang, J., et al. 2009. A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity against upper genital tract pathologies in mice. Vaccine 27:2967-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.WHO. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overviews and estimates. World Health Organization, Geneva, Switzerland.
- 114.WHO. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overviews and estimates. World Health Organization, Geneva, Switzerland.
- 115.WHO. 2007. Global strategy for prevention and control of sexually transmitted infections: 2006-2015. World Health Organization, Geneva, Switzerland.
- 116.Williams, D. M., B. G. Grubbs, E. Pack, K. Kelly, and R. G. Rank. 1997. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect. Immun. 65:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yen, T. Y., S. Pal, and L. M. de la Maza. 2005. Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry 44:6250-6256. [DOI] [PubMed] [Google Scholar]
- 118.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, and R. C. Brunham. 2009. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J. Immunol. 182:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu, H., et al. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 78:2272-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang, Y.-X., S. J. Stewart, and H. D. Caldwell. 1989. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 57:636-638. [DOI] [PMC free article] [PubMed] [Google Scholar]