Abstract
Gram-negative bacteria, including Salmonella enterica serovar Typhimurium, exploit type III secretion systems (T3SSs) through which virulence proteins are delivered into the host cytosol to reinforce invasive and replicative niches in their host. Although many secreted effector proteins and membrane-bound structural proteins in the T3SS have been characterized, the functions of many cytoplasmic proteins still remain unknown. In this study, we found that IacP, encoded by Salmonella pathogenicity island 1, was important for nonphagocytic cell invasion and bacterial virulence. When the iacP gene was deleted from several Salmonella serovar Typhimurium strains, the invasion into INT-407 epithelial cells was significantly decreased compared to that of their parental strains, and retarded rearrangements of actin fibers were observed for the iacP mutant-infected cells. Although IacP had no effect on the secretion of type III translocon proteins, the levels of secretion of the effector proteins SopB, SopA, and SopD into the culture medium were decreased in the iacP mutant. In a mouse infection model, mice infected with the iacP mutant exhibited alleviated pathological signs in the intestine and survived longer than did wild-type-infected mice. Taken together, IacP plays a key role in Salmonella virulence by regulating the translocation of T3SS effector proteins.
The injection of bacterial proteins by the type III secretion system (T3SS) into the host cytoplasm has been broadly applied to study pathogen-host interactions ranging from the invasion of plant and animal pathogens to a symbiont interaction of Rhizobium (22, 42). The T3SS is composed of more than 20 different structural proteins that form needle-like appendages through which effector proteins are delivered directly into host cells to manipulate various host cell signaling events. Moreover, cytoplasmic chaperones are involved in the stability and efficient translocation of effector proteins (14). Salmonella enterica serovar Typhimurium, a facultative intracellular pathogen, has evolved two distinct T3SSs encoded by Salmonella pathogenicity island 1 (SPI-1), responsible for the invasion of nonphagocytic cells, and by SPI-2, required for intracellular survival and replication inside the Salmonella-containing vacuole (SCV). The expressions of the two T3SSs are inversely regulated during the pathogenic process. Although the expression of the SPI-1 T3SS at systemic sites has remained controversial, some effector proteins of SPI-1 (e.g., SipA and SopB) are persistently expressed and secreted under favorable conditions for SPI-2 expression during the biogenesis and maturation of the SCV (17).
After the SPI-1 T3SS is activated upon host cell contact, the translocators SipB and SipC appear to be inserted into the host cell membrane, where they form a translocation pore, which is connected to the needle complex. A variety of effector proteins encoded within and outside SPI-1 can be translocated into a host cytoplasm and cooperatively induce membrane ruffling (11) and macropinocytosis (16). Among SPI-1 effector proteins, SopE, SopE2, and SopB trigger the actin rearrangement in host cells by activating small GTPases, including Rac1, Cdc42, and RhoG, directly or indirectly (39). A Salmonella serovar Typhimurium mutant carrying null mutations in these effector proteins failed to invade epithelial cells. After bacterial invasion, an activated membrane was subsequently recovered by SptP, another effector protein possessing GTPase-activating protein activity (13).
The iacP gene, which is located downstream of sicA- sipBCDA in the SPI-1 locus, was initially identified as a putative acyl carrier protein (ACP) by sequence similarity (26). ACP is an abundant small acidic and highly conserved protein that is essential for various biosynthetic pathways (5). In the process of fatty acid (FA) biosynthesis in Escherichia coli, ACP sequentially delivers the acyl intermediates for FA elongation as a cofactor of FA synthase (20). For the enzymatic activity of ACP, a prosthetic group 4′-phosphopantetheine (4′-PP) that was covalently incorporated into apo-ACP serves as the binding site of acyl groups. It was reported previously that the substitution of serine 36 in Escherichia coli ACP eliminated the attachment site of the 4′-PP and inhibited FA incorporation (27).
In addition to lipid biosynthesis, acyl-ACP is required for various bacterial virulence processes: the synthesis of the lipid A moiety of lipopolysaccharide (LPS) (43) and the N-acylhomoserine lactones as signal molecules in quorum sensing (52) and the posttranslational modification of bacterial toxins such as E. coli hemolysin (HlyA) (24). The activation of HlyA requires posttranslational acylation at two internal lysine residues by ACP and the acyl transferase HlyC. The conformation of acylated HlyA is matured into a molten globular form comprised of disordered regions, which is necessary for the hemolytic effects of a toxin to occur (21).
As a Salmonella serovar Typhimurium mutant that lacks an entire SPI-1 locus was found to grow as well as the wild type, it is predicted that IacP would be responsible for the modification of other proteins in the T3SS (26). However, it is not known which proteins are targeted by IacP or how the invasion process during SPI-1 activation is affected in the iacP mutant. In this study, we report that IacP promotes SopB, SopA, and SopD secretion during cell entry, thus contributing to the virulence of Salmonella serovar Typhimurium.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All Salmonella serovar Typhimurium strains used in this study are listed in Table 1. Unless otherwise noted, Salmonella serovar Typhimurium bacteria were incubated at 37°C in Luria-Bertani (LB) medium with 0.3 M NaCl for SPI-1 activation. When necessary, l-arabinose was added to induce the expression of plasmid-borne genes, and the following antibiotics were added to the cultures: ampicillin (Ap) (100 μg/ml), chloramphenicol (Cm) (30 μg/ml), kanamycin (Km) (50 μg/ml), and streptomycin (Sm) (50 μg/ml).
TABLE 1.
Salmonella strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| UK1 | Wild type | 38 |
| SL1344 | Wild type | 54 |
| LT2 | Wild type | 45 |
| ATCC 14028s | Wild type | ATCC |
| CAS152 | CS401 ΔsspB (sipB); Smr | 50 |
| YKJ013 | UK1 ΔsipB | 29 |
| YKJ032 | SL1344 ΔiacP | This study |
| YKJ033 | LT2 ΔiacP | This study |
| YKJ034 | UK1 iacP::Cm; Cmr | This study |
| YKJ035 | UK1 ΔiacP | This study |
| YKJ036 | ATCC 14028s ΔiacP | This study |
| YKJ074 | UK1 iacP::HA; Cmr | This study |
| YKJ204 | SL1344 sopE::Cm; Cmr | This study |
| YKJ205 | SL1344 ΔiacP sopE::Cm; Cmr | This study |
| YKJ029 | UK1 sopB::Km; Kmr | This study |
| YKJ099 | UK1 ΔiacP sopB::Km; Kmr | This study |
| YKJ042 | UK1 sopB::3×FLAG; Kmr | This study |
| YKJ098 | UK1 ΔiacP sopB::3×FLAG; Kmr | This study |
| YKJ119 | UK1 sptP::HA; Cmr | This study |
| YKJ123 | UK1 ΔiacP sptP::HA; Cmr | This study |
| Plasmids | ||
| pMW118 | Low-copy-no. plasmid; Apr | Nippon Gene |
| pBAD24 | Expression plasmid containing the arabinose-inducible promoter PBAD; Apr | 18 |
| pYKJ029 | pMW118 Psip; Apr | This study |
| pYKJ032 | pMW118 Pint-iacP; Apr | This study |
| pYKJ033 | pMW118 Psip-iacP::HA (piacP); Apr | This study |
| pYKJ034 | pBAD24 PBAD-iacP::HA; Apr | This study |
| pYKJ035 | pMW118 Psip-iacP(S38A)::HA; Apr | This study |
| pYKJ036 | pMW118 Psip-iacP(E51A)::HA; Apr | This study |
| pYKJ290 | pMW118 Psip-sipC::FLAG; Apr | This study |
| pYKJ291 | pMW118 Psip-sipD::FLAG; Apr | This study |
| pYKJ038 | pMW118 Psip-sipA::FLAG; Apr | This study |
| pYKJ304 | pBAD24 PBAD-sopA::FLAG; Apr | This study |
| pYKJ305 | pMW118 PsopD::FLAG; Apr | This study |
| pYKJ065 | pMW118 PsopE::HA; Apr | This study |
Construction of Salmonella serovar Typhimurium strains.
The disruption or epitope tagging of specific genes was conducted by using the red recombinase system (9, 51) with the appropriate primers listed in Table 2. Briefly, Cmr cassettes of pKD3 and pSU314 flanked by an Flp recombination target (FRT) site were amplified with the following primers: iacP mut-L and iacP mut-R for YKJ034, tagiacP HA-L and tagiacP HA-R for YKJ074, sopE mut-L and sopE mut-R for YKJ204, and sptP HA-L and sptP HA-R for YKJ119. Kmr cassettes of pKD4 and pSUB11 were amplified with the following primers: sopB mut-L and sopB mut-R for YKJ029 and tagsopB 3FL-L and tagsopB 3FL-R for YKJ042. Purified PCR products were electroporated into Salmonella serovar Typhimurium bacteria possessing red recombinase (pKD46), and transformants were incubated at 37°C for 1 h and then plated onto LB plates containing the appropriate antibiotics. Insertions of the antibiotic resistance gene were verified by PCR and DNA sequencing. If necessary, the resistance gene cassette was excised by using pCP20 carrying the Flp recombinase to generate YKJ032, YKJ033, YKJ035, and YKJ036. YKJ052, YKJ205, YKJ156, YKJ099, YKJ098, and YKJ123 were generated by the P22-mediated transduction of M587, YKJ204, YKJ029, YKJ042, and YKJ119 into UK1, YKJ032, and YKJ035.
TABLE 2.
Primer sequences used in this study
| Primers | Sequencea | Description |
|---|---|---|
| Chromosome | ||
| iacP mut-L | 5′-AGTCAAAAAAGTGATCACCTCTTGTATTGCCGTTGATGTTGTGTAGGCTGGAGCTGCTTC | YKJ034 |
| iacP mut-R | 5′-ACACGGCATATATCCGCAAAGGTCATCATATCAGGAAGATCATATGAATATCCTCCTTAG | YKJ034 |
| tagiacP HA-L | 5′-TATATGCCGTGTTGTTAAAAAAAGTCTTGAGTCCAGGGTGTATCCGTATGATGTTCCTGA | YKJ074 |
| tagiacP HA-R | 5′-TTACTTTCCTCTTGAATTATATCTTTTATAAGATTGCTTCCATATGAATATCCTCCTTAG | YKJ074 |
| sopE mut-L | 5′-TCCAAAAACAGGAAACCACACTACTAAAGAAAAATCAACCGTGTAGGCTGGAGCTGCTTC | YKJ204 |
| sopE mut-R | 5′-TGTTTTGTATATATTTATTAGCAATGTTTTCTAGTATCAGCATATGAATATCCTCCTTAG | YKJ204 |
| sopB mut-L | 5′-CATTTTTTTAAAGTTCCTGGTGCATAAAAGTCACATCCTTGTGTAGGCTGGAGCTGCTTC | YKJ029 |
| sopB mut-R | 5′-GTTTTATCTTTACCGTCCTCATGCACACTCACCGTGGACACATATGAATATCCTCCTTAG | YKJ029 |
| tagsopB3FL-L | 5′-TTGGCAGTCAGTAAAAGGCATTTCTTCATTAATCACATCTGACTACAAAGACCATGACGG | YKJ042 |
| tagsopB3FL-R | 5′-ATCCTTGCTATTGACTTCCTTCATTTCCAGCATAGCTTACCATATGAATATCCTCCTTAG | YKJ042 |
| sptP HA-L | 5′-AAAAGCAATGCAAGCCCAGTTGCTTATGACGACGGCAAGCTATCCGTATGATGTTCCTGA | YKJ119 |
| sptP HA-R | 5′-AGAGAAAAACAAAAGACTTTCTATCGCGGCAAACAAATAACATATGAATATCCTCCTTAG | YKJ119 |
| Plasmids | ||
| Psip-L | 5′-CCGGCATGCAGCATGGCTGACTACAGTTT | pYKJ029 |
| Psip-R | 5′-GTCGACTACTTACTCCTGTTATCTGTCACC | pYKJ029 |
| comiacP-L | 5′-CCGGTCGACATGAATATGGATATTGAAGCAA | pYKJ033 |
| comiacP HA-R | 5′-CCGGAGCTCTTAAAGAGCGTAATCTGGAACATCGTATGGGTACACCCTGGACTCAAGACTTTTT | pYKJ033 |
| iacP pro-L | 5′-CCGGCATGCGCGAGTCACACCATTCGACTA | pYKJ032 |
| iacP S38A-L | 5′-GAGGATCTTTACGCTGACGCCTTGGATTTAATTGATATTG | pYKJ035 |
| iacP S38A-R | 5′-CAATATCAATTAAATCCAAGGCGTCAGCGTAAAGATCCTC | pYKJ035 |
| iacP E51A-L | 5′-GTATTTGGTCTTAGTGAGGCCTTTGACATTAGTTGCAATG | pYKJ036 |
| iacP E51A-R | 5′-CATTGCAACTAATGTCAAAGGCCTCACTAAGACCAAATAC | pYKJ036 |
| BiacP-L | 5′-CCGGAATTCACCATGAATATGGATATTGAAGCAA | pYKJ034 |
| BiacP-R | 5′-CCGGCATGCTTAAAGAGCGTAATCTGGAACATCGTATGGGTACACCCTGGACTCAAGACTTTTT | pYKJ034 |
| sopE-L | 5′-CCGGCATGCCAATGCCAGAACGGCAAG | pYKJ065 |
| sopE HA-R | 5′-CCGGAGCTCTTAAAGAGCGTAATCTGGAACATCGTATGGGTAGGGAGTGTTTTGTATATATTTATTAGC | pYKJ065 |
| BsopA-L | 5′-CCGGAATTCACCATGAAGATATCATCAGGCGCAA | pYKJ304 |
| BsopA FL-R | 5′-CCGGCATGCCTACTTATCGTCGTCATCCTTGTAATCCGCCCAGGCCAGTGGCAGGAT | pYKJ304 |
| sopD-L | 5′-CCGGCATGCTTATAGTCACCACAAAGGATTACCAAC | pYKJ305 |
| sopD FL-R | 5′-CCGGAGCTCCTACTTATCGTCGTCATCCTTGTAATCTGTCAGTACTATATTACGACTGCA | pYKJ305 |
| sipC FL-L | 5′-CCGGTCGACATGTTAATTAGTAATGTGGGAA | pYKJ290 |
| sipC FL-R | 5′-CCGGAGCTCTTACTTATCGTCGTCATCCTTGTAATCAGCGCGAATATTGCCTGCGA | pYKJ290 |
| sipD FL-L | 5′-CCGGTCGACATGCTTAATATTCAAAATTATTCCGCTT | pYKJ291 |
| sipD FL-R | 5′-CCGGAGCTCTTACTTATCGTCGTCATCCTTGTAATCTCCTTGCAGGAAGCTTTT | pYKJ291 |
| sipA FL-L | 5′-CCGGTCGACATGGTTACAAGTGTAAGGACTCAGC | pYKJ038 |
| sipA FL-R | 5′-CCGGAGCTCTTACTTATCGTCGTCATCCTTGTAATCACGCTGCATGTGCAAGCCA | pYKJ038 |
For chromosome primers, FRT sequences are underlined. For plasmid primers, underlined sequences represent restriction enzyme recognition sites.
Construction of recombinant plasmids.
All plasmids and primers used in this study are listed in Tables 1 and 2. The promoter region of the sicA-sipBCDA-iacP operon was amplified by PCR from the UK1 chromosome and cloned into plasmid pMW118 to generate pYKJ029. For native expression, the iacP, sipC, sipD, and sipA genes were amplified and subcloned into pYKJ029, generating pYKJ033, pYKJ290, pYKJ291, and pYKJ038, respectively. To generate pYKJ032, an approximately 1-kb upstream region of the iacP gene was amplified by PCR and then cloned into plasmid pMW118. To construct pYKJ065, the sopE gene and upstream promoter region were amplified from the SL1344 chromosome and cloned into pMW118. This strategy was applied to the construction of pYKJ305 (sopB) except that the UK1 chromosome was used as a PCR template. For the arabinose-inducible expression of iacP (pYKJ034) and sopA (pYKJ304), amplified genes were placed under the control of the pBAD promoter of the pBAD24 plasmid. Site-directed substitutions of serine at position 38 (pYKJ035) and glutamate at position 51 (pYKJ036) with alanine in IacP were generated by using the QuikChange mutagenesis kit (Agilent Technologies) with pYKJ033 as a PCR template. Plasmid constructs were verified by DNA sequencing analysis.
Preparation of membrane and secreted proteins of Salmonella serovar Typhimurium.
For the activation of the T3SS-related genes, Salmonella serovar Typhimurium bacteria were grown under the growth conditions described above for 3 h. Membrane proteins of Salmonella serovar Typhimurium were prepared as previously described (29). Briefly, isolated whole membranes were placed onto a six-step sucrose gradient, centrifuged at 100,000 × g for 36 h, and then divided into 14 fractions. Fractions of the inner and outer membrane proteins were collected and precipitated with 10% (vol/vol) trichloroacetic acid (TCA). All steps were performed at 4°C. For the preparation of secreted proteins, the culture supernatant was passed through a 0.45-μm filter and then recovered by precipitation with 10% (vol/vol) TCA and acetone as described elsewhere previously (7). Equal amounts of sample proteins were subjected to SDS-PAGE and visualized by Western blotting.
Isolation and silver staining of Salmonella serovar Typhimurium LPS.
LPS of Salmonella serovar Typhimurium cells grown under SPI-1-inducing conditions for 3 h was prepared by using an LPS extraction kit (Intron Biotechnology, South Korea) according to the manufacturer's protocols. Extracted LPS was routinely analyzed on 14% SDS-PAGE gels and subsequently visualized by LPS-modified silver staining as described previously (49).
Invasion assays.
A gentamicin protection assay was conducted as described elsewhere previously (37). Briefly, the human embryonic intestinal epithelial cell line INT-407 (ATCC CCL-6) was seeded onto a 24-well plate and then infected with Salmonella serovar Typhimurium strains (multiplicity of infection [MOI] of 10) for 15 min at 37°C. After washing three times with fresh medium, cells were incubated in medium containing gentamicin (100 μg/ml) for 1 h to remove the extracellular bacteria. Cells were treated in 1% Triton X-100 for 15 min, after which intracellular bacteria were enumerated by serial dilution.
Translocation assay.
The protein translocation into cultured INT-407 cells was determined as previously described, with slight modifications (6). Briefly, 4 × 106 INT-407 cells seeded into two 100-mm-diameter culture dishes were infected with Salmonella serovar Typhimurium (MOI of 10) for 15 min. The cells were then further incubated in Dulbecco's modified Eagle's medium (DMEM) containing gentamicin (100 μg/ml) for 1 h, followed by three washes with phosphate-buffered saline (PBS). Next, 75 μl of PBS and 1% Triton X-100 (translocated protein) were added to one culture dish, while 75 μl of PBS and 1% SDS (internalized bacterial protein) were added to another culture dish. Following incubation at room temperature (RT) for 20 min, the cells were collected by using a cell scraper and centrifuged at 15,000 × g for 10 min to remove the cellular debris. The lysates were then loaded onto a 12% SDS-PAGE gel, and the translocated protein was detected by Western blotting.
Subcellular fraction and total proteins in infected INT-407 cells.
INT-407 cells were seeded into two 100-mm-diameter culture dishes (2 × 106 cells/dish) and then infected with bacteria at an MOI of 1:10 for 15 min. Next, the cells were washed with fresh DMEM and further incubated with DMEM supplemented with gentamicin (100 μg/ml) for 1 h. Following washes with PBS, membrane and nonmembrane fractions were prepared by using a ProteoExtract native membrane protein extraction kit (EMD) according to the manufacturer's instructions. To detect the intracellular level of phosphorylated Akt (phospho-Akt) in the infected cells, cells on the dishes were collected by using a scraper, lysed by brief sonication in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 1 mM EDTA) containing a protease inhibitor cocktail, and cleared by centrifugation. The total protein concentrations in the isolated fractions were determined and normalized via a Bradford protein assay (Bio-Rad).
Western blotting and antibodies.
A nitrocellulose membrane was blocked with 5% (wt/vol) skim milk in TBS-T (20 mM Tris, 0.2 M NaCl [pH 7.5], 0.1% Tween 20) for 1 h and then incubated with one of the following primary antibodies at the proper dilution for 1 h: a monoclonal anti-hemagglutinin (HA) antibody (Cell Signaling), a polyclonal anti-SipB antibody (28), a polyclonal anti-OmpW antiserum (a gift from H. Y. Kang, Pusan University, South Korea), a monoclonal anti-DnaK antibody (Assay Designs), a polyclonal anti-LPS antibody (BD), a monoclonal anti-FLAG antibody (Sigma-Aldrich), a monoclonal anti-β-tubulin antibody (Sigma-Aldrich), a monoclonal anti-caveolin-1 antibody (BD), a monoclonal anti-phospho-Akt antibody (Cell Signaling), and a polyclonal anti-Akt antibody (Sigma-Aldrich). After washing three times, the second antibody of goat anti-mouse IgG horseradish peroxidase (HRP) conjugate or goat anti-rabbit IgG HRP conjugate (Bio-Rad) was added, and the membranes were incubated for 1 h. The blots were then developed by using a BM chemiluminescence blotting substrate (POD) (Roche).
Immunofluorescence study.
A study of the invasion of Salmonella serovar Typhimurium bacteria into epithelial INT-407 cells was performed as described above, with some modifications. Briefly, Salmonella serovar Typhimurium bacteria were incubated with Hoechst 33342 dye (Invitrogen) for 20 min prior to infection. Monolayers of INT-407 cells (1 × 104 cells/well) grown on glass coverslips in 24-well plates were infected with Salmonella serovar Typhimurium at an MOI of 1:10 for 15 min. After further incubation with medium containing gentamicin, the samples on the coverslips were fixed with 3.7% paraformaldehyde in PBS for 20 min, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 10 min, and then blocked in 3% (wt/vol) bovine serum albumin in PBS for 1 h. To visualize phosphorylated Akt, anti-phospho-Akt (Ser473) and subsequent Alexa Fluor 488-conjugated goat anti-rabbit antibodies (Invitrogen) were used for 1 h of incubation at RT. Following mounting, the specimens were analyzed with a confocal laser scanning microscope (LSM700; Carl Zeiss). To examine actin fibers of the infected cells, they were fixed with paraformaldehyde without gentamicin incubation, as significant differences in accumulations of actin fibers were previously observed immediately after bacterial entry (25). To label F-actin, samples was incubated for 1 h with tetramethyl rhodamine isocyanate (TRITC)-conjugated phalloidin (Sigma-Aldrich), mounted using Fluoromount-G (SouthernBiotech) antifade medium, and then observed by using a fluorescence microscope (Axio Imager A1; Carl Zeiss) coupled with a charge-coupled-device (CCD) camera (AxioCam MRc5; Carl Zeiss).
In vivo virulence assays.
For the histochemistry of the small intestine and determinations of the bacterial burden in the spleen, 5-week-old female C3H/HeN mice (Labanimal, South Korea) were orally infected with 1 × 108 CFU of Salmonella serovar Typhimurium. Seven days later, the mice were sacrificed, and tissue samples from the intestine were embedded in paraffin. Subsequent hematoxylin and eosin (H/E) staining was performed as described previously (32). For the pathological scoring of infections, H/E-stained samples were examined and scored as described previously (8). The following features of the surface epithelium were evaluated: a sum of no pathological change (score of 0), regenerative change (mild, score of 1; moderate, score of 2; severe, score of 3), desquamation (patchy, score of 1; diffuse, score of 2), and polymorphonuclear leukocytes (PMNs) in the epithelium (score of 1). For the lumen, the sum of empty cells (score of 0), necrotic epithelial cells (scant, score of 1; moderate, score of 2; dense, score of 3), and PMNs (scant, score of 2; moderate, score of 3; dense, score of 4) were scored; for the mucosa, a sum of no pathological change (score of 0), crypt abscesses (rare [<15%], score of 1; moderate [15% to 50%], score of 2; abundant [>50%], score of 3), the presence of mucinous plugs (score of 1), and the presence of granulation tissue (score of 1) were evaluated; and for the submucosa, a sum of no pathological change (score of 0), mononuclear cell infiltrates (1 small aggregate, score of 0; more than 1 aggregate, score of 1; large aggregates plus increased numbers of single cells, score of 2), PMN infiltrate (none, score of 0; single, score of 1; aggregates, score of 2), and edema (mild, score of 0; moderate, score of 1; severe, score of 2) were evaluated. Spleens were removed from three mice in each group and homogenized in PBS. CFU were enumerated by plating serial dilutions of homogenized samples. For the survival assay, 5-week-old female C3H/HeN mice were infected with 1 × 107 CFU Salmonella serovar Typhimurium or PBS and then monitored daily. The survival rate was evaluated as the percentage of mice surviving after oral challenge.
Statistical analysis.
A Student's t test (SPSS 18.0) was used to identify significant differences in the bacterial invasion rates, and a Kaplan-Meier survival curve was plotted by using GraphPad Prism 5.
RESULTS
IacP is required for the invasion of nonphagocytic cells.
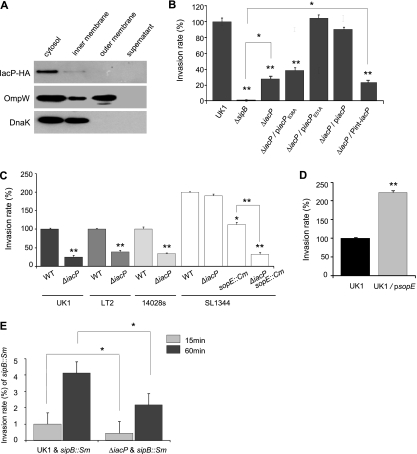
To investigate the expression and subcellular localization of IacP in Salmonella serovar Typhimurium, we chromosomally tagged IacP at the C terminus with HA using the red recombinase system. Salmonella serovar Typhimurium strains carrying an HA-tagged IacP protein were cultured for 3 h under SPI-1-inducing conditions and then subcellularly fractionated by six-step sucrose density gradient centrifugation into the cytoplasm, inner/outer membrane, and supernatant fractions. Figure 1A shows that IacP-HA (∼10 kDa) localized primarily within the cytoplasm.
FIG. 1.
IacP contributes to Salmonella serovar Typhimurium invasion into nonphagocytic cells. (A) Salmonella serovar Typhimurium bacteria grown under SPI-1-inducing conditions were fractionated on a sucrose gradient. HA-tagged IacP was detected in the cytoplasmic fraction. As a control for the membrane and cytoplasmic proteins, the blot was reprobed with antibodies against OmpW and DnaK. (B to E) To determine the invasion rate, bacterial strains were allowed to infect an INT-407 cell for 15 min or 60 min, after which a gentamicin protection assay was conducted. The intracellular bacteria were enumerated by the plating of serial dilutions, and the percent bacterial invasion rate was calculated in comparison to that of the wild type (WT), which was set as 100% (in the case of strain SL1344, the invasion rate of UK1 was set as 100%). All experiments were performed on separate days at least three times. Bars correspond to the means ± standard deviations (SD). *, P < 0.01; **, P < 0.001 (compared with invasion by the respective wild-type or control strain, determined by a Student's t test).
As the SPI-1 T3SS is essential for nonphagocytic cell invasion, we examined the effect of IacP during the invasion of INT-407 intestinal epithelial cells. INT-407 cells were infected by a Salmonella serovar Typhimurium wild-type strain, a sipB mutant, and an iacP mutant with or without various iacP-bearing plasmids grown under SPI-1-inducing conditions. As shown in Fig. 1B, the iacP mutant was less invasive than wild-type Salmonella serovar Typhimurium after 15 min of infection although not to the same extent as the sipB mutant. To complement the iacP-null mutant, we generated two plasmid constructs carrying the iacP gene under the control of the sicA-sipBCDA operon promoter (piacP) or under the control of ∼1,000 bp of the upstream sequence from the start codon of iacP (Pint-iacP). The iacP mutant possessing the piacP plasmid could be internalized into INT-407 cells similarly to the wild type, whereas the Pint-iacP plasmid was unable to recover the invasion of the iacP mutant (Fig. 1B). These findings suggest that the iacP gene is not regulated independently but may be cotranscribed as a sicA-sipBCDA-iacP operon during bacterial entry.
To characterize the specific role of IacP required for bacterial invasion, we replaced the putative 4′-PP attachment site, the serine at position 38, with alanine (IacPS38A) by site-directed mutagenesis. When INT-407 cells were infected with Salmonella serovar Typhimurium possessing substituted plasmids for 15 min, the IacPS38A plasmid failed to complement the invasion-defective phenotype of the iacP mutant, while no differences in invasion rates were observed between the wild type and the mutant harboring the IacPE51A plasmid used as a control (Fig. 1B). This result implies that the linkage between the 4′-PP and Ser38 of IacP is required for invasion into INT-407 cells.
To confirm the effect of iacP-mediated cell invasion in other Salmonella serovar Typhimurium strains, we introduced the iacP mutation into widely used laboratory strains such as ATCC 14028s, LT2, and SL1344. As shown in Fig. 1C, iacP mutants of ATCC 14028s and LT2 invaded INT-407 cells less than did their respective parental strains after 15 min of infection. However, the invasion defect could not be reproduced in SL1344 lacking the iacP gene (Fig. 1C), which is consistent with a previous report showing that the mutation of iacP did not alter the assembly of the T3SS apparatus and cell invasion of SL1344 (48).
As a mechanism for pathogenic adaptation, many lysogenic phages that participate in bacterial pathogenesis have been transferred between independent lineages of Salmonella (10). The Salmonella serovar Typhimurium effector SopE, which is encoded by a Fels-like prophage in SL1344 and translocated through the SPI-1 T3SS, activates cellular Rho GTPases such as Rac1 and Cdc42 to induce membrane ruffling (12). The sopE gene was not found in strain LT2, ATCC 14028s, or UK1 by PCR using specific primers (data not shown); however, the addition of the sopE plasmid into strain UK1 caused a 2-fold increase in bacterial entry (Fig. 1D). Thus, we reasoned that the IacP function might be masked by SopE in strain SL1344. As shown in Fig. 1C, the rate of internalization of the SL1344 sopE mutant into INT-407 cells was decreased to half that of wild-type SL1344 (19), and an additional iacP mutation resulted in a significant decrease in the invasion rate, which was identical to that of the iacP mutant of UK1. Together, these results suggested that Salmonella serovar Typhimurium IacP is required for early invasion into nonphagocytic cells and is not replaceable by other ACPs responsible for the biosynthesis of essential cellular lipids.
IacP is not necessary for LPS biosynthesis in Salmonella serovar Typhimurium.
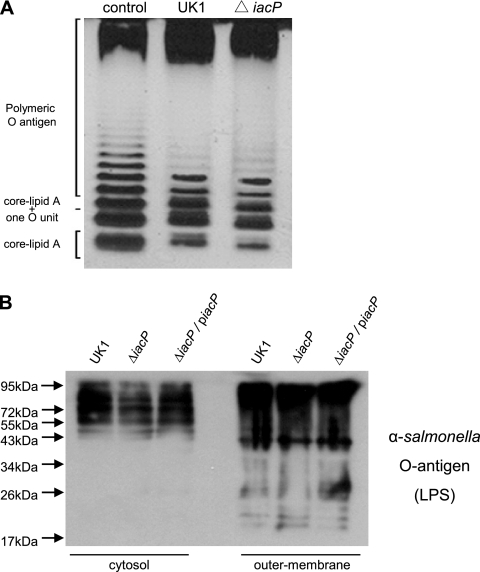
In many Gram-negative bacteria, LPS on the bacterial surface has been implicated as a virulence factor required for the adherence and invasion of epithelial cells (47). LPS is composed of three major parts: lipid A, the core oligosaccharide, and O antigen. As the acyl-ACP of E. coli is required for the biosynthesis of the lipid A component (43), we examined whether the lipid A portion was influenced by the iacP mutation. However, we found a typical LPS ladder pattern in the purified LPS and the isolated outer membrane fraction of the iacP mutant grown under SPI-1-inducing conditions (Fig. 2). These findings suggested that IacP is not required for the biosynthesis of LPS but rather is involved in the regulation of virulence factors necessary for SPI-1-mediated invasion.
FIG. 2.
Comparisons of the LPS profiles prepared from the wild type and the iacP mutant. (A) Silver-stained image of LPSs purified from the wild type and the iacP mutant grown under SPI-1-inducing conditions. Purified LPS from Salmonella serovar Typhimurium (Sigma-Aldrich) was used as a standard. (B) Cytosolic and outer membrane fractions isolated from the Salmonella serovar Typhimurium wild type, the iacP mutant, and the complement strain were immunoblotted with an anti-LPS antibody.
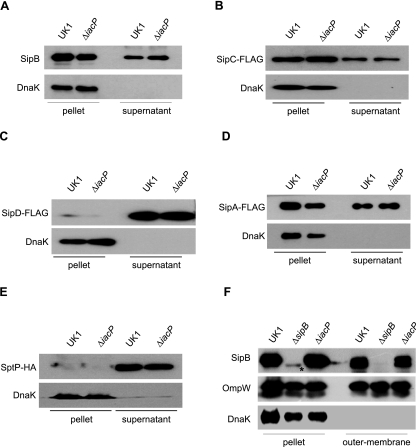
IacP did not influence the secretion of SipB, SipC, SipD, SipA, and SptP.
The SPI-1 effector proteins SipB, SipC, SipD, and SipA are encoded in the same operon of IacP and are required for bacterial internalization. To test the possibility that IacP was involved in the optimal secretion of these proteins under SPI-1-inducing conditions, we compared the secretion levels of SipB, SipC, SipD, and SipA. When measured by Western blotting using anti-SipB and anti-FLAG antibodies, SipB, SipC, SipD, and SipA were expressed and efficiently secreted into the culture supernatant in both the wild type and the iacP mutant (Fig. 3A to D). Similarly, the secretion of SptP, which is located downstream of the iacP gene, was not affected by the iacP mutation (Fig. 3E). These results indicate that IacP does not affect the expression and secretion of the SPI-1 translocon (SipB, SipC, and SipD) or other effector proteins (SipA and SptP).
FIG. 3.
IacP did not influence the secretion of SipB, SipC, SipD, SipA, and SptP. Wild-type Salmonella serovar Typhimurium and the iacP mutant were grown under SPI-1-inducing conditions for 3 h. (A to E) The levels of SipB, SipC-FLAG, SipD-FLAG, SipA-FLAG, and chromosomally HA-tagged SptP in the pellet and culture supernatant were determined by Western blotting using anti-SipB, anti-FLAG, and anti-HA antibodies. Anti-DnaK antibody was used as a control for cytoplasmic proteins. (F) Salmonella subcellular fractionation was undertaken to compare the outer membrane localizations of SipB in the wild type, the iacP mutant, and complement strains. As a control for membrane and cytoplasmic proteins, the blot was reprobed with antibodies to OmpW and DnaK. The asterisk denotes the nonspecific band.
The posttranslational acylation of some membrane-associated proteins is required for membrane targeting (46). Because SipB was observed on a bacterial surface for the attachment of host cells and may be inserted into the plasma membrane to form the translocation pore with SipC (33), we next examined whether the membrane localization of SipB was altered in the iacP mutant. As shown in Fig. 3F, the SipB localization on the membrane of the iacP mutant was equivalent to that of the wild type, indicating that the outer membrane localization of SipB did not appear to be modified by IacP.
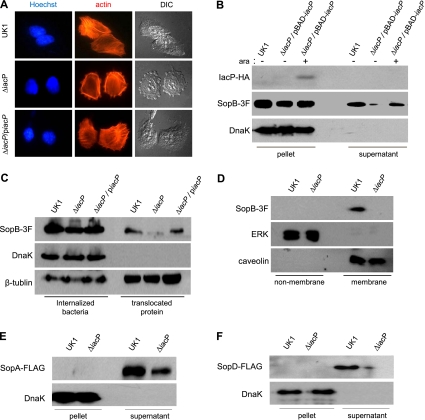
Levels of secretion of SopB, SopA, and SopD were decreased in the iacP mutant.
Actin cytoskeleton rearrangement in host cells is a characteristic of Salmonella serovar Typhimurium invasion and is accomplished by the translocation of several effector proteins through the SPI-1 T3SS. To determine if the decreased invasion of the iacP mutant was due to delayed actin remodeling, actin filaments were visualized by staining INT-407 cells with rhodamine-conjugated phalloidin immediately after Salmonella serovar Typhimurium infection for 15 min. As shown in Fig. 4A, the extended actin stress fibers at the cell periphery and the profound membrane protrusion were evidently shaped in INT-407 cells infected with the wild type. Actin fibers, however, were diffusely observed inside the cytoplasm, and the cellular morphology remained unchanged in many iacP mutant-infected cells. These findings indicate that IacP might be involved in the modification of T3SS effectors required for actin cytoskeleton remodeling (36). Among these effector proteins, the secretion of five proteins (SipA, SipC, SopE, SopE2, and SptP) was not affected by IacP (Fig. 3 and data not shown for SopE2). To examine the effect of IacP on SopB secretion, we used a 3×FLAG epitope tag at the C terminus of SopB in the wild type and the iacP mutant. The level of secretion of SopB into the culture supernatant was dramatically reduced in the iacP mutant relative to that of the wild type or the iacP mutant possessing plasmid pBAD-iacP::HA, in which the transcription of the iacP gene was controlled by the arabinose-inducible promoter (Fig. 4B). This result was reproduced in Salmonella serovar Typhimurium without epitope tagging by using an anti-SopB antibody (data not shown). We then compared the translocations of SopB in INT-407 cells infected with the wild type and the iacP mutant. Consistent with the secretion profile shown in Fig. 4B, the iacP mutation resulted in a significant decrease (−3.9-fold) in SopB translocation into INT-407 cells (Fig. 4C). Moreover, it was reported previously that the inositol phosphatase activity of SopB is achieved in the host membrane of the bacterial entry site and nascent SCV (34). When Salmonella-infected INT-407 cells were fractionated into membranes and nonmembranes, SopB was not observed in the membrane fraction of the iacP mutant-infected cells (Fig. 4D). This might result from the decrease in SopB translocation from the iacP mutant.
FIG. 4.
IacP promotes the T3SS-mediated secretion and translocation of SopB. (A) After bacterial infection for 15 min, filamentous actins were stained with rhodamine phalloidin. The nucleus and intracellular Salmonella serovar Typhimurium bacteria were stained with Hoechst 33342 dye (original magnification, ×1,260). (B) The secretion of SopB tagged with 3×FLAG (SopB-3F) in cultured supernatants was examined by Western blotting. l-Arabinose (0.2 mM) was added to induce the expression of IacP. Anti-DnaK antibody was used as a control for cytoplasmic proteins. (C) INT-407 cells infected with Salmonella serovar Typhimurium were treated with Triton X-100 (internalized bacteria) or SDS (translocated protein). Anti-β-tubulin antibody was used as a loading control. (D) To determine the subcellular localization of SopB, the membranes of infected INT-407 cells were isolated. Extracellular signal-regulated kinase (ERK) and caveolin were used as nonmembrane and membrane controls, respectively. (E and F) The culture supernatants of Salmonella serovar Typhimurium possessing SopA-FLAG (E) and SopD-FLAG (F) plasmids were immunoblotted with anti-FLAG antibody. Anti-DnaK antibody was used as a control for cytoplasmic proteins.
As other effector proteins, SopA and SopD, like SopB, are synthesized and translocated during the late stage of infection and contribute to the invasion of host cells (2, 17, 44), we next examined the secretion of SopA or SopD in the iacP mutant. Although no SopA or SopD was detected in the bacterial cytosol for some unknown reason, the secretion of both proteins was obviously decreased in the iacP mutant (Fig. 4E and F). These results suggest that IacP promotes the invasion of Salmonella serovar Typhimurium by enhancing SopB, SopA, and SopD secretion and translocation to trigger actin cytoskeleton rearrangements in host cells.
IacP is important for Salmonella serovar Typhimurium virulence.
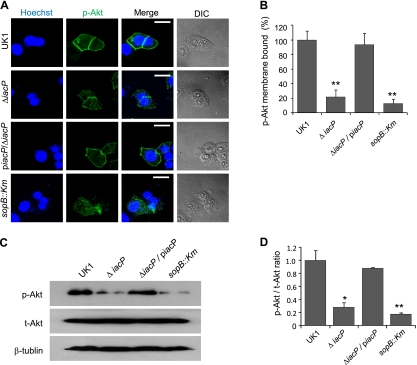
Kuijl et al. recently showed that translocated SopB induces the phosphorylation of Akt, which subsequently activates Rab14 and AS160 phosphorylation to inhibit phagosomal maturation (31). To further examine the Akt phosphorylation of host cells infected with the iacP mutant, we stained the infected INT-407 cells with anti-phospho-Akt (p-Akt) antibody. As shown in Fig. 5A and B, p-Akt was accumulated around the plasma membrane of the host cells infected by the wild-type and the iacP-piacP strains, whereas Akt activation was markedly decreased in iacP mutant-infected cells. Consistent with the confocal images, the amount of p-Akt was also decreased in the total extract from iacP-infected cells (Fig. 5C and D).
FIG. 5.
Akt phosphorylation is decreased in INT-407 cells infected with the iacP mutant. (A) To visualize phosphorylated Akt at the plasma membrane, INT-407 cells infected with Salmonella serovar Typhimurium were stained with anti-phospho-Akt. Scale bar, 40 μm. (B) Images from three different confocal planes (at least 30 cells) per sample were analyzed to determine the quantification of phosphorylated Akt bound to the membrane. Bars correspond to means ± SD. (C) Total cellular proteins of INT-407 cells infected with the wild type or its isogenic mutants were subjected to Western blot analysis. β-Tubulin was used as a loading control. (D) Quantification of the amount of phosphorylated Akt and total Akt in bacterially infected cells in C. Each band was densitometrically normalized to β-tubulin, and the phospho-Akt/total Akt ratio is expressed as the mean ± SD. *, P < 0.05; **, P < 0.001 (statistically significant difference from the control group). p-Akt, phosphorylated Akt; t-Akt, total Akt.
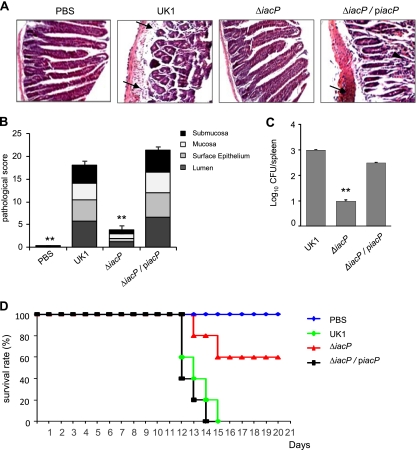
Furthermore, given the fact that the inositol phosphatase activity of SopB is required for SCV trafficking (1), we investigated whether the decreased translocation of SopB in the iacP mutant resulted in an attenuation of virulence in mice. C3H/HeN mice were orally infected with Salmonella serovar Typhimurium, and the pathological changes in the lining of the small intestine were investigated 7 days after infection by histochemistry. As shown in Fig. 6A and B, shortened and diffused desquamations of the epithelial brush border were observed for mice infected with the wild-type and iacP-piacP strains. Inflamed small intestines were densely filled with infiltrated neutrophils and necrotic epithelial cells. However, the iacP mutant evoked significantly less damage in the small intestine of C3H/HeN mice, showing no difference compared with the PBS control group. These results suggest that the iacP mutant is less virulent than the wild type and is likely to be expeditiously cleared by the host defense system after oral ingestion. This was confirmed by the bacterial load from the spleen at 7 days after oral infection. As shown in Fig. 6C, the level of bacterial colonization in the spleen was significantly lower in mice administered the iacP mutant than in the wild-type-infected mice.
FIG. 6.
IacP is required for full virulence of Salmonella serovar Typhimurium. (A) The intestinal linings of C3H/HeN mice orally infected with the wild type, the iacP mutant, and the complement strain were analyzed by histochemistry after 7 days of infection. The sections were counterstained with hematoxylin and eosin and mounted onto microscope slides (original magnification, ×200). The arrow indicates infiltrated PMNs, and the arrowhead indicates the diffused desquamation of epithelial cells. (B) The pathological scores are stacked averages from mice per group at 7 days after infection with Salmonella serovar Typhimurium. Bars correspond to means ± SD of the total score. (C) The bacterial load from mouse spleen was determined 7 days after oral infection with Salmonella serovar Typhimurium strains. Bars correspond to means ± SD. **, P < 0.001 compared with the mouse group infected with the wild-type strain. (D) The survival of each C3H/HeN mouse group following an oral injection of Salmonella serovar Typhimurium was recorded at daily intervals until the end of the study. The data are from a single representative experiment of three independent trials.
We next monitored the survival of C3H/HeN mice after the oral administration of Salmonella serovar Typhimurium. Two groups of mice challenged with the wild-type and iacP-piacP strains succumbed to infection until 15 days postinfection. In addition, one mouse each from the group infected with the iacP mutant died on day 13 and day 15 after infection; however, the remaining three mice recovered from the bacterial infection and survived for the rest of the study (Fig. 6D). These results indicated that IacP is required for the infection and virulence of Salmonella serovar Typhimurium in mice.
DISCUSSION
During the invasion of a nonphagocytic cell by Salmonella serovar Typhimurium, the SPI-1 T3SS is activated upon host cell contact and is used to export an arsenal of effector proteins into the host cytoplasm. In this study, we first showed that the SPI-1 cytosolic protein IacP is important for bacterial virulence by regulating the secretion of T3SS effector proteins.
IacP is encoded from a sicA-sipBCDA-iacP operon and expressed in the bacterial cytosol under SPI-1-inducing conditions. While the growth of Salmonella serovar Typhimurium lacking the iacP gene could not be distinguished from that of the wild type under SPI-1-inducing conditions (data not shown), the iacP mutant invaded INT-407 epithelial cells less efficiently than did the parental strain, and its activity was not limited to a particular strain of Salmonella serovar Typhimurium. Moreover, the substitution of the putative attachment site of 4′-PP (Ser38) was sufficient to cause a loss of function of the iacP gene. These data indicated that IacP might play a role as a specific ACP by associating with the 4′-PP group during Salmonella entry and that IacP might be involved in the posttranslational modification of invasion-related molecules that have a function at the bacterial or host membrane rather than fatty acid biosynthesis. LPS is necessary for bacterial adhesion and invasion in epithelial cells, and the lipid A component of LPS is synthesized in an ACP-dependent manner (43). However, we did not find significant differences in the biosyntheses and outer membrane localizations of the isolated LPSs from the wild type and the iacP mutant.
We next investigated the secretion pattern of effector proteins in the SPI-1 locus that are located upstream or downstream of flanking genes (SipB, SipC, SipD, SipA, and SptP). In the iacP mutant grown under SPI-1-inducing conditions, these proteins were equally expressed and secreted into the culture medium, indicating that these proteins were not targeted by IacP. In the present study, the invasion rate of the iacP mutant was significantly higher than that of the sipB mutant. In addition, we found that the membrane ruffling in INT-407 cells induced by the iacP mutant was insufficient to restore the internalization of a coinfected sipB mutant (Fig. 1E). Moreover, as shown in Fig. 1C, the deletion effect of iacP in the SL1344 strain was masked by SopE, which manipulated the actin rearrangement of host cells. Collectively, these data supported the notion that IacP might be involved in the modification of T3SS effectors required for actin cytoskeleton remodeling but not T3SS translocator proteins. Consistent with these results, we demonstrated that the rearrangement of actin fibers was delayed in INT-407 cells infected with the iacP mutant by staining the actin filaments. These findings could be ascribed to a decreased level of secretion of the SPI-1 effector protein SopB in the iacP mutant. SopB translocation and membrane localization in host cells infected with the iacP mutant were also dramatically reduced compared to wild-type-infected cells.
SopB activates the RhoG exchange factor SGEF (Src homology 3 domain-containing guanine nucleotide exchange factor) required for cytoskeletal rearrangement and enhanced bacterial entry (39). Upon the transient transfection of HeLa cells with a plasmid harboring sopB, SopB is associated with host cell membranes, and residues 117 to 167 of SopB are necessary for membrane targeting (35). We tested the hypothesis that IacP is involved in the modification of this domain. The expression and secretion of SopB lacking residues 117 to 167 in the wild-type strain were the same as those of full-length SopB, whereas the level of secretion of SopB without the membrane-targeting region was reduced in the iacP mutant (data not shown). In addition, ubiquitin-mediated localization, which directs the differential cellular function of SopB, is preceded by the plasma membrane accumulation of SopB, suggesting that the region at residues 117 to 167 of SopB is independent of the IacP modification and that the subcellular distribution of SopB in infected cells may be mediated by host factors (40).
This study found that IacP regulated the secretion of effector proteins encoded outside SPI-1, such as SopA or SopD, which are involved in bacterial adhesion, invasion, and virulence. This implies that IacP activity is not confined to the modification of some effectors and may be involved in the modification of some membrane-associated protein in the T3SS apparatus which controls the translocation of SPI-1 effectors. The hierarchical secretion of the T3SS between the membrane-bound translocon and secreted effectors has been well defined in previous studies of InvE of Salmonella serovar Typhimurium (30), the YopN/TyeA heterodimer of Yersinia enterocolitica (23), MxiC of Shigella flexneri (4), and SepL of enteropathogenic Escherichia coli (53). In addition, an alteration in the helical packing of the T3SS needle subunit upon host cell contact was reported previously to explain the signal responsible for the triggering of effector secretion (3, 41). If this signal is transmitted from the needle to the membrane-bound receiver protein in the T3SS base, IacP may be involved in the membrane localization of that protein, contributing to the establishment of a secretion hierarchy in the SPI-1 T3SS. However, further studies are needed to elucidate the IacP-dependent modification of membrane proteins, including InvE.
In INT-407 cells infected with the iacP mutant, the level of SopB-mediated phosphorylation of Akt was found to be significantly lower than that in wild-type-infected cells. These findings indicate that the amount of SopB translocated from the iacP mutant is was insufficient to activate the cytosolic Akt in host cells. The sopB mutation of Salmonella serovars Typhimurium and Dublin resulted in the attenuation of both gastroenteritis in calves (56) and inflammation in bovine ileal loops (15). Moreover, the sopB mutants augmented antigen uptake and presentation by dendritic cells (55). Therefore, we expected the iacP disruption to render Salmonella spp. avirulent. The pathological alterations observed in the intestine of C3H/HeN mice were prominent in a histochemical study of the iacP mutant-infected mouse group in which no flattened villi or signs of inflammation were observed. In our mouse survival assay, a group of C3H/HeN mice subjected to oral challenge with the iacP mutant survived longer than did wild-type-infected mice. Even though a small amount of SopB secretion was consistently observed for the iacP mutant, the level of attenuation achieved by the deletion of the iacP gene was greater than that of a single mutation in sopB (data not shown). This can be explained by the possibility that IacP might be involved in the translocation of various T3SS effector proteins, which is essential for the survival and replication of bacteria following host cell invasion. Taken together, our results indicate that IacP acts as a virulence factor by regulating the secretion and the translocation of type III effector proteins during host cell entry.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of South Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010-0012804) and was supported by a Korea University grant (grant K0715481).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 24 January 2011.
REFERENCES
- 1.Bakowski, M. A., et al. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe 7:453-462. [DOI] [PubMed] [Google Scholar]
- 2.Bakowski, M. A., J. T. Cirulis, N. F. Brown, B. B. Finlay, and J. H. Brumell. 2007. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell. Microbiol. 9:2839-2855. [DOI] [PubMed] [Google Scholar]
- 3.Blocker, A. J., et al. 2008. What's the point of the type III secretion system needle? Proc. Natl. Acad. Sci. U. S. A. 105:6507-6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botteaux, A., M. P. Sory, L. Biskri, C. Parsot, and A. Allaoui. 2009. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71:449-460. [DOI] [PubMed] [Google Scholar]
- 5.Byers, D. M., and H. Gong. 2007. Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem. Cell Biol. 85:649-662. [DOI] [PubMed] [Google Scholar]
- 6.Cisz, M., P. C. Lee, and A. Rietsch. 2008. ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J. Bacteriol. 190:2726-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collazo, C. M., and J. E. Galan. 1996. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect. Immun. 64:3524-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombes, B. K., et al. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa-Bossi, N., S. Uzzau, D. Maloriol, and L. Bossi. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 39:260-271. [DOI] [PubMed] [Google Scholar]
- 11.Finlay, B. B., S. Ruschkowski, and S. Dedhar. 1991. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 99(Pt. 2):283-296. [DOI] [PubMed] [Google Scholar]
- 12.Friebel, A., et al. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035-34040. [DOI] [PubMed] [Google Scholar]
- 13.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 14.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 15.Galyov, E. E., et al. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-del Portillo, F., and B. B. Finlay. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacomodonato, M. N., et al. 2007. SipA, SopA, SopB, SopD and SopE2 effector proteins of Salmonella enterica serovar Typhimurium are synthesized at late stages of infection in mice. Microbiology 153:1221-1228. [DOI] [PubMed] [Google Scholar]
- 18.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardt, W. D., H. Urlaub, and J. E. Galan. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 95:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath, R. J., and C. O. Rock. 1996. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J. Biol. Chem. 271:1833-1836. [DOI] [PubMed] [Google Scholar]
- 21.Herlax, V., and L. Bakas. 2007. Fatty acids covalently bound to alpha-hemolysin of Escherichia coli are involved in the molten globule conformation: implication of disordered regions in binding promiscuity. Biochemistry 46:5177-5184. [DOI] [PubMed] [Google Scholar]
- 22.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iriarte, M., et al. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Issartel, J. P., V. Koronakis, and C. Hughes. 1991. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature 351:759-761. [DOI] [PubMed] [Google Scholar]
- 25.Jepson, M. A., B. Kenny, and A. D. Leard. 2001. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell. Microbiol. 3:417-426. [DOI] [PubMed] [Google Scholar]
- 26.Kaniga, K., D. Trollinger, and J. E. Galan. 1995. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J. Bacteriol. 177:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating, D. H., M. R. Carey, and J. E. Cronan, Jr. 1995. The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J. Biol. Chem. 270:22229-22235. [DOI] [PubMed] [Google Scholar]
- 28.Kim, B. H., H. G. Kim, J. S. Kim, J. I. Jang, and Y. K. Park. 2007. Analysis of functional domains present in the N-terminus of the SipB protein. Microbiology 153:2998-3008. [DOI] [PubMed] [Google Scholar]
- 29.Kim, H. G., et al. 2008. N-terminal residues of SipB are required for its surface localization on Salmonella enterica serovar Typhimurium. Microbiology 154:207-216. [DOI] [PubMed] [Google Scholar]
- 30.Kubori, T., and J. E. Galan. 2002. Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184:4699-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuijl, C., et al. 2007. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450:725-730. [DOI] [PubMed] [Google Scholar]
- 32.Kum, W. W., et al. 2009. Lack of functional P-selectin ligand exacerbates Salmonella serovar Typhimurium infection. J. Immunol. 182:6550-6561. [DOI] [PubMed] [Google Scholar]
- 33.Lara-Tejero, M., and J. E. Galan. 2009. Salmonella enterica serovar Typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect. Immun. 77:2635-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallo, G. V., et al. 2008. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J. Cell Biol. 182:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus, S. L., L. A. Knodler, and B. B. Finlay. 2002. Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell. Microbiol. 4:435-446. [DOI] [PubMed] [Google Scholar]
- 36.McGhie, E. J., L. C. Brawn, P. J. Hume, D. Humphreys, and V. Koronakis. 2009. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12:117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinney, J. S., H. Zhang, T. Kubori, J. E. Galan, and S. Altman. 2004. Disruption of type III secretion in Salmonella enterica serovar Typhimurium by external guide sequences. Nucleic Acids Res. 32:848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno, M., J. P. Audia, S. M. Bearson, C. Webb, and J. W. Foster. 2000. Regulation of sigma S degradation in Salmonella enterica var Typhimurium: in vivo interactions between sigma S, the response regulator MviA (RssB) and ClpX. J. Mol. Microbiol. Biotechnol. 2:245-254. [PubMed] [Google Scholar]
- 39.Patel, J. C., and J. E. Galan. 2006. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 175:453-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel, J. C., K. Hueffer, T. T. Lam, and J. E. Galan. 2009. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell 137:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poyraz, O., et al. 2010. Protein refolding is required for assembly of the type three secretion needle. Nat. Struct. Mol. Biol. 17:788-792. [DOI] [PubMed] [Google Scholar]
- 42.Preston, G. M. 2007. Metropolitan microbes: type III secretion in multihost symbionts. Cell Host Microbe 2:291-294. [DOI] [PubMed] [Google Scholar]
- 43.Raetz, C. R., C. M. Reynolds, M. S. Trent, and R. E. Bishop. 2007. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76:295-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffatellu, M., et al. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanderson, K. E., and J. R. Roth. 1988. Linkage map of Salmonella typhimurium, edition VII. Microbiol. Rev. 52:485-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanley, P., V. Koronakis, and C. Hughes. 1998. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. 62:309-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St Geme, J. W., III. 2002. Molecular and cellular determinants of non-typeable Haemophilus influenzae adherence and invasion. Cell. Microbiol. 4:191-200. [DOI] [PubMed] [Google Scholar]
- 48.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 50.Tsolis, R. M., et al. 2000. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uzzau, S., N. Figueroa-Bossi, S. Rubino, and L. Bossi. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 98:15264-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Val, D. L., and J. E. Cronan, Jr. 1998. In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J. Bacteriol. 180:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, D., A. J. Roe, S. McAteer, M. J. Shipston, and D. L. Gally. 2008. Hierarchal type III secretion of translocators and effectors from Escherichia coli O157:H7 requires the carboxy terminus of SepL that binds to Tir. Mol. Microbiol. 69:1499-1512. [DOI] [PubMed] [Google Scholar]
- 54.Wray, C., and W. J. Sojka. 1978. Experimental Salmonella typhimurium infection in calves. Res. Vet. Sci. 25:139-143. [PubMed] [Google Scholar]
- 55.Yrlid, U., and M. J. Wick. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, S., et al. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]